Abstract
Basement membranes (BMs) are thin dense sheets of extracellular matrix that surround most tissues. When the BMs of neighboring tissues come into contact, they usually slide along one another and act to separate tissues and organs into distinct compartments. However, in certain specialized regions, the BMs of neighboring tissues link, helping to bring tissues together. These BM connections can be transient, such as during tissue fusion events in development, or long-term, as with adult tissues involved with filtration, including the blood brain barrier and kidney glomerulus. The transitory nature of these connections in development and the complexity of tissue filtration systems in adults have hindered the understanding of how juxtaposed BMs fasten together. The recent identification of a BM-BM adhesion system in C. elegans, termed B-LINK (BM linkage), however, is revealing cellular and extracellular matrix components of a nascent tissue adhesion system. We discuss insights gained from studying the B-LINK tissue adhesion system in C. elegans, compare this adhesion with other BM-BM connections in Drosophila and vertebrates, and outline important future directions towards elucidating this fascinating and poorly understood mode of adhesion that joins neighboring tissues.
Introduction
Basement membranes (BMs) are conserved specialized extracellular matrices that arose at the time of animal multicellularity and take the form of thin, but dense sheets that underlie all epithelia and endothelia, and surround muscle, fat, and glial cells [1, 2]. The major components of BMs are the large heterotrimeric proteins laminin and type IV collagen, which are ~80–160nm long and ~400nm long, respectively [3]. Laminin heterotrimers are composed of a single α, β, and γ chain. Vertebrates encode 5α, 4β, and 3γ laminin subunits, forming 16 confirmed heterotrimers [2]. Vertebrates encode six type IV collagen genes (α1-α6), which assemble into three type IV collagen trimers [4]. Laminin and type IV collagen self-associate to form independent polymeric networks, which are linked and further altered by a host of different molecules such as nidogen, and the heparan sulfate proteoglycans perlecan and agrin [2, 5]. Since their discovery by Bowman in 1840, our understanding of BMs’ many functions and dynamic nature has grown significantly [6]. For example, BM proteins are known to instruct cell polarity, regulate cell fate decisions, direct cell migrations, and harbor growth factors that mediate a plethora of cellular activities [1, 7]. BMs also have key structural roles and help shape organs, protect tissues from damaging mechanical forces, mediate filtration, and compartmentalize tissues [2, 8–10]. Given the essential roles for BMs in cell and tissue function, the emergence of BM might have been a prerequisite for animal multicellularity and the formation of diverse tissues [11, 12].
The BMs of neighboring tissues often make contact with one another. In most cases, BMs keep neighboring tissues separate, allowing them to slide along one another [8]. In specific sites, however, the BMs of juxtaposed tissues link together. Broadly, these BM-BM connections can be grouped into two classes. The first are transient associations, which occur during development and involve a brief connection between BMs. These short-lived BM-BM unions often align tissues and are then degraded to allow for precise tissue fusion events. Instances where transient BM-BM associations occur include uterine-vulval attachment in C. elegans, imaginal disc eversion in Drosophila, and mouth formation in vertebrates [9, 13, 14]. The second class of BM-BM connections is maintained long-term. These also form during development, but involve a BM-BM bond that persists and is an essential aspect of adult organ structure or function. Examples where long-term BM-BM attachments are found include the glomerular filtration unit of the kidney, the alveoli in the lung, and the blood brain barrier [15–18].
The molecular mechanisms that direct and maintain BM-BM linkages remain largely unknown, and even revealing their existence during transient interactions is challenging. However, recent work in C. elegans has begun to identify the cellular and extracellular components that mediate the formation and maintenance of these tissue junctions [9]. Understanding the mechanisms of BM-BM associations are important, as many tissues with BM connections facilitate crucial filtration and exchange roles, such as in the blood brain barrier, kidney, and lungs [16–19]. BM-BM adhesions are also sites of genetic disorders in human disease, including renal failure in Alport Syndrome [20]. Here, we review what is known about a recently identified BM linkage complex (B-LINK) that forms during transient and long-term BM linkages in C. elegans. We then document the occurrence and similarities between sites of BM-BM connections in other organisms and outline future studies to expand our understanding of this newly recognized form of tissue adhesion.
Transient BM-to-BM associations in developmental morphogenesis
A BM-BM adhesion facilitating cell invasion in C. elegans: the discovery of the B-LINK
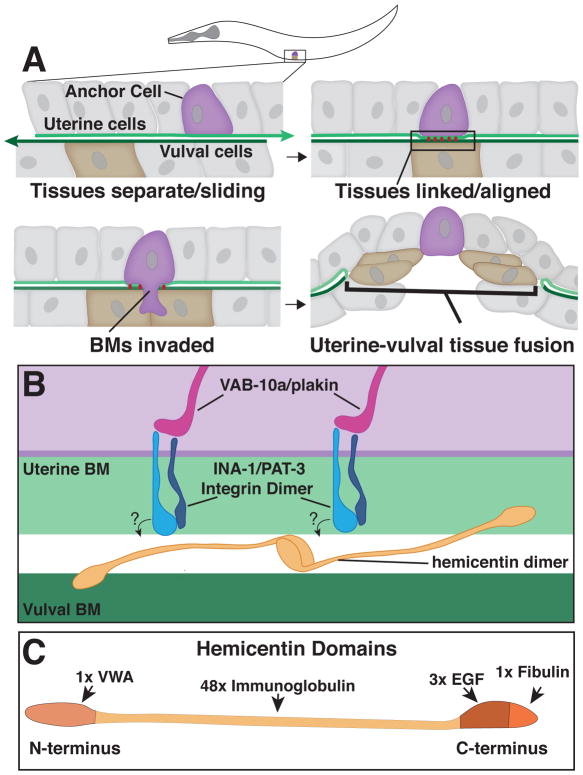
A specialized adhesion coupling adjacent BMs, named B-LINK for BM linkage, was first discovered at the site of anchor cell invasion in C. elegans (Fig. 1A)[9]. The anchor cell is a specialized invasive uterine cell, which breaches the juxtaposed uterine and vulval BMs to initiate uterine-vulval connection—a tissue fusion event required for mating and egg-laying [21]. The first evidence of a BM-BM connection came from tissue shifting experiments. By manually moving the uterine tissue in relation to the vulval tissue it was discovered that several hours prior to invasion the uterine and vulval BMs are not attached and slide freely along each other. However, approximately two hours before invasion, the uterine and vulval BMs no longer slide and instead link together under the anchor cell (Fig. 1A)[9]. These observations suggested that the anchor cell directs a BM-BM attachment prior to invasion.
Fig. 1. Anchor Cell invasion in C. elegans and the B-LINK BM-BM adhesion system.
A. A schematic of an L3 stage worm indicating the position of the anchor cell. Top Left – Prior to invasion, the uterine and vulval BMs are separate and the tissues slide along one another. Top Right - The B-LINK complex links the basement membranes (BMs) directly under the anchor cell prior to invasion, aligning the uterine and vulval tissues. Bottom Left – The anchor cell breaches the linked BMs. Bottom Right – The BMs are cleared and the uterine and vulval tissues fuse. B. A magnified inset of the B-LINK complex. Intracellularly, VAB-10A/plakin links the INA-1/PAT-3 integrin heterodimer to the cytoskeleton. Hemicentin is secreted by the anchor cell and may organize into multimers between the BMs that could directly link the BMs. Hemicentin organization is dependent on INA-1/PAT-3 integrin, but it is unknown if this interaction is direct or indirect as indicated by the questions marks. C. Hemicentin is comprised of four major domains: A Von Willebrand Factor type A (VWA) domain, 48 Immunoglobulin repeats, three Epidermal Growth Factor-like (EGF) repeats, and a fibulin family domain.
A screen for matrix components that the anchor cell secretes revealed that the anchor cell deposits the large extracellular matrix protein hemicentin into large aggregates between the uterine and vulval BMs just prior to invasion (Morrissey et al., 2014). Hemicentin is an atypical member of the fibulin family of matrix proteins that has a single Von Willebrand A (VWA) domain at the amino terminus, followed by 48 tandem immunoglobulin domains, three epidermal growth factor (EGF) repeats, and a single fibulin-like carboxyl-terminal module (Fig. 1B and 1C) [22–24]. Notably, in the absence of hemicentin, the BMs under the anchor cell no longer link together (Morrissey et al., 2014). How hemicentin connects neighboring BMs has not yet been established. As hemicentin’s VWA domain binds other matrix proteins [25], and the epidermal growth factor and fibulin-like domains can mediate self-assembly [26], it is possible that hemicentin bridges neighboring BMs directly (Fig. 1B). Hemicentin-mediated linkage of the uterine and vulval BMs helps align the proper site for invasion and facilitates more rapid invasion by allowing the anchor cell to cross both BMs simultaneously[9].
