Abstract
Objective
When an autogenous vein is harvested and used for arterial bypass, it suffers physical and biological injuries that may set in motion the cellular processes that lead to wall thickening, fibrosis, stenosis and ultimately graft failure. While the injurious effects of surgical preparation of the vein conduit have been extensively studied, little is known about the influence of the clinical environment of the donor leg from which the vein is obtained.
Methods
We studied the cellular responses of fresh saphenous vein samples obtained before implantation in 46 subjects undergoing elective lower extremity bypass surgeries. Using an ex vivo model of response to injury, we quantified the outgrowth of cells from explants of the adventitial and medial layers of the vein. We correlated this cellular outgrowth with the clinical characteristics of the subjects, including the WIfI classification of the donor leg for ischemia, wounds, and infection, as well as smoking and diabetes.
Results
Cellular outgrowth from the adventitial layer was significantly faster and more robust than from the medial layer. The factors of leg ischemia (P< 0.001), smoking (P=0.042), and leg infection (P=0.045) were associated with impaired overall outgrowth from the adventitial tissue, on multivariable analysis. Only ischemia (P=0.046) was associated with impaired outgrowth of smooth muscle cells (SMCs) from the medial tissue. Co-culture of adventitial and SMC cells propagated from vein explants revealed that adventitial cells significantly inhibited the growth of SMCs, while SMCs promoted the growth of adventitial cells. The AA genotype of the −838C>A p27 polymorphism (previously associated with superior graft patency) enhanced these effects, while the factor of smoking attenuated adventitial cell inhibition of SMC growth. Comparing gene expression, the cells cultured from the media overexpress KEEG pathways associated with inflammation and infection, while those from the adventitia overexpress gene families associated with development and stem/progenitor cell maintenance.
Conclusions
The adverse clinical environment of the leg may influence the biological behavior of the cells in the vein wall, especially the adventitial cells. Chronic ischemia was the most significant factor that retards adventitial cell outgrowth. The cells arising from the vein adventitia may be key players in determining a healthy adaptive, or a pathologic response to the injuries associated with vein grafting.
Introduction
Within the first one to two years after implantation of an arterial vein graft, up to 30% fail due to intimal hyperplasia, stenosis, and thrombosis. This deranged and pathologic response to injury is the leading cause of graft failure in these early years. Many research efforts have been applied to understand how the injuries associated with the handling and surgical preparation of a vein graft might affect its later function and evolution into a successful arterial conduit1–8. In fact, the vein conduit is most commonly obtained from the same diseased, ischemic leg that is undergoing bypass. This led us to ask the question: might the local environment of the leg influence the subsequent response to injury of the vein? Unlike the previous studies of vein preparation techniques, these clinical factors, which are unique and intrinsic to each patient, have not been examined. To that end, we systematically examined the ex vivo cellular behavior of samples of human saphenous veins obtained from leg bypass procedures, and correlated their cellular growth and proliferation with the clinical environment of the source leg.
Methods
A prospective, observational study
The subjects and veins studied in this report were a subset of a larger, ongoing prospective observational study of patients undergoing elective leg bypass with autogenous vein, for indications of peripheral arterial occlusive disease (PAD). All elective infrainguinal bypasses using autogenous vein were eligible for study. Exclusion criteria were: operations for non-atherosclerotic or aneurysmal disease, concurrent aorto-bifemoral or femoral-femoral bypass, use of a prosthetic bypass conduit, active treatment for malignancy (i.e. chemotherapy), chronic hemodialysis, perioperative thrombosis of the graft, or a diagnosed systemic inflammatory disease (e.g. lupus or other treatment with immunosuppressive drugs) or thrombophilia. The procedures and protocols were approved by the Institutional Review Boards for human studies at the University of Washington, and the VA Puget Sound Health Care System, Seattle, WA. All subjects gave their informed consent. The methods for vein harvesting were not prescribed, but all surgeons utilized the recognized standards of practice, avoiding excessive dissection of the vein and overdistention. At the satisfactory conclusion of the bypass, if the surgeon found that a remnant of harvested saphenous vein was not utilized (and would have been discarded), this fresh sample was conveyed to our laboratory for study within 2 hours.
Clinical data were recorded prospectively for each patient, including the presence of diabetes, medications, the status of the limb, and if they were current smokers (within the 30 days prior to surgery). The preoperative status of the subject’s leg in respect to wounds, infection, and ischemia, was categorized using the published WIfI (Wound, Ischemia, foot Infection) definitions9, 10. The leg wound and infection classifications were simplified as dichotomous variables (infection, wounds: Yes/No). The WIfI ischemia classifications utilize three different measures to categorize ischemia: ankle-arm index (AAI), absolute ankle pressure, and toe pressure, to more accurately capture the true level of ischemia in diabetics and others with incompressible arteries. For familiarity, this report refers to the AAI cut-points of the WIfI scale, although all of the ischemia parameters were used to classify the patients. Ischemia was analyzed as a dichotomous variable (WIfI grades 0 and 1 vs. 2 and 3; AAI < > 0.6), and also as three categories (WIfI grades 0 and 1, AAI ≥ 0.6; WIfI 2, 0.6 ≥ AAI > 0.4; and WIfI 3, AAI < 0.4). The indication for surgery was determined by the Rutherford classification, and categorized as claudication (Rutherford 1-3) or critical limb ischemia (Rutherford 4-6)11.
Ex Vivo Cellular Studies
We utilized an ex vivo model of vascular injury that reliably reflects the major cellular responses to injury that are seen in vivo.12 The residual segments of fresh saphenous vein were delivered fresh to the laboratory and processed within 2 hours. For details of the processing and testing of the veins and their cells, please see the Online Supplemental Materials. In brief, vein samples were opened longitudinally, the endothelium removed, and the vein was micro-dissected to separate the adventitial layer from the intima/medial layer13. An image of this dissection plane can be found in our previous publication.14 From each vein, these separate, paired layers were prepared into small explants of uniform size (2.5 mm2), placed into culture medium, and observed for 8 days for the outgrowth of cells from the tissue explant. Fifteen to thirty replicate explants were prepared for each vein. Cells were successfully grown from all veins.
We quantified the rate of outgrowth of cells from these explants with two parameters: Cells per Explant, and Migration-Positive Explants. To measure Cells per Explant, on each of 8 days of explant culture we counted the total number of cells that emerged from the explant (up to a maximum of 100 cells/explant, averaged over the replicates). Cells per Explant measures the overall vigor of cell outgrowth from the tissue: a combination of initial migration out of the tissue, and subsequent post-migration proliferation. To measure Migration-Positive Explants, on each of 8 days of culture we counted the percentage of replicates that showed at least one attached, spread cell outside the explant (up to the maximum of 100%). Migration-Positive Explants is a narrower measure of the migration component of this tissue response to injury12, 15, 16. After the outgrown cells around the explants became confluent (2-3 weeks), these cells were then collected and propagated for subsequent co-culture and other experiments. See the Online Supplemental Materials for these technical details.
