Abstract
Originally, uptake-mediated termination of monoamine (e.g., serotonin, dopamine) signaling was believed to only occur via high-affinity, low-capacity, transporters (“uptake1”) such as the serotonin or dopamine transporters, respectively. Now the important contribution of a second low-affinity, high-capacity, class of biogenic amine transporters has been recognized, particularly in circumstances when uptake1 transporter function is reduced (e.g., antidepressant treatment). Pharmacologic or genetic reductions in uptake1 function can change locomotor, anxiety-like, or stress coping behaviors. Comparable behavioral investigations into reduced low-affinity, high-capacity transporter function are lacking, in part, due to a current dearth of drugs that selectively target particular low-affinity, high-capacity transporters, such as the plasma membrane monoamine transporter. Therefore, the most direct approach involves constitutive genetic knockout of these transporters. Other groups have reported that knockout of the low-affinity, high-capacity organic cation transporters 2 or 3 alters anxiety-like and stress coping behaviors, but none have assessed behaviors in plasma membrane monoamine transporter knockout mice. Here, we evaluated adult male and female plasma membrane monoamine transporter wildtype, heterozygous, and knockout mice in locomotor, anxiety-like, and stress coping behavioral tests. A mild enhancement of anxiety-related behavior was noted in heterozygous mice. Active coping behavior was modestly and selectively increased in female knockout mice. These subtle behavioral changes support a supplemental role of plasma membrane monoamine transporter in serotonin and dopamine uptake, and suggest sex differences in transporter function should be examined more closely in future investigations.
Keywords: plasma membrane monoamine transporter, knockout mice, serotonin, dopamine
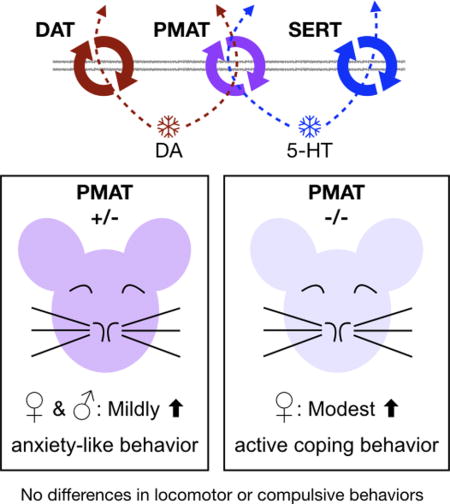
Graphical abstract
The plasma membrane monoamine transporter (PMAT, Slc29a4) contributes to dopamine and serotonin uptake. Given no selective PMAT inhibitors are currently available, the best way to examine its influence on behavior is with genetic knockouts. We discovered subtle changes in anxiety-like (both sexes) and active coping behaviors (females only) when PMAT expression was reduced or ablated, respectively, suggesting sex differences should be explored further in future transporter function studies.
Introduction
Duration of monoamine neurotransmitter signaling in brain is regulated primarily by active uptake; faster uptake results in shorter signaling duration. Drugs that inhibit monoamine uptake (e.g., cocaine, escitalopram) can have pronounced effects on motor activity, anxiety, and/or mood. Genetic loss of uptake function through knockout of specific transporters in rodents likewise generates animals with altered locomotor, anxiety-like, and/or stress coping phenotypes (see reviews (Haenisch & Bönisch, 2011; Commons et al., 2017)). Through these pharmacologic and genetic means, substantial information has been gained about high-affinity, low-capacity (i.e., uptake1) transporters, including the serotonin, dopamine, and norepinephrine transporters (SERT, DAT, and NET, respectively). For example, intensive study of uptake1 transporters, particularly in knockout mice, has revealed two additional monoamine clearance processes; together, these are aptly termed “uptake2” (for review see Daws, 2009). One process involves “unfaithful” uptake of monoamines by a different uptake1 transporter, e.g., serotonin uptake by DAT (Zhou et al., 2002) and dopamine uptake by NET (Morón et al., 2002). The other process is mediated by a class of low-affinity, high-capacity cation transporters, including the plasma membrane monoamine transporter (PMAT), which is the focus of this paper (Schmitt et al., 2003; Daws et al., 2006; Baganz et al., 2008) (see (Daws, 2009) for review). Herein, reference to the low-affinity, high-capacity biogenic amine transporters consisting of PMAT and the three organic cation transporters (OCTs) will be made by using the term “uptake2” for the sake of brevity, though we point out that this term also includes “unfaithful” uptake of monoamines by uptake1 transporters.
Compared to uptake1, far less is known about the behavioral consequences of reduced uptake2 function. In part, this is due to a lack of selective uptake2 inhibitors. Compounds that inhibit uptake2 transporters either broadly inhibit PMAT plus OCT1, OCT2, and OCT3 (Schömig et al., 1993; Koepsell et al., 2007; Horton et al., 2013), or have other primary effects that significantly complicate attributing any observed behavioral changes specifically to uptake2 inhibition. For example, corticosterone is a potent inhibitor of uptake by OCT3, but also elicits a host of both genomic and non-genomic effects through actions at the glucocorticoid receptor (Wu et al., 1998; Gasser, 2006; Baganz et al., 2010; Oakley & Cidlowski, 2013). Similarly, lopinavir is selective for PMAT over OCTs (Duan et al., 2015) but has poor bioavailability, particularly in brain, and as a protease inhibitor for treating HIV its use is further complicated by various undesirable side effects such as metabolic disruptions (Kumar et al., 1999; Pistell et al., 2010; Patel et al., 2014). Genetic knockout of uptake2 transporters therefore currently affords the most straightforward method of understanding how uptake2 function influences activity and emotion-related behaviors.
