Abstract
Objective:
To test the hypothesis that specific echocardiographic measurements of right ventricle (RV) mechanics at 36 weeks post-menstrual age (PMA) are associated with severity of bronchopulmonary dysplasia (BPD).
Study design:
A subset of 93 preterm infants (born between 27 and 29 weeks gestation) was retrospectively selected from a prospectively enrolled cohort. BPD was defined using the National Institutes of Health workshop definition, with modifications for oxygen reduction testing and altitude. The cohort was divided into no BPD and BPD using previously published methodology for analyses. Echocardiographic measurements of RV function (tricuspid annular plane systolic excursion, fractional area of change, systolic to diastolic ratio, tissue Doppler myocardial performance index, RV strain), RV remodeling/morphology (end-systolic left ventricular (LV) eccentricity index and RV afterload (pulmonary artery acceleration time measures) were evaluated at 36 weeks PMA. Multivariable logistic regression determined associations between RV measurements and BPD severity.
Results:
Compared with the no BPD cohort, the BPD group had lower birth weight z-scores (P = .04) and trended toward male predominance (p = 0.08). After adjusting for birth weight z-scores, gestational age and sex, there were no differences in echocardiographic measurements between groups except for the EI (scaled odds ratio (0.1-unit increase) of 1.49 (1.13 – 2.12, p = 0.01).
Conclusions:
Among conventional and emerging echocardiographic measurements of RV mechanics, EI was the only parameter independently associated with BPD severity in this study. The EI may be a useful echocardiographic measurement to characterize RV mechanics in patients with BPD at 36 weeks PMA.
Keywords: Bronchopulmonary Dysplasia, Echocardiography, Right Ventricular Mechanics, Pulmonary Vascular Disease
Pulmonary vascular disease (PVD), and its most severe form, pulmonary hypertension (PH) are commonly associated with bronchopulmonary dysplasia (BPD) following preterm birth.1 Poor outcomes may be partially mediated by altered right ventricular (RV) mechanics associated with PVD, even without a formal diagnosis of PH.2 Screening for abnormalities in RV mechanics has potential utility in understanding disease pathophysiology, prognostication and clinical management of infants with established BPD.
Multiple echocardiographic parameters for characterizing RV mechanics in preterm infants at risk for BPD have been described. Examples include measurements of RV function (tricuspid annular plane systolic excursion, fractional area of change, systolic to diastolic ratio, tissue Doppler myocardial performance index, RV strain), RV remodeling/morphology (end-systolic left ventricular (LV) eccentricity index (EI) and RV afterload (pulmonary artery acceleration time measures).3 However, little is known about which of these measures is most strongly associated with the severity of BPD. The aim of this study was to evaluate the strength of associations between echocardiographic measurements of RV mechanics and BPD severity in a cohort of preterm infants at 36 weeks post-menstrual age (PMA) who participated in a prospective study and underwent a protocolized echocardiographic assessment.1 We hypothesized that echocardiographic indices of RV mechanics would be associated with BPD and its severity.
Methods
A random subset of infants with and without BPD was retrospectively selected from a larger prospective study that included 277 subjects1. The prospective cohort was enrolled between July 2006 and March 2012 at hospitals affiliated with the University of Colorado Anschutz Medical Campus and Indiana University School of Medicine. Patients were recruited before seven days of age and inclusion criteria included gestational age (GA) less than 34 weeks and birthweight between 500 and 1,250 grams. Exclusion criteria included congenital heart disease (except patent ductus arteriosus (PDA), patent foramen ovale, atrial septal defect less than 1 cm, and ventricular septal defect less than 2 mm), lethal congenital abnormality, and cases deemed to be futile (anticipated death before discharge during the enrollment window).
The study population for this retrospective analysis was created using a multi-step process. First, BPD status and severity was determined at 36 weeks PMA using a modification of the National Institutes of Health workshop definition4 with application of the oxygen reduction test as described by Walsh et. al.1, 5 Patients who required supplemental oxygen for < 28 days after birth were classified as “no BPD” if they were on room air at 36 weeks PMA or < 30% supplemental oxygen (<35% at Denver altitude) and passed the oxygen reduction test. Patients who required supplemental oxygen for > 28 days had BPD. If patients were on room air or < 30% supplemental oxygen (<35% at Denver altitude) and passed the oxygen reduction test at 36 weeks PMA, they were classified as “mild BPD.” If patients were on < 30% supplemental oxygen (<35% at Denver altitude) and failed the oxygen reduction test at 36 weeks PMA, they were classified as “moderate BPD.” Patients requiring positive pressure support or receiving ≥30% supplemental oxygen (≥35% at Denver altitude) at 36 weeks PMA were classified as “severe BPD”. Disease severity categories were adjusted for altitude (applied to the result of the oxygen reduction test for those who performed the test) for subjects enrolled in Denver (1,600 m) as previously described.1, 6
To obtain a representative sample of the spectrum of BPD, 25 patients were randomly selected from each BPD severity group. The gestational age was restricted to 27 to 29 weeks to minimize the confounding effect of gestational age on outcomes and ensure well-balanced group membership (ie, between none, mild, moderate and severe BPD). A power analysis was performed to verify the adequacy of the sample size to detect differences between groups. A priori, the variable of RV FAC was selected for the power analysis. Means and standard deviations in patients with and without BPD were used (personal communication from Philip T. Levy, Washington University School of Medicine). With the sample size employed in the study, the power was calculated to be > 0.999.
For analytic purposes, the cohort was finally divided into a BPD vs. no BPD binary outcome using an additional modification to the National Institutes of Health workshop definition proposed by Poindexter et al.7 Using data from the Prematurity and Respiratory Outcomes Program (PROP), Poindexter et al omitted the need for 28 days of supplemental oxygen and thus eliminated the “mild BPD” classification.7 We similarly grouped patients on room air or having passed an oxygen reduction test at 36 weeks PMA as “no BPD” and all others as “BPD” for all analyses.
