Abstract
Rationale:
The Food and Drug Administration is considering severely restricting the nicotine in cigarettes, to reduce smoking. A study showed that non-daily, intermittent smokers (ITS) randomized to very-low-nicotine-content cigarettes (VLNCCs) reduced their cigarette consumption.
Objectives:
To assess whether increased smoking intensity of VLNCCs compensated for some of the reduced cigarette consumption.
Methods:
After a two-week baseline smoking their own-brand cigarettes, 118 ITS were randomized to VLNCCs (~ 1 mg nicotine/gram tobacco), and 120 to normal-nicotine-content cigarettes (NNCCs; ~16 mg/g) for 10 weeks. Laboratory measures of smoking intensity – total puff volume, and carbon monoxide (CO) boost – assessed single cigarettes smoked in up to three laboratory topography sessions. Field measures assessed returned cigarette butts, averaged over up to five two-week intervals: the mass of tobacco burned (computed from residual mass of butts), and the intensity of smoking (by scanning of returned filters). Analysis was by mixedmodel random effects models using baseline values as covariates.
Results:
ITS in the VLNCC group puffed less smoke in topography sessions (−38.50 mL [−75.21, −1.78]; p<0.04), but showed no difference in CO boost. Participants in the VLNCC group burned 0.02 [0.04, 0.002] grams less tobacco per cigarette (p<0.03). Analysis of filters showed their smoking intensity declined over time, compared to NNCC participants (p<0.04). “Cheating” by smoking normal cigarettes did not moderate these effects.
Conclusions:
ITS did not increase their smoking intensity when switched to VLNCCs; indeed, their smoking intensity decreased. Reductions in cigarette consumption seen when ITS are switched to VLNCCs were not compensated by increased smoking intensity.
Keywords: Tobacco, nicotine, smoking, compensation, low-nicotine, non-daily smoking, smoking topography
Tobacco smoking is the leading cause of preventable mortality. It is typically maintained by nicotine dependence (Benowitz 2010; Stolerman and Jarvis 1995) which has led to a policy proposal to reduce smoking prevalence by reducing nicotine levels in tobacco to a level too low to initiate or maintain dependence (Benowitz and Henningfield 1994; Henningfield et al. 1998). This proposal is being seriously considered by the US Food and Drug Administration (FDA), which now regulates tobacco (Tobacco Product Standard for Nicotine Level of Combusted Cigarettes 2018).
A number of studies have shown that switching to low-nicotine-content cigarettes (VLNCCs) reduced cigarette consumption in relatively heavy daily smokers (Benowitz et al. 2007; Donny et al. 2007, 2015). These studies have reported on short-term reductions that are not likely to have any material impact on health, but have been taken as proof-of-principle that VLNCCs can reduce smoking. In any case, these studies have omitted the 25–33% of adult US smokers who are non-daily or intermittent smokers (ITS) (Jamal et al. 2016; Reyes-Guzman et al. 2017; Substance Abuse and Mental Health Services Administration 2014). ITS suffer health risks from smoking (Inoue-Choi et al. 2017; Schane et al. 2010). Although ITS do absorb normal amounts of nicotine from each cigarette (Shiffman et al. 2014) and have low quit rates (Tindle and Shiffman 2011), they do not suffer craving or withdrawal when abstaining (Shiffman et al. 2015) and show few signs of dependence (Shiffman et al. 2012b). Thus, it has not been clear whether ITS’ smoking is motivated by nicotine-seeking or how ITS would respond to being switched to VLNCCs.
To address the effect of VLNCCs on ITS’ smoking we conducted a study that showed that ITS, like daily smokers, show reductions in cigarette consumption when randomized to VLNCCs (Shiffman et al. 2018). Average daily cigarette consumption was reduced by an average 51% in ITS randomized to smoke VLNCCs, while it remained relatively unchanged (2% average decrease) in ITS assigned to matched normal-nicotine-content cigarettes (NNCCs). After an initial decline, cigarette consumption in the VLNCC group leveled off, suggesting that subjects may have adapted to the VLNCC cigarettes.
