Abstract
Objective:
To assess the extent to which social and family factors explain variability in cognitive, language, and motor development among very preterm (VPT; <30 weeks of gestation) children from 2 to 5 years of age.
Study design:
As part of a longitudinal study, VPT children recruited as neonates were assessed at 2 (n=87) and 5 (n=83) years using standardized tests of cognitive, language, and motor ability alongside demographically-matched full term (FT) children (n=63). For VPT children, developmental change scores were calculated for each domain to assess within-individual variability to 5 years of age. Multivariate regression and mixed-effect models examined social risk index, parenting stress, family functioning, and maternal intellectual ability as predictors of developmental variation among VPT children.
Results:
VPT children demonstrated poorer cognitive, language, and motor abilities than FT children at 2 (P ≤ .001) and 5 (p<.002) years of age. Social adversity was associated with cognitive (p<.001) and language (p<.001) outcomes at both ages, with parenting stress also related to cognitive outcomes (p=.03). Infant medical risk was associated with motor outcome at 5 years (p=.01). VPT children showed considerable within-individual variation between assessments. Among VPT children, neonatal white matter abnormalities predicted worsening cognitive (p=.04) and motor development (p=.01). Social risk index predicted worsening language development (p=.04), but this association was subsequently explained by dysfunctional maternal affective involvement (p=.01) and lower maternal intellectual ability (p=.05).
Conclusions:
Both clinical and socioenvironmental factors are associated with cognitive, language and motor developmental variation among VPT children from infancy to early school age.
Keywords: Very preterm, follow-up, cognition, language, motor, development, social adversity
Very preterm birth, before 30 weeks of gestation, disproportionately occurs among socially disadvantaged mothers.1,2 However, few studies have investigated cumulative measures of social adversity on the developmental trajectories of VPT children.3–7 Findings from three previous studies suggest links between social risk index scores and cognitive development. Manley et al demonstrated that low birthweight children gained 11 cognitive points from age 18 months to 5 years when the social factors of maternal education, paternal education, and parental employment were present compared with low birthweight children raised in households without these factors.10 Mangin et al found that VPT children with moderate-severe neonatal white matter abnormalities (WMA) from high-social risk households have the poorest cognitive trajectories. 9
As prior VPT cohorts9,10 had fewer single parent, ethnic minority, and low socio-economic families than typical American cohorts,8,11 previous findings may not generalize to more disadvantaged samples12 and have not accounted for shared variance in social background among preterm multiples despite these families experiencing greater social hardships and parenting stress.13 Parenting stress and family dysfunction are associated with VPT birth14 and social risk15 yet the extent to which these factors underlie developmental variation among VPT children is unclear. In addition, maternal intelligence co-varies with education and occupation status16,17 and predicts cross-sectional cognitive, language and motor outcomes in term and VPT children.11,18 However, maternal intelligence has not been considered in the longitudinal development of VPT children.16,19 This study examines the cognitive, language and motor development of VPT children to 5 years of age and the extent to which social risk, parenting stress, and family dysfunction explains developmental variability, accounting for maternal intelligence and factors associated with prematurity.
Methods
Children were prospectively assessed at 2 (n= 120) and 5 years of age (n= 124). The VPT group (n=104) was born at ≤ 30 weeks of gestation from 2007–2011 and hospitalized in the St. Louis Children’s Hospital Neonatal Intensive Care Unit (NICU). At 2 years of corrected age, 87 (84%) VPT infants underwent developmental assessment. Eighty-three (80%) VPT children returned for follow-up at a chronological age of 5 years. VPT children lost to follow-up were more likely to be born to young mothers (≤ 18 years; p=.02) and with public health insurance (p=.002). The full term (FT) control group consisted of 33/37 (89%) children (born 37–41 weeks of gestation) who were recruited as infants from an adjoining hospital’s obstetric service and assessed at 2 years of age. At the time of data analysis, 11 FT children were 5 years old and returned for follow-up, alongside 30 additional controls recruited from the local communities of the VPT group. FT children were matched to VPT children based upon the distribution of sample characteristics, including age, sex, and ethnicity (Table 1). Comparison with 2010 Census tract data indicated that sample demographics were representative of the St. Louis city population.20 Study exclusion criteria included: parent unable to give informed consent, neonatal chromosomal/congenital abnormality or suspected/proven congenital infection. Additional exclusion criteria for FT infants included positive maternal urine drug screen and neonatal acidosis. Study procedures were approved by Washington University Intuitional Review Board. Written informed consent was obtained from all caregivers.
Table 1.
Infant Clinical and Social Background Characteristics of All Study Children
| VPT (n = 93)a | FT (n = 63)b | p | |
|---|---|---|---|
| Gestational age (weeks), m (SD) | 26.59 (1.8) | 39.48 (1.0) | <.001 |
| Birthweight (grams), m (SD) | 943.14 (257.6) | 3371.67 (484.3) | <.001 |
| Male, % | 44.1 | 46.0 | .81 |
| Multiple birth, % | 32.3 | - | |
| NICU Room Type, % | |||
| Private room | 52.7 | - | |
| Open ward | 46.2 | - | |
| Antenatal steroids not administered , % | 8.6 | - | |
| Postnatal dexamethasone administered, % | 9.7 | - | |
| Confirmed Sepsis, % | 32.3 | - | |
| Necrotizing enterocolitis, % | 6.5 | - | |
| Patent ductus arteriosus, % | 51.6 | - | |
| Prolonged oxygen supplementation, % | 52.7 | - | |
| Periventricular leukomalacia grade 3/4, % c | 3.5 | - | |
| Intraventricular hemorrhage grade 3/4, % c | 5.9 | - | |
| Moderate/severe white matter abnormality, % c | 36.6 | - | |
| Maternal Social Background, % | |||
| African American | 38.7 | 54.0 | .06 |
| ≤18 years at delivery | 6.5 | 1.6 | .24 |
| Not a High School graduate | 8.6 | 19.7 | .05 |
| Single parent household | 34.4 | 24.6 | .20 |
| Public health insurance | 62.4 | 57.1 | .51 |
| Social risk index, m (SD) | 1.46 (1.4) | 1.60 (1.4) | .53 |
| Early intervention service utilization, % | 63.0 | 2.4 | <.001 |
| Age (years) at 2-year follow-up, m (SD) | 2.37 (0.3) | 2.41 (0.3) | .58 |
| Age (years) at 5-year follow-up, m (SD ) | 5.62 (0.4) | 5.64 (0.5) | .83 |
93=87 VPT subjects seen at age 2 and 5 years, 6 additional VPT subjects relocated at age 5 years
63=33 FT subjects recruited as infants, 30 FT subjects recruited at age 5 years
From term-equivalent MRI scan
Infant and Child Factors.