Additional screening for defects in BM-BM adhesion identified an integrin heterodimer most similar to laminin binding integrins (INA-1/PAT-3), and the C. elegans ortholog of vertebrate plakin (VAB-10A), an integrin binding cytolinker, as components of this adhesion system (Fig. 1B) [9, 27–29]. Integrin organizes hemicentin into adhesive puncta under the anchor cell, while plakin might link integrin to the intermediate filament cytoskeleton and stabilize the connection of the cell to the hemicentin punctae [9, 30]. It is not yet known if hemicentin is an integrin ligand, or if their interaction might be indirect. Together these findings indicate that a single cell, the anchor cell, coordinates the precise spatial and temporal linkage of two neighboring BMs by forming a specialized BM-BM adhesion or B-LINK [9].
The invading anchor cell removes the tethered BMs shortly after their connection in part through secretion of the matrix metalloproteinase protein ZMP-1. Expression of both the zmp-1 gene and hemicentin is regulated by FOS-1, the C. elegans ortholog of the vertebrate Fos family of transcription factors [31]. Thus, the formation of the B-LINK and its removal is controlled through a coordinated transcriptional program that facilitates tissue fusion in C. elegans.
Imaginal Discs: BM-BM interactions in Drosophila wing disc morphogenesis
Most tissues in the adult fruit fly Drosophila melonogaster, such as the wing, head, thorax, limbs, and genitalia are formed from imaginal discs, sac-like epithelial structures found inside the larva [32]. When the larva pupates and undergoes metamorphosis, larval tissue degenerates and the imaginal discs rapidly expand giving rise to adult structures. During the third instar stage of larval development, the wing discs evert, moving from the inside of the larva to the outside through the larval epithelium. Disc eversion allows the larval wing tissues to unfold and extend so that wing development progresses normally [33]. In order for wing discs to evert properly, the peripodial epithelium and stalk cells of the disc invade and fuse with the larval epithelium (Fig. 2)[13]. Similar to anchor cell invasion in C. elegans, both tissues are encased by BM. These BMs become juxtaposed, and then are breached and cleared in order for the fusion of the tissues and wing development to proceed normally.
Fig. 2. A BM-BM association during Drosophila imaginal disc eversion.
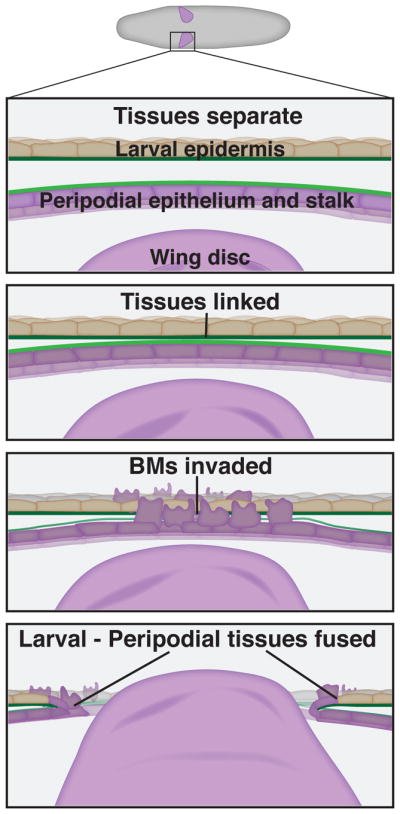
From top to bottom: A schematic diagram of a Drosophila larva indicates the position of the wing imaginal disc. Initially, the peripodial epithelia and stalk cells and the larval epithelial are not in contact with one another. Just prior to eversion the two tissues connect, linking through their BMs. The peripodial epithelial cells and stalk cells undergo a pseudo-epithelial-mesenchymal transition and the BMs are invaded in a JNK and matrix metalloproteinase dependent manner. After the BMs are cleared, the peripodial and stalk cells fuse with the larval epidermis, creating a hole that allows the wing disc to evert.
Sectioning of fixed tissues revealed that the peripodial epithelium and stalk cells appear to affix to the larval epidermis prior to BM removal, suggesting a BM-BM linkage (Fig. 2)[13]. While matrix proteins that may mediate this connection have not been identified, the Jun N-terminal kinase (JNK) signaling pathway is required for the BM-BM association between the wing disc and larval epidermis [13]. The JNK signaling cascade acts upstream of Fos, the key transcription factor that controls hemicentin secretion and promotes B-LINK formation under the AC in C. elegans (Morrissey et a., 2014). Additionally, Drosophila Fos and JNK signaling regulate expression of the two Drosophila matrix metalloproteinases, MMP1 and MMP2, which are secreted by the peripodial epithelium and stalk cells to help remove the attached BMs [34, 35]. Together these observations suggest that aspects of a conserved tissue fusion program might be shared between C. elegans and Drosophila, however, the possible mechanisms mediating BM-BM adhesion during Drosophila wing eversion remain unknown.
BM-BM interactions during tissue fusions in vertebrates
Anchor cell invasion and imaginal disc eversion are both examples of tissue fusion events where tissues fuse through BM-mediated contacts. Such tissue fusion events are also common during vertebrate development, including primary mouth formation, optic fissure closure, and nephrogenesis [36–38]. Examining these complex and transient tissue fusions is experimentally challenging, and thus very little is known about molecular aspects of the BM-BM interactions. JNK signaling promotes both primary mouth opening and wing disc eversion, however, the mechanisms regulating BM-BM associations and attachment are unknown [39–41],
One promising avenue for insight into mechanisms regulating BM-BM interactions are human disease genes associated with tissues requiring BM-BM connections. For example, defects in optic fissure closure leave an opening in the iris, retina or optic nerve, known as a coloboma [42, 43]. Mutations in approximately 50 genes are associated with human coloboma disease [38]. Interestingly, one of these is the BM protein SMOC-1 (SPARC-related modular calcium binding 1)[44, 45], which is related to the C. elegans gene ost-1/SPARC, a matricellular protein whose overexpression can remodel the BM during anchor cell invasion [46]. Characterization of the function of SMOC-1 as well as other genes implicated in coloboma may reveal aspects of BM-BM interactions during optic fissure closure as well as other BM-BM adhesions.
Short-term BM-BM adhesions that hold tissues in place: Fin folds and somites in zebrafish
In addition to tissue fusion events, transient BM-BM interactions briefly hold tissues together during morphogenesis. One example involves development of the fin folds in zebrafish. Fin folds are epidermal structures that develop from an apical ectodermal ridge and are composed of two juxtaposed epithelial sheets that grow and extend away from the body of the animal [47]. During the outgrowth of fin folds, extracellular matrix is deposited into the space between the layered epithelial sheets, which align into two sheets of back-to-back BM (Fig. 3A and 3B)[47–49]. Strands of electron dense extracellular matrix, termed cross fibers composed of unknown extracellular matrix molecules, spans the space between the BMs and penetrates through the BMs to attach onto the epidermal cells. These cross fibers have been proposed to maintain the structure of the early fin fold (Fig. 3B). Later, the cross fibers disappear as actinotricia, collagenous extracellular matrix fibers that run along the length of the fin folds, form and stabilize the fin fold structure [47, 50].
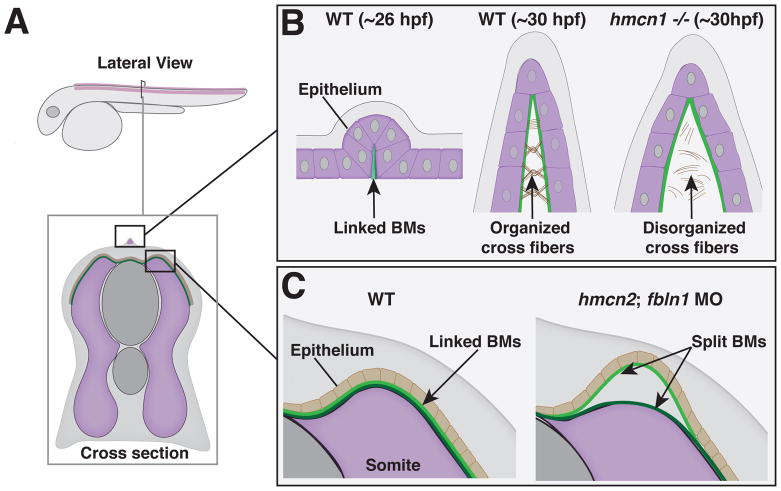
Fig. 3. Temporary BM-BM adhesions in zebrafish development.
A. A schematic view of a zebrafish larva ~28 hours post fertilization (hpf) with insets detailing temporary BM adhesions in the fin fold (B) and somites (C). B. Left – At ~26 hpf the back-to-back epithelia of the nascent fin fold secrete BMs that becomes fused early in fin fold development. Middle – Normal development of the fin fold at ~30 hpf with cross fibers assembled in between BMs. Right – Disorganized cross fibers and blistering exhibited by hemicentin 1 (hmcn1) −/− mutant fin folds. C. Left – BMs of the somites and epithelia are linked in normal development. Right – In hemicentin 2 (hmcn2)/fibulin 1 (fbln1) double morphants the BMs split, resulting in temporary blisters.