However, using the number of cigarettes as the metric for consumption and exposure is limiting, because the intensity with which a cigarette is smoked, and, accordingly, the smoker’s intake of nicotine and exposure to toxicants in smoke, can vary (Hasenfratz et al. 1993). One smoker might take only a few puffs from one cigarette, while another smokes a cigarette down to the filter, and a third lets the cigarette burn down in the ashtray without puffing at all; a smoker might puff intensively, taking large volumes of smoke, or less intensively, achieving lower total smoke volume (Ashton et al. 1979). Although ITS are not nicotine-dependent, the fact that they reduced their smoking when randomized to VLNCCs (Shiffman et al. 2018), along with the fact that some sought out e-cigarettes when assigned to VLNCCs, shows that ITS do smoke in order to get nicotine, and thus raises the question whether they might compensate for cigarettes’ reduced nicotine content by increasing their smoking intensity. An increase in smoking intensity might not only undermine the intention of a very-low-nicotine-cigarette policy, but could also expose ITS to increased health risk, as the smoke from cigarettes with reduced nicotine still contains the tobacco- and combustion-related toxicants that are responsible for smoking-related harm.
To better understand the smoking behavior resulting from a switch to VLNCCs, it is necessary to have measures of smoking intensity to complement the measures of number of cigarettes. In this paper, we analyze multiple measures of smoking intensity obtained from ITS participating in the Shiffman et al. (2018) study.
In analyses of smoking intensity, we also considered whether subjects had obtained nicotine from sources other than the study cigarettes. ITS assigned to VLNCCs were more likely to admit to ‘cheating’ by smoking conventional cigarettes, and analyses of nicotine metabolites also suggested more frequent cheating with conventional cigarettes in the VLNCC group (Shiffman et al. 2018). Accordingly, we consider such behaviors as potential moderators of smoking intensity differences between VLNCC and NNCC subjects. We also considered age as a potential moderator, as Cassidy et al. (2018) recently reported that younger daily smokers’ cigarette consumption and satisfaction were more greatly reduced when switched to VLNCCs.
Methods
Subjects.
Participants, recruited from advertisements in the Pittsburgh area, were ITS aged 18 and older. To be eligible, participants had to report smoking non-daily (defined as 4–27 days per month, with no restrictions on number of cigarettes smoked) for ≥1 year, and smoking at any rate for ≥3 years. Smokers identifying cost as the primary reason for nondaily smoking or reporting intention to try to quit in the coming 3 months were excluded. (See Shiffman et al. 2018 for additional inclusion and exclusion criteria.)
Randomized participants (N=238), the subjects of these analyses, averaged 37.9 years of age (SD = 13.8; range 19–80) and were 54.6% female; 41.2% college graduates; 63.9% White and 25.6% African American; 5.5% were Hispanic. Subjects reported having smoked for an average of 16.8 years (SD = 12.3), smoking 3.7 (SD = 1.4) days per week and consuming 3.4 (SD = 2.6) cigarettes per day on those days. Three quarters had a score of 0 on the Fagerström Test for Nicotine Dependence (Heatherton et al. 1991) at baseline. About half the participants (49.6%) had previously smoked daily for at least 6 months, and half smoked menthol cigarettes (50.4%). See Shiffman et al. (2018) for demographics of each study group, as well as data on drop-outs compared to retained participants.
Procedures.
In this 12-week double-blind randomized clinical trial, subjects were provided with cigarettes of their preferred brand for a two-week baseline, followed by a 10-week experimental period in which they were randomized, double-blind and stratified by menthol preference, to receive experimental cigarettes, either VLNCCs or NNCCs, which they were to smoke for the remainder of the study. The study was approved by the University of Pittsburgh Institutional Review Board and participants provided written informed consent. Subjects received financial compensation at each visit, for a maximum total of $455.
Experimental cigarettes were provided by the National Institute of Drug Abuse and approved for use as an Investigational Tobacco Product by the FDA, with VLNCCs yielding 0.07 mg of nicotine per cigarette (NRC200, 0.93 mg/g content, NRC201, 1.00 mg/g) and NNCCs yielding 0.8 mg per cigarette (NRC600, 15.7 mg/g, NRC601, 17.3 mg/g). Participants who normally smoked menthol cigarettes were provided with menthol experimental cigarettes. The tobacco’s nicotine content was modified genetically, rather than by filtration or ventilation, which are more easily circumvented.