Surprisingly few studies, however, have behaviorally characterized mice constitutively lacking an uptake2 transporter. Mice lacking OCT1, OCT2, or OCT3 were generated over a decade ago (Jonker et al., 2001; Zwart et al., 2001; Jonker et al., 2003), whereas PMAT was genetically knocked out in mice only a few years ago (Duan & Wang, 2013). Expression of OCT1 is predominantly in the liver (Jonker et al., 2001; Roth et al., 2012), and this may be why OCT1 deficient mice have not, to our knowledge, been behaviorally evaluated. Mice with double knockout of OCT1 and OCT2 are viable (Jonker et al., 2003), but similarly have not been assessed for behavioral perturbations. Knockout of OCT2 alone in mice reduced anxiety-like behaviors and enhanced passive coping, without affecting overall locomotor activity (Bacq et al., 2012). Conflicting reports of increased (Vialou et al., 2008) or decreased (Wultsch et al., 2009) anxiety-related behaviors have been reported, in the absence of any locomotor alterations, in mice lacking OCT3. The behavioral consequences of constitutive PMAT deficiency have not yet been examined.
Expression of PMAT is greater in brain than in other organs, and PMAT is more highly expressed in brain than other uptake2 transporters (Engel et al., 2004; Dahlin et al., 2007; Duan & Wang, 2010; Miura et al., 2017). Therefore, loss of PMAT function might have more pronounced behavioral effects than those observed in mice lacking an OCT. Moreover, the polyspecific uptake2 transporters exhibit differential affinities for monoamine neurotransmitters. PMAT preferentially transports serotonin and dopamine, whereas OCTs display higher affinities for histamine, epinephrine, and norepinephrine (Duan & Wang, 2010; Miura et al., 2017). Thus, serotonergic and dopaminergic signaling in brains of PMAT-deficient mice might be prolonged, though this has not yet been directly investigated. Both serotonin and dopamine are strongly implicated in the pathophysiology of neuropsychiatric disorders, particularly depression and anxiety (Perona et al., 2008; Daws, 2009; la Mora et al., 2010; Zweifel et al., 2011; Chaudhury et al., 2012; Fernandez & Gaspar, 2012; Horton et al., 2013; Russo & Nestler, 2013). Consequently, we hypothesized that mice with reduced or ablated PMAT function would exhibit disrupted anxiety-like and active coping behaviors, similar to some reports in constitutive SERT or DAT knockout mice that have prolonged serotonergic or dopaminergic signaling, respectively (Holmes et al., 2003; Shen et al., 2004; Pogorelov et al., 2005; Perona et al., 2008; but see Lira et al., 2003; Weiss et al., 2007; Wellman et al., 2007). To test this hypothesis, we evaluated the behavioral phenotype of mice constitutively deficient in PMAT using locomotor, anxiety-like, and stress coping measures.
Materials and Methods
Animals
Mice with targeted disruption of Slc29a4 (PMAT) were generously donated by Dr. Joanne Wang (Duan & Wang, 2013). Thereafter, all mice were bred in house and maintained on a C57BL/6J background. Mice were housed in a temperature-controlled vivarium maintained at 24°C, on 7090 Teklad sani-chip bedding (Envigo, East Millstone, NJ), and given Teklad LM-485 mouse/rat sterilizable diet 7012 chow (Envigo) and water ad libitum. After weaning, mice were group housed with same-sex littermates at 2-5 mice per cage. Adult (≥90 days of age) male and female mice with PMAT alleles intact (+/+), reduced (+/−), or knocked out (−/−) were used for all experiments. Animal numbers ranged from 9-25 per sex per genotype; exact Ns for each measure are provided in corresponding figure legends. Food and water were provided ad libitum, and mice were housed in a 12:12 light:dark cycle with lights on at 0800 h. No procedures involved pain, and every effort was made to minimize the discomfort of the animals. All experiments were approved by the University of Texas Health Science Center at San Antonio Institutional Animal Care and Use Committee, and complied with the National Research Council’s Guide for the Care and Use of Laboratory Animals, 8th Ed.
Genotyping
Genomic DNA was extracted from tail snips or ear punches using Proteinase K (Roche, Basel, Switzerland) in Tris-sodium dodecyl sulfate-EDTA digestion buffer. PCR analysis of genomic DNA (3.8 μL) was performed in 1X PCR buffer containing 1.74 mM MgCl2 and 34.7 μM dNTPs, with 0.46 μL of Platinum Taq (Invitrogen, Carlsbad, CA) per 22 μL reaction. Primers (Integrated DNA Technologies, Coralville, IA) designed by Duan and Wang (Duan & Wang, 2013) were used for amplification of the wildtype allele, between exons 3 and 4, and/or the knockout allele, at the neomycin resistance gene (Neo): Exon 3 forward – 5’ CGA CTA TCT TCA CCA CAA GTA CCC AG 3’; Exon 4 reverse – 5’ GAG GCT CAT GTC AAA TAC GAT GGA G 3’; Neo F – 5’ CTT GCT CCT GCC GAG AAA GTA TC 3’; Neo R – 5’ TCA GAA GAA CTC GTC AAG AAG GCG 3’. Each PCR proceeded as follows: 95°C for 5 min; 34 cycles of 94°C for 30 s, 59°C for 30 s, and 72°C for 90 s; 72°C for 5 min; hold at 4°C. Agarose gel (1%) electrophoresis in Tris-acetate-EDTA buffer was used to visual PCR products, in reference to a 1 kb Plus DNA ladder (Invitrogen, cat. no. 10787018), with the wildtype allele presenting at 847 bp, and the knockout allele at 447 bp (Duan & Wang, 2013). All genotypes were verified by at least 2 independent PCR reactions.
Behavior testing
In an effort to minimize animal numbers, mice were utilized in at least two different behavior tests. The first behavior test was always either the elevated plus maze or locomotor activity. Animals that underwent the marble burying test did so after one or both of these, and the forced swim test always occurred last to avoid introducing a stress confound on the preceding test(s). Tests were separated by at least 48 h to minimize carry-over effects of testing on subsequent behavioral evaluations. Males and females were always tested on different days, to further minimize potential behavioral confounds. Mice were moved in their home cages from their colony room to the testing room at least 1 h prior to test commencement, and returned to their colony room at the end of each test.