Most vertebrates harbor two paralogues of hemicentin-hemicentin 1 and hemicentin 2. In zebrafish, the hemicentin 1 gene is expressed in the apical median fin fold at the time extracellular matrix is deposited into the space between the epithelial sheets when the BM and cross fibers are being established [14]. Strikingly, hemicentin 1 mutants display specific blistering of the developing fins, suggesting a role in BM-BM linkage [14]. Consistent with this notion, transmission electron microscopy indicated that although BMs form normally in hemicentin 1 mutants, the extracellular matrix between the BMs is disorganized (Fig. 3B). Hemicentin might help anchor the cross fibers to the BM [50], however, the specific localization of the hemicentin 1 protein is unknown and thus its precise function is unclear. The hemicentin 1 blistering phenotype is only temporary--emerging 48 hours post fertilization, but disappearing and recovering by 120 hours. Thus, the functional requirement of hemicentin 1 protein in linking the BMs in fin folds seems to be early and short-lived, perhaps reflecting a role for some other mechanism stabilizing the fin folds later in their development.
The hemicentin 2 protein has an important, yet distinct role in transiently holding two tissues together through a BM-BM adhesion. Hemicentin 2 is expressed in the somites and helps mediate the attachment of the somite BM with the epidermal BM to stabilize an association between these two tissues. Knockdown of hemicentin 2 by itself does not cause a phenotype. However, loss of hemicentin 2 in combination with the absence of fibulin 1, disrupts the BM-BM adhesion between the somite and epidermis, and results a blistering phenotype along the trunk of the embryo (Fig. 3C)[50]. Similar to the fin fold, this blistering is temporary and the animals recover, suggesting a transient function for hemicentin 2. Fibulin 1 is a member of the fibulin class of matrix proteins, of which hemicentin (sometimes called fibulin 6) is an atypical member [23, 24]. In C. elegans, the hemicentin and fibulin-1 proteins directly interact, and hemicentin is required for fibulin-1 localization to build a long-term BM-BM adhesion (discussed in the next section). Together, these observations in zebrafish indicate that BM-BM adhesions can also be used for transient associations between tissues, and that hemicentin and fibulin 1 are possible components of a shared tissue adhesion system that joins neighboring BMs.
Transient BM-BM interactions: Summary and Outlook
Dynamic tissue fusion events involving short-lived BM-BM contact occur routinely in development. Transient BM-BM tissue stabilization processes, as have been observed during zebrafish development, might also be common, but overlooked, as their functions are only manifest in mutant backgrounds. In this section only four instances of transient BM-BM contacts are summarized, though additional instances do exist and more likely remain to be identified (Table 1). Because of the difficulty of imaging, manipulating, and genetically dissecting these transient interactions, our understanding of the nature of most BM-BM contacts is limited. In the future, it will be important to develop more effective experimental models, especially in vertebrates, that combine live-cell imaging with the ability to molecularly and physically perturb the tissues to examine the properties of the BM-BM contact. In vitro culture models will be particularly helpful with imaging, manipulation, and testing the function of genes that might be involved with BM-BM associations. In addition, the generation of transgenic animals with GFP-tagged BM components, as has been developed in C. elegans and Drosophila [51, 52], will allow precise examination of BM attachment events. Finally, it will be useful to broadly examine the localization and function of hemicentin and fibulin family members in other tissues and animals to determine if they mediate other BM-BM tissue connections.
Table 1.
Short-term BM-BM linkages that occur during development
| Process | Organism | Tissues linked by BMs | Outcome |
|---|---|---|---|
| Anchor Cell invasion [21] | C. elegans | Uterine and vulval tissue | Tissue fusion |
| Imaginal Disc eversion [13] | D. Melanogaster | Peripodial epithelium and larval epithelium | Tissue fusion |
| Fin fold development [14] | D. rerio | Two sides of the fin fold | Tissue stabilization |
| Somites [50] | D. rerio | Somites and epithelia | Tissue stabilization |
| Opitc fissure closure [42] | Vertebrates | Two optic shelves | Tissue fusion |
| Distal Nephron precursor invasion [36] | Vertebrates | Developing nephron and collecting tubule | Tissue fusion |
| Mouth formation [37] | Deutrostome animals | Endoderm and ectoderm | Tissue fusion |
Long-term BM-BM adhesions in organ structure and filtration
A uterine-seam cell BM-BM attachment in C. elegans supports the uterus during egg-laying
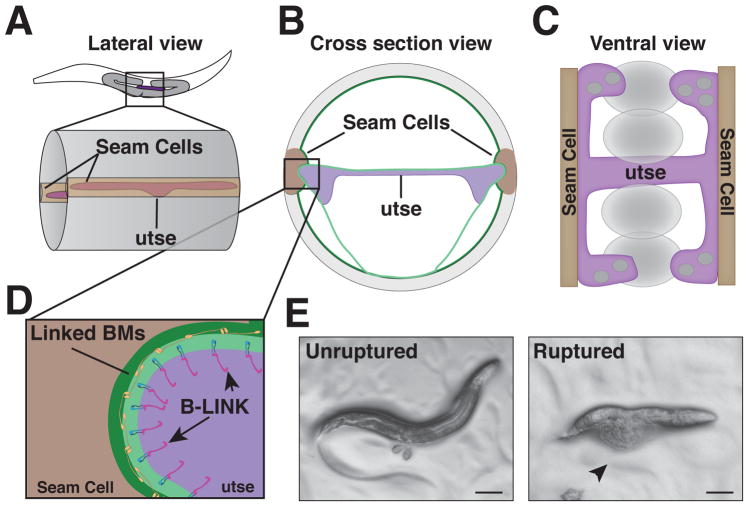
In contrast to transient BM-BM interactions, long-term BM-BM adhesions have roles in proper tissue formation and function. Although not commonly recognized, these connections are found in many adult tissues. One example of a long-term BM-BM linkage occurs in C. elegans at the junction between the seam cells of the hypodermis (skin) and an H-shaped uterine cell, the utse (Fig. 4)[9]. C. elegans has two rows of seam cells, one positioned along the lateral midline on each side of the hypodermis. The utse functions like a hammock for the uterus--the sides of the H attach to the left and right rows of hypodermal seam cells through a BM-BM contact, while the cross bar of the utse spans the center of the uterine tissue (Fig. 4A–4C)[53, 54]. The utse and its BM-BM connection to the seam cells is essential for resisting the forces from the uterine and vulval muscles that contract during egg-laying [54, 55].
Fig. 4. The utse-hypodermal BM-BM linkage in C. elegans.
A. A mid-body lateral view of an L4 stage worm where the utse and seam cells have linked through their BMs. B. A cross sectional view of the mid body showing the utse connecting to the seam cells on either side of the body. C. A ventral view of the utse-seam cell BM-BM adhesion demonstrating the H-shaped utse and how it acts as a hammock to support the uterine tissue and developing embryos (shown as ovals) during muscle contractions. D. An expanded view of inset in (B) highlighting the B-LINK mediated connection between tissues. E. Left – A wild type worm showing eggs laid as a result of normal egg laying muscle contraction. Right – A worm with a disrupted B-LINK exhibiting external intestine and gonads (arrowhead), which is characteristic of the Rup phenotype as a result of egg laying muscle contraction.
Molecular characterization has revealed that this BM-BM linkage is another B-LINK, composed of the matrix protein hemicentin, the integrin heterodimer INA-1/PAT-3, and the cytolinker plakin (VAB-10A) (Fig. 4D) [9, 22]. These observations strongly support the idea of a conserved tissue adhesion system. A notable difference between the uterine-vulval and utse-seam B-LINKs, however, is that once formed, the utse-seam B-LINK is maintained throughout the life of the animal. There are apparent molecular distinctions as well, as the two isoforms of the C. elegans fibulin-1 ortholog, fibulin-1C and fibulin-1D, localize to the utse-seam B-LINK, but do not appear to localize to the anchor cell B-LINK [9, 56]. Localization of fibulin-1 to the utse-seam B-LINK is dependent on hemicentin, and in turn fibulin-1 organizes the dense assembly of hemicentin at the B-LINK (Muriel et al., 2005). Thus, there is an association between these related fibulin family member matrix molecules in both C. elegans and zebrafish during BM-BM adhesion. Loss of utse-seam cell B-LINK components results in prolapse of the worm’s internal organs through the vulval opening during egg-laying, a phenotype known as ruptured vulva (Rup) (Fig. 4E). Genome-wide RNAi screens have identified over 200 genes that cause a Rup phenotype [57–59], suggesting that many additional proteins contribute to BM-BM adhesion and might function at the B-LINK.