At each of a possible 10 visits after enrollment, subjects returned butts from cigarettes they smoked since the previous visit. They also tracked cigarette consumption via real-time phone communication using Interactive Voice Response, using the phone’s touch-tone keypad, and retrospectively via calendar-based Timeline Follow-Back (Sobell et al. 1985) at each visit; cigarette consumption data have been reported in Shiffman et al. (2018). At a subset of visits, participants additionally provided data on smoking topography, as described below.
Measures
Demographic data, including age, were collected at enrollment. Urinary cotinine values were used to identify participants whose post-randomization cotinine levels were too high to be plausible in light of their reported consumption of research cigarettes, and who were therefore deemed to have “cheated” by significant consumption of ordinary normal-nicotine commercial cigarettes. We used the approach outlined by Benowitz (2015), as modified by Foulds et al. (2018) and adapted for ITS (see Shiffman et al. 2018). Participants whose cotinine values met this test were considered “cheaters,” and analyses examined whether this status moderated observed treatment effects on smoking intensity.
We had four measures of smoking intensity, two collected in the laboratory in smoking topography sessions, and two collected in the field, reflecting subjects’ real-world smoking:
Laboratory measures:
Smoking topography.
After the two-week own-brand baseline, subjects smoked one of their cigarettes in a laboratory setting furnished like a living room. They could smoke as much or as little of the cigarette as they wished, smoking through a hand-held instrumented holder that measured flow of smoke through the mouthpiece (CReSS Pocket; Borgwaldt KC Inc, Richmond, VA). This was repeated, with the assigned VLNCCs or NNCCs 2, 6, and 10 weeks after randomization. The device measured the number of puffs and the total volume taken over all puffs Eight participants declined to smoke, each at a single topography session, either because they felt ill (n=4), because they had not been smoking for 2 weeks or more (n=2), or both (n=2); this most often occurred at the last visit (62%). As noted in Shiffman and Scholl (2017), analyses showed that procedural and mechanical issues invalidated sessions where the estimated total volume exceeded 1.2L. Accordingly, these sessions (72/841, 8.6%) were excluded from both volume and carbon monoxide (CO) boost analyses. Baseline own-cigarette values were regarded as non-comparable to post-randomization smoking of experimental cigarettes, because topography measures can vary with the draw resistance of the cigarette, and the measure is also affected by the seal of the cigarette within the holder, which can be affected by the cigarette diameter, which varies among own-brand cigarettes. Nevertheless, baseline values were expected to be a meaningful covariate, and indeed were correlated with post-randomization values (r= 0.54, p<0.0001) and were, accordingly, included in analyses as a covariate.
CO boost.
In the topography sessions, exhaled CO was assessed before and after smoking, using a hand-held monitor (Vitalograph Inc, Lenexa, KS), and the increase in CO (“CO boost,” in parts per million) was calculated. CO boost has been used as a biochemical indicator of smoke exposure (Benowitz et al. 2009). As different cigarettes vary in CO delivery, and CO boost is also affected by the fit of the cigarette in the topography device cigarette holder, and thus can affected by cigarette diameter, baseline and post-randomization CO boost were regarded as non-comparable, but baseline CO boost, which correlated with post-randomization CO boost (r=0.71, p<0.0001) was included in the analyses as a covariate.
Field measures:
Mass of tobacco burned.
Subjects were directed to save all their cigarette butts in special bags provided for the purpose, each labeled for a day in the field. As the weight of the cigarettes and the bag was known, by counting the butts and weighing the returned bag, we could calculate the weight of the tobacco that was burned. Note that this captures how much tobacco was burned, not necessarily what was puffed or inhaled. Because own-brand cigarettes vary in tobacco mass, and may differ from experimental cigarettes, baseline and post-randomization values were not comparable. However, baseline tobacco mass burned correlated with postrandomization vales (r=0.49, p<0.0001), and, accordingly, was included in analyses as a covariate.