Elevated plus maze
Behavior in the elevated plus maze was assessed under dim white light (44 lux), between 1730 and 2000 h. The plus maze was constructed of white acrylic, with arms measuring 30 cm long × 5 cm wide, and closed arm walls 15.5 cm high. The floor of the maze was elevated 51 cm off the ground, and all surfaces of the maze were cleaned between each animal with water. Elevated plus maze behaviors were video recorded for offline analysis with AnyMaze (v5.2; Stoelting, Wood Dale, IL), in which 85% of the animal’s entire body area was required to be present in an arm to qualify as an entry. Mice that fell off an open arm during the 5 min test were excluded from analyses (female +/+: 2 of 13 excluded; female +/−: 3 of 12 excluded; female −/−: 5 of 24 excluded; male +/+: 3 of 14 excluded; male +/−: 4 of 22 excluded; male −/−: 4 of 22 excluded). These incidents are likely attributable to the absence of a ledge surrounding the open arms, which have been added by others to encourage open arm exploration (Rodgers & Johnson, 1995) and minimize falls (Lee & Rodgers, 1990; Cruz et al., 1994) in rodents, but consequently can also reduce the aversiveness of the open arms (Fernandes & File, 1996).
Locomotor activity
Locomotor activity was assessed using custom activity chambers previously described (Koek et al., 2012), containing 4 equidistant infrared beams spanning the width of clear Plexiglas chambers (15 cm wide × 30 cm long × 15 cm high). Chambers were located in sound-attenuating boxes equipped with quiet fans for air circulation. Beam breaks were recorded by Multi-Varimex software (v2.10; Columbus Instruments, Columbus, OH) over a 4 h span between 1130 and 1530 h. Fecal boli were counted at test completion, then all chamber surfaces were cleaned with water.
Marble burying test
Marble burying was evaluated as we have previously described (Gould et al., 2011), as an index of compulsive or repetitive tendencies, and occurred under dim lighting between 1730 and 2000 h. Briefly, blue decorative marbles were arranged in a 3 × 5 grid pattern atop 5-6 cm of wood chip bedding within a clear acrylic chamber (26 cm wide × 47 cm long × 20 cm high). Mice were then placed in the chamber and left undisturbed for 30 min, at which time pictures of the chambers were taken prior to removal of the mouse, to avoid any disturbance of the bedding. These photos were used to quantify the number of marbles that were >25% visible; this number was subtracted from 15 to quantify how many marbles were >75% buried.
Forced swim test
Mice underwent a single, 6 min forced swim between 1200 and 1430 h in clear acrylic cylinders (19 cm inner diameter × 25 cm high) containing 15 cm of room temperature water (23.5 ± 1.5°C) (Castagné et al., 2011; Can et al., 2012; Koek et al., 2017). Tests were video recorded for offline manual scoring of swimming, immobility, and climbing behaviors during the entire 6 min test by an observer blind to genotype and sex using Solomon Coder (beta 17.03.22; solomoncoder.com). Immobility was defined as the absence of all movement, except that minimally necessary to stay afloat, for at least 1 s. Climbing was defined as the mouse being oriented perpendicular to the edge of the cylinder, actively moving both forepaws against the wall, and fully extending the hind paws. Swimming was defined as active movement with forward propulsion beyond that necessary to stay afloat, but not meeting the criterion for climbing. After test completion, mice were immediately removed from the water, gently dried with a paper towel, and individually placed in clean cages situated half-on a warmed heating pad to facilitate their drying. Fecal boli were counted at test completion, then cylinders were rinsed and refilled with clean water for each animal tested.
Statistical analyses
Locomotor data over time were analyzed with a 3 way (time × sex × genotype) repeated measures ANOVA, with Geisser Greenhouse correction for within-subjects analyses, using NCSS (v11.0.13; NCSS, LLC, Kaysville, UT). All other data were analyzed with a two-way ANOVA (sex × genotype), with Dunnett’s post-hoc tests compared with same sex +/+ mice applied where appropriate, using GraphPad Prism (v7.0c; GraphPad Software, La Jolla, CA). Significance was set a priori at p<0.05, and all data were graphed with GraphPad Prism. Individual data points are shown, along with the mean and S.E.M.
Results
Elevated plus maze
Time spent in the open arms of the elevated plus maze was not significantly different across genotype or sex (Fig. 1A), though a non-significant trend was noted for sex (F(1,80)=3.01, p=0.09). Evaluation of latency to enter the open arms revealed significant main effects of sex (F(1,80)=5.58, p=0.02) and genotype (F(2,80)=5.01, p=0.009), but Dunnett’s post-hoc comparisons did not indicate any significant differences from +/+ mice (Fig. 1B). Similar to open arm time, only a main effect of sex (F(1,80)=4.47, p=0.04) was detected for time spent in the closed arms (Fig. 1C). Distance traveled in the entire elevated plus maze during the 5 min test revealed a significant main effect of genotype (F(2,80)=7.28, p=0.001), but relative to +/+ mice no significant differences were detected by post-hoc tests. (Fig. 1D). No significant interactions were detected for any of these measures in the elevated plus maze.
Figure 1. PMAT deficiency mildly affects anxiety-like behaviors.
