Abstract
Objective:
To assess whether adiposity measures differed according to joint categories of sleep duration and sleep variability in a sample of Mexican adolescents.
Study design:
A sample of 528 Mexico City adolescents aged 9 to 17 years wore wrist actigraphs for 6–7 days. Average age-specific sleep duration was categorized as sufficient or insufficient. Sleep variability, the standard deviation of sleep duration, was split at the median into stable versus variable. Adiposity measures -- BMI-for-age Z score (BMIz), triceps skinfolds, waist circumference, and percent body fat -- were collected by trained assistants. We regressed adiposity measures on combined sleep duration and variability categories. Log binomial models were used to estimate prevalence ratios (PR) and 95% confidence intervals (CI) for obesity (>2 BMIz) by joint categories of sleep duration and variability, adjusting for sex, age, and maternal education.
Results:
Approximately 40% of the adolescents had insufficient sleep, and 13% were obese. Relative to sufficient-stable sleepers, adolescents with insufficient-stable sleep had higher adiposity across all four measures (eg, adjusted difference in BMIz was 0.68, 95% CI 0.35, 1.00); and higher obesity prevalence (PR=2.54; 95% CI 1.36, 4.75). Insufficient-variable sleepers had slightly higher BMIz than sufficient-stable sleepers (adjusted difference=0.30, 95% CI 0.00, 0.59).
Conclusions:
Adolescents with consistently insufficient sleep could be at higher obesity risk. The finding that insufficient-variable sleepers had only slightly higher adiposity suggests that opportunities for “catch-up” sleep may be protective.
Keywords: BMI-for-age Z score, triceps skinfolds, waist circumference, percent body fat, catchup sleep
Adolescent obesity continues to be a pressing global public health problem, and Mexico is one of the countries with the highest prevalence, with 13–15% of Mexican adolescents classified as obese.1 Although the causes of excess adiposity are complex, diet and physical activity are among the most well-studied lifestyle predictors of adolescent obesity.2 Nonetheless, as dietary and physical activity interventions have had mixed results for obesity prevention or weight loss3,4, other potentially modifiable determinants of obesity have garnered attention.
Short sleep duration has been identified as a predictor of obesity development in adolescents.5–7 Recent meta-analyses of longitudinal studies reported greater probability of developing obesity or greater changes in BMI z-scores among children and adolescents with short sleep duration at baseline.6,7 This association is of concern in adolescents, who are prone to chronic sleep deprivation.8 Another common feature of adolescent sleep, high variability in night-to-night sleep duration,9 may be associated with obesity risk independently of average sleep duration. Two studies among US adolescents found that within-individual variability in actigraphy-assessed sleep duration was positively related to abdominal adiposity10 and body mass index.11 In contrast, other studies have reported null associations between sleep variability and adiposity measures.12–14 One possible explanation of the discrepant findings could be the presence of effect modification, such that a positive association between sleep variability and adiposity is more evident among adolescents with insufficient sleep. We therefore evaluated whether adiposity measures differed according to joint categories of sleep duration and sleep variability in a sample of Mexican adolescents. We hypothesized that relative to adolescents with sufficient-stable sleep, adolescents with insufficient and variable sleep would have higher adiposity, and the insufficient-variable group would have the highest adiposity measures.
METHODS
The study population includes adolescent participants from 3 sequentially-enrolled cohorts of the Early Life Exposure in Mexico to ENvironmental Toxicants (ELEMENT) study.15,16 Between 1997 and 2004, 1079 mother/child dyads were recruited from prenatal clinics of the Mexican Social Security Institute in Mexico City, which serves low- to middle-income populations formally employed in the private sector. Beginning in 2015, a subset of 550 participants from the original birth cohorts 2 and 3 who were in the midst of pubertal transition (ages 9 to 17 years) participated in a follow-up study. The present study is a cross-sectional analysis of measurements made during this wave of follow-up. The institutional review boards at the Mexico National Institute of Public Health and the University of Michigan approved research protocols, and informed consent was obtained from all participants.
Trained research assistants measured height (in cm, BAME Model 420; Catalogo Medico, weight (in kg, BAME Model 420; Catalogo Medico), triceps skinfolds (in mm, Lange calipers; Beta Technology) and waist circumference (in cm, QM2000; QuickMedical). They measured percent body fat by bioelectrical impedance (InBody USA). All measurements were taken twice and averaged. BMI-for-age Z scores (BMIz) were calculated based on the World Health Organization reference,17 with a z score>2 defined as obese.
At the clinic visit, adolescents were given an actigraph (ActiGraph GT3X+; ActiGraph LLC, Pensacola, FL) to wear on their non-dominant wrist continuously for 7 days. Nightly sleep duration was estimated from the actigraphic data with the use of a fused lasso (least absolute shrinkage and selection operator)-based calculator package developed in R (R Foundation for Statistical Computing, Vienna, Austria). The obtained estimates were highly correlated with manual sleep duration detection in a validation subset of 50 participants (r=0.95). We used nightly sleep duration averaged over the wear time and sleep variability as reflected by the individual standard deviation of the average sleep duration to assign adolescents into four mutually exclusive groups by sleep duration and sleep variability: 1) sufficient-stable; 2) sufficient-variable; 3) insufficient-stable; and 4) insufficient-variable. To classify adolescents into sufficient versus insufficient sleepers, we compared their average sleep duration with agespecific sleep duration recommendations of the American Academy of Sleep Medicine.18 To classify adolescents into stable or variable sleepers, we split them at the median of the individual standard deviations.