Co-culture experiments tested the effects of Medial SMCs on Adventitial Cell growth, and vice versa. Paired Adventitial and SMCs propagated from explants from the same vein (19 veins in total) were cultured on opposing sides of a membrane that permitted cell-cell contacts and the diffusion of proteins, but not cell migration. Controls included the same cell type on both sides of the membrane. The effect of a different, opposing cell type on growth was calculated as the percent change in the day4/day1 ratio (% change = ((growth ratio with different cell types/growth ratio of control)−1)*100). See the Online Supplemental Materials for the technical details.
RNA Sequencing
Six pairs of passaged adventitial and medial SMCs were cultured for 2 days in 2% fetal bovine serum (FBS), then stimulated with 10 ng/ml PDGF-BB in 2% FBS for 24 hours. RNA was harvested, purified, and then sequenced. See the Online Supplemental Materials for the technical details.
Immunohistochemistry
Rings of fresh saphenous veins were formalin-fixed, embedded in paraffin, and doubly stained: red for CD34 (a progenitor and endothelial cell marker), and black for CD31 (an endothelial cell marker). The relative areas of red and black staining were quantified from 4 sample areas in each section, and were compared between veins from legs with mild versus severe ischemia (WIfI ischemia 0 and 1 vs. 2 and 3). See the Online Supplemental Materials for the technical details.
Statistical Analyses
The patient’s clinical characteristics and the ex vivo cellular responses of their veins over time were correlated by 2-way repeated measures ANOVA, using the SPSS statistics package (IBM, Armonk, NY, version 24). An alpha of 0.05 was used for statistical significance. Co-culture experiments were analyzed by paired or unpaired t-tests as appropriate. Histologic quantifications were compared with the Mann-Whitney test.
Results
Population characteristics
Fresh samples of the saphenous conduit were collected from 46 subjects, between 2013-2016. All samples were from the greater saphenous vein. In three subjects the saphenous vein was harvested from the leg contralateral to the bypass, but in all cases the clinical characteristics of the donor leg were used for analysis. Table 1 summarizes the subject’s clinical characteristics. The AA genotype of the single nucleotide polymorphism −838C>A p27 was present in 7 subjects (15.2%), which is typical of its general prevalence17.
Table 1.
Clinical Characteristics of Study Population
| VEIN-PATIENTS STUDIED N=46 |
||
|---|---|---|
| Characteristic | Number of Subjects or Median | Percent, or Interquartile Range |
| Age | 65.5 | 62.25-69 |
| White Race | 40 | 87.0% |
| Male Sex | 44 | 95.7% |
| Current Smoker | 20 | 43.5% |
| Diabetes | 27 | 58.7% |
| Leg Wounds Present | 25 | 54.3% |
| Leg Infection Present | 16 | 34.8% |
| WIfI Ischemia grade 2 or 3 | 33 | 71.7% |
| Rutherford Category 1-3 (claudication) | 17 | 37% |
| Rutherford Category 4-6 (critical limb ischemia) | 29 | 63% |
| Serum Creatinine (mg/dL) | 0.99 | 0.83-1.11 |
| C-reactive protein (mg/L) | 20 | 5.5-65 |
| Statin therapy | 39 | 84.8% |
| Anti-Platelet therapy | 39 | 84.8% |
Clinical factors associated with cellular outgrowth from the vein
Three significant observations were made in examining the relationships between cellular outgrowth, and patient clinical factors. First, the cellular outgrowth from explants of the adventitial layer was significantly faster and more robust than from the explants of the media layer of the vein. The global measure of outgrowth (Cells Per Explant) from the adventitial tissue was significantly greater than from the medial tissue (p<0.001, 2-way ANOVA), and the measure of migration (Migration-Positive Explants) trended in the same direction, especially in the early days of culture (Figure 1).
Figure 1.
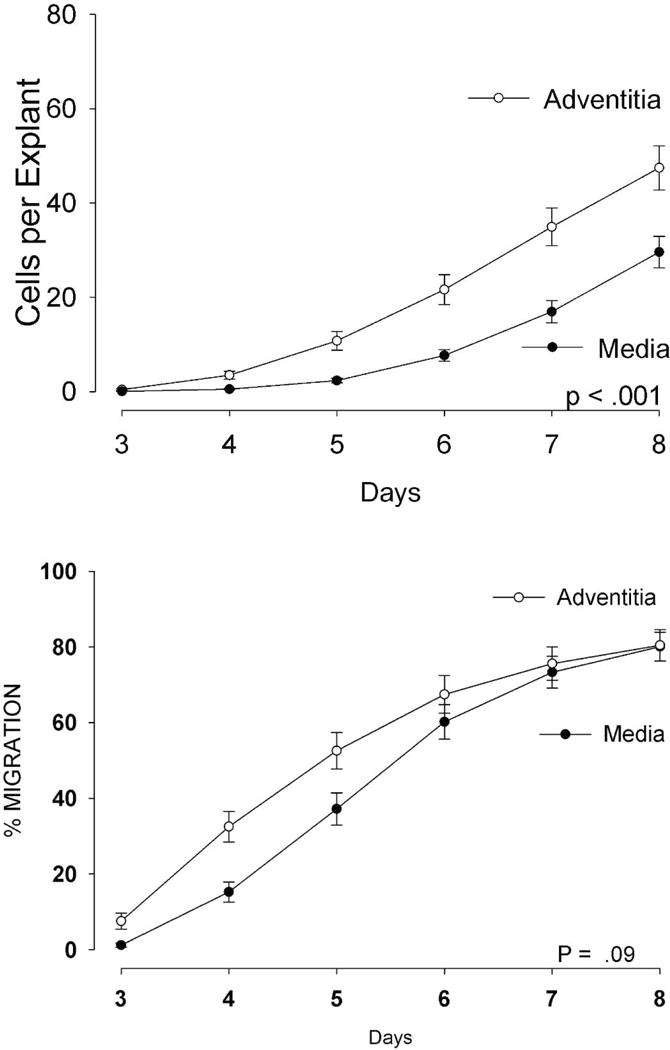
Outgrowth of cells from adventitial vein explants is significantly greater than from medial explants. N=46 pairs, means ± SEM are displayed, P<.001, 2-way ANOVA.
Secondly, the biological impact of clinical factors (e.g. ischemia, smoking) was more pronounced for the adventitial tissues than the medial tissues. Figure 2 shows that Cells Per Explant from the adventitial tissue was significantly retarded by ischemia, smoking, and the presence of leg infection or wounds. In contrast, for the medial tissue, overall outgrowth was only retarded by ischemia and leg infection.
Figure 2.