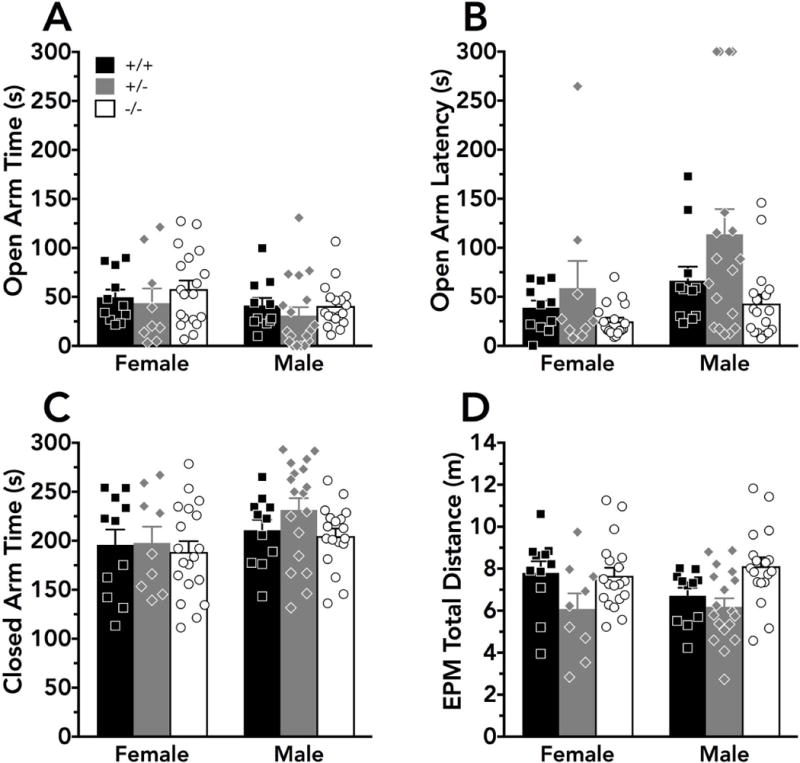
Anxiety-related behaviors were assessed in the elevated plus maze by measuring A) time spent in the open arms; B) latency to first enter an open arm; C) time spent in the closed arms; and D) total distance traveled in the elevated plus maze during the 5 min test. For B, a latency of 300 s indicates that the mouse did not enter an open arm for the duration of the test. Significant main effects of genotype (p<0.01) were detected for B) latency to first enter an open arm and D) total distance travelled, but post-hoc comparisons did not indicate significant differences compared to wildtype mice. Wildtype mice are indicated as black bars or squares, heterozygous mice as grey bars or diamonds, and knockout mice as white bars or circles. Individual data points are indicated by black squares (wildtypes; female N=11, male N=11), grey diamonds (heterozygotes; female N=9, male N=18), or white circles (knockouts; female N=19, male N=18), and bars indicate the mean + S.E.M.
Locomotor activity
Cumulative locomotor activity, measured by infrared beam breaks in 5 min bins over 4 h, did not reveal any sex (F(1,101)=0.131, p=0.72) or genotype (F(2, 101)=2.04, p=0.14) differences (Fig. 2A). When examining locomotor activity over time within subjects, there was a significant interaction between sex and time (F(47, 4747)=2.24, p=0.02), but no interaction between genotype and time (F(94, 4747)=1.24, p=0.22), or between time, sex, and genotype (F(94,4747)=1.20, p=0.25) (Fig. 2C,D). Significant main effects of sex (F(1,97)=4.11, p=0.05) and of genotype (F(2,97)=3.66, p=0.03) were detected with respect to fecal boli measured at the conclusion of the 4 h locomotor test (Fig. 2B), but post-hoc tests did not reveal any significant differences relative to +/+ mice within either sex.
Figure 2. Locomotor activity is unaltered by PMAT deficiency.
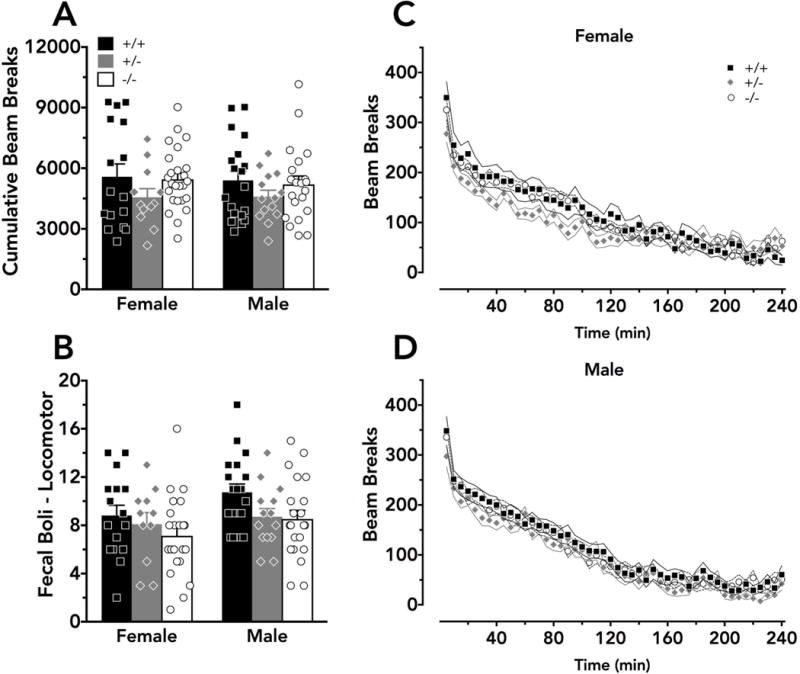
Locomotor activity was measured in 5 min bins by infrared beam breaks over a 4 h consecutive span. A) Cumulative beam breaks did not differ across sex or genotype. Significant main effects of sex and genotype were detected for B) fecal boli measured at the conclusion of the locomotor activity assay, but no significant differences were indicated by Dunnett’s post-hoc tests. A significant interaction between sex and time on locomotor activity was detected, and graphs are separated into C) female and D) male data for clarity. A,B) Means + S.E.M. for wildtype mice are indicated as black bars, heterozygous mice as grey bars, and knockout mice as white bars, with individual data points indicated by black squares (wildtypes), grey diamonds (heterozygotes), or white circles (knockouts). C,D) Means ± S.E.M. for each time bin, starting at 5 min, are indicated by black squares and solid black lines (wildtypes), grey diamonds and solid grey lines (heterozygotes), or white circles and dashed black lines (knockouts). Ns for all graphs are: wildtypes - female N=16, male N=19; heterozygotes - female N=12, male N=14; knockouts - female N=25, male N=21; except in b N=11 for heterozygous female and N=22 for knockout female because fecal boli counts were not recorded for 4 animals.
Marble burying test
With respect to the percent of marbles buried following 30 min in the marble burying test (Fig. 3), no significant effect of sex (F(1,95)=0.850, p=0.36) or genotype (F(2,95)=1.58, p=0.21) was detected.