The glomerular BM: A BM-BM filtration barrier within the kidney
The most well documented long-term BM-BM adhesions in vertebrates occur at organ-blood barriers, where the endothelium of blood vessels links to specialized epithelia through adjoining BMs. One of the best-characterized examples of this is the glomerular BM in the vertebrate kidney. The glomerulus is a network of capillaries located within the Bowman’s capsule of the kidney that filters solutes out of the blood while excluding large plasma proteins such as albumin [60, 61]. The glomerulus is composed of a three-part glomerular filtration unit made up of the fused glomerular BM that sits between a layer of fenestrated glomerular endothelial cells on one side and a layer of highly specialized epithelial cells called podocytes on the other (Fig. 5A). The podocytes are octopus like cells that extend multiple processes from the cell body that branch into foot processes and wrap around the capillaries. Between these foot processes are spaces, known as slit diaphragms, where the fluid passes during filtration (Fig. 5A) [62].
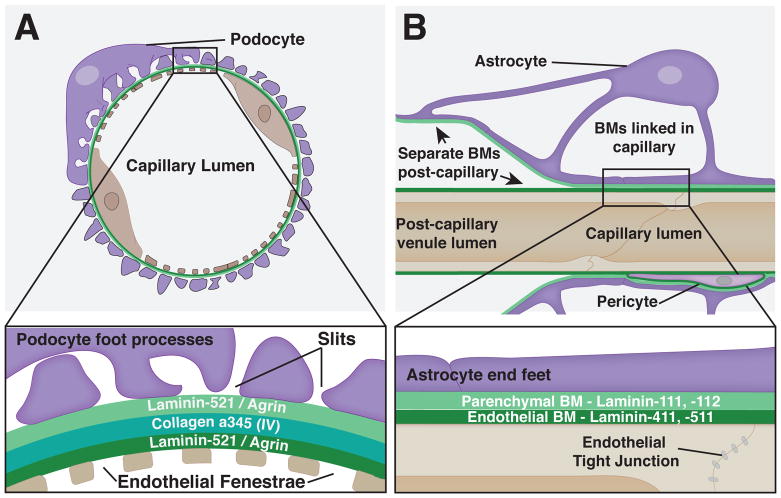
Fig. 5. Long-term BM-BM linkages in vertebrates.
A. The glomerular BM in the kidney. Top – A cross section view of a glomerular capillary with fenestrated endothelial cells on the inner surface and podocyte foot processes on the outer surface. Bottom – A close up view shows the tight linkage of the BMs as well as the nanoscale localization of the laminin and collagen trimers that make up the glomerular BM. B. The blood brain barrier (BBB). Top – Transverse view of a BBB capillary shows BMs covering the non-fenestrated endothelia and astrocyte end feet. BMs are linked in the capillary, but are not connected in the post capillary venule, where they are separated by a perivascular space. Bottom– A magnified view shows the distinct make up of the two linked BMs as well as the tight junctions of the endothelial cells.
During early glomerular development, both the endothelial cells and podocytes secrete their own independent BMs. Shortly after their formation, these independent BMs initiate fusion, which continues during glomerulogenesis. Interestingly, the glomerular BM undergoes changes in its composition as the glomerulus matures. Notably, the laminin and type IV collagen isoforms are replaced. Initially when the BMs are formed they contain the laminin heterotrimer, α1β1γ1 (LN111), and laminin α5β1γ1 (LN511) as well as the collagen α1α1α2(IV) trimer. However, during glomerular development the laminin isoforms are first replaced with laminin-521 and then the collagen isoform is exchanged for the α3α4α5 trimer (Fig. 5A) [17, 63, 64]. It has been suggested that this change in composition may make the glomerular BM more resistant to mechanical forces and proteolytic degradation [65, 66]. This alteration in BM constitution might also promote differentiation of the glomerular endothelial cells and podocytes [64, 67].
How the endothelial and podocyte BMs are linked is unresolved. Super-resolution imaging of mature mouse glomerular BM using stochastic optical reconstruction microscopy (STORM) with antibodies near the N- and C- termini of the major BM components agrin, laminin-521, and collagen α3α4α5(IV) revealed that agrin and laminin each form into layers adjacent to the podocyte and endothelial cells, a position consistent with roles in unlinked BMs (Fig. 5A). In contrast, collagen α3α4α5(IV) localizes near the center of the mature glomerular BM, suggesting a possible function in stabilizing the connection of the two BMs (Fig. 5A). Consistent with this possibility, the expression of collagen α3α4α5(IV) correlates with fusion of the BMs in development [16, 17]. Furthermore, Alport Syndrome, a genetically heterogenous human disease arising from mutations that perturb collagen α3α4α5(IV), results in thin glomerular BMs that often split into separate endothelial and podocyte BMs [68, 69]. A role for fibulin family members in endothelial cell and podocyte BMs has not been explored but is possible, as hemicentin 1 and fibulin 1 have been detected within the glomerular BM by proteomic analysis [70, 71].
Studies observing fluorescent tracer molecules in mouse knockouts of BM genes have suggested that the glomerular BM directly contributes to glomerular filtration through its negative charge and size selectivity [17, 72]. Consistent with this notion, mutations in laminin and type IV collagen, which cause Pierson and Alport syndrome respectively, result in glomerular barrier defects and proteinuria, the leakage of protein into the urine [72, 73]. Although the glomerular BM has an apparent role in filtration [72, 74], all layers of the glomerular capillary-- the endothelia, the podocytes and the BM--function as an integrated and highly interactive unit. Loss or defects in one component lead to alteration or decline of others [17], complicating analysis of specific functions for molecules and tissues. For example, Lamb2 mutant mice that lack the laminin-521 heterotrimer exhibit altered matrix composition due to ectopic expression of other laminin subunits, and loss of specialized cellular morphology as evidenced by effacement of podocyte foot processes, which result in compromised barrier function and defective kidney filtration (Suh and Miner 2013). The dynamic interconnection between cell matrix, cell morphology, and tissue function makes it challenging to gain a clear understanding of the specific roles of matrix molecules in linking the neighboring podocyte and endothelial BMs. Complex interactions between tissues and matrix have also hindered our understanding of mechanisms mediating the formation of another long-term BM-BM adhesion, the blood brain barrier, which we discuss next.
Blood Brain Barrier: A BM-BM linkage that regulates and protects the brain
The blood brain barrier (BBB) is a highly selective semipermeable barrier that forms around the vasculature within the central nervous system. The BBB regulates nutrient and ion exchange for proper brain physiology and restricts the movement of toxins and pathogens to protect the brain [75–77]. Specialized vascular endothelial cells, which form a key component of the BBB, have low transcytosis activity and are interconnected with tight junctions that restrict paracellular diffusion (Fig. 5B). The BBB is also composed of the endothelial BM with its embedded pericyte cells. Both the endothelia and pericytes secrete laminin-111 and -112. The last component of the BBB is a neighboring BM containing laminin-411 and -511, called the parenchymal BM, which is secreted by astrocytes (Fig. 5B) [15]. Much like the podocytes in the glomerulus, the astrocytes contact the underlying BM with foot processes called end feet, but astrocyte end feet form a continuous layer without slits called the glia limitans (Fig. 5B) [77, 78]. The juxtaposed parenchymal and endothelial BMs specifically link within the capillaries of the central nervous system, but are separated by a perivascular space at the post-capillary venules (Fig. 5B) [15, 76, 79, 80]. The BM-BM-linked capillary region of the BBB has unique properties as it is the site of nutrient transport, and is also a location that specifically excludes T-cell trafficking [15, 76].
Despite the apparent importance of BM-BM adhesion to the BBB structure in the capillaries, it is not known what controls endothelial and parenchymal BM fusion. Similar to the glomerular BM, a challenging factor in discovering these mechanisms is the complex interactions between cell types, BM components, and tissue function (see Daneman and Prat 2015 for a complete review). For example, type IV collagen and laminin strengthen tight junctions in cultured endothelial cells. Mouse knockouts of endothelial or astrocyte derived laminin increase endothelial permeability and cause cerebral hemorrhaging, destroying the tissue and thus obfuscating other possible functions [81–87]. Both fibulin-1 and hemicentin-1 are expressed by endothelial cells of mice [88, 89], where they could function in linking these BMs. However, no knockout studies have been conducted to date to test their potential roles. Collagen α3α4α5(IV) is not present at the BBB, suggesting that distinct mechanisms may mediate or modify BM-BM connections in different tissue settings.
Vertebrates have numerous tissues with BM-BM linkages
Ultrastructural studies in vertebrates have revealed that many tissues harbor BM-BM linkages (Table 2). Other documented vascular BM-BM linkages include: (1) the choroid plexus within the ventricles of the brain that mediates the exchange of solutes and nutrients between the blood and cerebrospinal fluid [90–92]; (2) Bruch’s membrane, a thickened and highly specialized BM-BM attachment located between the retinal pigment epithelium and the choroid capillaries of the eye that regulates the exchange of nutrients, oxygen, and waste between the retina and blood [93, 94]; and (3) the alveolar BM-capillary BM linkage in the lung, which facilitates efficient gas exchange between the blood and airways [95–97]. Additional non-vascular sites of putative BM-BM linkage occur in the inner ear [98]. One example is Reissner’s membrane (vestibular membrane), which connects the cochlear duct and vestibular duct through their respective BMs. Reissner’s membrane appears to function as a selective diffusion barrier between the perilymph and endolymph fluids in the cochlea, which each have unique ionic compositions important for their functions [99].