The original study protocol was approved by the institutional review boards at each participating institution and written informed consent was obtained from parents or guardians of all enrolled participants. Demographics, select morbidities, and outcomes were systematically collected for each study participant during their birth hospitalization.
A standardized imaging protocol was utilized to obtain echocardiographic images at 36 weeks PMA. Echocardiographic data have previously been published from this cohort,1, 8,10 but the echocardiographic measurements obtained in this study had not been previously assessed at 36 weeks PMA in relation to BPD. Echocardiograms were performed using a Vivid 7 (GE Healthcare, Milwaukee, WI) or an iE33 (Philips Medical Systems, Andover, MA) platform and reviewed offline using commercially available software (Cardiovascular Review Station version 2.14.03; AGFA Healthcare, Mortsel, Belgium). For this analysis, each measurement was made by a single investigator to prevent interreader variability, who was also blinded to BPD status at the time the interpretations were made. All measurement techniques were performed according to previously published guidelines and methods.1, 11, 12 Images were only included for analysis if the full extent of the structure or boundary of interest was contained within the imaging sector without prominent artifact (i.e., at least “good” quality images based on previously published grading systems).13 The measurements obtained included:
TAPSE:
The difference between the systolic and diastolic position of the tricuspid annular plane measured from the 2-dimensional apical 4 chamber view.11
RV and RA FAC:
The fractional change in areas from atrial and ventricular systole to diastole computed as a percent change from the apical four chamber view.11
Pulsed wave blood pool and Tissue Doppler derived MPI:
Standard measurements of tricuspid valve inflow, RV free wall velocities, and isovolumetric contraction (IVCT) and relaxation (IVRT) were performed in the apical four chamber views. Ejection time (ET) was measured in the pulmonary valve outflow short axis and apical four chamber view for the pulsed Doppler and tissue Doppler MPI, respectively. The MPI for both methodologies was calculated by computing (IVRT + IVCT)/ET.14–16 Differences in isovolumetric times and ejection times measured in different views with heart rate differences greater than 10% were excluded.
RV systolic to diastolic ratio:
Ratio of time in the cardiac cycle spent in systole compared with time spent in diastole, measured using the tricuspid valve continuous Doppler wave in the apical 4 chamber view.17
PAAT and PAAT/ET:
From the spectral Doppler trace at the pulmonary valve annulus, the PAAT was defined as the interval (in milliseconds [ms]) between the onset of systolic pulmonary arterial ejection and peak flow velocity; the ET was defined as the interval between the beginning of RV ejection and flow cessation.18, 19
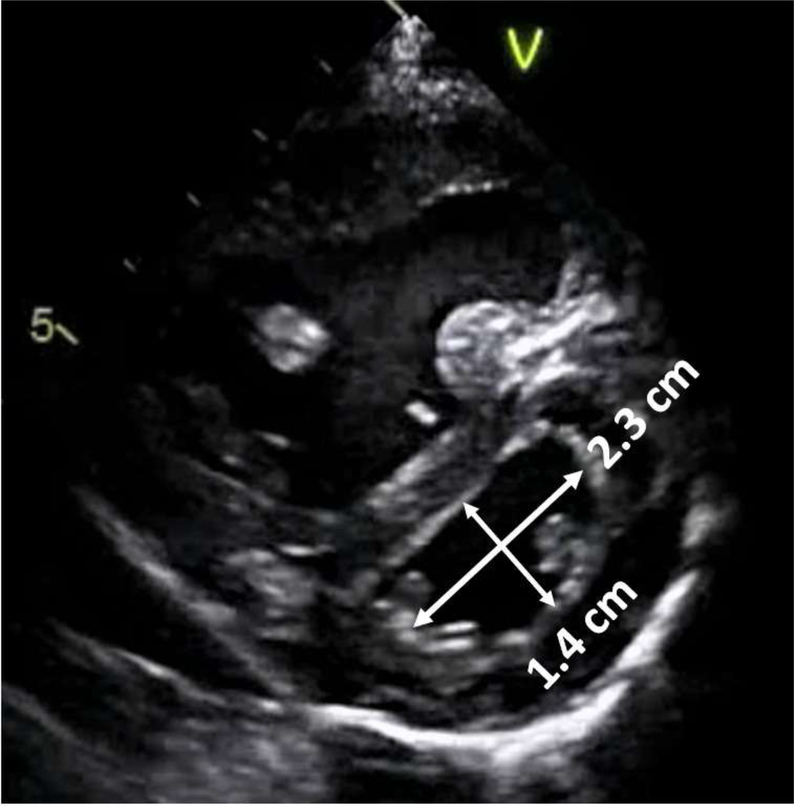
Left Ventricular (LV) EI (Figure 1):
Fig. 1.
The eccentricity index. The eccentricity index in this example is 2.3/1.4 = 1.6.