Figure 3. Marble burying behavior is not disrupted in PMAT knockouts.
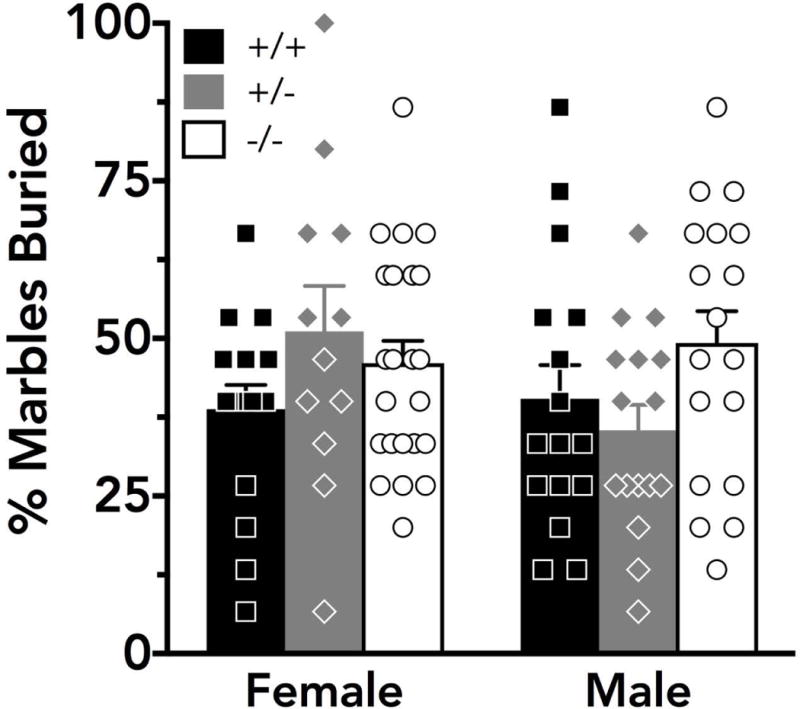
The percent of 15 total marbles buried at least 75% after a 30 min test was not significantly different across sex or genotype. Black, grey, and white bars indicate the mean + S.E.M. for wildtype (female N=16, male N=16), heterozygous (female N=12, male N=16), and knockout mice (female N=23, male N=18), respectively. Black squares, grey diamonds, and white circles indicate individual data points for wildtype, heterozygous, and knockout animals, respectively.
Forced swim test
A non-significant trend for an interaction between genotype and sex was noted for time spent immobile during the 6 min of the forced swim test (F(2,82)=2.58, p=0.08) (Fig. 4A). No main effect of genotype was detected for time spent immobile (F(2,82)=0.576, p=0.56), though there was a main effect of sex (F(1,82)=18.39, p<0.001) (Fig. 4A). A significant interaction between sex and genotype was observed for the time spent swimming (F(2,82)=3.12, p=0.05), and post-hoc tests indicated that −/− females swam significantly more than +/+ females (Fig. 4B). In contrast, only main effects of sex were detected for time spent climbing (F(1,82)=7.48, p=0.008) (Fig. 4C) and latency to first immobility (F(1,82)=5.22, p=0.02) (Fig. 4D). No main effects of sex (F(1,82)=0.0330, p=0.86) or genotype (F(2,82)=0.695, p=0.50) were observed for total fecal boli at the conclusion of the test (Fig. 4E).
Figure 4. Forced swim test behavior is moderately altered in female PMAT knockout mice.
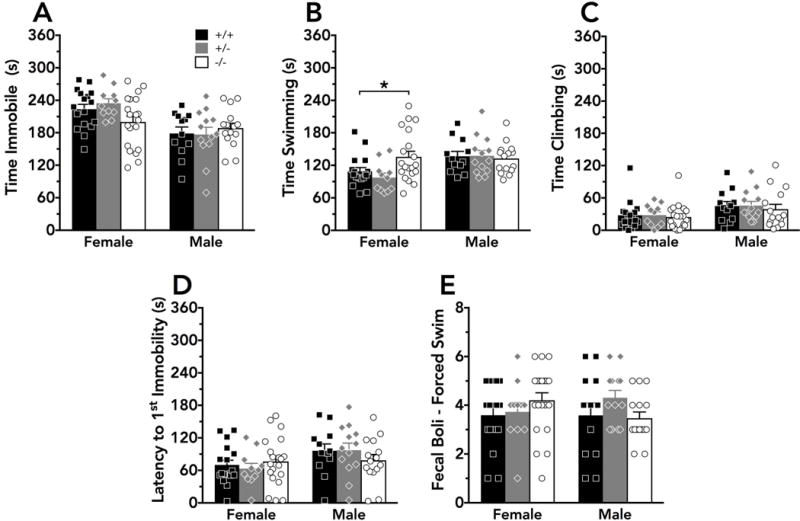
During a 6 min forced swim test, mice displayed no significant differences in A) time spent immobile, but a non-significant trend (p=0.08) for an interaction between genotype and sex was noted. A significant interaction was noted for B) time spent swimming, with post-hoc tests revealing increased swimming in female knockouts compared to female wildtypes. No significant genotype effects were detected for C) time spent climbing; D) latency to the first immobility bout; or E) fecal boli present at the conclusion of the test. *p<0.05. Respectively, black bars/squares, grey bars/diamonds, and white bars/circles indicate wildtype (female N=17, male N=12), heterozygous (female N=11, male N=13), and knockout (female N=20, male N=15) mouse means + S.E.M. and individual data points.
Discussion
Here we sought to investigate the behavioral consequences of constitutive PMAT reduction or ablation in mice, as a current lack of PMAT-selective inhibitors devoid of pronounced off-target effects (e.g., the antiretroviral lopinavir) preclude a pharmacological approach. Given previous findings in mice constitutively lacking uptake1-mediated transport of serotonin or dopamine, we hypothesized that reductions in PMAT function would similarly perturb anxiety-related behaviors and active coping responses. Overall, we found that constitutive deficiency of PMAT exerted remarkably subtle effects on anxiety-related behaviors, produced no significant changes in overall locomotor or compulsive/repetitive behaviors, and sex-selectively influenced stress coping behaviors.