Table 2.
Long-term BM-BM linkages that connect adult tissues
| Linkage | Organism | Tissues linked by BMs |
|---|---|---|
| UTSE [9] | C. elegans | Uterus and hypodermis |
| Glomerular BM [16] | Vertebrates | Podocytes and vascular endothelia |
| Alveolar BM [18] | Vertebrates | Alveoli and vascular endothelia |
| Blood Brain Barrier [15] | Vertebrates | Neural tissue and vascular endothelia |
| Blood Cerebrospinal Fluid Barrier [103] | Vertebrates | Vasculature and choroid plexus |
| Bruch’s Membrane [93] | Vertebrates | Vasculature and retinal pigmented epithelium |
| Reissner’s Membrane [98] | Vertebrates | Vestibular duct and cochlear duct |
| Spiral Ligament [98] | Vertebrates | Outer wall of the cochlear duct and vascular endothelia |
| Stria Vascularis [98] | Vertebrates | Stria vascularis and vascular endothelia |
Similar to other long-term BM-BM linkages in vertebrates, very little is known about the mechanisms that mediate these diverse connections. There are, however, intriguing correlations in extracellular matrix gene expression and dysfunction in human diseases. For example, collagen α3α4α5(IV), which might help link the juxtaposed BMs in the glomerulus, is also expressed in the BMs of the choroid plexus, Bruch’s membrane in the eye, the inner ear BMs, and the alveolar BM [63, 100–103]. Alport syndrome, a defect in collagen α3α4α5(IV), which leads to the loss of linkage of the podocyte and endothelial BMs in the kidney glomerulus, causes hearing loss and eye abnormalities [68, 69, 101, 104–106]. Antibodies thought to recognize both forms of vertebrate hemicentin localize to Bruch’s membrane in the retina of mice [24]. Notably, several studies have implicated hemicentin 1 as a potential age related macular degeneration gene [107–109], a disease that can be associated with the decline of Bruch’s membrane.
Long term BM-BM interactions: Summary and Outlook
Long-term BM-BM adhesions are a common feature of vertebrate tissue barriers found in the kidney, lung, eyes, ears, and brain that regulate the exchange and filtration of diverse compounds, including pathogens, nutrients, ions, proteins, and gases. In at least some of these BM-BM linkages the barrier/filtration properties of the tissue appear to be bestowed in part by the BMs, such as in the glomerular BM in the kidney and at the BBB in the brain [61, 87]. In all cases, the primary function of the BM-BM connection is to bring two distinct tissues into tight association to allow exchange of components. As these linkages must resist tissue shifting and compression forces, as well as hemodynamic forces in the vasculature [110–112], these BM-BM linkages must be strongly adhesive. The molecular composition of these BM-BM linkages are, however, largely unknown. Studies on the integrin adhesome, a cell-matrix adhesion, have revealed approximately 60 core proteins that regulate integrin-matrix adhesion and signaling [113]. This suggests that many additional components of this newly identified BM-BM adhesion system await discovery. The matrix is a promising place to begin uncovering molecular details of how BMs are linked. The role of hemicentin and potentially fibulin in the worm has provided the first molecular insight into extracellular modifications of linked BMs (Muriel et al. 2005; Vogel and Hedgecock 2001). In vertebrates, collagen α3α4α5(IV) is present at many sites of BM adhesion and localizes between the two linked BMs in the glomerulus, making it tempting to speculate that it plays a broad role in regulating these linkages [2, 22, 56, 66, 114].
One of the challenges in identifying additional components of BM-BM connections is the complexity of tissues where long-term BM-BM adhesions occur in vertebrates. These linkages arise at integrated barriers between tissues where matrix composition affects integrity of both tissues as well as the function of the barriers. Further, these BM-BM connections are difficult to dynamically visualize and experimentally challenge by applying force on them to test their integrity. Mutations in collagen α3α4α5(IV) that cause Alport syndrome have been implicated in linking BMs only in the case of the glomerular BM. Even in this instance, it is unclear if the BM-BM adhesion defects are a direct result of loss of collagen α3α4α5(IV), or an indirect effect of progression of glomerular dysfunction [115].
Deepening our understanding of mechanisms that stably connect BMs will require multiple approaches, as well as a broader awareness of this unique linkage. More thorough examination of the BMs in human diseases affecting the BBB, retina, and other BM-BM linked barriers might uncover additional genes regulating BM-BM adhesion. Proteomic methods in vertebrates combined with super-resolution imaging may also identify additional matrix proteins localized at the junction of linked BMs [116]. The function of these proteins could be further examined through mouse knockout studies. The B-LINK structure at the utse-seam cell connection in C. elegans is a highly promising site for discovering conserved components. The primary function of the utse-seam cell BM-BM adhesion is to resist the mechanical forces involved with egg laying. Notably, this linkage is not complicated by additional barrier functions found in long-term vertebrate BM-BM adhesions. Further, the ruptured vulval phenotype (Rup) that results from failure of this specific linkage is easy to detect in genetic and RNAi knockdown screens. With over 200 genes identified that cause the Rup phenotype in large-scale RNAi screening [57–59], cell biological studies can be pursued to examine their potential functions in regulating BM-BM adhesion. Extending these findings to vertebrates will likely advance our understanding of mechanisms mediating BM-BM adhesions.
Conclusion and Perspective on BM-BM adhesions
Given the similarities and importance of tissue adhesion through neighboring BMs, we expect that the B-LINK identified in C. elegans is a conserved linkage in animals. Furthermore, the resemblances in the transient and long-term BM-BM linkages also suggest that these connections are likely built on shared components that may be modified for particular functions. As outlined here, a broad strategy involving in vivo models, live-cell imaging, proteomics, super-resolution imaging, tissue manipulation, and human disease database mining will facilitate rapid progress on this highly understudied tissue adhesion system. Investigation of BM-BM linkage is important as BM-BM adhesions are found at medically vital tissues such as the blood brain barrier, the lung alveoli, and kidney glomerulus, which break down in many human diseases. Furthermore, investigation into BM-BM linkages will provide insight into a fascinating and poorly understood cell biological question—how cells build, monitor, and maintain an extracellular structure outside the reach of the cell surface.
Acknowledgments
We thank Ranjay Jayadev for comments on the manuscript, and Meghan Morrissey for earlier discussion on basement membrane linkages. D.R.S. was supported by R21HD084290 and NIGMS R35 MIRA GM118049.