Infant medical records were obtained and a medical risk index score (range: 0–10) was created from dichotomized (present= 1, absent= 0) factors: intrauterine growth restriction, did not receive antenatal steroids, received dexamethasone, oxygen at 36 weeks, necrotizing enterocolitis, confirmed sepsis, patent ductus arteriosus, retinopathy of prematurity, ≥3 standard deviation (SD) decrease in weight-for-height/length from birth to term-equivalent age, and >75th percentile for duration of parenteral nutrition.11 Based upon previous findings in this cohort,21 NICU room type (private room or open ward) was also examined as a potential covariate of interest. VPT infants underwent magnetic resonance imaging (MRI) at term-equivalent postmenstrual age using a Siemens Magnetom Trio 3T scanner with previously documented sequences.22 MRI images were qualitatively scored for the presence and severity of cystic lesions, focal signal abnormality, myelination delay, thinning of the corpus callosum, lateral ventricle dilatation, and cerebral volume reduction.23 Total WMA scores (range: 0–15) were categorized into none (0–2), mild (3–4), moderate (5–6), and severe (≥7).23 Mothers reported the use of physical, occupational or speech/language intervention services from birth to 5 years on a customized questionnaire.
Neurodevelopmental Abilities.
The Bayley Scales of Infant and Toddler Development-Third Edition (Bayley-III)24 assessed cognitive, language, and motor abilities at 2 years. Three children had missing Bayley-III scores due to non-compliance. At 5 years, cognition was assessed with the Wechsler Preschool and Primary Scale of Intelligence-Third Edition (WPPSI-III).25 Two VPT children were assessed with the Differential Abilities Scale due to low functioning.26 At 5 years, the Clinical Evaluation of Language Fundamentals-Preschool 2 (CELF-P2)27 provided a Core Language Score and the Movement Assessment Battery for Children, Second Edition (MABC-2)28 evaluated fine and gross motor abilities. The MABC-2 Total Standard Score (m=10, SD=3) was transformed to a standardized mean of 100 and SD of 15 to enable comparisons with other measures. Missing 5-year data was due to developmental impairment (n=2), non-compliance (n=8), and English not first language (n= 1).
Social and Family Factors
Based upon previous studies,9,29,30 an adapted social risk index (range: 0–5) was created from maternal factors that were dichotomized (present= 1, absent= 0) and summed: ≤18 years at delivery, African-American, no high school degree, public health insurance, and single parent household. At the 2 and 5 year follow-up, the Parenting Stress Index (PSI) assessed parenting stress related to the parenting role, dysfunctional parent-child interactions, and negative perceptions of the child.31 Mothers also completed the Family Assessment Device (FAD) at both time points to evaluate general family dysfunction, affective responsiveness, affective involvement, behavior control, communication, problem solving, and family role dysfunction.32 At the 5 year follow-up, maternal intellectual ability was assessed with the Wechsler Test of Adult Reading (WTAR).33 The WTAR was selected to balance participant burden and because it is co-normed with the Wechsler Adult Intelligence Scales-III (WASI-III). WTAR standard scores were converted to demographically-predicted WASI-III Full Scale IQ (FSIQ) scores based on a representative US sample. 33
Statistical Analyses.
Data analysis was completed in three steps. First, VPT and FT differences on cognitive, language and motor tasks were examined using independent t-tests, subsequently adjusted for covariates using Linear Mixed-effect Models (LMMs) with Restricted Maximum Likelihood estimation. Omega squared is reported as a measure of effect size.34 Mother-and-subject-within-mother was also included as a random factor with random intercept in the LMMs to account for shared variance between preterm twins and triplets clustered within a family. Unadjusted and adjusted rates of delay (defined as a standardized score ≤ 80 for Bayley-III,35 WPPSI-III,25 and CELF-P227; ≤ 5th percentile for MABC-228) were examined using chi-square tests and logistic regression, respectively. Second, LMMs examined links between prematurity and social risk index on outcomes at 2 and 5 years accounting for infant medical risk, sex, and family clustering. Models were then extended to include PSI Total Stress percentiles and FAD General Functioning scores. Third, a two-step multivariate approach examined longitudinal associations between social and family factors and developmental variability among VPT children from 2 to 5 years (n= 83). For each domain, a “developmental change” score was created (i.e., standardized score at 5 years minus the standardized score at 2 years) to describe developmental trajectories10,36 in terms of improvement (positive values) or decline (negative values). Stepwise multivariate linear regression models identified predictors of developmental change scores using infant medical risk, sex, neonatal WMA and social risk index in step 1 of model development. PSI (Parenting Distress, Parent-Child Dysfunctional Interaction, and Difficult Child) and FAD (Affective Responsiveness, Affective Involvement, Behavior Control, Communication, Problem Solving, and Family Roles) subscale scores collected at the 2-year follow-up were added in step 2, and maternal intellectual ability and the use of developmental intervention services were added in step 3. Key factors were then adjusted for family clustering using LMMs.
Results
Cross-Sectional Neurobehavioral Outcomes at Ages 2 and 5 Years:
Compared with FT children, VPT children had lower cognitive tests scores and increased rates of delay by 5 years (Table 2). Higher levels of social risk were related to poorer cognitive outcomes at 2 and 5 years; explaining up to 11.8% of the variance (Table 3; available at www.jpeds.com). There was no interaction between prematurity and social risk at either age. Extending the LLMs to include PSI Total Stress percentiles demonstrated that greater parenting stress was associated with poorer cognitive ability at 2 years (Estimate= −0.08, p=.03), explaining 26.9% of the variance. This association was independent of social risk that was also significant (Estimate= −2.75, p=.03). Similar results were found at 5 years, with greater parenting stress related to poorer cognitive ability (Estimate= −0.12, p=.05) and accounting for 18% of the variance, independent of social risk (Estimate= −3.50, p=.01). There was no interaction between social risk and parental stress either at 2 (p=.20) or 5 years (p=.70). FAD General Functioning scores were not associated with cognition at 2 (p=.15) or 5 years (p=.59).