Covariates included age, sex, pubertal status, maternal education, physical activity, screen time, sedentary time spent commuting (bus, car, etc.), alcohol consumption habits, and smoking behavior. Sexual maturation status, assessed by Tanner staging and testicular volume assessment (for boys), were completed by trained physicians during the visit using standard methods.19 Girls were also asked whether they had started menstruating. To assess pubertal status, we classified participants into those who had reached the latter stages of puberty by the visit (testicular volume ≥15 mm for boys and onset of menarche for girls) and those who had not. Maternal education was categorized as <9 years, 9 to <12 years, 12 years, or >12 years. Physical activity and sedentary behavior were assessed with a questionnaire adapted for and validated in Mexican adolescents 20. To calculate average hours of physical activity per week, we added the selfreported time in hours spent in all potential physical activities (e.g. soccer, volleyball, running). We categorized into quartiles the average number of hours spent doing physical activity per week, average number of hours of screen time per week, and average hours spent sitting while commuting per week. We classified alcohol and smoking behaviors into dichotomous variables: for alcohol, whether they had self-reported consuming alcohol in the past year, and for smoking, whether they had ever tried smoking.
Statistical Analyses
A total of 550 adolescents participated in the peri-pubertal visit. Of those, 539 consented to wear the wrist actigraphs and returned them at the end of the 7-day period. After exclusion of adolescents with <4 days of continuous actigraph wear (n=11), the final analytic sample included 528 (96%) adolescents.
To describe the study population and to assess potential confounders, we first calculated summary statistics of sleep characteristics (average ± SD sleep duration, the proportion not meeting sleep duration recommendations, and average ± SD sleep variability) stratified by categories of sociodemographic and lifestyle characteristics. Next, we reported the proportion of adolescents in each of the four sleep categories according to categorical sociodemographic and lifestyle characteristics. To evaluate the differences in adiposity measures for each sleep category, as compared with the sufficient-stable sleepers (reference group), we used multiple linear regression models in which the adiposity measure was the outcome and sleep categories were the dummy predictors. To account for potential confounders, we adjusted for all covariates listed above, but for sake of parsimony only retained sex, age, maternal education, and smoking behavior in the models (inclusion of other variables did not alter estimates). Further, we did not adjust for dietary variables such as carbohydrate or total energy intake, as they may be mediators on the causal pathway; and adjusting for mediators could result in biased estimates.21 To formally test for effect modification between sleep sufficiency (dichotomous) and sleep variability (continuous), we ran a linear regression model that also included product terms for sleep sufficiency*sleep variability. Finally, we used log binomial models to calculate adjusted prevalence ratios and 95% confidence intervals for obesity prevalence comparing each sleep category with the sufficient-stable category, and adjusting for the same potential confounders. Statistical analyses were conducted in Stata 14.0 (College Station, TX).
RESULTS
The mean ± SD age of the adolescents was 14.4 ± 2.1 years. The mean sleep duration was 516 ± 58 minutes (8.6 ± 1.0 hours); it did not differ on average from weekdays (Sunday through Thursday nights) to weekends (516 ± 65 minutes and 517 ± 73 minutes, respectively). Based on AASM age-specific sleep recommendations, 41% of the sample had insufficient sleep on average (Table 1). Older adolescents had shorter sleep duration than younger participants, although the younger age groups were more likely to not meet the age-specific sleep recommendations. For example, 65% of the children <12 years of age had insufficient sleep for their age, twice as many as adolescents aged 16 years or older. Age was positively associated with sleep variability; there was a difference of almost 30 minutes in sleep variability between the youngest and the oldest adolescents. Sufficient sleep and sleep variability were associated with smoking and pubertal status (Table 1), although after age adjustment only the association between smoking and higher sleep variability persisted (data not shown). Finally, higher amount of sedentary commuting time (e.g. bus, car) was related to shorter sleep duration.
Table 1.