Heterozygous mice appeared to drive the main effects of genotype detected on open arm latency and on total distance travelled in the elevated plus maze. These outcomes indicate a mild enhancement of anxiety-related behavior across sexes in mice with reduced or ablated PMAT function. Quantification of mobility (swimming and climbing) and immobility in the forced swim test as indicators of active and passive coping behavior, respectively, revealed that swimming behavior in the forced swim test was selectively enhanced by PMAT knockout in females. A similar non-significant trend for interaction between genotype and sex was noted for time spent immobile. Thus, PMAT ablation specifically increased active coping behavior in females but not males. Number of fecal boli, a measure used in rodents to indicate stress (Taché & Bonaz, 2007; Crumeyrolle-Arias et al., 2014), after the 6 min forced swim test were not different between PMAT genotypes. However, there was a main effect of genotype on fecal boli after 4 h in the locomotor assay, with PMAT deficiency appearing to reduce fecal boli in a low stress condition. Given evidence of PMAT expression in the intestines (Zhou, Xia, & Wang, 2007; Han et al., 2015; Wagner et al., 2016; Mimura et al., 2017), this reduced fecal output may be an indicator of altered digestive functionality rather than an indicator of stress response. In sum, constitutive deficiency of PMAT in mice produces remarkably mild effects on anxiety-related behaviors, and enhances active coping behaviors specifically in female knockouts.
Though these data are the first, to our knowledge, to assess the behavioral repercussions of loss of PMAT function, far more is known about the cellular localization and function of PMAT. Since discovery of PMAT in 2004 by Wang and colleagues (Engel et al., 2004), impressive advances have been made in characterizing the PMAT protein. The Wang lab has extensively assessed how protein domains, membrane potential, and extracellular pH affect PMAT kinetic parameters (Zhou, Xia, Engel, et al., 2007; Itagaki et al., 2012). In PMAT knockout mice generated by the Wang lab, they confirmed a prominent role of PMAT in the choroid plexus, where it transports monoamines and other organic cations out of the cerebrospinal fluid (Duan & Wang, 2013). Multiple research groups have continued to explore cation transport by PMAT at the blood-brain barrier, particularly at the choroid plexus (Okura et al., 2011; Duan & Wang, 2013; Wu et al., 2015; Usui et al., 2016; Hu et al., 2017). PMAT is also suspected to contribute to intestinal transport and accumulation of cationic drugs, such as metformin and atenolol, and this is supported by in vitro evidence (Zhou, Xia, & Wang, 2007; Han et al., 2015; Wagner et al., 2016; Mimura et al., 2017). Continued study of PMAT’s gastrointestinal contributions, particularly given the reduced defecation that we observed as a function of PMAT deficiency, could expand investigative avenues for this protein beyond drug absorption into the realm of gastrointestinal disorders.
In addition to the unanticipated reduction in defecation by PMAT knockouts following the 4 h locomotor activity assay, the subtle shifts in anxiety-like behaviors and female-specific increase in active stress coping were unexpectedly mild. Measures of activity, both in the locomotor assay and in distance travelled in the elevated plus maze, argue that this increased swimming behavior is not confounded by any overall enhanced activity in female knockouts. Because mice were tested on 2 or more behavior measures at least 48 h apart, a potential for test carry-over effects remains (McIlwain et al., 2001), despite the intentional progression of tests from least to most stressful. Though our behavioral findings only partially support our original hypothesis, they still fit with the current understanding of PMAT and other high-capacity biogenic amine transporters as contributing to more phasic, ‘as-needed’ clearance (see (Daws, 2009)). This overflow engagement of PMAT complements the primary uptake roles of the low-capacity SERT and DAT that are more consistently engaged by tonic signaling conditions. Importantly, PMAT knockout mice do not display compensatory changes in in blood chemistry or in whole brain SERT, DAT, NET, or OCT3 mRNA expression (Duan & Wang, 2013), suggesting the increased active coping behavior observed in females is likely not a consequence of compensatory upregulation of other transporters. However, brain region-specific expression levels and function of monoamine transporters have not yet been quantified in PMAT-deficient mice, so a possibility remains for more localized compensatory upregulation. Alternatively, the modest anxiety-related perturbations noted in heterozygotes, but not knockouts, could suggest recruitment of an as-yet-unidentified transporter in the latter. This might explain why knockout behavior more closely resembles that of wildtypes in the elevated plus maze, whereas partial deletion of PMAT in heterozygotes might not be sufficient to elicit this compensatory upregulation and thereby could reveal a more accurate representation of constitutive PMAT deficiency. Such mice could provide valuable models of reduced PMAT function (Shirasaka et al., 2017), as recently two SLC29A4 polymorphisms conferring loss-of-function have been discovered in humans (Adamsen et al., 2014). Indeed, deficiency of PMAT may not become overtly evident in behaviors until monoaminergic systems are sufficiently perturbed. Future studies that evaluate how these mice perform under conditioned behavioral paradigms (e.g., drug self-administration) or respond to chronic stressors could unmask the necessity of PMAT as a compensatory monoamine transporter.