Table 2.
Neurodevelopmental Outcomes of VPT and FT Children at Ages 2 and 5 Years
| VPT | FT | p | d/OR | Adjusted pc | Adjusted pd | |
|---|---|---|---|---|---|---|
| Age 2 Years a | n = 87 | n = 33 | ||||
| Cognition | n = 87 | n = 33 | ||||
| Cognition score, m (SD) | 85.98 (9.30) | 92.73 (11.8) | .001 | 0.64 | <.001 | <.001 |
| Cognitive delay, % (n) | 33.3 (29) | 15.2 (5) | .05 | 2.80 | .02 | .06 |
| Language | n = 85 | n = 33 | ||||
| Language score, m (SD) | 88.38 (11.3) | 100.09 (17.7) | .001 | 0.79 | <.001 | <.001 |
| Language delay, % (n) | 24.7 (21) | 9.1 (3) | .06 | 3.28 | .06 | .22 |
| Motor | n = 86 | n = 32 | ||||
| Motor score, m (SD) | 83.41 (11.0) | 100.28 (15.6) | <.001 | 1.25 | <.001 | <.001 |
| Motor delay, % (n) | 36.0 (31) | 6.3 (2) | .001 | 8.46 | .007 | .03 |
| Age 5 Years | n = 83 | n = 41 | ||||
| Cognition | n = 82 | n = 41 | ||||
| Cognition score, m (SD) | 88.23 (14.4) | 104.49 (17.6) | <.001 | 1.01 | <.001 | <.001 |
| Cognitive delay, % (n) a | 30.5 (25) | 4.9 (2) | .001 | 8.55 | .003 | .004 |
| Language | n = 77 | n = 40 | ||||
| Language score, m (SD) | 88.43 (17.7) | 103.56 (13.5) | <.001 | 0.96 | <.001 | <.001 |
| Language delay, % (n) a | 27.3 (21) | 2.5 (1) | .001 | 14.63 | .008 | .02 |
| Motor | n = 75 | n = 39 | ||||
| Motor score, m (SD) | 77.67 (16.1) | 95.26 (14.2) | <.001 | 1.16 | <.001 | <.001 |
| Motor delay, % (n) b | 52.0 (39) | 7.7 (3) | <.001 | 13.00 | <.001 | <.001 |
Note. Bonferroni corrected p-value = .05/12 = .004.
Delay defined as a standardized score ≤ 80;
Delay defined as standardized score ≤ 5th percentile;
Adjusted for social risk index and sibling clustering;
Adjusted model excluding VPT children with moderate/severe WMA (n=32 at age 2, n=28 at age 5)
Table 3;
Online Only. Effects of Prematurity and Social Factors on Neurodevelopmental Outcomes at Age 2 and 5 Years
| Cognition | Language | Motor | ||||
|---|---|---|---|---|---|---|
| Estimate (S.E) |
p | Estimate (S.E) |
p | Estimate (S.E) |
p | |
| Age 2 Years (n = 120) | ||||||
| Prematurity | 11.21 (3.3) | .001 | 22.25 (4.4) | <.001 | 19.41 (4.7) | <.001 |
| Infant Medical Risk | −0.58 (0.6) | .31 | −0.43 (0.7) | .57 | −1.05 (0.8) | .17 |
| Sex (m=1) | −1.97 (1.5) | .20 | −3.30 (2.0) | .12 | −2.52 (2.0) | .21 |
| Social Risk Index | −2.38 (0.9) | <.001 | −1.52 (1.9) | <.001 | −0.64 (1.2) | .08 |
| Social Risk Index: Interaction with birth group |
−2.39 (1.4) | .09 | −5.77 (1.9) | .003 | −2.16 (1.9) | .27 |
| Age 5 Years (n = 124) | ||||||
| Prematurity | 18.99 (4.3) | <.001 | 15.57 (4.8) | .002 | 15.84 (4.6) | .001 |
| Infant Medical Risk | −1.09 (0.9) | .21 | −1.08 (1.0) | .27 | −2.50 (1.0) | .01 |
| Sex (m=1) | −1.92 (2.5) | .45 | −2.04 (2.5) | .42 | −3.23 (2.8) | .25 |
| Social Risk Index | −3.59 (1.3) | <.001 | −3.68 (1.5) | <.001 | −1.02 (1.4) | .07 |
| Social Risk Index: Interaction with birth group |
−3.17 (2.0) | .12 | −2.01 (2.3) | .39 | −2.07 (2.2) | .36 |
Note. Linear Mixed-Effect Model with family clustering entered as a random factor with intercept.
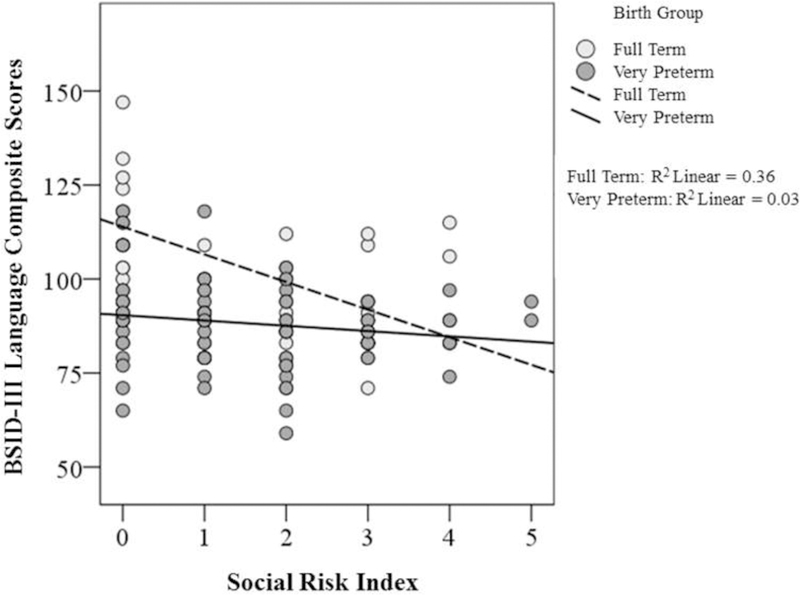
VPT children had lower language scores than FT children at 2 and 5 years, with more VPT children in the delayed range by 5 years (Table 2). Higher levels of social risk were associated with poorer language abilities at 2 and 5 years (Table 3 ), with the proportion of variance explained by social risk increasing from 6.7% at 2 years to 21.5% at 5 years. There was a significant interaction between prematurity and social risk at 2 years. The interaction term suggested that although FT children with lower levels of social risk obtained higher Bayley-III language scores than FT children from disadvantaged backgrounds, VPT children did not similarly benefit from being raised in lower risk households (Figure 2; available at www.jpeds.com). No associations were found between parenting stress or family dysfunction and language outcomes at 2 or 5 years (p>.05).
Figure 2;
Online Only. Interaction between Prematurity and Social Adversity. Illustration of the interaction between prematurity and social risk index on Bayley-III Language Composite Scores obtained by VPT and FT children age 2. This finding suggests that while FT children raised in households characterized by lower levels of social risk obtained higher language scores at age 2, VPT children did not demonstrate a similar advantage for language outcomes when raised in households characterized by lower levels of social risk.
VPT children had lower motor composite scores and higher rates of motor delays compared with FT children at 2 and 5 years (Table 2). Higher levels of social risk were not associated with adverse motor outcomes, but the extent of infant medical risk was inversely related to lower motor scores at 5 years and explained 18.2% of the variance in motor outcome (Table 3). Among individual medical factors, only confirmed sepsis was associated with poorer motor scores at 5 years (β=−.23, p=.05). However, sepsis explained a smaller proportion of variance in outcome than the composite medical risk index (β=−.28, p=.01).
Developmental Variability among VPT Children from 2 to 5 Years:
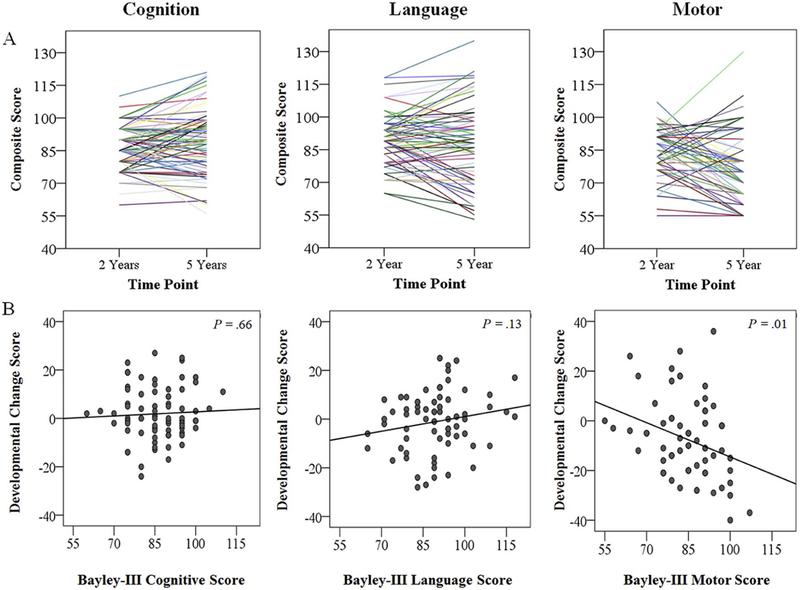
Figure 1, A shows the standardized composite scores for each VPT child at 2 and 5 years. To determine the extent to which developmental trajectories were dependent upon ability at 2 years, developmental change scores (descriptive statistics, Table 4; available online) were examined in relation to Bayley-III scores. Using a bivariate linear regression, there was no clear association between developmental change scores and cognitive ability at 2 years (β=.06, p=.66, Figure 1B). Although Figure 1B suggests that VPT children had a tendency to improving language scores from 2 to 5 years, this association was not significant (β=.20, p=.13). Developmental change scores were negatively associated with 2-year Bayley-III motor scores (β=−.46, p=.01, Figure 1B), suggesting a general decline in motor skills to 5 years, particularly among VPT children who obtained higher 2-year Bayley-III motor scores. Supplementary paired-sample t-tests indicated this negative association was primarily driven by decline in fine motor skills, as illustrated by lower fine motor subscale scores at 5 years (m= 4.4 ± 2.8) compared with 2 years (m= 8.00 ± 2.0, p< .001). Decline in gross motor subscale scores (mage2= 6.77 ± 2.1, mage5= 6.16 ± 3.2, p=.07) was less prominent.
Figure 1.
Neurobehavioral Development in VPT Children. Figure 1 shows within-individual variation in composite scores obtained by VPT children at 2 and 5 years for cognitive, language, and motor domains (Panel A). Figure 1 also shows the extent to which composite scores obtained on the 2-year Bayley-III scores relate to developmental change scores calculated for cognitive, language and motor domains (Panel B).
Table 4;
Online Only. Descriptive Statistics of Developmental Change Scores for Cognitive, Language and Motor Domains for Very Preterm Children Between Age 2 to 5 Years (n = 83)
| Domain | M (SD) | Median | Range |
|---|---|---|---|
| Cognition | 1.91 (10.7) | 0.00 | −24.0 – 27.0 |
| Language | −1.06 (12.3) | 0.00 | −28.0 – 25.0 |
| Motor | −7.49 (15.9) | −10.00 | −40.0 – 36.0 |
Predictors of VPT Children’s Development from 2 to 5 years:
Table 5 summarizes the predictors of developmental change scores for cognitive, language, and motor domains among VPT children (n=83). For cognitive development, as the severity of neonatal WMA increased there was a corresponding decline in cognitive development to 5 years. This association persisted after excluding VPT children with moderate-severe WMA (β= −0.32, p=.03). Because there was a small but significant correlation between neonatal WMA and maternal FSIQ (r= −.26, p=.03), alternative models were created without WMA (to examine whether maternal FSIQ might predict change in cognitive development), but these did not alter study findings. Utilization of intervention services was also related to cognitive decline, but this association was attenuated after accounting for family clustering (Table 5). Eighty percent of preterm twins and triplets received physical, occupational, or speech/language services compared with 54.8% of preterm singletons (p=.02). Interestingly, 58% of preterm multiples had cognitive or language delay at 2 years compared with 36% of preterm singletons (p=.05). Exposure to social adversity, parenting stress or family dysfunction did not predict variation in cognitive development among VPT children between 2 and 5 years. Additional supplementary analysis did not suggest that NICU room type was associated with change in cognitive development.