From the parasternal short axis view, the left ventricle was viewed in cross-section at the level of the papillary muscles. The distance from the anterior wall to the inferior/posterior wall is divided by the distance from the septal wall to the lateral/posterior wall at end-systole. The normal value for the EI is one.20
Speckle-derived myocardial deformation imaging (i.e. strain):
Manual RV dominant border tracking (on the frame prior to tricuspid valve closing) was performed using an adapted institutional protocol21–23 and deformation percentages were rendered by an offline software (TomTec Imaging Systems GMBH, Unterschleissheim, Germany) to yield RV free wall (RVFWS) and global longitudinal strain (RVGLS) in the apical four chamber view24
Statistical Analyses
All non-echocardiographic data were prospectively collected and maintained using a REDcap (Research Electronic Data Capture) database hosted at the University of Colorado Denver.25 Summaries of continuous data are presented as mean and standard deviations or median and interquartile ranges, when appropriate. Categorical data are summarized as proportions. The Shapiro-Wilk test was used to assess normality of all continuous variables. Student t-tests, Wilcoxon rank sum tests, and Chi-square tests were used to analyze differences between BPD groups, as appropriate. For the primary analyses, multivariable logistic regression modeling was used to identify the relationship between each measurement of RV mechanics and BPD severity, controlling for known risk factors. To identify associations between echocardiographic measurements and BPD severity among patients without echocardiographic evidence of PH, secondary modelling was performed after patients with estimated PH were removed. PH was defined via the tricuspid regurgitation jet velocity (TRJV) and the modified Bernoulli equation if the systolic pulmonary artery pressure (sPAP)/systemic systolic blood pressure was ≥ 0.5, sPAP was > 40 mm Hg or there was evidence of septal flattening.1 The TRJV measurements had to meet predefined quality criteria to be used for analysis. The criteria required that the TRJV envelope consisted of clearly defined and reproducible descending and ascending limbs forming a peak velocity. Incomplete or poorly demarcated envelopes were not used to estimate PH. Odds ratios were scaled for improved interpretability for parameters with narrow ranges. Days of supplemental oxygen needed were log, base X, transformed. Reliability testing was performed on the EI. A random sample of 20% of echocardiograms included in the study were selected. For intra-observer reliability testing, EI measurements were repeated by the same investigator one year after the initial measurements were performed. For inter-observer reliability testing, a different investigator measured the EI independently. Reliability was assessed via calculation of median differences (with interquartile ranges), Pearson linear correlation coefficient and the intra-class correlation coefficient. Statistical significance was assessed using an alpha level of 0.05. All statistics were computed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
From the original cohort of 277 infants, 93 were randomly selected using statistical software for this study, 47 without the diagnosis of BPD and 46 with BPD as assessed at 36 weeks PMA. Patient characteristics are listed in Table I. In comparison with subjects with no BPD, patients with BPD had lower birth weight z-scores (mean (std) −0.52 (0.7) vs. −0.25 (0.6), p = 0.04) and were born on average 4 days earlier. There was a higher proportion of males in the BPD group, but the difference was not significant (56.5% vs. 38.3%, p = 0.08). Morbidities were more prevalent in those with BPD, including pneumonia (15.2% vs. 2.1%, p = 0.02), longer median days of mechanical ventilation (13.5 vs. 3 days, p < 0.01) and neonatal intensive care unit length of stay (90 vs. 73 days, p < 0.01). Eleven patients had PH (11.8% of cohort), which was more prevalent in the BPD group (21.7% vs. 2%, p < 0.01). The TRJV was of sufficient quality to measure in 15% of the cohort.
Table I.
Characteristics of the Study Cohort
| Variable | Overall (n = 93) | No BPD (n = 47) | BPD (n = 46) | P-value |
|---|---|---|---|---|
| Birth Weight (grams) | 994 (170) | 1067 (127) | 919 (177) | <0.01 |
| Birth Weight Z-Score | −0.38 (0.6) | −0.25 (0.61) | −0.52 (0.66) | 0.04 |
| Gestational Age (weeks) | 28 (0.8) | 28.1 (0.8) | 27.5 (0.6) | <0.01 |
| Gender (Male) | 44 (47.3%) | 18 (38.3%) | 26 (56.5%) | 0.08 |
| Small for Gestational Age | 4 (4.3%) | 3 (6.4%) | 1 (2.2%) | 0.62 |
| Antenatal Corticosteroids | 74 (79.6%) | 39 (83%) | 35 (76.1%) | 0.41 |
| PDA - Medical Treatment | 30 (32.3%) | 13 (27.7%) | 17 (37%) | 0.09 |
| PDA - Surgical Ligation | 7 (7.5%) | 0 (0%) | 7 (15.2%) | 0.01 |
| Pneumonia | 8 (8.6%) | 1 (2.1%) | 7 (15.2%) | 0.02 |
| Necrotizing Enterocolitis | 13 (14%) | 8 (17%) | 5 (10.9%) | 0.39 |
| Sepsis | 14 (15.1%) | 4 (8.5%) | 10 (21.7%) | 0.07 |
| Pulmonary Hypertension | 11 (11.8%) | 1 (2%) | 10 (21.7%) | <0.01 |
| Days of MV | 6 (2 — 15) | 3 (1 – 7) | 13.5 (4 – 25) | <0.01 |
| NICU length of stay (days) | 82 (72 — 97) | 73 (63 – 89) | 90 (81 – 109) | <0.01 |
| Discharged on Oxygen | 48 (51.6%) | 20 (42.6%) | 28 (60.9%) | 0.08 |
| Mortality | 3 (3.2%) | 0 (0%) | 3 (6.5%) | 0.08 |
Abbreviations: PDA, Patent ductus arteriosus; MV, Mechanical ventilation; NICU, Neonatal intensive care unit
Data are presented as N (%) for categorical variables and Mean (Std) or Median (25th percentile – 75th percentile) for continuous variables.