Filter optical density.
As a cigarette is puffed and smoke passes through the filter, it deposits material (“tar”) that darkens the filter. Quantifying this darkening can quantify smoking intensity and mouth-level exposure (Kozlowski 1981; O’Connor et al. 2007; Strasser et al. 2006). We used a desktop scanner (Canon 9000F Mark II, Canon U.S.A., Inc., Melville, NY) to optically scan the filters, which were cut 1 cm from the end. The resulting images were calibrated using a Kodak Q-13 reference strip (Eastman Kodak, Rochester, NY), and then processed using software developed by the US Centers for Disease Control to calculate each filter’s optical density. Tests with controlled machine-smoked cigarettes and analysis of condensate collected on filter pads (Polzin et al. 2006) have demonstrated the validity of the method, showing that optical density assessed this way correlates 0.98 with measured nicotine delivery. Up to two butts per subject-smoking-day were scanned (selected at random if more than two were available), resulting in an average of 62.14 (47.50) butts scanned per participant. Because optical density can vary with filter properties, baseline own-brand values were regarded as non-comparable to post-randomization values, but correlated highly with those values (r=0.86, p<0.0001) and were, accordingly, included as a covariate in the analyses.
Analysis
Analyses used hierarchical mixed-model approaches (SAS v9.4 PROC MIXED), with random intercepts and slopes to test differences between the VLNCC and NNCC groups, trends over time (linear and quadratic for the field measures), and their interaction. Analyses of these variables was pre-planned. The field measures, which were assessed at the day level, were averaged over two-week intervals post-randomization, and time was treated as a continuous variable. Topography-based measures were analyzed at three time-points post-randomization, and time was treated categorically. For all intensity measures, baseline values from own-brand cigarettes were modeled as covariates, rather than as part of the time-trend, because, as described above, these measures vary with the physical characteristics of the cigarettes, which changed from baseline (own-brand commercial cigarettes) to post-randomization (experimental cigarettes). Age (treated as a continuous variable) and cheater status (categorical) were examined as potential subject-level moderators of treatment effects.
Results
Smoking topography.
Participants in the VLNCC group showed puff volumes almost 40 mL (7%) lower than those in the NNCC group (p= 0.04, Table 1). This did not vary over time (p = 0.69). The treatment effect was not related to subject age (interaction p=0.20) or cheating status (p=0.62).
Table 1.
Average group differences in measures of smoking intensity, adjusted for baseline values
| VLNCC | NNCC | Difference (95% CI) | |
|---|---|---|---|
| Mean (SE) | Mean (SE) | ||
| Topography: Total puff volume (mL) | 501.20 (13.50) | 539.70 (12.83) | −38.50 (−75.21, −1.78)* |
| Topography: CO boost (ppm) | 2.24 (0.12) | 2.32 (0.11) | −0.08 (−0.41, 0.25) |
| Field: Mass of tobacco burned (g) | 0.58 (0.01) | 0.60 (0.01) | −0.02 (−0.04, −0.002)* |
p<0.05
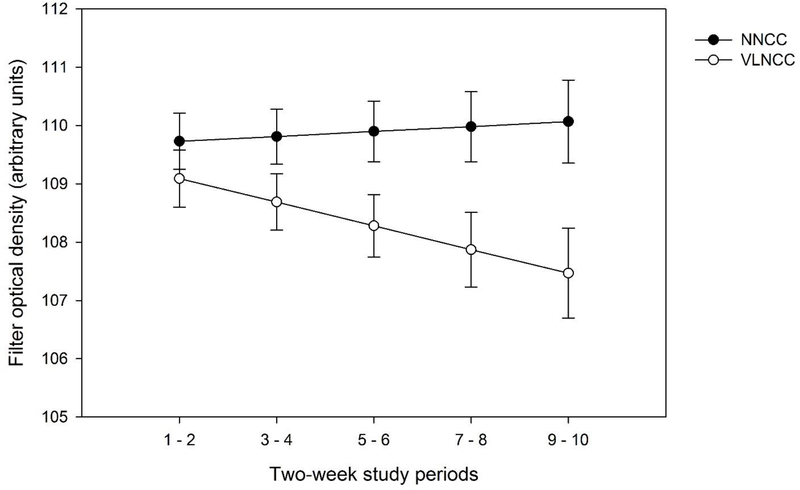
Note. Field measures based on optical density of the filters in returned cigarette butts are not included in the table because analysis showed a group x time interaction, shown in Figure 1.