Table 5.
Predictors of Cognitive, Language and Motor Development among VPT Children Between Age 2 and 5 Years (n = 83)
| Base Model | Final Model | LMM | |||
|---|---|---|---|---|---|
| β | p | β | p | p | |
| Cognitive Development | R2 (5, 77 ) = .11, p = .11 | R2 (6, 76 ) = .15, p = .04 | |||
| Medical Risk Index | −.07 | .51 | −.08 | .46 | .21 |
| Sex | .13 | .23 | .10 | .35 | .71 |
| White Matter Abnormalities | .25 | .03 | −.23 | .04 | .03 |
| Cognitive Ability at Age 2 | −.05 | .67 | −.05 | .69 | .99 |
| Social Risk Index | −.15 | .20 | −.16 | .16 | .34 |
| Intervention Service utilization | - | - | −.21 | .05 | .06 |
| Language Development | R2 (5, 52) = .14, p = .16 | R2 (7, 50 ) = .27, p = .02 | |||
| Medical Risk Index | −.11 | .41 | −.10 | .42 | .57 |
| Sex | .05 | .69 | .08 | .53 | .13 |
| White Matter Abnormalities | −.10 | .46 | −.03 | .83 | .63 |
| Language Ability at age 2 | .12 | .38 | .05 | .72 | .50 |
| Social Risk Index | −.27 | .04 | −.01 | .94 | .94 |
| Maternal Affective Involvement | - | - | −.32 | .01 | .03 |
| Maternal Intellectual Ability | - | - | .35 | .05 | .04 |
| Motor Development | R2 (5, 77) = .21, p = .002 | R2 (6, 76) = .27, p < .001 | |||
| Medial Risk Index | −0.14 | .18 | −0.19 | .07 | .38 |
| Sex | 0.09 | .37 | 0.07 | .47 | .19 |
| White Matter Abnormalities | −0.30 | .007 | −0.27 | .01 | .01 |
| Motor Ability at Age 2 | −0.38 | .001 | −0.37 | .001 | .005 |
| Social Risk Index | −0.06 | .56 | −0.05 | .64 | .45 |
| Maternal Behavioral Control | - | - | 0.25 | .01 | .21 |
Note. LMM p-value: Linear Mixed-Effect Model with family clustering entered as a random factor with intercept.
Higher levels of social risk were associated with declining developmental change scores for language among VPT children (Table 5). However, the association between social risk and declining language change scores was attenuated when FAD Affective Involvement scores and maternal FSIQs were added to the model. Maternal affective involvement and intellectual ability explained an additional 13% of the variance (total R2= .27, p=.02). Associations for FAD Affective Involvement and maternal FSIQ persisted after accounting for family clustering. Additional supplementary analysis did not suggest that NICU room type was associated with change in language development.
Consistent with cross-sectional outcomes reported at 2 and 5 years, social risk index was not associated with variability in motor development among VPT children (Table 5). However, the presence and severity of neonatal WMA and motor ability at 2 years were both negatively associated with motor change scores. Together, these factors explained 21% of the variance in change in motor ability from 2 to 5 years and these findings persisted after accounting for family clustering (p≤.01). Additional supplementary analysis did not suggest that NICU room type was associated with change in motor development.
Discussion
This study examined cognitive, language, and motor development in VPT children with a focus on the social and family factors that explain developmental variation among VPT children to 5 years of age. Consistent with prior studies,37,38 VPT children performed less well and had higher rates of delay on cognitive, language, and motor tasks than FT children at ages 2 and 5. In line with Manley et al, social risk index explained 7–12% of the variance in cross-sectional cognitive outcomes.10 Parenting stress was associated more strongly with cross-sectional cognitive outcomes, explaining 18–27% of the variance. There was no evidence of an interaction between social adversity and parenting stress, highlighting independent influences of demographic and parenting factors.39 High social risk mothers may be less well-adjusted to the parenting role, and in turn, less likely to provide supportive early learning enviroments.40,41 Higher parenting stress may also reflect the longer-term consequences of infant admission to the NICU, due to the perceived loss of the parenting role and disrupted parent-infant relationship,42,43 as well as the longer-term challenges associated with parenting a high-risk preterm child.44
Social risk also was associated with cross-sectional language outcomes. Both the LMM estimates and the proportion of variance explained (7–22%) in language increased from 2 to 5 years. The interaction between VPT birth and social risk indicated that FT children with lower levels of social risk had better language skills, and VPT children did not similarly benefit from being raised in lower risk households. This suggests that language disparities begin to widen among school-age children raised in environments characterized by varying degrees of social adversity, and that VPT children may be less sensitive to social advantage than FT children.45,46 In contrast to studies linking social risk with motor skills in VPT children,47 infant medical risks were associated with poorer motor outcomes. The influence of infant medical risk on motor skills48,49 may be due to the neural networks serving motor skill acquisition maturing in infancy,50,51 making them more vulnerable to biological insults during the neonatal period compared with later developing cognitive and language networks which may be more susceptible to social adversity in childhood.50, 51
In contrast to the hypothesis, neither exposure to social adversity, parenting stress nor family dysfunction at 2 years predicted variability in cognitive development by 5 years. Instead, moderate-severe neonatal WMA was negatively associated with developmental change scores for cognition. Although two prior studies have shown that parental stress52 and ethnic minority status36 predict cognitive decline in VPT children, these studies did not include measures of neonatal WMA. Moderate-severe WMA, as well as aberrant white matter microstructure assessed using diffusion tensor MRI, have been strongly linked to poorer cognitive trajectories in VPT children.7,53 However, our results show that neonatal WMA is also associated with intraindividual cognitive decline relative to standardized norms, likely reflecting the emergence of subtle cognitive problems as the demands placed on children increase by early school age. The use of developmental intervention services also was associated with intraindividual cognitive decline, although this finding was attenuated after adjusting for family clustering. In line with prior reports,13,54 preterm multiples were more likely to have received interventions than singletons, potentially due higher rates of delay at 2 years.