The importance of monoamine neurotransmitter uptake by transporters is underscored by marked psychological and behavioral effects resulting from reductions in their function. For example, uptake1 inhibitors such as cocaine, methylphenidate, bupropion, venlafaxine, and citalopram can induce changes in cognition, impulse control, alertness, mood, and anxiety (for review see (Liu & Molino, 2007; Daws, 2009; Haenisch & Bönisch, 2011)). Likewise, genetic knockout of uptake1 transporters in mice and rats alters anxiety- and depressive-like behaviors, and changes responses to cognitive tasks or rewarding drugs (Holmes et al., 2003; Shen et al., 2004; Perona et al., 2008; see Haenisch & Bönisch, 2011 for review). Such phenotypes, however, are sometimes at least partially moderated by compensatory upregulation of other biogenic amine transporters, such as by OCT3 in SERT heterozygous and knockout mice (Baganz et al., 2008), and by SERT and DAT in NET knockout mice (Solich et al., 2011). This moderation, which could be strain-, species-, and even context-dependent, might help explain discrepancies within the uptake1 knockout literature regarding directionality of anxiety-like and active coping behavioral changes elicited by SERT or DAT deletion (Holmes et al., 2003; Lira et al., 2003; Pogorelov et al., 2005; Weiss et al., 2007; Wellman et al., 2007; Olivier et al., 2008; Perona et al., 2008). Certainly, behavior in constitutive genetic knockouts does not necessarily correspond to the behavioral consequences of transient pharmacologic transporter inhibition. For example, the elevated anxiety-like phenotype of constitutive SERT knockout rodents (Holmes et al., 2003; Olivier et al., 2008) (but see Lira et al., 2003) contrasts with the robust effectiveness of SERT-inhibiting drugs in treating anxiety in adults (Bandelow et al., 2015). Constitutive reduction or knockout of specific monoamine transporters nonetheless provides valuable information about compromised transporter function, compensatory processes, monoamine signaling dynamics, and physiological and behavioral sequelae that can help direct future studies.
Supplementary Material
Acknowledgments
The authors would like to thank Melodi Bowman, Kyra Clarke, Dr. Valentina R. Garbarino, Dr. Georgianna G. Gould, Kristi Guerrero, Lauren Metzel, Dr. Nathan Mitchell, Robert Seaman, and Kelsey Toney for technical assistance. We thank Dr. Joanne Wang for providing us with the PMAT knockout mice. We also thank the Department of Laboratory Animal Resources at UT Health San Antonio for their animal care efforts. This work was supported by a 2017 NARSAD Young Investigator Grant (26249) from the Brain & Behavior Research Foundation and Vital Projects Fund, Inc., to TLG, and National Institute of Mental Health grants (R01 MH093320 and R01 MH106978) to LCD. TLG was supported by a National Institute on Drug Abuse grant (T32 DA031115) to Dr. Charles P. France. None of the funding sources were involved in study design, data collection or analyses, writing this manuscript, or deciding to publish these findings.
Abbreviations
- DAT
Dopamine transporter
- NET
norepinephrine transporter
- OCTs
organic cation transporters
- PMAT
plasma membrane monoamine transporter
- SERT
serotonin transporter
Footnotes
Data Accessibility Statement
The supporting data for this manuscript have been uploaded with the submission.
DR. T. LEE GILMAN (Orcid ID : 0000-0001-8398-0195)
Conflict of Interest Statement
The authors have no actual or potential conflicts of interest to disclose.
Author Contributions
TLG planned all experiments, analyzed all behavior data, graphed all results, and wrote and revised manuscript. TLG and CMG performed all behavior experiments. TLG, CMG, and MV genotyped all animals. MH-R maintained mouse colony, including breeding and weaning. MSB assisted with some behavior experiments. WK provided locomotor equipment, assisted with experimental planning and statistical analyses, and revised manuscript. LCD assisted with experimental planning, revised manuscript, and provided all other equipment necessary for experiments and reagents for genotyping.
References
- Adamsen D, Ramaekers V, Ho HT, Britschgi C, Rüfenacht V, Meili D, Bobrowski E, Philippe P, Nava C, Maldergem L, Bruggmann R, Walitza S, Wang J, Grünblatt E, Thöny B. Autism spectrum disorder associated with low serotonin in CSF and mutations in the SLC29A4 plasma membrane monoamine transporter (PMAT) gene. 2014;5:1–11. doi: 10.1186/2040-2392-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacq A, Balasse L, Biala G, Guiard B, Gardier AM, Schinkel A, Louis F, Vialou V, Martres MP, Chevarin C, Hamon M, Giros B, Gautron S. Organic cation transporter 2 controls brain norepinephrine and serotonin clearance and antidepressant response. Mol Psychiatry. 2012;17:926–939. doi: 10.1038/mp.2011.87. [DOI] [PubMed] [Google Scholar]
- Baganz N, Horton R, Martin K, Holmes A, Daws LC. Repeated swim impairs serotonin clearance via a corticosterone-sensitive mechanism: Organic cation transporter 3, the smoking gun. J Neurosci. 2010;30:15185–15195. doi: 10.1523/JNEUROSCI.2740-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, Koldzic-Zivanovic N, Jeske NA, Koek W, Toney GM, Daws LC. Organic cation transporter 3: Keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci USA. 2008;105:18976–18981. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B, Reitt M, Röver C, Michaelis S, Görlich Y, Wedekind D. Efficacy of treatments for anxiety disorders: a meta-analysis. Int Clin Psychopharmacol. 2015;30:183–192. doi: 10.1097/YIC.0000000000000078. [DOI] [PubMed] [Google Scholar]
- Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The mouse forced swim test. J Vis Exp Jove. 2012:e3638. doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagné V, Moser P, Roux S, Porsolt R. Rodent Models of Depression: Forced Swim and Tail Suspension Behavioral Despair Tests in Rats and Mice. wiley; 2011. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo J, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo M, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2012;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commons KG, Cholanians AB, Babb JA, Ehlinger DG. The Rodent Forced Swim Test Measures Stress-Coping Strategy, Not Depression-like Behavior. ACS Chem Neurosci. 2017;8:955–960. doi: 10.1021/acschemneuro.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Daugé V, Naudon L, Rabot S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–217. doi: 10.1016/j.psyneuen.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Be. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- Dahlin A, Xia L, Kong W, Hevner R, Wang J. Expression and immunolocalization of the plasma membrane monoamine transporter in the brain. Neuroscience. 2007;146:1193–1211. doi: 10.1016/j.neuroscience.2007.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC. Unfaithful neurotransmitter transporters: Focus on serotonin uptake and implications for antidepressant efficacy. Pharmacol Therapeut. 2009;121:89–99. doi: 10.1016/j.pharmthera.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daws LC, Montañez S, Munn JL, Owens WA, Baganz NL, Boyce-Rustay JM, Millstein RA, Wiedholz LM, Murphy DL, Holmes A. Ethanol inhibits clearance of brain serotonin by a serotonin transporter-independent mechanism. J Neurosci. 2006;26:6431–6438. doi: 10.1523/JNEUROSCI.4050-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther. 2010;335:743–753. doi: 10.1124/jpet.110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Wang J. Impaired monoamine and organic cation uptake in choroid plexus in mice with targeted disruption of the plasma membrane monoamine transporter (Slc29a4) gene. J Biol Chem. 2013;288:3535–3544. doi: 10.1074/jbc.M112.436972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan H, Hu T, Foti RS, Pan Y, Swaan PW, Wang J. Potent and selective inhibition of plasma membrane monoamine transporter by HIV protease inhibitors. Drug Metabolism and Disposition. 2015;43:1773–1780. doi: 10.1124/dmd.115.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem. 2004;279:50042–50049. doi: 10.1074/jbc.M407913200. [DOI] [PubMed] [Google Scholar]
- Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol Biochem Be. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- Fernandez S, Gaspar P. Investigating anxiety and depressive-like phenotypes in genetic mouse models of serotonin depletion. Neuropharmacology. 2012;62:144–154. doi: 10.1016/j.neuropharm.2011.08.049. [DOI] [PubMed] [Google Scholar]
- Gasser PJ. Corticosterone-Sensitive Monoamine Transport in the Rat Dorsomedial Hypothalamus: Potential Role for Organic Cation Transporter 3 in Stress-Induced Modulation of Monoaminergic Neurotransmission. J Neurosci. 2006;26:8758–8766. doi: 10.1523/JNEUROSCI.0570-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch B, Bönisch H. Depression and antidepressants: Insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol Therapeut. 2011;129:352–368. doi: 10.1016/j.pharmthera.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Han T, Proctor W, Costales C, Cai H, Everett R, Thakker D. Four Cation-Selective Transporters Contribute to Apical Uptake and Accumulation of Metformin in Caco-2 Cell Monolayers. J Pharmacol Exp Ther. 2015;352:519–528. doi: 10.1124/jpet.114.220350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Li Q, Murphy D, Gold E, Crawley J. Abnormal anxiety‐related behavior in serotonin transporter null mutant mice: the influence of genetic background. Genes Brain Behav. 2003;2:365–380. doi: 10.1046/j.1601-1848.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- Horton RE, Apple DM, Owens WA, Baganz NL, Cano S, Mitchell NC, Vitela M, Gould GG, Koek W, Daws LC. Decynium-22 enhances SSRI-induced antidepressant-like effects in mice: Uncovering novel targets to treat depression. J Neurosci. 2013;33:10534–10543. doi: 10.1523/JNEUROSCI.5687-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T, Zha W, Duan H, Wang J. Live tissue imaging of organic cation and anion transport at the blood-CSF barrier reveals specific transporter function and distinct transcellular pathways. The FASEB Journal. 2017;31:1062.14–1062.14. [Google Scholar]
- Itagaki S, Ganapathy V, Ho HTB, Zhou M, Babu E, Wang J. Electrophysiological characterization of the polyspecific organic cation transporter plasma membrane monoamine transporter. Drug Metabolism and Disposition. 2012;40:1138–1143. doi: 10.1124/dmd.111.042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Mol CA, Buitelaar M, Koepsell H, Smit JW, Schinkel AH. Reduced hepatic uptake and intestinal excretion of organic cations in mice with a targeted disruption of the organic cation transporter 1 (Oct1 [Slc22a1]) gene. Mol Cell Biol. 2001;21:5471–5477. doi: 10.1128/MCB.21.16.5471-5477.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker JW, Wagenaar E, Van Eijl S, Schinkel AH. Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol. 2003;23:7902–7908. doi: 10.1128/MCB.23.21.7902-7908.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, France C, Javors M. Morphine-induced motor stimulation, motor incoordination, and hypothermia in adolescent and adult mice. Psychopharmacology. 2012;219:1027–1037. doi: 10.1007/s00213-011-2432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koek W, Sandoval T, Daws L. Effects of the antidepressants desipramine and fluvoxamine on latency to immobility and duration of immobility in the forced swim test in adult male C57BL/6J mice. 2017;1 doi: 10.1097/FBP.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- Kumar GN, Dykstra J, Roberts EM, Jayanti VK, Hickman D, Uchic J, Yao Y, Surber B, Thomas S, Granneman GR. Potent inhibition of the cytochrome P-450 3A-mediated human liver microsomal metabolism of a novel HIV protease inhibitor by ritonavir: A positive drug-drug interaction. Drug Metab Dispos. 1999;27:902–908. [PubMed] [Google Scholar]
- de la Mora MP, Gallegos-Cari A, Arizmendi-García Y, Marcellino D, Fuxe K. Role of dopamine receptor mechanisms in the amygdaloid modulation of fear and anxiety: Structural and functional analysis. Prog Neurobiol. 2010;90:198–216. doi: 10.1016/j.pneurobio.2009.10.010. [DOI] [PubMed] [Google Scholar]
- Lee C, Rodgers RJ. Antinociceptive effects of elevated plus-maze exposure: influence of opiate receptor manipulations. Psychopharmacology. 1990;102:507–513. doi: 10.1007/BF02247133. [DOI] [PubMed] [Google Scholar]
- Lira A, Zhou M, Castanon N, Ansorge M, Gordon J, Francis J, Bradley-Moore M, Lira J, Underwood M, Arango V, Kung H, Hofer M, Hen R, Gingrich J. Altered depression-related behaviors and functional changes in the dorsal raphe nucleus of serotonin transporter-deficient mice. Biol Psychiat. 2003;54:960–971. doi: 10.1016/s0006-3223(03)00696-6. [DOI] [PubMed] [Google Scholar]
- