Abstract
Background and Objectives:
A randomized trial of concurrent recombinant tissue-type plasminogen activator (r-tPA) + thrombin-inhibition with Argatroban in stroke patients recently demonstrated safety and signal of efficacy compared to r-tPA alone, but patients having endovascular therapy (EVT) were excluded. The current study intended to study feasibility and safety of concurrent r-tPA and Argatroban in patients undergoing EVT.
Methods:
We conducted a single-arm, feasibility and safety study of patients that received standard-dose r-tPA, had intracranial large vessel occlusions and underwent EVT within 6-hours of stroke onset. During r-tPA, a 100 ng/kg Argatroban bolus, followed by 12-hour infusion, targeted an aPTT 2.25 times baseline. Feasibility was defined as ability to combine treatments without EVT time-metric delays, compared to cotemporaneous r-tPA+EVT treatments. Safety was incidence of symptomatic intracerebral hemorrhage (sICH), systemic hemorrhage or EVT complications.
Results:
All pre-planned 10 patients were enrolled. Arterial occlusions were middle cerebral artery (n=8), internal carotid artery (n=1) and posterior cerebral artery (n=1). All received Argatroban before EVT and completed infusions. There were no delays in time-metrics compared to non-study patients during the same period. Nine patients achieved excellent angiographic reperfusion (Thrombolysis In Cerebral Ischemia [TICI] ≥2b); with 7 complete (TICI=3). There were no sICH, systemic hemorrhage, or EVT complications. At 90-days, 6 (60%) patients had a modified Rankin Scale of 0–2 and none died.
Conclusion:
In patients treated with r-tPA and EVT, concomitant Argatroban is feasible, does not delay EVT provision, produces high rates of recanalization, is probably safe, and warrants further study.
INTRODUCTION
Rapid administration of recombinant tissue-type plasminogen activator (r-tPA) to appropriately selected patients remains the mainstay of early treatment of acute ischemic stroke (AIS). Endovascular thrombectomy (EVT) has recently become the standard treatment for AIS in patients with large vessel occlusion (LVO). American Heart Association/American Stroke Association (AHA/ASA) guidelines recommend achieving Thrombolysis In Cerebral Infarction (TICI) 2b or 3 score following EVT to maximize the probability of good functional clinical outcome.[1]
The recent landmark endovascular trials demonstrated 60–88% reperfusion, TICI 2b or 3.[2] However, approximately one third of patients did not achieve full reperfusion, emphasizing the unmet need of identifying more effective strategies to improve cerebral perfusion that can be used as an adjunctive to EVT.
Thrombin-inhibition with Argatroban has been demonstrated to be safe when used in conjunction with r-tPA to maximize recanalization and improve outcomes. Our previous phase IIa multicenter, single arm study assessed the safety of Argatroban started during standard-dose r-tPA infusion and continued for 48 hours. In this study 4.6% (95% CI, 0.9–12.9) of patients had symptomatic intracranial hemorrhage (sICH) and 37% achieved complete recanalization versus 18% of historical controls treated with r-tPA alone.[3,4] A subsequent phase IIb, randomized trial, compared two doses (low vs. high) of Argatroban with r-tPA versus r-tPA alone.[5] Rates of sICH were similar and the combined high+low dose Argatroban experienced higher rates of excellent (mRS 0–1) clinical outcomes (31%) compared with 21% in r-tPA alone. Furthermore, Bayesian analyses indicated a 79% probability that adjunctive Argatroban was superior to r-tPA alone. The trial was stopped prematurely (N=90 of 105) after beneficial results of EVT trials resulted in most eligible patients receiving EVT, which was a study exclusion.
The current study was designed to explore the feasibility and safety of adding Argatroban to r- tPA and EVT in AIS patients.
METHODS
ARTSS-IA was a pilot, single-center, phase IIa, single arm feasibility and safety study of intravenous Argatroban in combination with r-tPA in AIS patients with LVO who undergo EVT. Eligibility of r-tPA met guidelines.[6] Additional criteria included: CTA confirmation of intracranial LVO in either: the terminal internal carotid artery (ICA), middle cerebral artery (MCA, M1 or M2 territories), posterior cerebral artery (PCA), distal vertebral or basilar artery; Alberta Stroke Program Early CT Score (ASPECTS) on non-contrast head CT ≥6, and ability to start EVT within 6 hours of stroke onset or last seen well. See online supplement for complete inclusion/exclusion criteria. Written informed consent was obtained by the patient or legally authorized representative. The University’s Committee for the Protection of Human Subjects approved the study.
A flow chart of study procedures is illustrated (Figure). All patients received r-tPA, 0.9 mg/kg infused over 60 minutes (max dose 90mg) with 10% of the total dose administered as a 1- minute bolus. Argatroban began as 100 μg/kg bolus over 3–5 minutes followed by a continuous intravenous infusion of 3.0 μg/kg/min for 12 hours, including during EVT. Argatroban was titrated to achieve an aPTT of 2.25 times baseline (± 10%) - see online supplement. The study provided guidance (online supplement) for EVT-specific procedures based on EVT trial publications.
Figure:
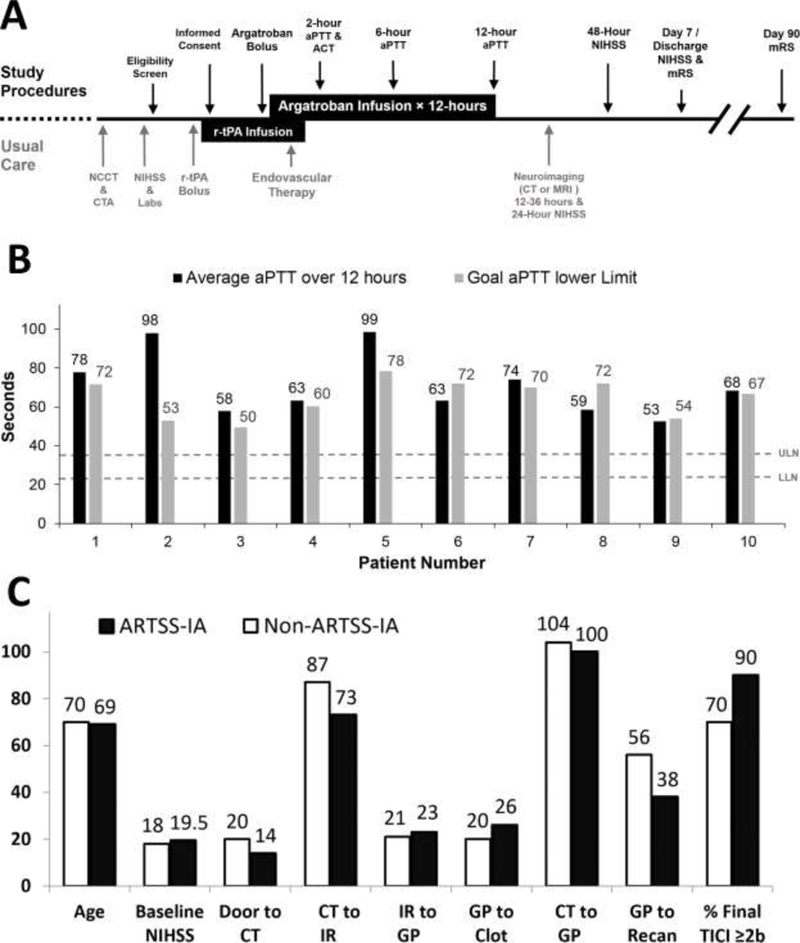
Panel A. Study schedule of observations and procedures. Panel B. Average aPTT lab values over 12 hours of Argatroban infusion. Dashed lines represent laboratory reference range for normal aPTT values - upper limit of normal (ULN - 36secs) and lower limit of normal (LLN - 23secs). Panel C. EVT procedural timing data. Abbreviations: ACT-Activated Clotting Time; CT- Computed Tomography; IR-Interventional Radiology suite; GP-Groin Puncture; Recan- Recanalization.