CO boost.
CO boost did not vary by group (p = 0.64; Table 1), time (p = 0.77), or their interaction (p = 0.33). CO boost was unaffected by subjects’ cheating status, but did vary with subject age (group x age interaction, p<0.01): As shown in Table 2, older subjects (dichotomized at age 40) had lower CO boost in the VLNCC group (simple main effect B = −.82 ppm, p < 0.01), but not in the NNCC group (p=0.34).
Table 2.
Interactions between treatment group and age (dichotomized at age 40, model-based estimates)
| CO boost (ppm) | Mass tobacco burned (g) | ||||
| Treatment Group | Age Group | Mean | SE | Mean | SE |
| VLNCC | < 40 | 2.52 | 0.15 | 0.61 | 0.01 |
| >= 40 | 1.67 | 0.21 | 0.54 | 0.01 | |
| NNCC | < 40 | 2.19 | 0.14 | 0.61 | 0.01 |
| >= 40 | 2.54 | 0.18 | 0.59 | 0.01 | |
Mass of tobacco burned.
Cigarette butts returned by participants in the VLNCC group indicated participants in this group had burned 0.02g (3%) less of the tobacco on average than participants in the NNCC group (p=0.03; Table 1). This did not vary over time (Linear: p = 0.24, Quadratic: p = 0.14). Cheating did not moderate the treatment effect (p=0.52), but age did (group x age p=0.01; Table 2): Among older subjects (dichotomized at age 40), assignment to VLNCCs resulted in decreased mass burned (B=0.05 g; p < 0.01); this was not true in the younger age group (p=0.74). Controlling for history of daily smoking, years smoked, or baseline CPD did not reverse this interaction, suggesting these covariates did not account for the effect of age.
Filter optical density.
Filter optical density, reflecting puffing intensity, showed a group by time interaction (linear p = 0.04). As seen in Figure 1, density declined in the VLNCC group (simple main effect, p = 0.03), while staying flat or tending towards increase in the NNCC group (p = 0.59). Neither cheating status (p = 0.59) nor age (p = 0.37) moderated this effect.
Figure 1. Change over time, by group, in optical density of cigarette filters in returned cigarette butts, adjusted for baseline values.
Model-based estimated optical density of filters, averaged over 2-week post-randomization intervals, and adjusted for baseline (own-cigarette) values. Optical density is measured in arbitrary units, with higher values reflecting darker filters, which in turn indicate that a greater amount of smoke was drawn through the filter.
Discussion
With ITS, as with daily smokers (Donny et al. 2015), there had been concern that switching to very-low nicotine content cigarettes might trigger compensatory increases in smoking intensity, which could undermine the intent of the low-nicotine-cigarette policy and potentially increase the harm of smoking. Using multiple measures of smoking intensity in a randomized trial of VLNCCs, these analyses demonstrated that smoking intensity did not increase among ITS, even as they were decreasing the number of cigarettes they smoked (Shiffman et al. 2018).
Measures of smoking intensity taken in a laboratory smoking topography session showed that ITS assigned to smoke VLNCCs actually had less mouth-level smoke exposure, puffing less than those in the NNC group, taking about 7% less total smoke volume. However, this did not result in any reliable difference in CO boost due to smoking, though the observed CO boost trended lower (by 3.6%) in the VLNCC group, rather than higher, suggesting that compensation did not occur.
Measures of smoking intensity during ad libitum smoking in the field indicated that smoking intensity decreased over time in the VLNCC group, while it remained unchanged among those smoking NNCCs. The amount of tobacco burned per cigarette (but not necessarily puffed or inhaled) was lower in the VLNCC group, but only by a very small amount: 3.4%. This effect was concentrated in older ITS, contrary to Cassidy et al.’s (2018) finding that younger smokers reacted more strongly to being switched to VLNCCs. However, Cassidy et al did not assess the amount of tobacco burned, so the results are not necessarily contradictory.