Average sleep duration and variability of 528 youth aged 9–18 y from Mexico City, according to sociodemographic predictors
| Sociodemographic predictors | N | Mean sleep duration (min)±SD | Did not meet sleep recommendations,1 % | Mean sleep variability (min)± SD | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 252 | 511 ± 55 | 41.3 | 91 ± 43 | |
| Female | 276 | 520 ± 60 | 40.2 | 95 ± 41 | |
| P value2 | 0.07 | 0.81 | 0.36 | ||
| Age group, years (y) | |||||
| 9.5 to <12 y | 93 | 525 ± 45 | 65.6 | 78 ± 37 | |
| 12 to <14 y | 154 | 522 ± 51 | 42.9 | 84 ± 39 | |
| 14 to <16 y | 98 | 513 ± 55 | 28.6 | 99 ± 42 | |
| 16 to 18 y | 183 | 508 ± 67 | 32.8 | 105 ± 44 | |
| P value | 0.007 | <0.0001 | <0.0001 | ||
| Testicular volume (boys only) | |||||
| <15 mm | 41 | 511 ± 42 | 58.5 | 68 ± 31 | |
| ≥15 mm (latter stages of puberty) | 199 | 511 ± 56 | 38.2 | 95 ± 44 | |
| P value | 0.94 | 0.02 | <0.0001 | ||
| Menarche status (girls only) | |||||
| Had not experienced | 45 | 532 ± 49 | 55.6 | 81 ± 35 | |
| Had experienced | 228 | 519 ± 62 | 37.3 | 97 ± 42 | |
| P value | 0.18 | 0.02 | 0.02 | ||
| Maternal education, years (y) | |||||
| 8 y or less (secondary or primary) | 61 | 520 ± 58 | 36.1 | 98 ± 46 | |
| 9 to 11 y (some high school) | 205 | 519 ± 57 | 39.5 | 96 ± 43 | |
| 12 y (completed high school) | 181 | 516 ± 59 | 37.6 | 90 ± 41 | |
| >12 y | 76 | 506 ± 58 | 51.3 | 88 ± 39 | |
| P value | 0.15 | 0.18 | 0.06 | ||
| Physical activity, quartiles | |||||
| Q1, 0 to 5.5 hours/week (h/wk) | 136 | 516 ± 61 | 39.0 | 98 ± 47 | |
| Q2, 5.8 to 9 h/wk | 128 | 522 ± 58 | 39.1 | 94 ± 42 | |
| Q3, 9.3 to 14 h/wk | 138 | 510 ± 55 | 42.0 | 89 ± 43 | |
| Q4, 14.3 to 29 h/wk | 126 | 517 ± 55 | 42.9 | 90 ± 36 | |
| P value | 0.63 | 0.88 | 0.10 | ||
| Screen time, quartiles | |||||
| Q1, 1 to 22.5 hours/week (h/wk) | 135 | 519 ± 53 | 42.2 | 89 ± 41 | |
| Q2, 23 to 32.5 h/wk | 130 | 516 ± 58 | 44.6 | 94 ± 42 | |
| Q3, 33 to 48 h/wk | 131 | 509 ± 59 | 42.8 | 93 ± 45 | |
| Q4, 48.5 to 116 h/wk | 132 | 520 ± 60 | 33.3 | 95 ± 41 | |
| P value | 0.92 | 0.25 | 0.29 | ||
| Sedentary time in commuting, quartiles | |||||
| Q1, 3.5 hours/week (h/wk) or less | 223 | 523.7 ± 54.6 | 36.8 | 93.7 | 43.1 |
| Q2, >3.5 h/wk to 5.5 h/wk | 57 | 516.9 ± 58.4 | 35.1 | 77.1 | 35.1 |
| Q3, >5.5 h/wk to 10.5 h/wk | 171 | 511.1 ± 57.1 | 43.2 | 98.1 | 45.0 |
| Q4, >10.5 h/wk to 31.5 h/wk | 77 | 504.7 ± 64.8 | 50.7 | 90.7 | 35.4 |
| P value | 0.004 | 0.12 | 0.72 | ||
| Consumed alcohol in the past year | |||||
| No | 61 | 524 ± 63 | 37.8 | 89 ± 37 | |
| Yes | 342 | 512 ± 59 | 38.0 | 98 ± 43 | |
| P value | 0.16 | 0.96 | 0.14 | ||
| Ever smoked cigarettes | |||||
| No | 391 | 518 ± 54 | 42.4 | 87 ± 40 | |
| Yes | 133 | 509 ± 67 | 32.3 | 110 ± 45 | |
| P value | 0.11 | 0.02 | <0.0001 | ||
Based on the American Academy of Sleep Medicine recommendations; the recommendation for children <13 years old is between 9 and 12 hours of sleep per 24 hour period, and the recommendation for children 13 to 18 years old is between 8 and 10 hours of sleep per 24 hour period
P values were obtained from linear regression models with average sleep duration as the outcome and sociodemographic correlate as the exposure. For ordinal characteristics, a P for trend was obtained by including in the model a continuous variable that represented the ordinal categories. For dichotomous characteristics, a Wald test was used.
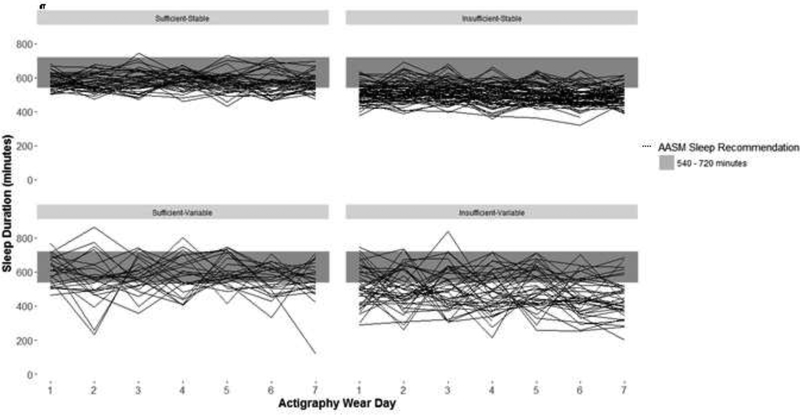
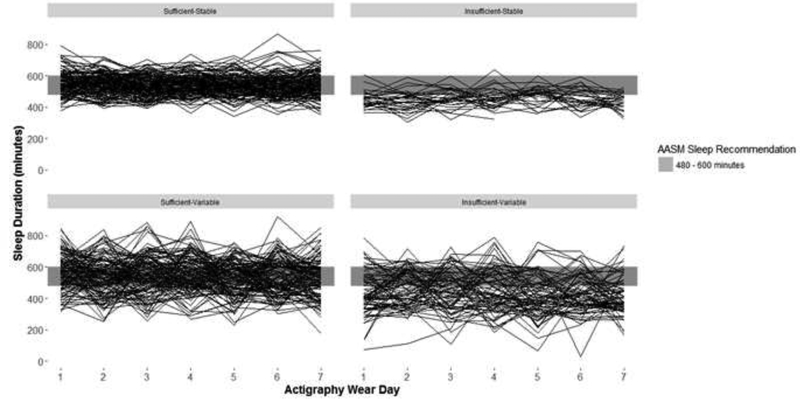
Sleep duration and sleep variability for adolescents younger than 13 years and those 13 years or older are presented in Figure, A and B, respectively. The distribution of adolescents across the four sleep categories – sufficient-stable, sufficient-variable, insufficient-stable, and insufficient-variable – was 31%, 28%, 19% and 22% respectively. Sex, age, pubertal stage, and smoking experience were distributed differently according to the joint sleep duration and variability categories (Table 2).