Feasibility of combining Argatroban with usual care was measured by success of starting the study medication before r-tPA completion; beginning of Argatroban before groin puncture (GP) and demonstration of similar EVT benchmark times compared to consensus and guideline recommendations. [1,6,7,8] Comparison was also made to patients being treated with r-tPA and EVT out of the study at our CSC during the same period.
Safety was measured by the incidence of sICH defined as using the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) criteria (≥4 NIHSS point increase and parenchymal hemorrhage type-2) [9]; incidence of groin, retroperitoneal hematoma; major systemic hemorrhage requiring transfusion; or arterial perforation or dissection. Successful recanalization rate was defined as TICI 2b or TICI 3. Functional outcome was measured by mRS at 90 days.
Sample size was determined based on medical criteria in terms of balancing patient exposure with the objective of gaining useful preliminary safety information. Since the current study was a pilot study, a total of 10 patients were enrolled. Safety stopping rules were created in order to identify a high rate of sICH using a binomial confidence interval approach (online supplement). If the sICH rate exceeded the lower limit of the 95% confidence interval for ≥10% rate, the study would terminate.
RESULTS
All pre-planned 10 patients were enrolled from 06–2015 to 05–2016 at a single comprehensive stroke center (CSC) (Table). LVOs were proximal MCA (n=6), tandem extra-cranial ICA + MCA (n=2), terminal ICA (n=1) and proximal PCA (n=1). Eight of 10 patients had r-tPA-Argatroban infusion overlap. The other two received r-tPA via a mobile stroke unit (MSU), which led to slight delays in Argatroban bolus (2 and 14 minutes).
Table.
Patient Characteristics (median[IQR]), reperfusion and timing results. All times are minutes and displayed as median(IQR).
| ARTSS-IA | NON-ARTSS-IA | P VALUE | |
|---|---|---|---|
| N=10 | N=22 | ||
| Age | 69(63,80) | 70(61,80) | 0.54 |
| NIHSS | 19.5(12,24) | 18(12,24) | 0.63 |
| ASPECTS | 9(8,9) | 8(7,10) | 0.51 |
| Arrival to rt-PA bolus* | 45(39,53) | 53(39,69) | 0.24 |
| rt-PA-Argatroban overlap | 4.5(1,11) | - | |
| CT to IR | 72.5(50,83) | 87(65,117) | 0.19 |
| IR to GP | 23(13,25) | 21(14,26) | 0.9 |
| CT to GP† | 100(63,102) | 104(87,139) | 0.21 |
| GP to Reperfusion‡ (TICI ≥2b) | 38(33,42) | 56(37,69) | 0.05 |
| Arrival to Reperfusion§ | 147(140,155) | 189(176,228) | 0.009 |
| TICI (%) | 0.27 | ||
| 0–2a | 10 | 30 | |
| 2b | 20 | 18 | |
| 3 | 70 | 52 | |
Abbreviations: CT-Computed Tomography;, IR-Interventional Radiology; GP-groin puncture. Recommended Metrics:
≥50% of cases <45 minutes1
75% of cases ≤110 minutes7
70% of cases <60 minutes7
<120 minutes.6
Argatroban was initiated before EVT and full 12-hour infusion completed in all patients. aPTT lab values were on target during the 12 hour Argatroban infusion (Figure). Embolectomy with stent retrievers was performed in all but 1 patient, a proximal PCA occlusion, which was deemed too hazardous for device passage. Two patients with tandem occlusions underwent angioplasty and stenting of the extracranial ICA. One patient received 300mg of clopidogrel during EVT and Argatroban infusion.
Twenty-two patients were included in the comparison, non-study group. Study patients experienced no time delays in thrombolysis compared to non-study patients (Table). In the study group 7/7 (100%) patients received r-tPA bolus in the emergency department within 60 minutes of arrival time. Three patients were treated in our MSU. Fewer, 13/22 (59%) non-study patients were treated within 60 minutes. Both study and non-study patients met 2018 AHA/ASA guideline to achieve door-to-needle times of <60 minutes in ≥50% of stroke patients treated with r-tPA.[1] New recommendations advise ≥50% receive r-tPA within 45 minutes of arrival. Five of 7 (71%) of Argatroban patients met that threshold compared to 8/22 (36%) of non-study patients.