References
- 1.Pozzi A, Yurchenco PD, Iozzo RV. The nature and biology of basement membranes. Matrix Biol. 2017;57–58:1–11. doi: 10.1016/j.matbio.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yurchenco PD. Basement membranes: cell scaffoldings and signaling platforms. Cold Spring Harb Perspect Biol. 2011;3(2) doi: 10.1101/cshperspect.a004911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glentis A, Gurchenkov V, Vignjevic DM. Assembly, heterogeneity, and breaching of the basement membranes. Cell Adhesion & Migration. 2014;8(3):236–245. doi: 10.4161/cam.28733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khoshnoodi J, Pedchenko V, Hudson Billy G. Mammalian collagen IV. Microscopy Research and Technique. 2008;71(5):357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarrazin S, Lamanna WC, Esko JD. Heparan Sulfate Proteoglycans. Cold Spring Harbor Perspectives in Biology. 2011;3(7) doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bowman W. On the minute structure and movements of voluntary muscle. Phil Trans R Soc Lond. 1840;130:457–501. [Google Scholar]
- 7.Sekiguchi R, Yamada KM. Basement Membranes in Development and Disease. 2018 doi: 10.1016/bs.ctdb.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown NH. Extracellular matrix in development: insights from mechanisms conserved between invertebrates and vertebrates. Cold Spring Harb Perspect Biol. 2011;3(12) doi: 10.1101/cshperspect.a005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrissey MA, Keeley DP, Hagedorn EJ, McClatchey ST, Chi Q, Hall DH, Sherwood DR. B-LINK: a hemicentin, plakin, and integrin-dependent adhesion system that links tissues by connecting adjacent basement membranes. Dev Cell. 2014;31(3):319–31. doi: 10.1016/j.devcel.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos-Lewis W, Page-McCaw A. Basement membrane mechanics shape development: Lesson from the fly. Matrix Biol. 2018 doi: 10.1016/j.matbio.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fidler AL, Darris CE, Chetyrkin SV, Pedchenko VK, Boudko SP, Brown KL, Gray Jerome W, Hudson JK, Rokas A, Hudson BG. Collagen IV and basement membrane at the evolutionary dawn of metazoan tissues. Elife. 2017;6 doi: 10.7554/eLife.24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fidler AL, Boudko SP, Rokas A, Hudson BG. The triple helix of collagens - an ancient protein structure that enabled animal multicellularity and tissue evolution. J Cell Sci. 2018;131(7) doi: 10.1242/jcs.203950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pastor-Pareja JC, Grawe F, Martin-Blanco E, Garcia-Bellido A. Invasive cell behavior during Drosophila imaginal disc eversion is mediated by the JNK signaling cascade. Dev Cell. 2004;7(3):387–99. doi: 10.1016/j.devcel.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 14.Carney TJ, Feitosa NM, Sonntag C, Slanchev K, Kluger J, Kiyozumi D, Gebauer JM, Coffin Talbot J, Kimmel CB, Sekiguchi K, Wagener R, Schwarz H, Ingham PW, Hammerschmidt M. Genetic analysis of fin development in zebrafish identifies furin and hemicentin1 as potential novel fraser syndrome disease genes. PLoS Genet. 2010;6(4):e1000907. doi: 10.1371/journal.pgen.1000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM. Endothelial Cell Laminin Isoforms, Laminins 8 and 10, Play Decisive Roles in T Cell Recruitment across the Blood–Brain Barrier in Experimental Autoimmune Encephalomyelitis. The Journal of Cell Biology. 2001;153(5):933. doi: 10.1083/jcb.153.5.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abrahamson DR. Origin of the glomerular basement membrane visualized after in vivo labeling of laminin in newborn rat kidneys. The Journal of Cell Biology. 1985;100(6):1988. doi: 10.1083/jcb.100.6.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrahamson DR, Hudson BG, Stroganova L, Borza DB, St John PL. Cellular origins of type IV collagen networks in developing glomeruli. J Am Soc Nephrol. 2009;20(7):1471–9. doi: 10.1681/ASN.2008101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaccaro CA, Brody JS. Structural features of alveolar wall basement membrane in the adult rat lung. The Journal of Cell Biology. 1981;91(2):427. doi: 10.1083/jcb.91.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sixt M, Bauer M, Lammermann T, Fassler R. b1 integrins: zip codes and signaling relay for blood cells. Curr Opin Cell Biol. 2006;(18):482–490. doi: 10.1016/j.ceb.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Kruegel J, Rubel D, Gross O. Alport syndrome--insights from basic and clinical research. Nat Rev Nephrol. 2013;9(3):170–8. doi: 10.1038/nrneph.2012.259. [DOI] [PubMed] [Google Scholar]
- 21.Sherwood DR, Sternberg PW. Anchor cell invasion into the vulval epithelium in C. elegans. Dev Cell. 2003;5(1):21–31. doi: 10.1016/s1534-5807(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 22.Vogel BE, Hedgecock EM. Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development. 2001;128(6):883. doi: 10.1242/dev.128.6.883. [DOI] [PubMed] [Google Scholar]
- 23.Argraves WS, Greene LM, Cooley MA, Gallagher WM. Fibulins: physiological and disease perspectives. EMBO Rep. 2003;4(12):1127–31. doi: 10.1038/sj.embor.7400033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu X, Xu M, Zhou X, Jones OB, Moharomd E, Pan Y, Yan G, Anthony DD, Isaacs WB. Specific structure and unique function define the hemicentin. Cell & Bioscience. 2013;3(1):27. doi: 10.1186/2045-3701-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whittaker CA, Hynes RO, Pollard TD. Distribution and Evolution of von Willebrand/Integrin A Domains: Widely Dispersed Domains with Roles in Cell Adhesion and Elsewhere. Molecular Biology of the Cell. 2002;13(10):3369–3387. doi: 10.1091/mbc.E02-05-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong C, Muriel JM, Ramirez S, Hutter H, Hedgecock EM, Breydo L, Baskakov IV, Vogel BE. Hemicentin assembly in the extracellular matrix is mediated by distinct structural modules. J Biol Chem. 2006;281(33):23606–10. doi: 10.1074/jbc.M513589200. [DOI] [PubMed] [Google Scholar]
- 27.Rezniczek GA, de Pereda JM, Reipert S, Wiche G. Linking Integrin α(6)β(4)-based Cell Adhesion to the Intermediate Filament Cytoskeleton: Direct Interaction between the β(4) Subunit and Plectin at Multiple Molecular Sites. The Journal of Cell Biology. 1998;141(1):209–225. doi: 10.1083/jcb.141.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu R, Jiang X, Huang Z, Zhang H. The spectraplakins of Caenorhabditis elegans: Cytoskeletal crosslinkers and beyond. Semin Cell Dev Biol. 2017;69:58–68. doi: 10.1016/j.semcdb.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 29.Baum PD, Garriga G. Neuronal Migrations and Axon Fasciculation Are Disrupted in ina-1 Integrin Mutants. Neuron. 19(1):51–62. doi: 10.1016/s0896-6273(00)80347-5. [DOI] [PubMed] [Google Scholar]
- 30.Gally C, Zhang H, Labouesse M. Functional and Genetic Analysis of VAB-10 Spectraplakin in Caenorhabditis elegans. Methods Enzymol. 2016;569:407–30. doi: 10.1016/bs.mie.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Sherwood DR, Butler JA, Kramer JM, Sternberg PW. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell. 2005;121(6):951–62. doi: 10.1016/j.cell.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 32.Aldaz S, Escudero LM. Imaginal discs. Current Biology. 20(10):R429–R431. doi: 10.1016/j.cub.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Fristrom FJD. The metamorphic development of the adult epidermis. In: Bate MAAM, editor. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press; 1993. pp. 843–897. [Google Scholar]
- 34.Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. 2006 doi: 10.1038/sj.emboj.7601401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proceedings of the National Academy of Sciences. 2007;104(8):2721. doi: 10.1073/pnas.0611666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kao RM, Vasilyev A, Miyawaki A, Drummond IA, McMahon AP. Invasion of distal nephron precursors associates with tubular interconnection during nephrogenesis. J Am Soc Nephrol. 2012;23(10):1682–90. doi: 10.1681/ASN.2012030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickinson AJ, Sive H. Development of the primary mouth in Xenopus laevis. Dev Biol. 2006;295(2):700–13. doi: 10.1016/j.ydbio.2006.03.054. [DOI] [PubMed] [Google Scholar]
- 38.Patel A, Sowden JC. Genes and pathways in optic fissure closure. Seminars in Cell & Developmental Biology. 2017 doi: 10.1016/j.semcdb.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 39.Dickinson AJ, Sive HL. The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth. Development. 2009;136(7):1071–81. doi: 10.1242/dev.032912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuji N, Matsuura T, Narama I, Yoshiki A, Ozaki K. Macrophage-Associated Gelatinase Degrades Basement Membrane at the Optic Fissure Margins During Normal Ocular Development in Mice. Investigative Ophthalmology & Visual Science. 2018;59(3):1368–1373. doi: 10.1167/iovs.17-21841. [DOI] [PubMed] [Google Scholar]