Figure A1.
Sleep duration and variability patterns in adolescents aged <13 years (N=178)
Figure B1.
Sleep duration and variability patterns in adolescents aged ≥13 years (N=360)
Table 2.
Categories of average sleep duration and variability in relation to sociodemographic predictors among 528 adolescents aged 9–18 y from Mexico City, Mexico
| Sociodemographic predictors | N | Sufficient-stable duration, % N=166 | Sufficient-variable duration, % N=147 | Insufficient-stable duration, % N=98 | Insufficient-variable duration, % N=117 | P value1 |
|---|---|---|---|---|---|---|
| Average sleep duration (min), mean ± SD | 549 ± 43 | 549 ± 40 | 487 ± 36 | 453 ± 41 | <0.0001 | |
| Sleep duration variability (min), mean ± SD | 61 ± 16 | 123 ± 32 | 57 ± 17.0 | 129 ± 34 | <0.0001 | |
| Sex | 0.046 | |||||
| Male | 252 | 29.3 | 29.4 | 22.6 | 18.7 | |
| Female | 276 | 33.3 | 26.5 | 14.9 | 25.4 | |
| Age group | <0.0001 | |||||
| 9.5 to <12 years | 93 | 20.4 | 14.0 | 41.9 | 23.7 | |
| 12 to <14 years | 154 | 37.7 | 19.5 | 22.7 | 20.1 | |
| 14 to <16 years | 98 | 34.7 | 36.7 | 10.2 | 18.4 | |
| 16 to <18 years | 183 | 30.1 | 37.2 | 7.7 | 25.1 | |
| Testicular volume (boys only) | <0.0001 | |||||
| <15 mm | 41 | 31.7 | 9.8 | 46.3 | 12.2 | |
| ≥15 mm (latter stages of puberty) | 199 | 29.7 | 32.2 | 18.6 | 19.6 | |
| Menarche status (girls only) | 0.005 | |||||
| Had not experienced | 45 | 31.1 | 13.3 | 31.1 | 24.4 | |
| Had experienced | 228 | 34.2 | 28.5 | 11.8 | 25.4 | |
| Mother’s education, years (y) | 0.37 | |||||
| 8 y or less (secondary or primary) | 61 | 34.4 | 29.5 | 11.5 | 24.6 | |
| 9 to 11 y (some high school) | 205 | 29.3 | 31.2 | 17.6 | 22.0 | |
| 12 y (completed high school) | 181 | 34.8 | 27.6 | 17.1 | 20.4 | |
| >12 y | 76 | 29.0 | 19.7 | 27.6 | 23.7 | |
| Physical activity, quartiles | 0.88 | |||||
| Q1, 0 to 5.5 hours/week (h/wk) | 136 | 28.7 | 32.4 | 19.1 | 19.9 | |
| Q2, 5.8 to 9 h/wk | 128 | 30.5 | 30.5 | 17.2 | 21.9 | |
| Q3, 9.3 to 14 h/wk | 138 | 34.8 | 23.2 | 19.6 | 22.5 | |
| Q4, 14.3 to 29 h/wk | 126 | 31.8 | 25.4 | 18.3 | 24.6 | |
| Screen time, quartiles | 0.42 | |||||
| Q1, 1 to 22.5 hours/week (h/wk) | 135 | 34.8 | 23.0 | 18.5 | 23.7 | |
| Q2, 23 to 32.5 h/wk | 130 | 30.0 | 25.4 | 21.5 | 23.1 | |
| Q3, 33 to 48 h/wk | 131 | 31.3 | 26.0 | 19.1 | 23.7 | |
| Q4, 48.5 to 116 h/wk | 132 | 29.6 | 37.1 | 15.2 | 18.2 | |
| Sedentary time in commuting, quartiles | 0.06 | |||||
| Q1, 3.5 hours/week (h/wk) or less | 223 | 30.9 | 32.3 | 17.9 | 18.8 | |
| Q2, >3.5 h/wk to 5.5 h/wk | 57 | 40.4 | 24.6 | 24.6 | 10.5 | |
| Q3, >5.5 h/wk to 10.5 h/wk | 171 | 30.4 | 26.3 | 18.1 | 25.2 | |
| Q4, >10.5 h/wk to 31.5 h/wk | 77 | 28.6 | 20.8 | 16.9 | 33.8 | |
| Consumed alcohol in the past year | 0.99 | |||||
| No | 61 | 31.2 | 31.2 | 14.8 | 23.0 | |
| Yes | 342 | 32.2 | 29.8 | 14.6 | 23.4 | |
| Ever smoked cigarettes | <0.0001 | |||||
| No | 391 | 35.0 | 21.5 | 21.7 | 21.7 | |
| Yes | 133 | 21.8 | 45.9 | 9.0 | 23.3 |
From a Chi-square test
Average BMIz (SD) was 0.51 (1.25); 25% of the adolescents were overweight and 13% were obese. Average body fat percentage, triceps skinfold and waist circumference were 26.9% (9.9%), 19.0 (6.8) mm, and 79.6 (11.5) cm, respectively. Adolescents classified as having insufficient-stable sleep had the highest BMIz, body fat percentage, triceps skinfold and waist circumference. The second highest adiposity measures were observed among those with insufficient-variable sleep, and sufficient-stable sleepers had the lowest adiposity measures. Similarly, in adjusted linear regression analysis, adolescents in the insufficient-stable sleep duration category had the largest differences in adiposity – BMIz, percent body fat, triceps