Higher levels of social risk were associated with worsening language trajectories among VPT children to 5 years. This finding is consistent with prior reports separately linking maternal education, ethnicity, and low SES with worsening language development in preterm children.5,36 We also found that the association between social risk and language decline was subsequently accounted for by lower levels of maternal affective involvement and maternal intellectual ability. This finding could reflect the extent to which maternal concern and nurturing behavior shapes the early language environment, above and beyond socio-demographic factors.55,56 Although we11 and others18 have reported that maternal intellectual ability is associated with concurrent language abilities in VPT and FT children, our results also show that maternal intellectual ability is related to change in language. Prior evidence suggests that mothers with greater cognitive ability may be using rich and diverse language with their children in the home.57
Intraindividual decline in motor development was associated with the presence and extent of neonatal WMA. Preterm insults to vulnerable white matter development commonly occur in regions important for motor skill acquisition.53 Troublingly, decline in motor abilities was particularly noticeable in VPT children who obtained higher Bayley-III motor scores at 2 years of age. Although regression toward the mean could partially account for this finding,10 it does not account for the fact that a small number of VPT children who performed less well at 2 years continued to demonstrate poorer motor trajectories to 5 years of age. Many studies suggest VPT children grow into their motor problems as difficulties with advanced fine and static/dynamic gross motor skills emerge and become more prominent relative to age-norms.58,59 Indeed, supplementary analysis indicated that worsening motor trajectories were primarily due to decline in fine motor skills by 5 years.
Limitations of this study include two follow-up assessments precluding growth curve analysis, the use of two cross-sectional control groups, and the fact that the Bayley-III assesses early development whereas the WPPSI-III, CELF-P2, and MABC-2 assess functioning. Also, this analysis did not include an observational measure of parenting sensitivity. Future work should include longer-term follow-up with repeated observations of parenting behaviors. Although our sample is of modest size (n=124), it is comparable with prior studies of VPT children.47,60 We also acknowledge that concerns exist regarding the identification of impairment using the Bayley-III.61 Although overestimation of abilities at 2 years of age could potentially explain declining motor skills in VPT children, the mean scores and rates of delay reported for our VPT cohort are poorer than other studies using the Bayley-III,62 and FT children’s scores were close to standardized norms. 61
From 2 to 5 years, VPT children showed within-individual developmental variation. Cerebral WMA identified on term-equivalent MRI was associated with worsening cognitive and motor development to 5 years. This suggests that poor developmental trajectories of high-risk VPT children emerge early due to, at least in part, aberrations in vulnerable neonatal white matter development; emphasizing the need for early referral and regular surveillance following NICU discharge. Although social risk was associated with worsening language trajectories, this association was explained by lower levels of maternal affective involvement and maternal intellectual ability. This highlights the extent to which potentially modifiable maternal factors, such as affective involvement, may support language development in VPT children. Thus, information on the ways in which parents can positively influence the development of their VPT infant should be provided as early as the NICU stay.
Acknowledgments
We thank Karen Lukas, Anthony Barton, and Jessica Perkins for study coordination; the IDDRC at Washington University for assistance with data collection; and Charlotte Herzmann, PhD, for feedback regarding preliminary data analysis. We also thank the children and their families for their participation in the study.
Funded by the National Institutes of Health (R01 HD057098, K02 NS089852 [to C.S.], UL1 TR000448 [to C.R. and C.S.], and K23 MH105179 [to C.R.]); Intellectual and Developmental Disabilities Research Center (IDDRC) at Washington University (U54 HD087011); Cerebral Palsy International Research Foundation; The Dana Foundation, and The Doris Duke Charitable Foundation.
Abbreviations:
- FAD
Family Assessment Device
- FSIQ
Full Scale Intelligence Quotient
- FT
Full Term
- GA
Gestational Age
- LMM
Linear Mixed-effects Model
- PSI
Parenting Stress Index
- WMA
White Matter Abnormalities
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Carmichael SL, Kan P, Padula AM, Rehkopf DH, Oehlert JW, Mayo J, et al. Social disadvantage and the black-white disparity in spontaneous preterm delivery among California births. PloS One 2017;12:e0182862 10.1371/journal.pone.0182862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lefmann T, Combs-Orme T, Orme JG. Examining the inter-correlated effects of low income, life stress, and race on birth outcomes: A representative state study. Soc Work Health Care 2017;56:450–469. [DOI] [PubMed] [Google Scholar]
- 3.Wong HS, Edwards P. Nature or Nurture: A Systematic Review of the Effect of Socio-economic Status on the Developmental and Cognitive Outcomes of Children Born Preterm. Matern Child Health J 2013;17:1689–1700. [DOI] [PubMed] [Google Scholar]
- 4.Anderson P, Doyle LW. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA 2003;289:3264–3272. [DOI] [PubMed] [Google Scholar]
- 5.Luu TM, Vohr BR, Schneider KC, Katz KH, Tucker R, Allan WC, et al. Trajectories of Receptive Language Development From 3 to 12 Years of Age for Very Preterm Children. Pediatrics 2009;124:333–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaekel J, Eryigit-Madzwamuse S, Wolke D. Preterm Toddlers’ Inhibitory Control Abilities Predict Attention Regulation and Academic Achievement at Age 8 Years. J Pediatr 2016;169:87–92. [DOI] [PubMed] [Google Scholar]