Argatroban EVT metrics were very similar or better than non-study patients (Table and Figure). Ninety percent (9/10) met the CT to GP time benchmark of ≤110 minutes, which exceeds the threshold of 75% as set-forth by a multi-societal EVT consensus statement.[8] In the non-study group, 12 (55%) patients met the CT to GP time metrics. Time interval from GP to recanalization was <60 minutes in 89% (8/9) patients vs. 63% (10/16) of non-study patients. Only 1 patient in the study group met the arrival to reperfusion time benchmark of <120 minutes.[6]
No patients suffered sICH. Two patients had asymptomatic ICH. There were no complications or serious adverse events noted during or after EVT: no groin or retroperitoneal hematoma, or major systemic hemorrhage requiring transfusion, arterial perforation or dissection. At 90-days, 6 (60%) patients were functionally independent (mRS 0–2) and none died.
DISCUSSION
The current study is the first safety and feasibility study of concurrent IV thrombolysis, EVT and Argatroban. We found no evidence that the addition of Argatroban to r-tPA and EVT increases risk of hemorrhage or death. There were no serious adverse events or complications attributed to combination treatment, and most importantly, no delays in r-tPA or EVT time metrics. Although the sample size was small, our data suggest combination therapy may result in high rates of both successful reperfusion and independent functional outcome at 90 days.
Adequate safety and potential efficacy of combination systemic anticoagulation and EVT has been previously described. A post-hoc analysis of stroke embolectomy (Multi-MERCI trial) compared cases that received IV-heparin (n=24, median dose 3000 units) to those who did not (n=27). There was no increased risk of ICH or death. Rather, procedural heparin was independently associated with good clinical outcome (mRS 0–2) at 90-days (OR 5.9; 95%CI 1.3–25.9, p=0.019).[10] Due to the similarities between unfractionated heparin and Argatroban, we did not anticipate significant risk differences with Argatroban compared to heparin in EVT patients.
Despite recent important endovascular advances, a large percentage of patients remain disabled, which may be in part explained by significant delays to endovascular facilities as well as moderately low rates of complete TICI-3 reperfusion. The latter may contribute to microcirculation thrombosis and no-reflow, which concurrent antithrombotic infusions may prevent. Therefore, amplification and maintenance of recanalization with widely available medical therapies remains a crucial target for AIS.
We had found a higher rate of early recanalization than expected with the combination of lower dose Argatroban + tPA in the non-randomized ARTSS-1 study. However, in ARTSS-IA, rates of successful recanalization prior to EVT and after systemic thrombolysis with tPA alone or tPA plus Argatroban were similar--about 10%. In a recent meta-analysis of 1561 AIS patients with LVO, successful early recanalization following systemic intravenous thrombolysis before EVT was 11%.[11] With regard to ultimate recanalization, all 9 ARTSS-IA patients undergoing embolectomy achieved excellent angiographic results (Thrombolysis In Cerebral Ischemia [TICI] ≥2b); with 7 achieving complete recanalization (TICI=3).
The rate of sICH in our studies was 5.2%, which is very similar to the rate (6.4%) of in the NINDS-tPA trial that led to FDA-approval of r-tPA. Therefore, we hypothesized that combination therapy of Argatroban with r-tPA is likely to be safe, and the higher-dose arm had demonstrated the most promise (no increase in bleeding with better clinical outcomes), hence its choice for the present study.[4,5]
There is strong rationale for combination thrombin-inhibition and thrombolytic medical therapy in AIS. In animal stroke models of MCA occlusion, Argatroban was shown to reduce the number of microthrombi in penumbral areas, accelerate recanalization, reduce infarction size and improve behavioral outcomes.[12–15]
Besides the small sample size that preclude definite conclusions about rates of recanalization or clinical outcomes, study limitations include single-arm and open-label design. To minimize bias, all reperfusion timing metrics were documented and collected by independent personnel for CSC reporting requirements.
CONCLUSIONS
The results of this study demonstrated feasibility, safety and high rate of reperfusion in acute stroke patients treated with concomitant Argatroban, r-tPA and EVT. Further studies are warranted to investigate the efficacy of the concomitant Argatroban treatment that might further increase the recanalization rates and good functional outcome in AIS patients with LVO who undergo EVT.