skinfolds and waist circumference – when compared with adolescents in the sufficient-stable sleep duration (reference) category (0.68 with 95% CI 0.35, 1.00; 4.98 with 95% CI 2.77, 7.18; 4.44 with 95% CI 2.79, 6.09; and 5.42 with 95% CI 2.48, 8.36, respectively). In contrast, BMIfor-age z-scores and triceps skinfolds were only marginally different among adolescents with insufficient-variable duration compared with sufficient-stable sleep duration (0.30 with 95% CI 0.0, 0.59, and 1.45 with 95% CI -0.05, 2.95). The adiposity measures in the sufficient-variable sleep duration group were not statistically significantly different than the reference group (Table 3). In models with interaction terms for sleep sufficiency and variability, there was evidence of effect modification between sleep sufficiency and variability in relation to adiposity (BMIz P for interaction=0.04; percent body fat P for interaction=0.007; triceps skinfold P for interaction=0.02; and waist circumference P for interaction=0.03). Furthermore, these results confirm the presence of a qualitative interaction, as there was no association between sleep variability and adiposity among children with sufficient sleep, and there was an inverse association between sleep variability and adiposity among children with insufficient sleep.
Table 3.
Categories of average sleep duration and variability in relation to anthropometric indicators of adiposity among 528 adolescents aged 9–18 y from Mexico City, Mexico
| Adiposity Measure |
||||
|---|---|---|---|---|
| N | Mean ± SD | Adjusted β (95% CI)1,2 | P value3 | |
| BMI-for-age z-scores | ||||
| Average sleep duration and variability category | ||||
| Sufficient-stable duration | 166 | 0.31 ± 1.26 | Reference | |
| Sufficient -variable duration | 147 | 0.33 ± 1.31 | 0.05 (-0.23, 0.33) | 0.75 |
| Insufficient-stable duration | 95 | 1.03 ± 1.18 | 0.68 (0.35, 1.00) | <0.0001 |
| Insufficient-variable duration | 115 | 0.61 ± 1.09 | 0.30 (0.00, 0.59) | 0.047 |
| Percent body fat | ||||
| Average sleep duration and variability category | ||||
| Sufficient-stable duration | 165 | 25.8 ± 10.6 | Reference | |
| Sufficient-variable duration | 146 | 26.2 ± 9.7 | 0.93 (-0.98, 2.84) | 0.34 |
| Insufficient-stable duration | 98 | 28.8 ± 9.4 | 4.98 (2.77, 7.18) | <0.0001 |
| Insufficient-variable duration | 117 | 27.7 ± 9.5 | 1.41 (-0.58, 3.41) | 0.16 |
| Triceps skinfolds, mm | ||||
| Average sleep duration and variability category | ||||
| Sufficient-stable duration | 165 | 18.1 ± 6.7 | Reference | |
| Sufficient -variable duration | 147 | 18.4 ± 7.2 | 0.17 (-1.26, 1.60) | 0.82 |
| Insufficient-stable duration | 98 | 20.5 ± 7.0 | 4.44 (2.79, 6.09) | <0.0001 |
| Insufficient-variable duration | 117 | 19.7 ± 6.0 | 1.45 (-0.05, 2.95) | 0.06 |
| Waist circumference, cm | ||||
| Average sleep duration and variability category | ||||
| Sufficient-stable duration | 166 | 78.4 ± 11.7 | Reference | |
| Sufficient -variable duration | 147 | 79.9 ± 11.9 | 0.63 (-1.91, 3.17) | 0.63 |
| Insufficient-stable duration | 98 | 80.8 ± 12.1 | 5.42 (2.48, 8.36) | <0.0001 |
| Insufficient-variable duration | 117 | 80.0 ± 10.1 | 1.58 (-1.08, 4.24) | 0.24 |
From separate linear regression models with adiposity measure as the outcome and indicator variables for sleep duration and variability category as the exposure
Each model adjusted for sex, age, maternal education, and smoking behavior
From a Wald test
Finally, the adjusted prevalence of obesity was 154% higher in adolescents with insufficient-stable sleep compared with those with sufficient-stable (PR=2.54 (95% CI 1.36, 4.75)). There was also evidence of effect modification in this model (P for interaction=0.02).