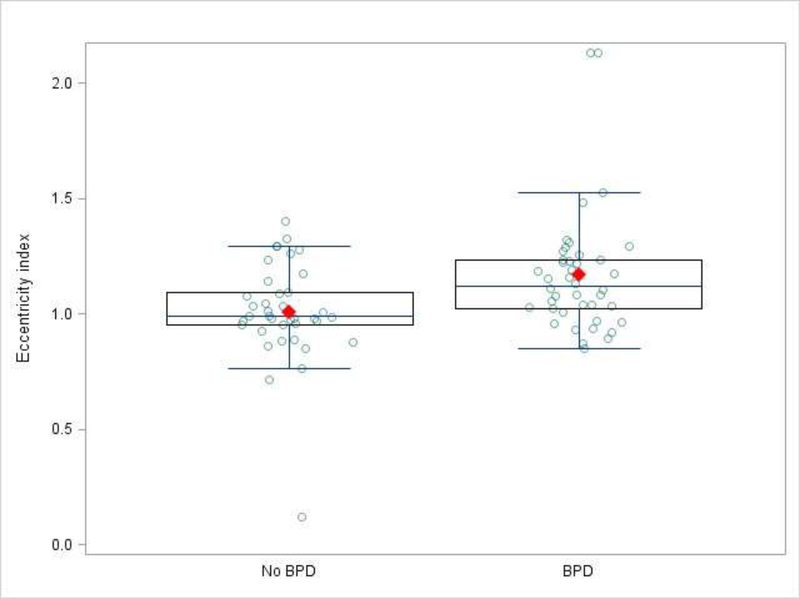
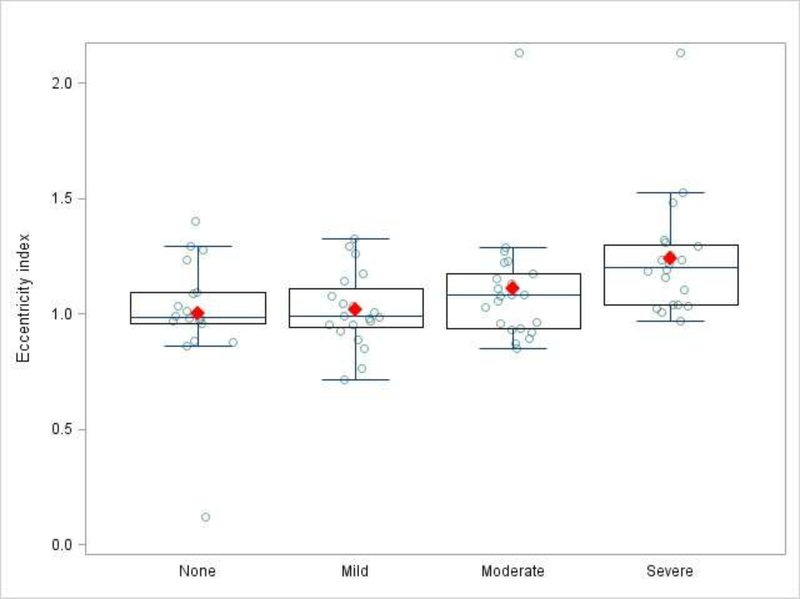
Echocardiographic data are summarized in Table II. The successful quantification of measures of RV mechanics ranged between 69% and 95%. Median frame rates for strain calculations were 91 frames per second with a median heart rate of 169 beats per minute. The median frame rate/heart rate ratio was 0.52. None of the measured parameters differed significantly between the BPD groups, except for the EI (Figure 2). Although TAPSE was greater in infants without BPD, the difference was not significant (8.66 vs. 8.01, p = 0.08). To correct for potential confounding variables, a multivariable logistic model was constructed with EI, GA, BWZ and sex (Table III; available at www.jpeds.com). The final model contained 80 patients with complete data. The association between EI and BPD group remained significant with an odds ratio of 1.49 (1.13 – 2.12, p = 0.01) for every 0.1 unit increase in EI. In a secondary sensitivity analysis that excluded subjects diagnosed with PH, the model was re-fit with 69 patients. The scaled odds ratio for EI in the cohort without PH remained significant at 1.40 (1.02 – 1.93, p = 0.04). We further examined trends in EI according to each of the 4 NIH categories of BPD severity (none, mild, moderate and severe) (Figure 3; available at www.jpeds.com). EI increased with increased BPD severity (moderate vs. none OR 1.3, p = 0.15; severe vs. none OR 1.8, p < 0.01). The EI intra-observer median difference was −0.035 (IQR −0.214 – 0.024) with an intra-class correlation coefficient of 0.76, p < 0.001. The inter-observer reliability median difference was 0.049 (IQR 0.009 – 0.109) with a Pearson linear correlation coefficient of 0.91, p < 0.001).
Table II.
Echocardiographic Parameters of RV Dysfunction
| Variable | Feasibility (%) | No BPD (n = 47) | BPD (n = 46) | P-value |
|---|---|---|---|---|
| Eccentricity index | 86 | 0.99 (0.95 — 1.09) | 1.12 (1.02 — 1.24) | 0.01 |
| TAPSE (mm) | 90 | 8.66 (6.87 — 10.04) | 8.01 (6.46 — 8.58) | 0.08 |
| RA Fractional Area Change | 90 | 0.57 (0.50 — 0.69) | 0.58 (0.50 — 0.65) | 0.98 |
| RV Fractional Area Change | 85 | 0.51 (0.47 — 0.56) | 0.52 (0.47 — 0.59) | 0.43 |
| RV End-Diastolic Area | 85 | 3.15 (2.68 — 3.50) | 2.75 (2.50 — 3.28) | 0.12 |
| RV End-Systolic Area | 85 | 1.55 (1.38 — 1.83) | 1.5 (1.23 — 1.78) | 0.49 |
| RV Systolic to Diastolic Ratio | 82 | 1.52 (1.25 — 1.82) | 1.61 (1.35 — 1.78) | 0.36 |
| Pulse Wave MPI | 81 | 0.16 (0.03 — 0.22) | 0.12 (0.05 — 0.24) | 0.67 |
| TDI MPI | 69 | 0.25 (0.19 — 0.36) | 0.25 (0.20 — 0.39) | 0.51 |
| Total RVFWS (%) | 73 | −12.74 (−17.00 — −10.47) | −14.27 (−21.27 — −10.81) | 0.21 |
| RV GLS (%) | 73 | −16.38 (−19.12 — −10.53) | −16.47 (−18.69 — −11.72) | 0.70 |
| PAAT (milliseconds) | 95 | 60 (50 — 80) | 60 (50 — 70) | 0.28 |
| PAAT:ET | 95 | 0.32 (0.24 – 0.39) | 0.29 (0.24 – 0.37) | 0.49 |
| Subjective Septal Flattening | 95 | 5 (10.6%) | 9 (19.6%) | 0.18 |
Abbreviations: TAPSE, Tricuspid annular plane systolic excursion; RA, Right atrium; RV, Right ventricle; MPI, Myocardial performance index; TDI, Tissue doppler imaging; RVFWS, Right ventricle free wall strain; GLS, Global longitudinal strain; PAAT; Pulmonary artery acceleration time; ET; Right ventricular ejection time
Fig. 2.