In brief, the convergent evidence from multiple measures of smoking intensity indicates that assignment to smoking VLNCCs did not result in an increase in per-cigarette smoking intensity and, indeed, tended to decrease it. Along with the earlier finding that switching to VLNCCs led to a decrease in the number of cigarettes smoked (Shiffman et al. 2018), this suggests that a policy that mandated that all cigarettes be VLNCCs would be highly unlikely to increase smoke exposure – and therefore toxicant exposure – among ITS. This mirrors the findings obtained from heavier daily smokers as well (Donny et al. 2015).
All of the measures of smoking intensity showed decreased smoking intensity, and thus smoke exposure, among ITS switched to VLNCCs, with some of the decreases being statistically significant. However, the decreases were quite small, and are unlikely to have any meaningful impact on health. The importance of the observed decreases is that they help rule out any potential increases in exposure.
A substantial fraction of ITS in this study (36% in VLNCC and 16% in NNCC groups) were presumed to have ‘cheated’ by smoking commercial cigarettes during the study, based on comparisons of their reported cigarette consumption and observed cotinine values (see Shiffman et al, 2018). It was hypothesized that compensation was less likely to be observed among those ‘cheaters’ in the VLNCC group, since they, in effect, compensated for reduced nicotine by smoking normal-nicotine cigarettes; that is, that there would be an interaction between cheating status and treatment assignment. However, no such interaction was observed on any of the measures, further confirming the lack of any compensation in this population.
Compensation for decreases in nicotine levels has traditionally been viewed as a hallmark of dependence, which leads to dependent smokers needing to maintain an accustomed level of nicotine in order to avoid nicotine withdrawal (Benowitz 2010; Stolerman and Jarvis 1995). As ITS show little or no sign of dependence (Shiffman et al. 2012b), and do not suffer withdrawal when not smoking at all (Shiffman et al. 2015), it is perhaps not surprising that they would not show compensation when nicotine delivery is reduced. However, the primary outcomes from this study (Shiffman et al. 2018) showed that being switched to VLNCCs caused ITS to smoke fewer cigarettes, while also causing them to seek nicotine from other sources (both normal cigarettes and e-cigarettes), demonstrated that ITS smoking is in fact motivated by nicotineseeking, likely for its acute direct effects (in contrast to daily smokers’ motive to avoid withdrawal if they didn’t smoke). (Shiffman et al. 2012a). Thus, even without dependence, it was still plausible that ITS would increase their smoking and/or their smoking intensity to compensate for the decreased nicotine in VLNCCs. These analyses demonstrated that this did not occur.
Among the study’s limitation was reliance on a relatively small sample from a single metropolitan area. Despite the limited sample size, the study demonstrated that switching to VLNCCs resulted in significant reductions in two measures of exposure. Further, even on the measures that did not show significant decreases, the 95% confidence interval of group differences indicated that any plausible increases had to be quite small (e.g., any increase in CO boost would be less than 0.25 ppm; Table 1). Also, the descriptive data did not indicate any increases, but rather decreases in smoking intensity among those using VLNCCs, even when the variations were not significant. Of the measures used in these analyses, only the change in CO concentration after smoking is a measure of actual biological exposure to smoke constituents; the others are limited to mouth-level exposure, at best. However, such measures are likely to reflect changes in smoking behavior, and have previously been used in that way (O’Connor et al. 2007; Strasser et al. 2006). Among the strengths of the study was the use of multiple measures, assessing both smoking in controlled laboratory sessions and in-field naturalistic smoking behavior. The measures based on returned cigarette butts provided particularly robust data, as they were based on many cigarettes per subject over a period of up to 10 weeks.
In summary, data from multiple measures support the conclusion that ITS assigned to smoke VLNCCs did not increase their smoking intensity, even as they decreased their cigarette consumption. Thus, the decreased cigarette consumption observed in this study (Shiffman et al, 2018) is not compensated for by increased smoking intensity, and reflects reduced intake of nicotine and of smoking-related toxicants.