DISCUSSION
Parallel to other populations, we showed a strong relationship between insufficient sleep and adiposity in this cohort of Mexican children. These findings represent an extension o of the prior literature as they suggest that a very specific group -- adolescents with chronic short sleep - may be at highest risk of obesity. Specifically, adolescents with insufficient-stable sleep as opposed to sufficient-stable sleep had a two-fold higher prevalence of obesity.
Our findings are distinct from those of the few other studies that independently examined sleep variability and duration with adiposity. In particular, two cross-sectional studies from the US showed positive associations between sleep variability and adiposity measures.10,11 The first, a US study of 305 older adolescents that used 7-night actigraphy to assess sleep, 10 reported a 7 cm2 higher visceral fat area with each 1-hr higher sleep variability, after adjustment for sleep duration. The second, also a US study of 247 adolescents, found a positive association between BMI and actigraphy-assessed sleep variability.11 However, the latter study did not control for sleep duration. In contrast, other cross-sectional studies found no differences in sleep variability in overweight or obese groups versus normal groups.12,24 Similarly, a longitudinal study in Danish children showed no association between baseline sleep variability based on self-reports and gain in adiposity 1 year later.14
Inconsistencies between our findings and those of prior reports may stem from differences in study designs and analytic approaches. For example, high sleep variability has been associated with low socioeconomic status (SES),25 and low SES is usually associated with higher adiposity in US and European settings. Therefore, residual confounding due to unadjusted SES might explain the findings of the previous studies. Second, previous studies did not consider sleep variability and sleep duration jointly; thus, true associations may have been masked. Third, reliance on self-report versus objective sleep measures in a few of the studies with null findings12,14 could have produced biased estimates.
In this study, adolescents who were chronically sleep-deficient, rather than insufficientvariable sleepers had the highest adiposity, despite similar amounts of sleep overall. One plausible explanation may have to do with the fact that insufficient-variable sleepers had nights with short sleep duration followed by nights with long sleep durations to compensate (“catch-up sleep”), and insufficient-stable sleepers had much less compensatory sleep. The notion of “catchup sleep” as protective of weight gain is supported by a few studies among Asian children or adolescents.26–28 Two independent studies from Korea and Hong Kong showed that in subgroups of schoolchildren with self-reported short sleep duration, those with longer weekend catch-up sleep had lower odds of obesity compared with those with less weekend catch-up sleep.27,28 Similarly, a Chinese study found that children who did not compensate for sleep on weekends or holidays had greater odds of overweight or obesity.26 Finally, one US study that compared sleep patterns in overweight and obese children versus normal weight children noted that children in the obese category were more likely to have short sleep on weekends.13 A protective role of catch-up sleep on adiposity gain may be related to the higher proportion of time is spent in stage 3 sleep, also known as slow-wave or deep sleep, observed followed a night of restricted sleep. It is during this sleep stage that growth hormone is released, which is an important hormone for linear growth and may protect against excess adiposity gain.29 Further evidence in adults demonstrates the short-term metabolic effects of ‘catch-up sleep’ following sleep restriction, and may also offer insight into mechanisms.30 In this small experimental study among sleep-deprived men, researchers found that after a typical work week, three nights of catch-up sleep on the weekend, as compared with 3 nights of continued restricted sleep, were associated with higher insulin sensitivity.
In accordance with the adolescent sleep literature,8 sleep deprivation was common in our study population. About 40% of the adolescents did not obtain sufficient sleep on average. Interestingly, in this Mexican cohort, the younger adolescents were more likely than older adolescents to not meet sleep recommendations, and sleep duration did not differ on weekends versus weekdays. Both of these findings are contrary to results from the US.8 Although speculative, cultural differences in sleeping arrangements (e.g. room or bed sharing with siblings) may partially explain the apparent discrepancy. Sleep duration variability, as observed in this study, had a similar magnitude to adolescents’ sleep variability in other reports. 10,31,32 Further, the higher sleep variability in adolescents of older ages compared with younger ages was also aligned with previous studies.33
This study has several limitations. First, the cross-sectional design of this study does not allow the examination of temporal associations between sleep characteristics and adiposity measures. Second, whereas high population density in Mexico City necessitates assignment of students to a two-shift school attendance, data on school start time were not available. However, as school shift assignment may not be strongly associated with adiposity, school start times are unlikely to be a strong confounder. Third, lack of information on obstructive sleep apnea, which is often comorbid with obesity, may confound the results. Finally, residual confounding may have occurred as a result of self-reported physical activity and sedentary behavior; in particular, television watching and vigorous activity may have been overestimated and moderate physical activity may have been underestimated.
Among adolescents with insufficient sleep, those with stable sleep rather than highly variable sleep had the highest prevalence of obesity. These results suggest potential metabolic benefits of intermittent sleep recovery for sleep-deprived adolescents.
Acknowledgments
E.J. received grant support from the National Institute of Diabetes and Digestive and Kidney Diseases (5T32 DK071212–12). G.D. received partial grant support from the National Institute of Neurological Disorders and Stroke (NIH/NINDS T32 NS007222) and from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/NICHD F32 HD091938). R.C. is named in or has developed patented and copyrighted materials owned by the University of Michigan and designed to assist with assessment or treatment of sleep disorders. R.C. serves on the boards of the American Academy of Sleep Medicine; the Association of Professional Sleep Societies (currently as President); the International Pediatric Sleep Society; and the nonprofit Sweet Dreamzzz; is an editor for UpToDate; has edited a book for Cambridge University Press; and served as a consultant for Zansors. The other authors declare no conflicts of interest.