- 41.Houssin Nathalie S, Bharathan Navaneetha K, Turner Stephen D, Dickinson Amanda JG. Role of JNK during buccopharyngeal membrane perforation, the last step of embryonic mouth formation. Developmental Dynamics. 2016;246(2):100–115. doi: 10.1002/dvdy.24470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsuji N, Kita K, Ozaki K, Narama I, Matsuura T. Organogenesis of mild ocular coloboma in FLS mice: failure of basement membrane disintegration at optic fissure margins. Exp Eye Res. 2012;94(1):174–8. doi: 10.1016/j.exer.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Ray HJ, Niswander L. Mechanisms of tissue fusion during development. Development. 2012;139(10):1701–11. doi: 10.1242/dev.068338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vannahme C, Smyth N, Miosge N, Gösling S, Frie C, Paulsson M, Maurer P, Hartmann U. Characterization of SMOC-1, a Novel Modular Calcium-binding Protein in Basement Membranes. Journal of Biological Chemistry. 2002;277(41):37977–37986. doi: 10.1074/jbc.M203830200. [DOI] [PubMed] [Google Scholar]
- 45.Rainger J, van Beusekom E, Ramsay JK, McKie L, Al-Gazali L, Pallotta R, Saponari A, Branney P, Fisher M, Morrison H, Bicknell L, Gautier P, Perry P, Sokhi K, Sexton D, Bardakjian TM, Schneider AS, Elcioglu N, Ozkinay F, Koenig R, Mégarbané A, Semerci CN, Khan A, Zafar S, Hennekam R, Sousa SB, Ramos L, Garavelli L, Furga AS, Wischmeijer A, Jackson IJ, Gillessen-Kaesbach G, Brunner HG, Wieczorek D, van Bokhoven H, FitzPatrick DR. Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice. PLOS Genetics. 2011;7(7):e1002114. doi: 10.1371/journal.pgen.1002114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrissey MA, Jayadev R, Miley GR, Blebea CA, Chi Q, Ihara S, Sherwood DR. SPARC Promotes Cell Invasion In Vivo by Decreasing Type IV Collagen Levels in the Basement Membrane. PLoS Genet. 2016;12(2):e1005905. doi: 10.1371/journal.pgen.1005905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dane PJ, Tucker JB. Modulation of epidermal cell shaping and extracellular matrix during caudal fin morphogenesis in the zebra fish Brachydanio rerio. Journal of Embryology and Experimental Morphology. 1985;87(1):145. [PubMed] [Google Scholar]
- 48.Yano T, Abe G, Yokoyama H, Kawakami K, Tamura K. Mechanism of pectoral fin outgrowth in zebrafish development. Development. 2012;139(16):2916. doi: 10.1242/dev.075572. [DOI] [PubMed] [Google Scholar]
- 49.Webb AE, Sanderford J, Frank D, Talbot WS, Driever W, Kimelman D. Laminin α5 is essential for the formation of the zebrafish fins. Developmental Biology. 2007;311(2):369–382. doi: 10.1016/j.ydbio.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 50.Feitosa NM, Zhang J, Carney TJ, Metzger M, Korzh V, Bloch W, Hammerschmidt M. Hemicentin 2 and Fibulin 1 are required for epidermal-dermal junction formation and fin mesenchymal cell migration during zebrafish development. Dev Biol. 2012;369(2):235–48. doi: 10.1016/j.ydbio.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Isabella AJ, Horne-Badovinac S. Rab10-Mediated Secretion Synergizes with Tissue Movement to Build a Polarized Basement Membrane Architecture for Organ Morphogenesis. Dev Cell. 2016;38(1):47–60. doi: 10.1016/j.devcel.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hagedorn EJ, Yashiro H, Ziel JW, Ihara S, Wang Z, Sherwood DR. Integrin acts upstream of netrin signaling to regulate formation of the anchor cell’s invasive membrane in C. elegans. Dev Cell. 2009;17(2):187–98. doi: 10.1016/j.devcel.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lints HDHR. WormAtlas. 2018. Reproductive system. [Google Scholar]
- 54.Newman AP, White JG, Sternberg PW. Morphogenesis of the C. elegans hermaphrodite uterus. Development. 1996;122(11):3617. doi: 10.1242/dev.122.11.3617. [DOI] [PubMed] [Google Scholar]
- 55.Schindler Adam J, Sherwood David R. Morphogenesis of the Caenorhabditis elegans vulva. Wiley Interdisciplinary Reviews: Developmental Biology. 2012;2(1):75–95. doi: 10.1002/wdev.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muriel JM, Dong C, Hutter H, Vogel BE. Fibulin-1C and Fibulin-1D splice variants have distinct functions and assemble in a hemicentin-dependent manner. Development. 2005;132(19):4223–34. doi: 10.1242/dev.02007. [DOI] [PubMed] [Google Scholar]
- 57.Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 58.Simmer F, Moorman C, van der Linden AM, Kuijk E, van den Berghe PVE, Kamath RS, Fraser AG, Ahringer J, Plasterk RHA. Genome-Wide RNAi of C. elegans Using the Hypersensitive rrf-3 Strain Reveals Novel Gene Functions. PLOS Biology. 2003;1(1):e12. doi: 10.1371/journal.pbio.0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ceron J, Rual JF, Chandra A, Dupuy D, Vidal M, van den Heuvel S. Large-scale RNAi screens identify novel genes that interact with the C. elegans retinoblastoma pathway as well as splicing-related components with synMuv B activity. BMC Developmental Biology. 2007;7(1):30. doi: 10.1186/1471-213X-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Miner JH. Glomerular basement membrane composition and the filtration barrier. Pediatr Nephrol. 2011;26(9):1413–7. doi: 10.1007/s00467-011-1785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miner JH. The glomerular basement membrane. Exp Cell Res. 2012;318(9):973–8. doi: 10.1016/j.yexcr.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Farquhar MG. The glomerular basement membrane: not gone, just forgotten. J Clin Invest. 2006;116(8):2090–3. doi: 10.1172/JCI29488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miner JH, Sanes JR. Collagen IV alpha 3, alpha 4, and alpha 5 chains in rodent basal laminae: sequence, distribution, association with laminins, and developmental switches. The Journal of Cell Biology. 1994;127(3):879. doi: 10.1083/jcb.127.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Abrahamson DR, St John PL, Stroganova L, Zelenchuk A, Steenhard BM. Laminin and type IV collagen isoform substitutions occur in temporally and spatially distinct patterns in developing kidney glomerular basement membranes. J Histochem Cytochem. 2013;61(10):706–18. doi: 10.1369/0022155413501677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chew C, Lennon R. Basement Membrane Defects in Genetic Kidney Diseases. Front Pediatr. 2018;6:11. doi: 10.3389/fped.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gunwar S, Ballester F, Noelken ME, Sado Y, Ninomiya Y, Hudson BG. Glomerular Basement Membrane: IDENTIFICATION OF A NOVEL DISULFIDE-CROSS-LINKED NETWORK OF α3, α4, AND α5 CHAINS OF TYPE IV COLLAGEN AND ITS IMPLICATIONS FOR THE PATHOGENESIS OF ALPORT SYNDROME. Journal of Biological Chemistry. 1998;273(15):8767–8775. doi: 10.1074/jbc.273.15.8767. [DOI] [PubMed] [Google Scholar]
- 67.Miner JH, Li C. Defective Glomerulogenesis in the Absence of Laminin α5 Demonstrates a Developmental Role for the Kidney Glomerular Basement Membrane. Developmental Biology. 2000;217(2):278–289. doi: 10.1006/dbio.1999.9546. [DOI] [PubMed] [Google Scholar]
- 68.Savige J. Alport syndrome: its effects on the glomerular filtration barrier and implications for future treatment. J Physiol. 2014;592(18):4013–23. doi: 10.1113/jphysiol.2014.274449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cosgrove D, Liu S. Collagen IV diseases: A focus on the glomerular basement membrane in Alport syndrome. Matrix Biol. 2017;57–58:45–54. doi: 10.1016/j.matbio.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lennon R, Byron A, Humphries JD, Randles MJ, Carisey A, Murphy S, Knight D, Brenchley PE, Zent R, Humphries MJ. Global analysis reveals the complexity of the human glomerular extracellular matrix. J Am Soc Nephrol. 2014;25(5):939–51. doi: 10.1681/ASN.2013030233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hobeika L, Barati MT, Caster DJ, McLeish KR, Merchant ML. Characterization of glomerular extracellular matrix by proteomic analysis of laser-captured microdissected glomeruli. Kidney Int. 2017;91(2):501–511. doi: 10.1016/j.kint.2016.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Suh JH, Miner JH. The glomerular basement membrane as a barrier to albumin. Nat Rev Nephrol. 2013;9(8):470–7. doi: 10.1038/nrneph.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Funk SD, Lin M-H, Miner JH. Alport syndrome and Pierson syndrome: Diseases of the glomerular basement membrane. Matrix Biology. doi: 10.1016/j.matbio.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jarad G, Cunningham J, Shaw AS, Miner JH. Proteinuria precedes podocyte abnormalities inLamb2−/− mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest. 2006;116(8):2272–9. doi: 10.1172/JCI28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and Function of Laminins in the Embryonic and Mature Vasculature. Physiological Reviews. 2005;85(3):979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 76.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19(12):1584–96. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7(1):a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: An electron microscopic 3D reconstruction. Glia. 2010;58(9):1094–1103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- 79.Owens T, Bechmann I, Engelhardt B. Perivascular Spaces and the Two Steps to Neuroinflammation. Journal of Neuropathology & Experimental Neurology. 2008;67(12):1113–1121. doi: 10.1097/NEN.0b013e31818f9ca8. [DOI] [PubMed] [Google Scholar]