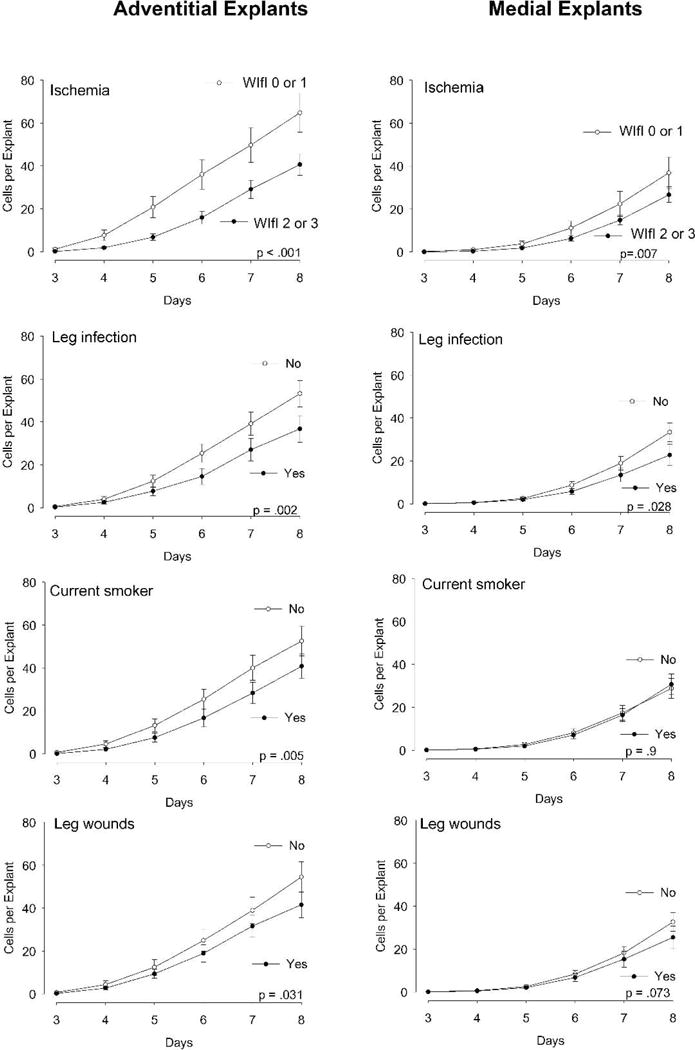
Measurements of global outgrowth of cells (Cells per Explant) from adventitial explants (left) and intimal/medial explants (right), analyzed according to the clinical characteristics of the patient and leg from which they were harvested. N=46 veins, mean ± SEM are displayed. The P value in each graph indicates the significant difference for the clinical factor, over all the days measured (2-way ANOVA).
Thirdly, the process of migration from the explants (Migration-Positive Explants) was much less affected by the clinical factors than was the overall measure of cumulative migration and proliferation (Cells Per Explant). None of the clinical factors showed a statistically significant association with the narrower measure of migration alone from the explants (Figure 3).
Figure 3.
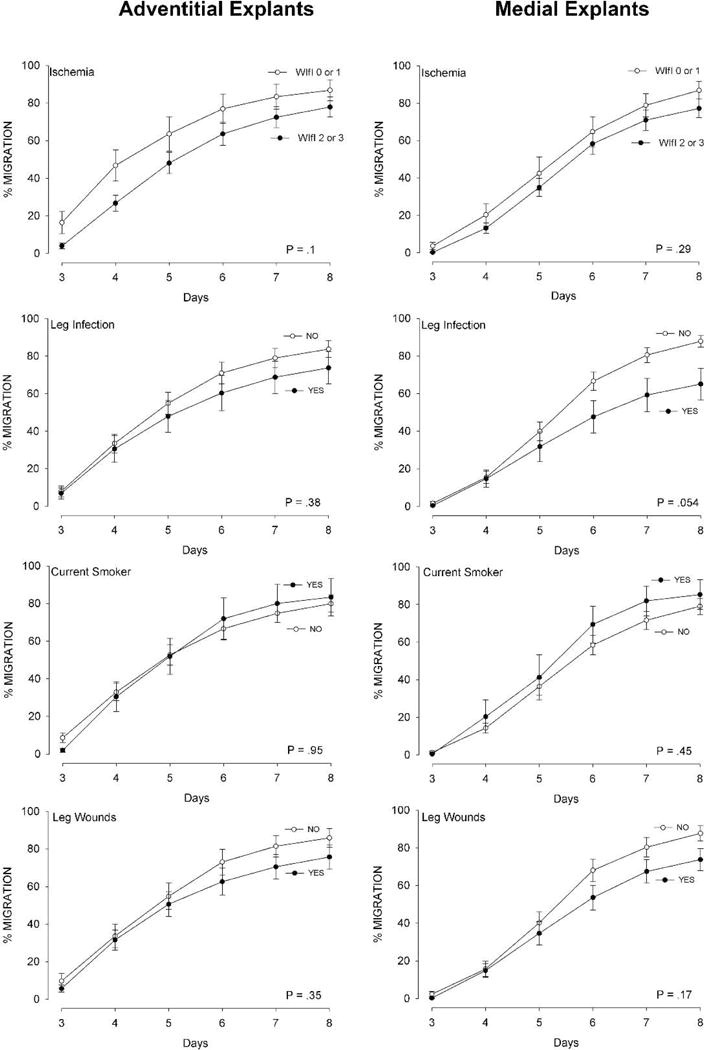
Measurements of migration of cells (% Migration Positive Explants) from adventitial explants (left) and intimal/medial explants (right), analyzed according to the clinical characteristics of the patient and leg from which they were harvested. N=46 veins, mean ± SEM are displayed. The P value in each graph indicates the significant difference for the clinical factor, over all the days measured (2-way ANOVA).
In subsequent analyses we focused on the metric of Cells Per Explant. Among the clinical factors, severe ischemia (WIfI grade 2 or 3), compared to milder ischemia, stood out with the statistically strongest association with the adventitial explants (Figure 2, p < 0.001, 2-way ANOVA). The other factors that were significantly associated with adventitial cell outgrowth were the presence of leg infection, wounds and smoking (Figure 2). The same patterns of outgrowth were borne out by the medial explants, except that smoking and leg wounds did not significantly impact the outgrowth of cells from the media. Neither age, diabetes, nor the p27 SNP (AA vs CC) showed a significant influence on overall cell outgrowth from either adventitia or media explants (data not shown).
We then analyzed the influence of ischemia on cell outgrowth (Cells Per Explant) with more granularity, employing three gradations of ischemia instead of two: the WIfI grades of 0 and 1 (nominally AAI ≥ 0.6), vs. grade 2 (AAI between 0.59 and 0.4), vs. grade 3 (AAI < 0.4). Figure 4 and statistical analysis by 2-way ANOVA show that those patients with milder ischemia (WIfI grade of 0 or 1, AAI ≥ 0.6) grew out significantly more cells than those with all lower AAI categories. There was no difference in cell outgrowth between the more ischemic WIfI grades of 2 and 3. This finding justified our choice of a dichotomous variable for ischemia, i.e. WifI grades 0 and 1 versus 2 and 3 (AAI < > 0.6).