The eccentricity index in those with and without bronchopulmonary dysplasia.
Table III.
Multivariable Logistic Regression Model of the Scaled Eccentricity Index+
| Parameter | Parameter Estimate | Standard Error | P-value | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|---|---|
| Scaled Eccentricity Index | 0.397 | 0.159 | 0.01 | 1.49 | (1.13, 2.12) |
| Lower Birthweight z-score | 2.55 | 0.71 | <0.001 | 12.8 | (3.19, 51.6) |
| Male Gender | −0.61 | 0.65 | 0.35 | 0.54 | (0.14, 1.87) |
| Lower Gestational Age | 2.25 | 0.59 | <0.001 | 9.51 | (3.00, 30.2) |
EI is scaled to yield an odds ratio for BPD group membership (vs. no BPD membership), for each 0.1 unit increase in EI.
Fig. 3.
The eccentricity index in those with none, mild, moderate and severe bronchopulmonary dysplasia.
Discussion
Abnormalities of the pulmonary vasculature in preterm infants are strongly associated with BPD and its severity.1, 26–29 However, the relative utility of specific echocardiographic parameters that identify preterm infants with BPD-associated PVD and its severity are uncertain. In this retrospective analysis of a prospectively enrolled cohort at high risk for BPD, we showed that the EI was the only objective parameter among the evaluated assessments of RV mechanics that was independently associated with BPD severity at 36 weeks PMA.
The EI can be readily standardized, is highly reproducible, and is independent from the angle of insonation.20, 30 Like other assessments of septal geometry, the EI reflects interventricular interactions, primarily driven by the RV/LV pressure differential,31 and the EI directly changes with perturbations of RV/LV pressures, such as in the setting of PH. Prior studies have found associations between EI and BPD-associated PH.30, 32 McCrary et al found that the EI was significantly elevated in BPD patients with non-invasively determined PH.30 Abraham et al also reported an association between EI and PH, especially at EIs ≥ 1.30 among premature infants with and without BPD.32 There were not enough patients in this study diagnosed with PH by echocardiography to perform analogous analyses. In addition to echocardiography, McCrary et al used pulmonary vasodilator and future catheterization data to diagnose PH, which may have strengthened their ability to perform sub-analyses within the BPD group.
The EI may also have a role in the early identification of abnormal RV pressure load due to PVD, even in the absence of overt PH. An RV pressure load that does not meet criteria for PH has been associated with changes in RV mechanics in BPD2 as well as increased risk of morbidity and mortality in adult studies.33–35 In the study by Abraham et al, patients with EIs between 1.15 and 1.29 had a median estimated RV systolic pressure that was 42% of the systemic blood pressure using the modified Bernoulli principle.32 Although the RV was presumably exposed to an abnormal pressure load, these patients did not have PH. In our study, we found a significant, independent association between the EI and BPD severity, even when all patients diagnosed with PH were removed from the analysis. Because we anticipate that some patients had PVD without PH, the EI may reflect RV adaptation to abnormal but sub-PH afterloads.
The association between EI and BPD in this study highlight additional questions about the relationship between EI and BPD without any PVD/PH. Choi et al found that preterm BPD patients without evidence PVD/PH may nevertheless have RV dysfunction between 35 and 37 weeks PMA.36 Haque et al found similar results using myocardial deformation imaging in a cohort of BPD patients without PH.37 The current paradigm of BPD suggests that preterm infants with BPD may have impairments in the growth, structure and function of the pulmonary vasculature which may lead to PH and ultimately RV dysfunction. However, it may be possible that preterm patients have abnormal ventricular morphology/function as a primary insult.38 Perhaps preterm patients with abnormalities in ventricular programming (i.e., a primary insult) develop changes in RV structure and function when exposed to intermittent or chronic hypoxia (i.e., a secondary insult) associated with BPD. Because BPD is not always associated with PVD/PH, this may be one plausible explanation whereby preterm patients with BPD have abnormalities in RV morphology/function without PVD/PH. If the EI can reflect changes in RV morphology prior to the development of RV dysfunction in patients with BPD, it may be of clinical utility independent of the presence of PVD/PH. Further longitudinal studies may be able to shed more light on this complex relationship.
The strong feasibility and intra/inter-rater reliability of EI demonstrated in this study suggest that the EI may be more feasible and reliable than other measurements often used to estimate RV pressure load, such as TRJV and septal flattening. Our group and others have previously demonstrated the challenges in using the TRJV to assess PH patients with BPD.39 In a retrospective analysis of 25 patients under 2 years old with BPD and highly suspected or confirmed PH, the systolic pulmonary artery pressure could be assessed in only 61% of the cases, and the correlation between non-invasive and invasive measurements of PH was suboptimal.39 Using the same criteria as this study, we previously reported that only eight percent of preterm patients with BPD had an acceptable TR jet to measure the TRJV.1 The TRJV was of sufficient quality to measure in 15% of the cohort. The use of septal flattening to predict PH is also subject to important limitations.3 A recent study showed sub-optimal inter-rater reliability among cardiologists reading echocardiograms of preterm patients at risk of BPD at 7 days PMA, although agreement was far stronger at 36 weeks PMA.40 Furthermore, septal flattening may not be recognized until the EI is ≥ 1.15.32
We found no statistically significant associations between many conventional measurements of RV mechanics and BPD at 36 weeks PMA. Haque et al recently reported no association between RVFAC, TAPSE and MPI with BPD at > 36 weeks PMA.37 However, our findings contrast with those of other prior reports in which there were associations between conventional measurements of RV mechanics and BPD.41–44 The discrepancy between our findings and prior studies may be partially due to known limitations of some of the measurements performed. TAPSE and TDI are sensitive to angles of insonation and RV and RA FAC are strongly impacted by volume loading.3 Variations in sample size, study design, analytic methods, and approach to confounding may also explain why our findings differ from other studies. Furthermore, there is substantial phenotypic heterogeneity in cases of severe BPD, and echocardiographic indices may differ greatly between infants with the same BPD severity with vastly different clinical presentations. Our analysis attempted to mitigate some of these challenges by selecting infants within a narrow gestational age range at birth, a relatively large sample size and controlling for covariates. Nonetheless, we failed to find associations between many of these measurements and BPD severity. Future prospective randomized controlled studies specifically designed to assess the role of conventional and emerging echocardiographic measurements of RV mechanics in the BPD population will be of significant value.