- 80.Kao RM. The luminal connection: from animal development to lumopathies. Organogenesis. 2013;9(2):111–7. doi: 10.4161/org.25225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, Sorokin L, Risling M, Cao Y, Tryggvason K. Deletion of the Laminin 4 Chain Leads to Impaired Microvessel Maturation. Molecular and Cellular Biology. 2002;22(4):1194–1202. doi: 10.1128/MCB.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yao Y, Chen ZL, Norris EH, Strickland S. Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Nat Commun. 2014;5:3413. doi: 10.1038/ncomms4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gautam J, Zhang X, Yao Y. The role of pericytic laminin in blood brain barrier integrity maintenance. Sci Rep. 2016;6:36450. doi: 10.1038/srep36450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tilling T, Korte D, Hoheisel D, Galla HJ. Basement Membrane Proteins Influence Brain Capillary Endothelial Barrier Function In Vitro. Journal of Neurochemistry. 2002;71(3):1151–1157. doi: 10.1046/j.1471-4159.1998.71031151.x. [DOI] [PubMed] [Google Scholar]
- 85.Hayashi Y, Nomura M, Yamagishi SI, Harada SI, Yamashita J, Yamamoto H. Induction of various blood-brain barrier properties in non-neural endothelial cells by close apposition to co-cultured astrocytes. Glia. 1998;19(1):13–26. [PubMed] [Google Scholar]
- 86.Wagner S, Tagaya M, Koziol JA, Quaranta V, del Zoppo GJ. Rapid Disruption of an Astrocyte Interaction With the Extracellular Matrix Mediated by Integrin α β During Focal Cerebral Ischemia/Reperfusion. Stroke. 1997;28(4):858. doi: 10.1161/01.str.28.4.858. [DOI] [PubMed] [Google Scholar]
- 87.Baeten KM, Akassoglou K. Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol. 2011;71(11):1018–39. doi: 10.1002/dneu.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One. 2010;5(10):e13741. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thomsen MS, Birkelund S, Burkhart A, Stensballe A, Moos T. Synthesis and deposition of basement membrane proteins by primary brain capillary endothelial cells in a murine model of the blood-brain barrier. J Neurochem. 2017;140(5):741–754. doi: 10.1111/jnc.13747. [DOI] [PubMed] [Google Scholar]
- 90.Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31(4):497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- 91.Mortazavi MM, Griessenauer CJ, Adeeb N, Deep A, Bavarsad Shahripour R, Loukas M, Tubbs RI, Tubbs RS. The choroid plexus: a comprehensive review of its history, anatomy, function, histology, embryology, and surgical considerations. Childs Nerv Syst. 2014;30(2):205–14. doi: 10.1007/s00381-013-2326-y. [DOI] [PubMed] [Google Scholar]
- 92.Lehtinen MK, Bjornsson CS, Dymecki SM, Gilbertson RJ, Holtzman DM, Monuki ES. The choroid plexus and cerebrospinal fluid: emerging roles in development, disease, and therapy. J Neurosci. 2013;33(45):17553–9. doi: 10.1523/JNEUROSCI.3258-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Booij JC, Baas DC, Beisekeeva J, Gorgels TG, Bergen AA. The dynamic nature of Bruch’s membrane. Prog Retin Eye Res. 2010;29(1):1–18. doi: 10.1016/j.preteyeres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 94.Caceres PS, Benedicto I, Lehmann GL, Rodriguez-Boulan EJ. Directional Fluid Transport across Organ-Blood Barriers: Physiology and Cell Biology. Cold Spring Harb Perspect Biol. 2017;9(3) doi: 10.1101/cshperspect.a027847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Low Frank N. The extracellular portion of the human blood-air barrier and its relation to tissue space. The Anatomical Record. 1961;139(2):105–123. [Google Scholar]
- 96.Low Frank N. The pulmonary alveolar epithelium of laboratory mammals and man. The Anatomical Record. 1953;117(2):241–263. doi: 10.1002/ar.1091170208. [DOI] [PubMed] [Google Scholar]
- 97.Makanya A, Anagnostopoulou A, Djonov V. Development and remodeling of the vertebrate blood-gas barrier. Biomed Res Int. 2013;2013:101597. doi: 10.1155/2013/101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsuprun V, Santi P. Proteoglycan arrays in the cochlear basement membrane. Hearing Research. 2001;157(1):65–76. doi: 10.1016/s0378-5955(01)00278-7. [DOI] [PubMed] [Google Scholar]
- 99.Zdebik AA, Wangemann P, Jentsch TJ. Potassium Ion Movement in the Inner Ear: Insights from Genetic Disease and Mouse Models. Physiology. 2009;24(5):307–316. doi: 10.1152/physiol.00018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen L, Miyamura N, Ninomiya Y, Handa JT. Distribution of the collagen IV isoforms in human Bruch’s membrane. British Journal of Ophthalmology. 2003;87(2):212. doi: 10.1136/bjo.87.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zehnder AF, Adams JC, Santi PA, et al. Distribution of type iv collagen in the cochlea in alport syndrome. Archives of Otolaryngology–Head & Neck Surgery. 2005;131(11):1007–1013. doi: 10.1001/archotol.131.11.1007. [DOI] [PubMed] [Google Scholar]
- 102.Kalluri R, Gattone VH, Hudson BG. Identification and Localization of Type IV Collagen Chains in the Inner Ear Cochlea. Connective Tissue Research. 1998;37(1–2):143–150. doi: 10.3109/03008209809028906. [DOI] [PubMed] [Google Scholar]
- 103.Urabe N, Naito I, Saito K, Yonezawa T, Sado Y, Yoshioka H, Kusachi S, Tsuji T, Ohtsuka A, Taguchi T, Murakami T, Ninomiya Y. Basement Membrane Type IV Collagen Molecules in the Choroid Plexus, Pia Mater and Capillaries in the Mouse Brain. Archives of Histology and Cytology. 2002;65(2):133–143. doi: 10.1679/aohc.65.133. [DOI] [PubMed] [Google Scholar]
- 104.Savige J, Liu J, DeBuc DC, Handa JT, Hageman GS, Wang YY, Parkin JD, Vote B, Fassett R, Sarks S, Colville D. Retinal Basement Membrane Abnormalities and the Retinopathy of Alport Syndrome. Investigative Ophthalmology & Visual Science. 2010;51(3):1621–1627. doi: 10.1167/iovs.08-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Savige J, Sheth S, Leys A, Nicholson A, Mack HG, Colville D. Ocular Features in Alport Syndrome: Pathogenesis and Clinical Significance. Clinical Journal of the American Society of Nephrology. 2015 doi: 10.2215/CJN.10581014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kashtan CE. Alport Syndrome: An Inherited Disorder of Renal, Ocular, and Cochlear Basement Membranes. Medicine. 1999;78(5) doi: 10.1097/00005792-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 107.Majewski J, Schultz DW, Weleber RG, Schain MB, Edwards AO, Matise TC, Acott TS, Ott J, Klein ML. Age-related macular degeneration--a genome scan in extended families. Am J Hum Genet. 2003;73(3):540–50. doi: 10.1086/377701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schultz DW, Klein ML, Humpert AJ, Luzier CW, Persun V, Schain M, Mahan A, Runckel C, Cassera M, Vittal V, Doyle TM, Martin TM, Weleber RG, Francis PJ, Acott TS. Analysis of the ARMD1 locus: evidence that a mutation in HEMICENTIN-1 is associated with age-related macular degeneration in a large family. Hum Mol Genet. 2003;12(24):3315–23. doi: 10.1093/hmg/ddg348. [DOI] [PubMed] [Google Scholar]
- 109.Thompson CL, Klein BE, Klein R, Xu Z, Capriotti J, Joshi T, Leontiev D, Lee KE, Elston RC, Iyengar SK. Complement factor H and hemicentin-1 in age-related macular degeneration and renal phenotypes. Hum Mol Genet. 2007;16(17):2135–48. doi: 10.1093/hmg/ddm164. [DOI] [PubMed] [Google Scholar]
- 110.Gimbrone MA, Anderson KR, Topper JN. The Critical Role of Mechanical Forces in Blood Vessel Development, Physiology and Pathology. Journal of Vascular Surgery. 1999;29(6):1104–1151. doi: 10.1016/s0741-5214(99)70252-1. [DOI] [PubMed] [Google Scholar]
- 111.Hsieh HJ, Liu CA, Huang B, Tseng AHH, Wang DL. Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. Journal of Biomedical Science. 2014;21(1):3. doi: 10.1186/1423-0127-21-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miller RT. Mechanical properties of basement membrane in health and disease. Matrix Biol. 2017;57–58:366–373. doi: 10.1016/j.matbio.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 113.Horton ER, Byron A, Askari JA, Ng DHJ, Millon-Fremillon A, Robertson J, Koper EJ, Paul NR, Warwood S, Knight D, Humphries JD, Humphries MJ. Definition of a consensus integrin adhesome and its dynamics during adhesion complex assembly and disassembly. Nat Cell Biol. 2015;17(12):1577–1587. doi: 10.1038/ncb3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Suleiman H, Zhang L, Roth R, Heuser JE, Miner JH, Shaw AS, Dani A. Nanoscale protein architecture of the kidney glomerular basement membrane. Elife. 2013;2:e01149. doi: 10.7554/eLife.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rumpelt HJ. Alport’s syndrome: specificity and pathogenesis of glomerular basement membrane alterations. Pediatric Nephrology. 1987;1(3):422–427. doi: 10.1007/BF00849248. [DOI] [PubMed] [Google Scholar]
- 116.Randles MJ, Humphries MJ, Lennon R. Proteomic definitions of basement membrane composition in health and disease. Matrix Biol. 2017;57–58:12–28. doi: 10.1016/j.matbio.2016.08.006. [DOI] [PubMed] [Google Scholar]