- 7.Lean RE, Melzer TR, Bora S, Watts R, Woodward LJ. Attention and Regional Gray Matter Development in Very Preterm Children at Age 12 Years. J Int Neuropsychol Soc 2017;23:539–550. [DOI] [PubMed] [Google Scholar]
- 8.Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Wilson-Costello D , et al. Poor Predictive Validity of the Bayley Scales of Infant Development for Cognitive Function of Extremely Low Birth Weight Children at School Age. Pediatrics 2005;116:333–341. [DOI] [PubMed] [Google Scholar]
- 9.Mangin KS, Horwood LJ, Woodward LJ. Cognitive Development Trajectories of Very Preterm and Typically Developing Children. Child Dev 2016; 1: 282–298 [DOI] [PubMed] [Google Scholar]
- 10.Manley BJ, Roberts RS, Doyle LW, Schmidt B, Anderson P, Barrington KJ, et al. Social Variables Predict Gains in Cognitive Scores across the Preschool Years in Children with Birth Weights 500 to 1250 Grams. J Pediatr 2015;166:870–876. [DOI] [PubMed] [Google Scholar]
- 11.Lean RE, Paul RA, Smyser CD, Rogers CE. Maternal intelligence quotient (IQ) predicts IQ and language in very preterm children at age 5 years. J Child Psychol Psychiatry 2018;59:150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reagan PB, Salsberry PJ. Race and ethnic differences in determinants of preterm birth in the USA: broadening the social context. Soc Sci Med 2005;60:2217–2228. [DOI] [PubMed] [Google Scholar]
- 13.Baptista J, Moutinho V, Mateus V, Guimaraes H, Clemente F, Almeida S et al. Being a mother of preterm multiples in the context of socioeconomic disadvantage: perceived stress and psychological symptoms. J Pediatr (Rio J) 2017; S0021–7557. 10.1016/j.jped.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 14.Treyvaud K, Lee KJ, Doyle LW, Anderson PJ. Very Preterm Birth Influences Parental Mental Health and Family Outcomes Seven Years after Birth. J Pediatr 2014;164:515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Devenish B, Hooley M, Mellor D. The Pathways Between Socioeconomic Status and Adolescent Outcomes: A Systematic Review. Am J Community Psychol 2017;59:219–238. [DOI] [PubMed] [Google Scholar]
- 16.Plomin R, Deary IJ. Genetics and intelligence differences: five special findings. Mol Psychiatry 2015;20:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seltzer MM, Floyd F, Greenberg J, Lounds J, Lindstromm M, Hong J. Life course impacts of mild intellectual deficits. Am J Ment Retard AJMR 2005;110:451–468. [DOI] [PubMed] [Google Scholar]
- 18.Ronfani L, Brumatti LV, Mariuz M, Tognin V, Bin M, Ferluga V, et al. The complex interaction between home environment, socioeconomic status, maternal IQ and early child neurocognitive development: a multivariate analysis of data collected in a newborn cohort study. PLoS One 2015;10:e0127052 10.1371/journal.pone.0127052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Soelen ILC, Brouwer RM, van Leeuwen M, Kahn RS, Hulshoff Pol HE, Boomsma DI. Heritability of verbal and performance intelligence in a pediatric longitudinal sample. Twin Res Hum Genet Off J Int Soc Twin Stud 2011;14:119–128. [DOI] [PubMed] [Google Scholar]
- 20.United States Census Bureau. QuickFacts. St. Louis City, Missouri: (County) [2010; cited 2018 May 30] Available from: https://www.census.gov/quickfacts/fact/table/stlouiscitymissouricounty/PST045216. [Google Scholar]
- 21.Pineda RG, Tjoeng TH, Vavasseur C, Kidokoro H, Neil JJ, Inder T. Patterns of altered neurobehavior in preterm infants within the neonatal intensive care unit. J Pediatr 2013;162:470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers CE, Smyser T, Smyser CD, Shimony J, Inder TE, Neil JJ. Regional white matter development in very preterm infants: perinatal predictors and early developmental outcomes. Pediatr Res 2015;79:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol 2013;34:2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bayley N Bayley Scales of Infant and Toddler Development, Third Edition (Bayley III). Pearson Clinical; 2005. [Google Scholar]
- 25.Wechsler D WPPSI-III: Administration and Scoring Manual San Antonio, TX: The Psychological Corporation; 2004. [Google Scholar]
- 26.Elliott CD. Differential Ability Scales Second Edition. San Antonio, TX: Harcourt Assessment; 2007. [Google Scholar]
- 27.Semel E, Wiig EH, Secord WA. Clinical Evaluation of Language Fundamentals Preschool (2nd Ed.). San Antonio, TX: Harcourt Assessment PsyCorp; 2004. [Google Scholar]
- 28.Henderson SE, Sugden DA, Barnett AL, others Movement Assessment Battery for Children-2: Movement ABC-2: Examiner’s Manual Pearson São Paulo; 2007. [Google Scholar]
- 29.Whitaker AH, Feldman JF, van Rossem R, et al. Neontatl Cranial Ultrasound Abnormalities in Low Birth Weight Infants: Relation to Cognitive Outcomes at Six Years of Age. PEDIATRICS 1996;98:719–729. [PubMed] [Google Scholar]
- 30.Treyvaud K, Inder TE, Lee KJ, Northam EA, Doyle LW, Anderson PJ. Can the home environment promote resilience for children born very preterm in the context of social and medical risk? J Exp Child Psychol 2012;112:326–337. [DOI] [PubMed] [Google Scholar]
- 31.Abidin RR. Parenting Stress Index (PSI) Charlottesville, VA: Pediatric Psychology Stress; 1990. [Google Scholar]
- 32.Epstein NB, Baldwin LM, Bishop D. The McMaster family assessment device. J Marital Fam Ther 1983;9:171–180. [Google Scholar]
- 33.Wechsler D Wechsler Test of Adult Reading San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 34.Xu R Measuring explained variation in linear mixed effects models. Stat Med 2003;22:3527–3541. [DOI] [PubMed] [Google Scholar]
- 35.Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res 2014;75:670–674. [DOI] [PubMed] [Google Scholar]
- 36.Greene MM, Patra K, Nelson MN, Silvestri JM. Evaluating preterm infants with the Bayley-III: Patterns and correlates of development. Res Dev Disabil 2012;33:1948–1956. [DOI] [PubMed] [Google Scholar]