Figure 4.
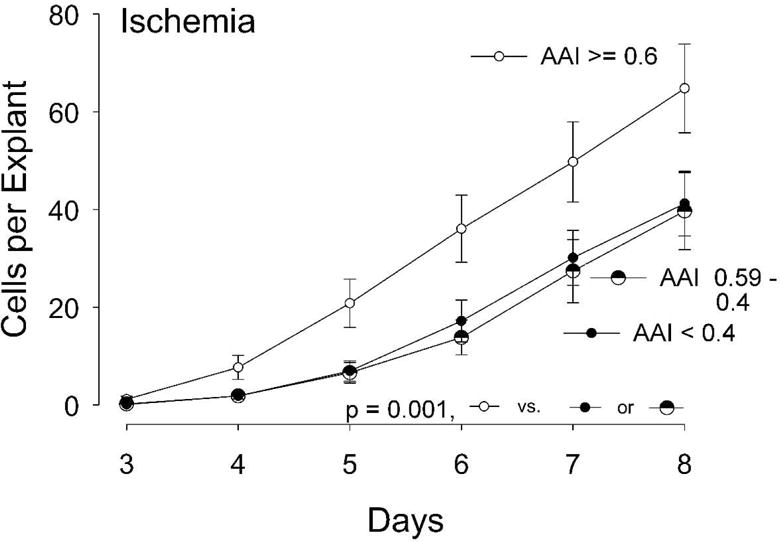
Outgrowth of cells from adventitial explants analyzed according to three levels of limb ischemia, per the WIfI definitions (WIfI grades 0 + 1, AAI ≥ 0.6, N=13; WIfI 2, AAI 0.59-0.4, N=12; and WIfI 3, AAI less than 0.4, N=21). The outgrowth by WIfI grades 0+1 were significantly different from both lower grades, 2 and 3 (P < 0.001, 2-way ANOVA over all the days measured).
To determine the influence of multiple factors, we performed 2-way ANOVA analysis of the effect of ischemia (WIfI 0 and 1 versus 2 and 3)) on Cells Per Explant, while including all the clinical covariates with a univariate P value of less than 0.2 found in Figure 2, namely: the factors of wounds, infection, and smoking. Table 2 shows that even after accounting for these other clinical covariables, adventitial cell outgrowth was significantly retarded in the presence of more severe ischemia, as well as by smoking and infection. WIfI ischemia grade had the strongest association. In contrast, the outgrowth from medial explants showed a significant association only with ischemia in the multivariable analysis (Table 2).
Table 2.
Relationship of clinical factors to cellular outgrowth over time – Multivariate Analysis
| Characteristic | P value, 2-way ANOVA | |
|---|---|---|
| Adventitia | Media | |
| WIfI Ischemia grade 2 or 3 vs 0 or 1 | < .001 | 0.046 |
| Smoking | 0.042 | 0.74 |
| Infection | 0.045 | 0.35 |
| Wounds | 0.23 | 0.98 |
Characteristics of the cells that grow out from adventitial and medial explants
The cells cultured from the medial and adventitial layers are quite different. In previous studies we found that the cells cultured from the medial layer explants are predominantly smooth muscle cells (SMCs), since greater than 90% of these cells are smooth muscle alpha-actin positive14. Under phase-contrast microscopy (see Supplemental Figure 1) the outgrowing adventitial cells appeared morphologically similar to SMCs, but the cultured adventitial cells are less than 10% positive for smooth muscle alpha-actin14.
The effect of ischemia might be explained by differences in the density of progenitor cells or vasa vasorum in the intact veins. To test this, we performed immunohistochemical studies of whole veins with mild ischemia (WIfI 0 or 1, n=7) and compared them to those with severe ischemia (WIfI 2 or 3, n=8). These were matched for smoking, and the other WIfI parameters. Supplemental Figure 2 (Online Supplemental Materials) illustrates a typical cross-section of vein in which we quantified the prevalence of CD34+ progenitor cells and endothelial cells within the wall of the veins. We found no differences in the prevalence of progenitor or endothelial cells between mild or severe ischemia (Supplemental Figure 3).
To better understand differences between the cells that grow out from the adventitia and media, we measured global gene expression by these cells after stimulation with PDGF. There were 1322 genes differentially expressed between medial and adventitial cells. These were fairly evenly divided: 609 genes were more highly expressed by adventitial cells and 713 were more highly expressed by SMCs (Online Supplemental Table 1). The genes with the highest fold differences between adventitial cells and SMCs (and with expression levels by at least one cell type in the top 25% of all genes) are presented in Online Supplemental Table 2. For SMCs, these include inflammatory factors such as interleukin 1β, CXCL8, and CXCL3. For adventitial cells, these include H19, WNT2, WISP2, and PRG4, which are factors involved in: development, progenitor cell maintenance or differentiation, and anti-inflammatory, anti-arthritic activities18–21.
KEEG (Kyoto Encyclopedia of Genes and Genomes) pathway analysis of all of the differentially expressed genes confirmed these impressions. For the 609 genes more highly expressed by adventitial cells, analysis indicated pathways associated with TGFβ, Hippo, Wnt, and Hedgehog signaling; arachidonic acid and glycerolipid metabolism; complement and coagulation cascades; and focal adhesion/extracellular matrix receptor interactions (Online Supplemental Table 3). KEEG analysis of the 713 genes more highly expressed by SMCs yielded pathways associated with inflammation and infection; vascular smooth muscle and cardiomyopathy; TNF and cytokine signaling; and NFκB, PI3K/Akt, and MAPK signaling (Online Supplemental Table 3). In summary, the cells arising from the medial tissue show a more inflammatory phenotype compared to those from the adventitia, which express genes associated with development and stem/progenitor cell maintenance.
Influence of clinical factors on the interactions between Adventitial Cells and SMCs
The data indicate that clinical characteristics that one would assume to be unhealthy for the vein (i.e. ischemia, smoking, and infection) are associated with retarded outgrowth of adventitial cells from the vein wall, with ischemia having the strongest association. One interpretation of these findings is that the migration and proliferation of certain adventitial cells could represent a salutary adaptive response to injury, which is inhibited by these detrimental clinical factors. To test this possibility, we cultured adventitial and SMCs from the same vein on opposite sides of a filter that prevents cell migration but allows cell contact and the flow of conditioned medium. We observed that the adventitial cells inhibited SMC growth, and conversely that SMCs stimulated the growth of adventitial cells (Figure 5).
Figure 5.