Acknowledgements
The authors are grateful to Allison Brown for assistance overseeing the study, to David Colarusso, Corinne Hogge, and Ian Jutsum, research assistants who conducted research sessions, to James Moorehead for data management and preparation, to Alexsys Hoesch for administrative assistance, to Dr. Esa Davis for medical oversight, and to Dr. Clifford Watson for providing the filter-scanning software and training and consultation on the scanning. We also appreciate the contributions of members of the study Data and Safety Monitoring Board, Peter Callas (University of Vermont), Matthew Carpenter (Medical University of South Carolina), Jonathan Foulds (Pennsylvania State University), and John Hughes (University of Vermont).
Funding
This work was supported by a grant (S. Shiffman) from National Cancer Institute (NCI) at the National Institutes of Health (NIH) and the Center for Tobacco Products (CTP) at the Food and Drug Administration (FDA), awarded as a supplement to grant number P30CA047904. The grant supported use of the UPMC Hillman Cancer Center Biostatistics Shared Resource Facility. NCI, FDA, and NIDA had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Footnotes
Conflicts of Interest
SS, through Pinney Associates, consults on tobacco cessation and harm reduction (including nicotine replacement therapy and digital vapor products; by contract, combusted cigarettes are excluded) to Niconovum USA, RJ Reynolds Vapor Company, and RAI Services Company, all subsidiaries of Reynolds American, Inc. and British American Tobacco. Previously, SS consulted to NJOY on e-cigarettes, and to GlaxoSmithKline Consumer Healthcare on smoking cessation medications and treatments. SS holds patents for a novel nicotine smoking-cessation medication that is not under commercial development. Other authors report no competing interests.
References
- Ashton H, Stepney R, Thompson JW (1979) Self-titration by cigarette smokers. BMJ 2: 357–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz N (2010) Nicotine addiction. N Engl J Med 362: 2295–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Hall SM, Stewart S, Wilson M, Dempsey D, Jacob Pr (2007) Nicotine and carcinogen exposure with smoking of progressively reduced nicotine content cigarette. Cancer Epidemiol Biomarkers Prev 16: 2479–2485. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Henningfield JE (1994) Establishing a nicotine threshold for addiction: The implications for tobacco regulation. N Engl J Med 331: 123–125. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob Pr (2009) Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol 192: 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Nardone N, Hatsukami DK, Donny EC (2015) Biochemical estimation of noncompliance with smoking of very low nicotine content cigarettes. Cancer Epidemiol Biomarkers Prev 24: 331–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy RN, Tidey JW, Cao Q, Colby SM, McClernon FJ, Koopmeiners JS, Hatsukami D, Donny EC (2018) Age moderates smokers’ subjective response to very-low nicotine content cigarettes: Evidence from a randomized controlled trial. Nicotine Tob Res. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, al’Absi M, Carmella SG, Cinciripini PM, Dermody SS, Drobes DJ, Hecht SS, Jensen J, Lane T, Le CT, McClernon FJ, Montoya ID, Murphy SE, Robinson JD, Stitzer ML, Strasser AA, Tindle H, Hatsukami DK (2015) Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med 373: 1340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller EJ, Stitzer ML (2007) Smoking in the absence of nicotine: Behavioral, subjective and physiological effects over 11 days. Addiction 102: 324–334. [DOI] [PubMed] [Google Scholar]