The PAAT and PAAT:ET have been shown to be useful in the detection of PH in adults.19 Recently, normative age and sex-matched PAAT values have been derived in healthy children.45 Levy et al reported the significant associations between PAAT and PAAT/ET with PH in a cohort of children with and without PH.18 The PAAT:ET ratio has been associated with BPD in prior studies.46–50 We did not find an association between PAAT and PAAT/ET with BPD severity in this study. Bokiniec et al studied 89 preterm infants with and without BPD and found no association between the PAAT or PAAT:ET and BPD at day of life one, day 28 or 36 weeks PMA.51 Much remains to be understood about the timing and mechanisms by which the premature RV adapts to an increasing pressure load. The positive association between EI and BPD reported herein contrasted with the lack of association between PAAT and BPD raise important questions for future studies, especially because the post-hoc correlation between the PAAT and EI in our cohort was not statistically significant (r = −0.11, p = 0.32). In the early stages of PH, the RV develops significantly increased contractility to maintain ventriculoarterial coupling.52 This may be one factor that contributes to a shortening PAAT in PH.19 It is unclear when this adaptive process occurs in relation to the morphologic changes in premature patients with BPD. This requires further study.
Our findings should be interpreted in the context of important limitations. First, this study may not represent a consecutive birth cohort given that informed consent was required prior to enrollment and not all eligible patients participated. Secondly, we employed a design that purposely restricted patients to 27 – 29 weeks GA to ensure adequate representation of different BPD phenotypes in our sample and control for the effect of GA on the development of BPD. Thus, our findings may not be directly generalizable to other patients with BPD born outside of this GA window. There were also limitations related to imaging technique and quality. Specifically, some patients were excluded from final analyses due to not having available highquality images on which the parameters of interest could be measured. Because reproducibility of deformation imaging has been shown to be strongest with frame rate/heart rate ratios ≥ 0.7,53 the strain findings reported herein should be interpreted with caution given lower frame rate/heart rate ratios. Strain rate was also not measured in this study, which may be less affected by loading conditions and more reflective of myocardial contractility than measurements of global longitudinal strain reported in this study.54 All measurements were performed by a single investigator to limit inter-reader variability. Post-hoc intra-observer and inter-observer reliability testing was performed only for the EI and not for other variables measured in this study. Thus, interpretation of the results and associations from our analyses should be interpreted with this limitation in mind. However, most measures reported in this study have been shown to be highly feasible and reproducible in premature infants using specific image acquisition and post-processing data analysis protocols.24, 30, 41, 55 Lastly, we were unable to account for all variables that may have impacted the EI such as PH medications, positive pressure ventilation and PDAs. The impact of these variables on right heart function and clinical course using serial measures of EI as a potentially useful metric over time is worth future study.
We conclude that among conventional and emerging echocardiographic measurements of RV mechanics, EI was the only parameter independently associated with BPD severity in this study. The EI may be a useful echocardiographic measurement to characterize RV mechanics in patients with BPD at 36 weeks PMA.
Acknowledgments
Supported by the National Institutes of Health/National Center for Research Resources (K23RR021921 [to P.M.]), the National Institutes of Health/National Heart, Lung, and Blood Institute (R01 HL085703 [to S.A.]), and the National Institutes of Health/National Center for Research Resources Colorado (CTSI UL1 TR000154). The authors declare no conflicts of interest.
Abbreviations:
- BPD
Bronchopulmonary dysplasia
- PH
pulmonary hypertension
- RV
right ventricular
- TAPSE
tricuspid annular plane systolic excursion
- RA
RV and right atrial
- FAC
fractional area change
- TDI
tissue Doppler imaging
- MPI
myocardial performance index
- TRJV
tricuspid regurgitation jet velocity
- BWZ
Birthweight z-score
- PMA
post-menstrual age
- REDcap
Research Electronic Data Capture
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mourani PM, Sontag MK, Younoszai A, Miller JI, Kinsella JP, Baker CD, et al. Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;191:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Son J-W. Early detection for right ventricular dysfunction in bronchopulmonary dysplasia without pulmonary hypertension. J Cardiovasc Ultrasound. 2016;24:268–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Breatnach CR, Levy PT, James AT, Franklin O, El-Khuffash A. Novel echocardiography methods in the functional assessment of the newborn heart. Neonatology. 2016;110:248–60. [DOI] [PubMed] [Google Scholar]
- [4].Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. [DOI] [PubMed] [Google Scholar]
- [5].Walsh MC, Wilson-Costello D, Zadell A, Newman N, Fanaroff A. Safety, reliability, and validity of a physiologic definition of bronchopulmonary dysplasia. J Perinatol. 2003;23:451–6 [DOI] [PubMed] [Google Scholar]