Supplementary Material
ACKNOWLEDGMENTS
None
SOURCE OF FUNDING
This study was supported by NIH grants P50NS044227, T32NS07412, UL1 RR024148 [TL1 RR024147 for the T32 program; KL2 RR0224149 for the K12 program], and Diane and Harold Farb Stroke Research Fund (DHFSRF) to UTHSC. Glaxo-Smith-Kline Inc. provided study medication without charge. Funders and medication suppliers had no role in study design, analysis, or writing.
Footnotes
DISCLOSURES
AB reports grants from NIH and other from DHFSRF to UTHSC and JG reports grants from NINDS, other from DHFSRF, during study conduct.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2018;49:e46–e110. [DOI] [PubMed] [Google Scholar]
- 2.Vidale S, Agostoni E. Endovascular treatment of ischemic stroke: an updated meta-analysis of efficacy and safety. Vasc Endovascular Surg. 2017;51:215–219. [DOI] [PubMed] [Google Scholar]
- 3.Barreto AD and Grotta JC. The Argatroban and tPA Stroke Study. Progress in Neurotherapeutics and Neuropharmacology 2008;3:35–47. [Google Scholar]
- 4.Barreto AD, Alexandrov AV, Lyden P, et al. The Argatroban and tissue-type plasminogen Activator Stroke Study: Final Results of a Pilot Safety Study. Stroke 2012;43:770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barreto AD, Ford GA, Shen L, et al. Randomized, Multicenter Trial of ARTSS-2 (Argatroban with Recombinant Tissue Plasminogen Activator for Acute Stroke). Stroke 2017;48:1608– 1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demaerschalk BM, Kleindorfer DO, Adeoye OM, et al. on behalf of the American Heart Association Stroke Council and Council on Epidemiology and Prevention. Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016;47:581–641 [DOI] [PubMed] [Google Scholar]
- 7.Specifications Manual for Joint Commission National Quality Measures, v2018A. Comprehensive Stroke Center - CSTK-11: Timeliness of Reperfusion. https://manual.jointcommission.org/releases/TJC2018A/assets/Manual/TableOfContentsTJC/TJCv2018A.pdf Accessed March 28, 2018.
- 8.Sacks D, Baxter B, Campbell BCV, et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. J Vasc Interv Radiol. 2018;29:441–453. [DOI] [PubMed] [Google Scholar]
- 9.Wahlgren N, Ahmed N, Davalos A, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS- MOST): An observational study. Lancet. 2007;369:275–282 [DOI] [PubMed] [Google Scholar]
- 10.Nahab F, Walker GA, Dion JE, et al. Safety of periprocedural heparin in acute ischemic stroke endovascular therapy: the multi MERCI trial. J Stroke Cerebrovasc Dis 2012;21:790– 793. [DOI] [PubMed] [Google Scholar]
- 11.Tsivgoulis G, Katsanos AH, Schellinger PD, Kohrman M et al. Successful Reperfusion with Intravenous Thrombolysis Preceeding Mechanical Thrombectomy in Large Vessel Occlusions. Stroke. 2018;49(1):232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyden P, Pereira B, Che B, et al. Direct thrombin inhibitor argatroban reduces stroke damage in two different models. Stroke. 2014;45:896–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai H, Umemura K, Nakashima M. Effect of argatroban on microthrombi formation and brain damage in the rat middle cerebral artery thrombosis model. Jpn J Pharmacol. 1995;69:143–148. [DOI] [PubMed] [Google Scholar]
- 14.Tamao Y, Kikumoto R. Effect of Argatroban, a selective thrombin inhibitor, on animal models of cerebral thrombosis. Semin Thromb Hemost. 1997;23(6):523–530. [DOI] [PubMed] [Google Scholar]
- 15.Jang IK, Gold HK, Leinbach RC, et al. In vivo thrombin inhibition enhances and sustains arterial recanalization with recombinant tissue-type plasminogen activator. Circ Res. 1990;67:1552–1561. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