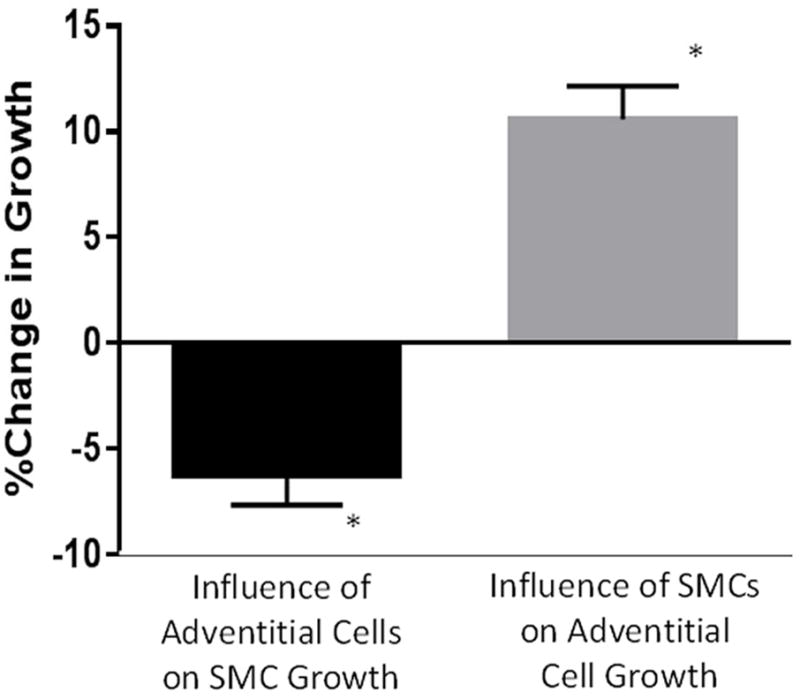
Adventitial cells inhibit SMC growth and SMCs stimulate adventitial cell growth. Each cell type was co-cultured across a membrane paired with its opposing type (or the same type for control). *P<.01 vs control for each bar graph, N=19 unique pairs.
We also wished to see if the clinical effects of their leg of origin persisted over time in cell culture. Long term cultures (passage 6) of the cells propagated from adventitial and intima/media explants showed no difference in their primary growth rates according to the degree of ischemia (data not shown). Nor did the factor of ischemia status have any influence on the counter-inhibitory/stimulatory effects of cultured adventitial and SMCs on each other’s growth (not shown). However, the factor of smoking appeared to have a sustained effect in the cultured cells: the inhibitory effect of adventitial cells on SMC growth was significantly diminished in smokers (Figure 6A). In co-culture experiments, we found that AA genotype SMCs stimulated adventitial cell growth significantly more than the CC genotype. The converse trend was also true – AA adventitial cells inhibited SMC growth more than CC adventitial cells – but that difference did not reach statistical significance (Figure 6B). Finally, in this experiment we confirmed our prior observation14 that AA adventitial cells grow slower than CC adventitial cells, but SMCs did not show this genotype-dependent response (Supplemental Figure 4).
Figure 6.
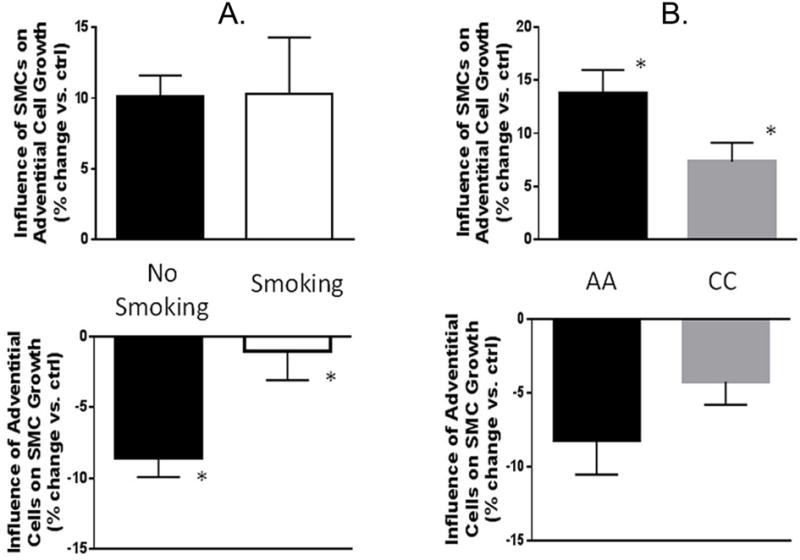
Effects of opposing cell types on growth, according to smoking status (panel A - left) and p27 SNP genotype (panel B - right). Current smoking decreases the inhibitory influence of adventitial cells on SMC growth, but not the stimulatory effect of SMCs on adventitial cells. N= 19 pairs of cells total, 13 for current smoking. *P<.01 smoking vs. no smoking. The protective AA p27 SNP genotype leads to more stimulation of adventitial cell growth by SMCs and a trend for more inhibition of SMC growth by adventitial cells. N=19 pairs of cells total, 10 for for AA. *P<.04, AA vs. CC.
Discussion
We have correlated the clinical characteristics of the patients and their legs from which the saphenous vein was harvested, with the ex vivo cellular behavior of the vein components using an ex vivo model that reflects two main vascular responses to injury: initial migration of cells, followed by proliferation of the migrated cells. The chronology and biology of this process has been confirmed in vivo in primates and rodents16, 22, 23. Overall, the adventitial layer of the vein showed significantly faster and greater overall outgrowth of cells than the intima/medial layer, which is similar to what has been observed for porcine arteries24. Retarded outgrowth of adventitial cells was associated with the clinical factors of ischemia of the leg, smoking, and the presence of infection in the leg. The severity of ischemia in the donor leg was the dominant factor, even after accounting for other clinical factors. In contrast, the outgrowth of smooth muscle cells from the medial layer showed a more blunted response to these clinical factors. The responses to ischemia could not be explained by the overall prevalence of CD34+ progenitor cells, or endothelial cells in the vasa vasorum of the intact veins. In addition, of the two metrics we measured, migration (Migration-Positive Explants) was not significantly affected by the clinical factors, while the more global measure of migration/proliferation (Cells Per Explant) was more sensitive. This suggests that the initial migratory responses to injury may be less influenced by the deleterious factors of ischemia, smoking, etc, while the subsequent proliferative phase of the response to injury is more susceptible to external conditions.
Once the cells had grown out from the explants, and were cultured, the persistence of clinical effects was variable. The primary growth rates of these cultured cells were no longer influenced by the ischemia of the leg from which they came. However, co-culture of the cells revealed that they reciprocally inhibited and promoted each other’s growth: adventitial cells inhibited SMCs, and SMCs promoted growth of adventitial cells. In co-culture, smoking and the genetic trait of the −838C>A p27 SNP showed a lasting influence. Where SMCs promoted adventitial proliferation, the effect was stronger in the protective, AA genotype. Where adventitial cells inhibited SMC proliferation, there was a trend for a larger effect in the AA compared to the CC genotype. And advential cell inhibition of SMC growth was greatly diminished in smokers.