Abbreviations:
- BMI
body mass index
- PR
prevalence ratio
- SES
socioeconomic status
- CI
confidence interval
- AASM
American Academy of Sleep Medicine
- ELEMENT
Early Life Exposure in Mexico to ENvironmental Toxicants
- SD
standard deviation
- SES
socioeconomic status
Footnotes
Ethics approval and consent to participate: The study was approved by the institutional review board at the University of Michigan and the National Institute of Public Health in Mexico, and informed consent was obtained from all participants.
Availability of Data and Materials: The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Shamah-Levi T, Cuevas-Nasu L, Dommarco-Rivera J, Hernandez-Avila M. Encuesta Nacional de Salud y Nutrición de Medio Camino 2016. (ENSANUT MC 2016). Inst Nac Salud Pública [Internet]. 2016;2016:151 Available from: http://promocion.salud.gob.mx/dgps/descargas1/doctos_2016/ensanut_mc_2016-310oct.pdf [Google Scholar]
- 2.Kumar S, Kelly AS. Review of Childhood Obesity: From Epidemiology, Etiology, and Comorbidities to Clinical Assessment and Treatment. Vol. 92, Mayo Clinic Proceedings. 2017. p. 251–65. [DOI] [PubMed] [Google Scholar]
- 3.Lobstein T, Jackson-Leach R, Moodie ML, Hall KD, Gortmaker SL, Swinburn BA, et al. Child and adolescent obesity: part of a bigger picture. Lancet (London, England) [Internet]. 2015. June 20 [cited 2017 Nov 18];385:2510–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25703114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Khudairy L, Loveman E, Colquitt JL, Mead E, Johnson RE, Fraser H, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years In: Rees K, editor. Cochrane Database of Systematic Reviews [Internet]. Chichester, UK: John Wiley & Sons, Ltd; 2017. [cited 2017 Nov 18]. p. CD012691 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28639320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell J a, Rodriguez D, Schmitz KH, Audrain-McGovern J Sleep duration and adolescent obesity. Pediatrics [Internet]. 2013;131:e1428–34. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3639456&tool=pmcentrez&rendertype=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatima Y, Doi SAR, Mamun AA. Longitudinal impact of sleep on overweight and obesity in children and adolescents: a systematic review and bias-adjusted meta-analysis. Obes Rev [Internet]. 2015. February [cited 2017 Nov 18];16:137–49. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25589359 [DOI] [PubMed] [Google Scholar]
- 7.Miller MA, Kruisbrink M, Wallace J, Ji C, Cappuccio FP. Sleep duration and incidence of obesity in infants, children, and adolescents: a systematic review and meta-analysis of prospective studies. Sleep [Internet]. 2018; Available from: https://academic.oup.com/sleep/advance-article/doi/10.1093/sleep/zsy018/4833233 [DOI] [PubMed] [Google Scholar]
- 8.Owens J, Adolescent Sleep Working Group ASW, Committee on Adolescence CO. Insufficient sleep in adolescents and young adults: an update on causes and consequences. Pediatrics [Internet]. 2014. September 1 [cited 2017 Nov 18];134:e921–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25157012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Redline S. Correlates of adolescent sleep time and variability in sleep time: the role of individual and health related characteristics. Sleep Med [Internet] 2011. March [cited 2017 Nov 22];12:239–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21316300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He F, Bixler EO, Liao J, Berg A, Imamura Kawasawa Y, Fernandez-Mendoza J, et al. Habitual sleep variability, mediated by nutrition intake, is associated with abdominal obesity in adolescents. Sleep Med [Internet]. 2015. December [cited 2017 Nov 18];16:1489–94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26611945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore M, Kirchner HL, Drotar D, Johnson N, Rosen C, Redline S. Correlates of adolescent sleep time and variability in sleep time: the role of individual and health related characteristics. Sleep Med [Internet]. 2011. March [cited 2017 Nov 18];12:239–45. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1389945711000177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo SI-C, Updegraff KA, Zeiders KH, McHale SM, Umaña-Taylor AJ, De Jesús SAR. Mexican American Adolescents’ Sleep Patterns: Contextual Correlates and Implications for Health and Adjustment in Young Adulthood. J Youth Adolesc [Internet]. 2015. February 22 [cited 2017 Nov 18];44:346–61. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25047598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spruyt K, Molfese DL, Gozal D. Sleep Duration, Sleep Regularity, Body Weight, and Metabolic Homeostasis in School-aged Children. Pediatrics [Internet]. 2011. February 1 [cited 2017. Nov 18];127:e345–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21262888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rangan A, Zheng M, Olsen NJ, Rohde JF, Heitmann BL. Shorter sleep duration is associated with higher energy intake and an increase in BMI z-score in young children predisposed to overweight. Int J Obes (Lond) [Internet]. 2017. September 8 [cited 2017 Nov 18]; Available from: http://www.nature.com/doifinder/10.1038/ijo.2017.216 [DOI] [PubMed] [Google Scholar]