- 37.Månsson J, Stjernqvist K. Children born extremely preterm show significant lower cognitive, language and motor function levels compared with children born at term, as measured by the Bayley-III at 2.5 years. Acta Paediatr 2014;103:504–511. [DOI] [PubMed] [Google Scholar]
- 38.Woodward LJ, Moor S, Hood KM, Champion PR, Foster-Cohen S, Inder TE, et al. Very preterm children show impairments across multiple neurodevelopmental domains by age 4 years. Arch Dis Child Fetal Neonatal Ed 2009;94:339–344. [DOI] [PubMed] [Google Scholar]
- 39.Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, Keidar A, et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants: Pain 2009;143:138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huhtala M, Korja R, Lehtonen L,Haataja L, Lapinleimu H, Rautava P, et al. Parental Psychological Well-Being and Behavioral Outcome of Very Low Birth Weight Infants at 3 Years. Pediatrics 2012;129:937–944. [DOI] [PubMed] [Google Scholar]
- 41.Miceli PJ, Goeke-Morey MC, Whitman TL, Kolberg KS, Miller-Loncar C, White RD. Brief Report : Birth Status, Medical Complications, and Social Environment : Individual Differences in Development of Preterm, Very Low Birth Weight Infants. J Pediatr Psychol 2000;25:353–358. [DOI] [PubMed] [Google Scholar]
- 42.Baía I, Amorim M, Silva S, Kelly-Irving M, de Freitas C, Alves E. Parenting very preterm infants and stress in Neonatal Intensive Care Units. Early Hum Dev 2016;101:3–9. [DOI] [PubMed] [Google Scholar]
- 43.Woodward LJ, Bora S, Clark CAC, Montgomery-Honger A, Pritchard VE, Spencer C, et al. Very Preterm Birth: Maternal Experiences of the Neonatal Intensive Care Unit. J Perinatol Off J Calif Perinat Assoc 2014;34:555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treyvaud K Parent and family outcomes following very preterm or very low birth weight birth: A review. Semin Fetal Neonatal Med 2014;19:131–135. [DOI] [PubMed] [Google Scholar]
- 45.Perkins SC, Finegood ED, Swain JE. Poverty and Language Development: Roles of Parenting and Stress. Innov Clin Neurosci 2013;10:10–19. [PMC free article] [PubMed] [Google Scholar]
- 46.Aylward GP. Methodological Issues in Outcome Studies of At-Risk Infants. J Pediatr Psychol 2002;27:37–45. [DOI] [PubMed] [Google Scholar]
- 47.Yaari M, Mankuta D, Harel-Gadassi A, et al. Early developmental trajectories of preterm infants. Res Dev Disabil 2017; 10.1016/j.ridd.2017.10.018 [DOI] [PubMed] [Google Scholar]
- 48.Linsell L, Malouf R, Morris J, Kurinczuk JJ, Marlow N. Prognostic factors for cerebral palsy and motor impairment in children born very preterm or very low birthweight: a systematic review. Dev Med Child Neurol 2016;58:554–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Griffiths A, Morgan P, Anderson PJ, Doyle LW, Lee KJ, Spittle AJ. Predictive value of the Movement Assessment Battery for Children - Second Edition at 4 years, for motor impairment at 8 years in children born preterm. Dev Med Child Neurol 2017;59:490–496. [DOI] [PubMed] [Google Scholar]
- 50.Smyser CD, Snyder AZ, Shimony JS, Mitra A, Inder TE, Neil JJ. Resting-State Network Complexity and Magnitude Are Reduced in Prematurely Born Infants. Cereb Cortex 2016;26:322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smyser CD, Inder TE, Shimony JS, Hill JE, Degnan AJ, Snyder AZ, et al. Longitudinal Analysis of Neural Network Development in Preterm Infants. Cereb Cortex 2010;20:2852–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brummelte S, Grunau RE, Synnes AR, Whitfield MF, Petrie-Thomas J. Declining cognitive development from 8 to 18months in preterm children predicts persisting higher parenting stress. Early Hum Dev 2011;87:273–280. [DOI] [PubMed] [Google Scholar]
- 53.Schadl K, Vassar R, Cahill-Rowley K, Yeom KW, Stevenson DK, Rose J. Prediction of cognitive and motor development in preterm children using exhaustive feature selection and cross-validation of near-term white matter microstructure. NeuroImage Clin 2018;17:667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clements KM, Barfield WD, Kotelchuck M, Lee KG, Wilber N. Birth Characteristics Associated With Early Intervention Referral, Evaluation for Eligibility, and Program Eligibility in the First Year of Life. Matern Child Health J 2006;10:433–441. [DOI] [PubMed] [Google Scholar]
- 55.Laranjo J, Bernier A. Children’s expressive language in early toddlerhood: Links to prior maternal mind-mindedness. Early Child Dev Care 2013;183:951–962 [Google Scholar]
- 56.Leffel K, Suskind D. Parent-Directed Approaches to Enrich the Early Language Environments of Children Living in Poverty. Semin Speech Lang 2013;34:267–278. [DOI] [PubMed] [Google Scholar]
- 57.Zauche LH, Thul TA, Mahoney AED, Stapel-Wax JL. Influence of language nutrition on children’s language and cognitive development: An integrated review. Early Child Res Q 2016;36:318–333. [Google Scholar]
- 58.de Kieviet JF, Piek JP, Aarnoudse-Moens CS, Oosterlaan J. Motor development in very preterm and very low-birth-weight children from birth to adolescence: a meta-analysis. JAMA 2009;302:2235–2242. [DOI] [PubMed] [Google Scholar]
- 59.Sansavini A, Pentimonti J, Justice L, Guarini A, Savini S, Alessandroni R, et al. Language, motor and cognitive development of extremely preterm children: Modeling individual growth trajectories over the first three years of life. J Commun Disord 2014;49:55–68. [DOI] [PubMed] [Google Scholar]
- 60.Pérez-Pereira M, Cruz R. A longitudinal study of vocabulary size and composition in low risk preterm children. First Lang 2018;38,72–94 [Google Scholar]
- 61.Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW, Victorian Infant Collaborative Group. Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med 2010;164:352–356. [DOI] [PubMed] [Google Scholar]
- 62.Spittle AJ, Spencer-Smith MM, Eeles AL, et al. Does the Bayley-III Motor Scale at 2 years predict motor outcome at 4 years in very preterm children? Dev Med Child Neurol 2013;55:448–452. [DOI] [PubMed] [Google Scholar]