This study offers two novel perspectives. First, rarely have the clinical characteristics of the patient or their donor leg been correlated with the biological behavior of cells of the vein conduit. A number of research studies have examined the histological appearance of the vein prior to implantation. Some found a correlation between patency and certain preoperative histological characteristics such as intimal/medial wall thickening25–27, while others have not28. Wilson et. al. found that the thicker the preoperative medial layer, the more the SMCs proliferated in ex vivo culture29. The strong clinical associations between graft patency and the factors of leg ischemia and smoking are well established30, 31 32. In this report, ischemia and smoking were associated with impaired adventitial cell outgrowth. Even though some of the harmful biological effects of smoking may take years to recover after cessation, we may justify our cut-off of 30 days with supportive evidence suggesting that the systemic inflammatory profiles may ameliorate significantly within days to weeks of smoking cessation33, 34. The presence of leg infection has not been isolated as a specific risk factor in most clinical studies of vein graft failure, although there is some indirect evidence of an association35. Clinical studies have also shown that patients with the AA genotype of the p27 SNP experience superior long term graft patency17. The co-culture experiments show that the AA genotype enhanced SMC promotion of adventitial growth, and adventitial inhibition of SMCs, while smoking retarded adventitial inhibition of SMCs.
The second novel perspective offers a general mechanistic theory: that a healthy, adaptive response to vascular injury may include increased activity of certain cells arising from the adventitia. Contrary to the conventional wisdom that all cellular proliferation and migration in response to vascular injury leads inevitably to intimal hyperplasia and stenosis, the migration and proliferation of certain adventitial cells may be a salutary response. Prior studies have shown that murine and porcine adventitial cells do migrate to the intima of vein grafts36–38 as has been observed for injured arteries39. Historically, it has been assumed that these adventitial cells pathologically contribute to intimal hyperplasia. However, support for a beneficial role for adventitial cells comes from Betz et al., who reported that adventitial cells inhibited SMC migration and proliferation in arterial tissue explants40 and from the results of Barker et al. that also suggest an inhibitory effect of the adventitia on intimal hyperplasia41. Our observations that ischemia inhibits adventitial cell outgrowth and that adventitial cells inhibit SMC proliferation are consistent with the hypothesis that certain adventitial cells may play a protective role by inhibiting the proliferation of SMCs - the dominant actors in intimal hyperplasia and graft failure42. Our data suggest that the effects of ischemia may predominate in the proliferative phase of the response to injury, not in the initial migration of cells.
We do not yet know the identity of the adventitial cells that migrate from adventitial tissue in these experiments, given the cellular heterogeneity of the adventitia. However, the adventitial cells are distinctly different from the medial SMCs. The adventitial cells are predominately smooth muscle α-actin negative, while the SMCs are predominately positive14. Compared to the adventitial cells, the SMCs overexpress genes in the areas of inflammation and infection, TNF and cytokine signaling; and NFκB, PI3K/Akt, and MAPK signaling. In contrast, the adventitial cells dominate in the expression of gene families associated with development and stem/progenitor cell maintenance. These characteristics suggest the possibility that CD34+ progenitor cells give rise to the migrating adventitial cells. The CD34+ progenitor cell has been shown to possess immunomodulatory, anti-inflammatory and tissue repair properties.43–46 The human venous CD34+ progenitor cell, like the cultured adventitial cell of the current study, does not express smooth muscle α-actin within the vein nor after in vitro culture47. This distinguishes these progenitor cells from other major cell types that populate the adventitia: SMCs, pericytes, and endothelial cells (after endothelial-mesenchymal transition). However, the challenge of identifying the source of the migrating adventitial cell is made more difficult by the fact that progenitor cells (as well as other vascular cells) change their phenotype once they emerge from the tissue and are cultured, losing their CD34 expression47. Only in genetically modified animal models has it been possible to track the true origins of cells from different vascular layers, as they change their phenotypes in response to injury48.
This study has important limitations. We do not yet know which resident adventitial cell(s) migrate from the tissue in response to injury. This is the subject of our ongoing work. And although the deleterious clinical factors we have identified are well known to impair graft patency, we cannot directly link graft patency with the measures of cellular outgrowth in this small sample. The microdissection technique separating the vein layers always leaves a small portion of the media with the adventitial layer, but this failing should blunt, rather than accentuate the differences we found between the responses of the two layers. The vast majority of the veins were derived from Caucasian males with relatively normal renal function, so we cannot make any inferences about the roles of gender,race, or kidney function. In the case of leg bypass surgery, some of the clinical factors can be interdependent. For example, the presence of wounds, infection, and the degree of ischemia may sometimes be linked. An extremely large sample size would be needed to define the independent effects of these different factors, or the gradations of severity of wounds or foot infection.
Vein harvest techniques are known to influence endothelial function, and although good practice standards were applied in the surgical harvest of the veins, those techniques were not standardized for this study. All but three of the bypass conduits were resected and re-implanted (three were done with the in situ technique). In the vast majority of cases the remnant of vein furnished for research was the most distal end of the vein, in keeping with the standard practice of conserving the portion of conduit with the largest diameter. We would argue that the ex vivo injury model used here represents a relatively severe injury in comparison to the subtleties of harvest technique, especially considering that we remove the lumenal endothelium in processing. In spite of the heterogeneity of harvesting techniques, all of the potentially deleterious clinical effects, such as ischemia and smoking, lead in the same direction of impaired adventitial cell outgrowth.
Conclusions
From the study of 46 human vein grafts we have found that cells grow out from the adventitial tissue more vigorously than from the media, and are more susceptible to the negative effects of leg ischemia, as well as smoking and leg infection. These adventitial cells have distinctly different gene expression profiles. If adventitial cells do play an important role in the vein’s response to injury, then this understanding opens the door to explore novel therapeutic approaches to prevent graft stenosis and improve patency, by targeting the responsible adventitial cells. Our continuing focus will be on these adventitial cells, their origins within the adventitial layer, and their modulation of the response to injury.
Supplementary Material
Clinical Relevance.
We prospectively studied saphenous veins from 46 infrainguinal bypasses, and found that ex vivo cellular outgrowth was significantly impaired by the deleterious clinical factors of leg ischemia, smoking, and infection – especially for the adventitial layer of the vein. The adventitial cells may play an important role in the healthy remodeling of a successful vein graft.
Take Home Message
The authors found that chronic leg ischemia, as well as smoking and leg infection, reduced adventitial cell outgrowth.
Recommendation
These data suggest that clinical factors influence adventitial cell biology and therefore may determine whether vein grafts remodel successfully or not.