- [6].Baker CD, Balasubramaniam V, Mourani PM, Sontag MK, Black CP, Ryan SL, et al. Cord blood angiogenic progenitor cells are decreased in bronchopulmonary dysplasia. Eur Respir J. 2012;40:1516–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Poindexter BB, Feng R, Schmidt B, Aschner JL, Ballard RA, Hamvas A, et al. Comparisons and limitations of current definitions of bronchopulmonary dysplasia for the prematurity and respiratory outcomes program. Ann Am Thorac Soc. 2015;12:1822–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Di Maria MV, Younoszai AK, Sontag MK, Miller JI, Poindexter BB, Ingram DA, et al. Maturational changes in diastolic longitudinal myocardial velocity in preterm infants. J Am Soc Echocardiogr. 2015;28:1045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Morrow LA, Wagner BD, Ingram DA, Poindexter BB, Schibler K, Cotten CM, et al. Antenatal determinants of bronchopulmonary dysplasia and late respiratory disease in preterm infants. Am J Respir Crit Care Med. 2017;196:364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wagner BD, Babinec AE, Carpenter C, Gonzalez S, O’Brien G, Rollock K, et al. Proteomic profiles associated with early echocardiogram evidence of pulmonary vascular disease in preterm infants. Am J Respir Crit Care Med. 2018;197:394–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: A report from the pediatric measurements writing group of the american society of echocardiography pediatric and congenital heart disease council. J Am Soc Echocardiogr. 2010;23:465–95; quiz 576–7. [DOI] [PubMed] [Google Scholar]
- [12].Mertens L, Seri I, Marek J, Arlettaz R, Barker P, McNamara P, et al. Targeted neonatal echocardiography in the neonatal intensive care unit: Practice guidelines and recommendations for training. Writing group of the American Society of Echocardiography (ASE) in collaboration with the European Association of Echocardiography (EAE) and the Association for European Pediatric Cardiologists (AEPC). J Am Soc Echocardiogr. 2011;24:1057–78. [DOI] [PubMed] [Google Scholar]
- [13].Colan SD, Shirali G, Margossian R, Gallagher D, Altmann K, Canter C, et al. The ventricular volume variability study of the Pediatric Heart Network: study design and impact of beat averaging and variable type on the reproducibility of echocardiographic measurements in children with chronic dilated cardiomyopathy. J Am Soc Echocardiogr. 2012;25:842–54.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tei C, Dujardin KS, Hodge DO, Bailey KR, McGoon MD, Tajik AJ, et al. Doppler echocardiographic index for assessment of global right ventricular function. J Am Soc Echocardiogr. 1996;9:838–47. [DOI] [PubMed] [Google Scholar]
- [15].Harada K, Tamura M, Toyono M, Yasuoka K. Comparison of the right ventricular Tei index by tissue Doppler imaging to that obtained by pulsed Doppler in children without heart disease. Am J Cardiol. 2002;90:566–9. [DOI] [PubMed] [Google Scholar]
- [16].Waggoner AD, Bierig SM. Tissue Doppler imaging: a useful echocardiographic method for the cardiac sonographer to assess systolic and diastolic ventricular function. J Am Soc Echocardiogr. 2001;14:1143–52. [DOI] [PubMed] [Google Scholar]
- [17].Friedberg MK, Silverman NH. The systolic to diastolic duration ratio in children with heart failure secondary to restrictive cardiomyopathy. J Am Soc Echocardiogr. 2006;19:1326–31. [DOI] [PubMed] [Google Scholar]
- [18].Levy PT, Patel MD, Groh G, Choudhry S, Murphy J, Holland MR, et al. Pulmonary artery acceleration time provides a reliable estimate of invasive pulmonary hemodynamics in children. J Am Soc Echocardiogr. 2016;29:1056–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang YC, Huang CH, Tu YK. Pulmonary hypertension and pulmonary artery acceleration time: A systematic review and meta-analysis. J Am Soc Echocardiogr. 2018;31:201–10.e3. [DOI] [PubMed] [Google Scholar]
- [20].Ryan T, Petrovic O, Dillon JC, Feigenbaum H, Conley MJ, Armstrong WF. An echocardiographic index for separation of right ventricular volume and pressure overload. J Am Coll Cardiol. 1985;5:918–27. [DOI] [PubMed] [Google Scholar]
- [21].Webb MK, Auerbach SR, Younoszai AK, Patel SS, Landeck BF 2nd. Strain and strain rate measured on echocardiogram 1–3 weeks after starting treatment is worse in acute dilated cardiomyopathy pediatric patients with poor outcomes at one year. Echocardiography. 2015;32:1688–96. [DOI] [PubMed] [Google Scholar]
- [22].Boruta RJ, Miyamoto SD, Younoszai AK, Patel SS, Landeck BF. Worsening in longitudinal strain and strain rate anticipates development of pediatric transplant coronary artery vasculopathy as soon as one year following transplant. Pediatr Cardiol. 2018;39:129–39. [DOI] [PubMed] [Google Scholar]
- [23].Zoeller BB, Miyamoto SD, Younoszai AK, Landeck BF 2nd. Longitudinal strain and strain rate abnormalities precede invasive diagnosis of transplant coronary artery vasculopathy in pediatric cardiac transplant patients. Pediatr Cardiol. 2016;37:656–62. [DOI] [PubMed] [Google Scholar]
- [24].Levy PT, Holland MR, Sekarski TJ, Hamvas A, Singh GK. Feasibility and reproducibility of systolic right ventricular strain measurement by speckle-tracking echocardiography in premature infants. J Am Soc Echocardiogr. 2013;26:1201–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Khemani E, McElhinney DB, Rhein L, Andrade O, Lacro RV, Thomas KC, et al. Pulmonary artery hypertension in formerly premature infants with bronchopulmonary dysplasia: Clinical features and outcomes in the surfactant era. Pediatrics. 2007;120:1260–9. [DOI] [PubMed] [Google Scholar]
- [27].del Cerro MJ, Sabate Rotes A, Carton A, Deiros L, Bret M, Cordeiro M, et al. Pulmonary hypertension in bronchopulmonary dysplasia: clinical findings, cardiovascular anomalies and outcomes. Pediatr Pulmonol. 2014;49:49–59. [DOI] [PubMed] [Google Scholar]
- [28].Islam JY, Keller RL, Aschner JL, Hartert TV, Moore PE. Understanding the short- and long-term respiratory outcomes of prematurity and bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2015;192:134–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics. 2010;126:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McCrary AW, Malowitz JR, Hornick CP, Hill KD, Cotten CM, Tatum GH, et al. Differences in eccentricity index and systolic-diastolic ratio in extremely low-birth-weight infants with bronchopulmonary dysplasia at risk of pulmonary hypertension. Am J Perinatol. 2016;33:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Skinner GJ. Echocardiographic assessment of pulmonary arterial hypertension for pediatricians and neonatologists. Front Pediatr. 2017;5:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Abraham S, Weismann CG. Left ventricular end-systolic eccentricity index for assessment of pulmonary hypertension in infants. Echocardiography. 2016;33:910–5. [DOI] [PubMed] [Google Scholar]
- [33].Kovacs G, Maier R, Aberer E, Brodmann M, Scheidl S, Tröster N, et al. Borderline pulmonary arterial pressure is associated with decreased exercise capacity in scleroderma. Am J Respir Crit Care Med. 2009;180:881–6. [DOI] [PubMed] [Google Scholar]
- [34].Maron BA, Brittain EL, Choudhary G, Gladwin MT. Redefining pulmonary hypertension. Lancet Respir Med. 2017. [DOI] [PubMed] [Google Scholar]
- [35].Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: Insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation. 2016;133:1240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Choi YE, Cho HJ, Song ES, Jeong IS, Yoon N, Choi YY, et al. Clinical utility of echocardiography for the diagnosis and prognosis in children with bronchopulmonary dsyplasia. J Cardiovasc Ultrasound. 2016;24:278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Haque U, Stiver C, Rivera BK, Richards B, Ma N, Cua CL, et al. Right ventricular performance using myocardial deformation imaging in infants with bronchopulmonary dysplasia. J Perinatol. 2017;37:81–7. [DOI] [PubMed] [Google Scholar]
- [38].Aye CYL, Lewandowski AJ, Lamata P, Upton R, Davis E, Ohuma EO, et al. Disproportionate cardiac hypertrophy during early postnatal development in infants born preterm. Pediatr Res. 2017;82:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Mourani PM, Sontag MK, Younoszai A, Ivy DD, Abman SH. Clinical utility of echocardiography for the diagnosis and management of pulmonary vascular disease in young children with chronic lung disease. Pediatrics. 2008;121:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Carlton EF, Sontag MK, Younoszai A, DiMaria MV, Miller JI, Poindexter BB, et al. Reliability of echocardiographic indicators of pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. J Pediatr. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Levy PT, Dioneda B, Holland MR, Sekarski TJ, Lee CK, Mathur A, et al. Right ventricular function in preterm and term neonates: reference values for right ventricle areas and fractional area of change. J Am Soc Echocardiogr. 2015;28:559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sehgal A, Malikiwi A, Paul E, Tan K, Menahem S. Right ventricular function in infants with bronchopulmonary dysplasia: Association with respiratory sequelae. Neonatology. 2016;109:289–96. [DOI] [PubMed] [Google Scholar]
- [43].Seo YH, Choi HJ. Clinical utility of echocardiography for early and late pulmonary hypertension in preterm infants: Relation with bronchopulmonary dysplasia. J Cardiovasc Ultrasound. 2017;25:124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Yates AR, Welty SE, Gest AL, Cua CL. Myocardial tissue Doppler changes in patients with bronchopulmonary dysplasia. J Pediatr. 2008;152:766–70, 70 e1. [DOI] [PubMed] [Google Scholar]
- [45].Koestenberger M, Grangl G, Avian A, Gamillscheg A, Grillitsch M, Cvirn G, et al. Normal reference values and z scores of the pulmonary artery acceleration time in children and its importance for the assessment of pulmonary hypertension. Circ Cardiovasc Imaging. 2017;10. [DOI] [PubMed] [Google Scholar]
- [46].Subhedar NV, Shaw NJ. Changes in pulmonary arterial pressure in preterm infants with chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 2000;82:F243–F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gill AB, Weindling AM. Raised pulmonary artery pressure in very low birthweight infants requiring supplemental oxygen at 36 weeks after conception. Arch Dis Child Fetal Neonatal Ed. 1995;72:F20–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gill AB, Weindling AM. Pulmonary artery pressure changes in the very low birthweight infant developing chronic lung disease. Arch Dis Child. 1993;68:303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Subhedar NV, Hamdan AH, Ryan SW, Shaw NJ. Pulmonary artery pressure: early predictor of chronic lung disease in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;78:F20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Su BH, Watanabe T, Shimizu M, Yanagisawa M. Doppler assessment of pulmonary artery pressure in neonates at risk of chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 1997;77:F23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bokiniec R, Wlasienko P, Borszewska-Kornacka M, Szymkiewicz-Dangel J. Echocardiographic evaluation of right ventricular function in preterm infants with bronchopulmonary dysplasia. Echocardiography. 2017;34:577–86. [DOI] [PubMed] [Google Scholar]
- [52].Vonk Noordegraaf A, Westerhof BE, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. 2017;69:236–43. [DOI] [PubMed] [Google Scholar]
- [53].Sanchez AA, Levy PT, Sekarski TJ, Hamvas A, Holland MR, Singh GK. Effects of frame rate on two-dimensional speckle tracking-derived measurements of myocardial deformation in premature infants. Echocardiography. 2015;32:839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Breatnach CR, Levy PT, Franklin O, El-Khuffash A. Strain rate and its positive forcefrequency relationship: Further evidence from a premature infant cohort. J Am Soc Echocardiogr. 2017;30:1045–6. [DOI] [PubMed] [Google Scholar]
- [55].Richardson C, Amirtharaj C, Gruber D, Hayes DA. Assessing myocardial function in infants with pulmonary hypertension: The role of tissue doppler imaging and tricuspid annular plane systolic excursion. Pediatr Cardiol. 2017;38:558–65. [DOI] [PubMed] [Google Scholar]