- Foulds J, Hobkirk A, Wasserman E, Richie J, Veldheer S, Krebs NM, Reinhart L, Muscat J (2018) Estimation of compliance with exclusive smoking of very low nicotine content cigarettes using plasma cotinine. Prev Med. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenfratz M, Baldinger B, Battig K (1993) Nicotine or tar titration in cigarette smoking behavior? Psychopharmacology (Berl) 112: 253–8. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO (1991) The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 86: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Benowitz NL, Slade J, Houston TP, Davis RM, Deitchman SD (1998) Reducing the addictiveness of cigarettes. Council on Scientific Affairs, American Medical Association. Tob Control 7: 281–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue-Choi M, Liao LM, Reyes-Guzman C, Hartge P, Caporaso N, Freedman ND (2017) Association of long-term, low-intensity smoking with all-cause and cause-specific mortality in the National Institutes of Health-AARP Diet and Health Study. JAMA Intern Med 177: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM (2016) Current Cigarette Smoking Among Adults - United States, 2005–2015. Mmwr-Morbid Mortal W 65: 12051211. [DOI] [PubMed] [Google Scholar]
- Kozlowski LT (1981) Applications of some physical indicators of cigarette smoking. Addict Behav 6: 213–219. [DOI] [PubMed] [Google Scholar]
- O’Connor RJ, Kozlowski LT, Hammond D, Vance TT, Stitt JP, Cummings KM (2007) Digital image analysis of cigarette filter staining to estimate smoke exposure. Nicotine Tob Res 9: 865–71. [DOI] [PubMed] [Google Scholar]
- Polzin G, Xizheng Y, McCraw J, Ashley D, Watson C (2006) Comparison of optical, ultraviolet, and mass spectrometric methods for the determination of cigarette smoking patterns: Can lower cost/higher thoughtput methods provide reliable data. Poster presented at The 13th World Conference on Tobacco OR Health, Washington, DC. [Google Scholar]
- Reyes-Guzman CM, Pfeiffer RM, Lubin J, Freedman ND, Cleary SD, Levine PH, Caporaso NE (2017) Determinants of light and intermittent smoking in the United States: results from three pooled national health surveys. Cancer Epidemiol Biomarkers Prev 26: 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schane RE, Ling PM, Glantz SA (2010) Health effects of light and intermittent smoking: a review. Circulation 121: 1518–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Benowitz NL (2014) A comparison of nicotine biomarkers and smoking patterns in daily and nondaily smokers. Cancer Epidemiol Biomarkers Prev 23: 1264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Scholl SM, Tindle HA (2012a) Smoking motives of daily and nondaily smokers: a profile analysis. Drug Alcohol Depend 126: 362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Dunbar MS, Tindle HA, Ferguson SG (2015) Nondaily smokers’ experience of craving on days they do not smoke. J Abnorm Psychol 124: 648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Kurland B, Scholl S, Mao J (2018) Non-daily smokers’ changes in cigarette consumption with very-low-nicotine-content cigarettes: A randomized double-blind clinical trial. JAMA Psychiatry. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Scholl SM (2017) Increases in cigarette consumption and decreases in smoking intensity when non-daily smokers are provided with free cigarettes. Nicotine Tob Res: Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S, Tindle H, Li X, Scholl S, Dunbar M, Mitchell-Miland C (2012b) Characteristics and smoking patterns of intermittent smokers. Exp Clin Psychopharmacol 20: 264–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Maisto SA (1985) Time-line follow-back assessment methods In: Lettieri DJ, Sayers MA, Nelson JE (eds) NIAA treatment handbook series: Vol 2, Alcoholism treatment assessment research instruments. National Institute on Alcoholism and Drug Abuse DHHS Publications, Washington, D.C. [Google Scholar]
- Stolerman IP, Jarvis MJ (1995) The scientific case that nicotine is addictive. Psychopharmacology (Berl) 117: 2–10. [DOI] [PubMed] [Google Scholar]
- Strasser AA, O’Connor RJ, Mooney ME, Wileyto EP (2006) Digital image analysis of cigarette filter stains as an indicator of compensatory smoking. Cancer Epidemiol Biomarkers Prev 15: 2565–9. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2014) Results from the 2013 National Survey on Drug Use and Health: National Findings In: NSDUH Series H-48 HPNS- (ed). Substance Abuse and Mental Health Service Administration, 2014, Rockville, MD. [Google Scholar]
- Tindle HA, Shiffman S (2011) Smoking cessation behavior among intermittent smokers versus daily smokers. Am J Public Health 101: e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacco Product Standard for Nicotine Level of Combusted Cigarettes; HHS Advance notice of proposed rulemaking, 83 Fed. Reg. 11818 (March 16, 2018) (to be codified at 21 C.F.R. pt. 1130).