Acknowledgments
This work was supported by National Institutes of Health grant HL30946 (M.S. and R.D.K.), a VA Research and Development Merit Review Award (M.S.), JSPS KAKENHI Grant 16K19962 (S.K.), and Institute of Translational Health Science (ITHS) grant support (UL1 RR025014 from NCRR/NIH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kikuchi S, Kenagy RD, Gao L, Wight TN, Azuma N, Sobel M, et al. Surgical marking pen dye inhibits saphenous vein cell proliferation and migration in saphenous vein graft tissue. J Vasc Surg. 2016;63(4):1044–50. doi: 10.1016/j.jvs.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dreifaldt M, Souza DS, Loesch A, Muddle JR, Karlsson MG, Filbey D, et al. The “no-touch” harvesting technique for vein grafts in coronary artery bypass surgery preserves an intact vasa vasorum. J Thorac Cardiovasc Surg. 2011;141(1):145–50. doi: 10.1016/j.jtcvs.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Adcock OT, Jr, Adcock GL, Wheeler JR, Gregory RT, Snyder SO, Jr, Gayle RG. Optimal techniques for harvesting and preparation of reversed autogenous vein grafts for use as arterial substitutes: a review. Surgery. 1984;96(5):886–94. [PubMed] [Google Scholar]
- 4.Sakaguchi T, Asai T, Belov D, Okada M, Pinsky DJ, Schmidt AM, et al. Influence of ischemic injury on vein graft remodeling: role of cyclic adenosine monophosphate second messenger pathway in enhanced vein graft preservation. J Thorac Cardiovasc Surg. 2005;129(1):129–37. doi: 10.1016/j.jtcvs.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Wise ES, Hocking KM, Luo W, Feldman DL, Song J, Komalavilas P, et al. Traditional graft preparation decreases physiologic responses, diminishes viscoelasticity, and reduces cellular viability of the conduit: A porcine saphenous vein model. Vasc Med. 2016;21(5):413–21. doi: 10.1177/1358863X16649040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harskamp RE, Alexander JH, Schulte PJ, Brophy CM, Mack MJ, Peterson ED, et al. Vein graft preservation solutions, patency, and outcomes after coronary artery bypass graft surgery: follow-up from the PREVENT IV randomized clinical trial. JAMA Surg. 2014;149(8):798–805. doi: 10.1001/jamasurg.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wise ES, Brophy CM. The Case for Endothelial Preservation via Pressure-Regulated Distension in the Preparation of Autologous Saphenous Vein Conduits in Cardiac and Peripheral Bypass Operations. Front Surg. 2016;3:54. doi: 10.3389/fsurg.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wise ES, Hocking KM, Eagle S, Absi T, Komalavilas P, Cheung-Flynn J, et al. Preservation solution impacts physiologic function and cellular viability of human saphenous vein graft. Surgery. 2015;158(2):537–46. doi: 10.1016/j.surg.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cull DL, Manos G, Hartley MC, Taylor SM, Langan EM, Eidt JF, et al. An early validation of the Society for Vascular Surgery lower extremity threatened limb classification system. J Vasc Surg. 2014;60(6):1535–41. doi: 10.1016/j.jvs.2014.08.107. [DOI] [PubMed] [Google Scholar]
- 10.Mills JL, Sr, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI) J Vasc Surg. 2014;59(1):220–34. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Rutherford RB, Flanigan DP, Gupta SK, Johnston KW, Karmody A, Whittemore AD, et al. Suggested standards for reports dealing with lower extremity ischemia. J Vasc Surg. 1986;4:80–94. [Google Scholar]
- 12.Kenagy RD, Hart CE, Stetler-Stevenson WG, Clowes AW. Primate smooth muscle cell migration from aortic explants is mediated by endogenous platelet-derived growth factor and basic fibroblast growth factor acting through matrix metalloproteinases 2 and 9. Circulation. 1997;96(10):3555–60. doi: 10.1161/01.cir.96.10.3555. [DOI] [PubMed] [Google Scholar]
- 13.Kenagy RD, Civelek M, Kikuchi S, Chen L, Grieff A, Sobel M, et al. Scavenger receptor class A member 5 (SCARA5) and suprabasin (SBSN) are hub genes of coexpression network modules associated with peripheral vein graft patency. J Vasc Surg. 2016;64(1):202–9. doi: 10.1016/j.jvs.2014.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kenagy RD, Kikuchi S, Chen L, Wijelath ES, Stergachis AB, Stamatoyannopoulos J, et al. A single nucleotide polymorphism of cyclin-dependent kinase inhibitor 1B (p27Kip1) associated with human vein graft failure affects growth of human venous adventitial cells but not smooth muscle cells. J Vasc Surg. 2018;67(1):309–17. doi: 10.1016/j.jvs.2016.12.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenagy RD, Clowes AW. Blockade of smooth muscle cell migration and proliferation in baboon aortic explants by interleukin-1beta and tumor necrosis factor-alpha is nitric oxide-dependent and nitric oxide-independent. J Vasc Res. 2000;37(5):381–9. doi: 10.1159/000025754. [DOI] [PubMed] [Google Scholar]
- 16.Kenagy RD, Vergel S, Mattsson E, Bendeck M, Reidy MA, Clowes AW. The role of plasminogen, plasminogen activators, and matrix metalloproteinases in primate arterial smooth muscle cell migration. Arterioscler Thromb Vasc Biol. 1996;16(11):1373–82. doi: 10.1161/01.atv.16.11.1373. [DOI] [PubMed] [Google Scholar]
- 17.Conte MS, Owens CD, Belkin M, Creager MA, Edwards KL, Gasper WJ, et al. A single nucleotide polymorphism in the p27(Kip1) gene is associated with primary patency of lower extremity vein bypass grafts. J Vasc Surg. 2013;57(5):1179–85. doi: 10.1016/j.jvs.2012.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu YJ, Lu SH, Xu B, Yang RC, Ren Q, Liu B, et al. Hemangiopoietin, a novel human growth factor for the primitive cells of both hematopoietic and endothelial cell lineages. Blood. 2004;103(12):4449–56. doi: 10.1182/blood-2003-06-1825. [DOI] [PubMed] [Google Scholar]
- 19.Reis M, Liebner S. Wnt signaling in the vasculature. Exp Cell Res. 2013;319(9):1317–23. doi: 10.1016/j.yexcr.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 20.Alquraini A, Garguilo S, D’Souza G, Zhang LX, Schmidt TA, Jay GD, et al. The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: an anti–inflammatory role of PRG4 in synovial fluid. Arthritis Res Ther. 2015;17:353. doi: 10.1186/s13075-015-0877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Y, Li G, Zhang JF. The role of long non-coding RNA H19 in musculoskeletal system: A new player in an old game. Exp Cell Res. 2017;360(2):61–5. doi: 10.1016/j.yexcr.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 22.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49(3):327–33. [PubMed] [Google Scholar]
- 23.Zou Y, Qi Y, Roztocil E, Nicholl SM, Davies MG. Patterns of kinase activation induced by injury in the murine femoral artery. J Surg Res. 2007;142(2):332–40. doi: 10.1016/j.jss.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi Y, Patel S, Niculescu R, Chung W, Desrochers P, Zalewski A. Role of matrix metalloproteinases and their tissue inhibitors in the regulation of coronary cell migration. Arterioscler Thromb Vasc Biol. 1999;19(5):1150–5. doi: 10.1161/01.atv.19.5.1150. [DOI] [PubMed] [Google Scholar]
- 25.Marin ML, Veith FJ, Panetta TF, Gordon RE, Wengerter KR, Suggs WD, et al. Saphenous vein biopsy: a predictor of vein graft failure. J Vasc Surg. 1993;18(3):407–14. [PubMed] [Google Scholar]
- 26.Perek B, Malinska A, Stefaniak S, Ostalska-Nowicka D, Misterski M, Zabel M, et al. Predictive factors of late venous aortocoronary graft failure: ultrastructural studies. PLoS One. 2013;8(8):e70628. doi: 10.1371/journal.pone.0070628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kouzi-Koliakos K, Kanellaki-Kyparissi M, Marinov G, Knyazhev V, Tsalie E, Batzios C, et al. Prebypass histological and ultrastructural evaluation of the long saphenous vein as a predictor of early graft failure. Cardiovasc Pathol. 2006;15(6):336–46. doi: 10.1016/j.carpath.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 28.James DC, Durrani T, Wixon CL, Hughes JD, Westerband A, Mills JL. Preimplant vein intimal thickness is not a predictor of bypass graft stenosis. J Surg Res. 2001;96(1):1–5. doi: 10.1006/jsre.2000.6046. [DOI] [PubMed] [Google Scholar]
- 29.Wilson YG. Vein quality in infrainguinal revascularisation: assessment by angioscopy and histology. Ann R Coll Surg Engl. 1998;80(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- 30.Goodney PP, Nolan BW, Schanzer A, Eldrup-Jorgensen J, Bertges DJ, Stanley AC, et al. Factors associated with amputation or graft occlusion one year after lower extremity bypass in northern New England. AnnVascSurg. 2010;24(1):57–68. doi: 10.1016/j.avsg.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Willigendael EM, Teijink JA, Bartelink ML, Peters RJ, Buller HR, Prins MH. Smoking and the patency of lower extremity bypass grafts: a meta-analysis. J Vasc Surg. 2005;42(1):67–74. doi: 10.1016/j.jvs.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 32.Cheshire NJ, Wolfe JH, Barradas MA, Chambler AW, Mikhailidis DP. Smoking and plasma fibrinogen, lipoprotein (a) and serotonin are markers for postoperative infrainguinal graft stenosis. Eur J Vasc Endovasc Surg. 1996;11(4):479–86. doi: 10.1016/s1078-5884(96)80185-7. [DOI] [PubMed] [Google Scholar]
- 33.Reichert V, Xue X, Bartscherer D, Jacobsen D, Fardellone C, Folan P, et al. A pilot study to examine the effects of smoking cessation on serum markers of inflammation in women at risk for cardiovascular disease. Chest. 2009;136(1):212–9. doi: 10.1378/chest.08-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodrigues FM, Ramos D, Xavier RF, Ito JT, Souza AP, Fernandes RA, et al. Nasal and systemic inflammatory profile after short term smoking cessation. Respir Med. 2014;108(7):999–1006. doi: 10.1016/j.rmed.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen LL, Lipsitz SR, Bandyk DF, Clowes AW, Moneta GL, Belkin M, et al. Resource utilization in the treatment of critical limb ischemia: The effect of tissue loss, comorbidities, and graft-related events. J Vasc Surg. 2006;44(5):971–5. doi: 10.1016/j.jvs.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 36.Tomas JJ, Stark VE, Kim JL, Wolff RA, Hullett DA, Warner TF, et al. Beta-galactosidase-tagged adventitial myofibroblasts tracked to the neointima in healing rat vein grafts. J Vasc Res. 2003;40(3):266–75. doi: 10.1159/000071890. [DOI] [PubMed] [Google Scholar]
- 37.Shi Y, O’Brien JE, Jr, Mannion JD, Morrison RC, Chung W, Fard A, et al. Remodeling of autologous saphenous vein grafts. The role of perivascular myofibroblasts. Circulation. 1997;95(12):2684–93. doi: 10.1161/01.cir.95.12.2684. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y, O’Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94(7):1655–64. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 39.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, et al. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89(12):1111–21. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 40.Betz E, Fallier-Becker P, Wolburg-Buchholz K, Fotev Z. Proliferation of smooth muscle cells in the inner and outer layers of the tunica media of arteries: an in vitro study. J Cell Physiol. 1991;147(3):385–95. doi: 10.1002/jcp.1041470302. [DOI] [PubMed] [Google Scholar]
- 41.Barker SG, Tilling LC, Miller GC, Beesley JE, Fleetwood G, Stavri GT, et al. The adventitia and atherogenesis: removal initiates intimal proliferation in the rabbit which regresses on generation of a ‘neoadventitia’. Atherosclerosis. 1994;105(2):131–44. doi: 10.1016/0021-9150(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 42.Owens CD, Gasper WJ, Rahman AS, Conte MS. Vein graft failure. J Vasc Surg. 2015;61(1):203–16. doi: 10.1016/j.jvs.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Peault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21(8):1299–308. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Worsdorfer P, Mekala SR, Bauer J, Edenhofer F, Kuerten S, Ergun S. The vascular adventitia: An endogenous, omnipresent source of stem cells in the body. Pharmacol Ther. 2017;171:13–29. doi: 10.1016/j.pharmthera.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 45.Klein D. Vascular Wall-Resident Multipotent Stem Cells of Mesenchymal Nature within the Process of Vascular Remodeling: Cellular Basis, Clinical Relevance, and Implications for Stem Cell Therapy. Stem Cells Int. 2016;2016:1905846. doi: 10.1155/2016/1905846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Orlandi A. The contribution of resident vascular stem cells to arterial pathology. Int J Stem Cells. 2015;8(1):9–17. doi: 10.15283/ijsc.2015.8.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Krankel N, Katare R, et al. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121(15):1735–45. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Majesky MW, Horita H, Ostriker A, Lu S, Regan JN, Bagchi A, et al. Differentiated Smooth Muscle Cells Generate a Subpopulation of Resident Vascular Progenitor Cells in the Adventitia Regulated by Klf4. Circ Res. 2017;120(2):296–311. doi: 10.1161/CIRCRESAHA.116.309322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