- 15.Lewis RC, Meeker JD, Peterson KE, Lee JM, Pace GG, Cantoral A, et al. Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere. 2013;93:2390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ettinger AS, Lamadrid-Figueroa H, Mercado-García A, Kordas K, Wood RJ, Peterson KE, et al. Effect of calcium supplementation on bone resorption in pregnancy and the early postpartum: a randomized controlled trial in Mexican Women. Nutr J [Internet]. 2014;13:116 Available from: http://nutritionj.biomedcentral.com/articles/10.1186/1475-2891-13-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85:660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paruthi S, Brooks LJ, D’Ambrosio C, Hall WA, Kotagal S, Lloyd RM, et al. Recommended amount of sleep for pediatric populations: A consensus statement of the American Academy of Sleep Medicine. J Clin Sleep Med. 2016;12:785–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chavarro JE, Watkins DJ, Afeiche MC, Zhang Z, Sánchez BN, Cantonwine D, et al. Validity of Self-Assessed Sexual Maturation Against Physician Assessments and Hormone Levels. J Pediatr. 2017;186:172–178.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernández B, Gortmaker SL, Laird NM, Colditz GA, Parra-Cabrera S, Peterson KE. Validez y reproducibilidad de un cuestionario de actividad e inactividad fisica para escolares de la ciudad de Mexico. Salud Publica Mex. 2000;42:315–23. [PubMed] [Google Scholar]
- 21.Schisterman EF, Cole SR, Platf RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abarca-Gómez L, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, Acuin C, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet (London, England) [Internet]. 2017;390:2627–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29029897%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC5735219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernández-Cordero S, Cuevas-Nasu L, Morán-Ruán MC, Méndez-Gómez Humarán I, Ávila-Arcos MA, Rivera-Dommarco JA. Overweight and obesity in Mexican children and adolescents during the last 25 years. Nutr Diabetes [Internet]. 2017. March 13 [cited 2017 Nov 19];7:e247 Available from: http://www.ncbi.nlm.nih.gov/pubmed/28287630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spruyt K, Alaribe CU, Nwabara OU. Daily dynamics in sleep and behavior of young African-American children: A convoluted dyad?! Int J Psychophysiol [Internet]. 2016. January [cited 2017 Sep 8];99:57–66. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0167876015300404 [DOI] [PubMed] [Google Scholar]
- 25.Marco CA, Wolfson AR, Sparling M, Azuaje A. Family Socioeconomic Status and Sleep Patterns of Young Adolescents. Behav Sleep Med. 2011;10:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim CW, Choi MK, Im HJ, Kim OH, Lee HJ, Song J, et al. Weekend catch-up sleep is associated with decreased risk of being overweight among fifth-grade students with short sleep duration. J Sleep Res [Internet]. 2012. October 1 [cited 2017 Nov 19];21:546–51. Available from: http://doi.wiley.com/10.1111/j.1365-2869.2012.01013.x [DOI] [PubMed] [Google Scholar]
- 27.Wing YK, Li SX, Li AM, Zhang J, Kong APS. The Effect of Weekend and Holiday Sleep Compensation on Childhood Overweight and Obesity. Pediatrics [Internet]. 2009;124:e994–1000. Available from: http://pediatrics.aappublications.org/cgi/doi/10.1542/peds.2008-3602 [DOI] [PubMed] [Google Scholar]
- 28.Zhang B, Hao Y, Zhou J, Jia F, Li X, Tang Y, et al. The association between sleep patterns and overweight/obesity in Chinese children: a cross-sectional study. Neuropsychiatr Dis Treat [Internet]. 2015. August [cited 2017 Dec 3];11:2209–16. Available from: http://www.dovepress.com/the-association-between-sleep-patterns-andoverweightobesity-in-chines-peer-reviewed-article-NDT [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewitt MS. The Role of the Growth Hormone/Insulin-Like Growth Factor System in Visceral Adiposity. Biochem Insights [Internet]. 2017;10:117862641770399 Available from: http://journals.sagepub.com/doi/10.1177/1178626417703995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Killick R, Hoyos CM, Melehan KL, Dungan GC, Poh J, Liu PY. Metabolic and hormonal effects of “catch-up” sleep in men with chronic, repetitive, lifestyle-driven sleep restriction. Clin Endocrinol (Oxf) [Internet]. 2015. October [cited 2017 Nov 22];83:498–507. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25683266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Könen T, Dirk J, Schmiedek F. Cognitive benefits of last night’s sleep: daily variations in children’s sleep behavior are related to working memory fluctuations. J Child Psychol Psychiatry [Internet]. 2015. February 1 [cited 2017 Nov 22];56:171–82. Available from: http://doi.wiley.com/10.1111/jcpp.12296 [DOI] [PubMed] [Google Scholar]
- 32.Telzer EH, Goldenberg D, Fuligni AJ, Lieberman MD, Gálvan A. Sleep variability in adolescence is associated with altered brain development. Dev Cogn Neurosci [Internet]. 2015. August [cited 2017 Nov 22];14:16–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26093368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker SP, Sidol CA, Van Dyk TR, Epstein JN, Beebe DW. Intraindividual variability of sleep/wake patterns in relation to child and adolescent functioning: A systematic review. Sleep Med Rev [Internet]. 2017. August [cited 2017 Nov 22];34:94–121. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1087079216300661 [DOI] [PMC free article] [PubMed] [Google Scholar]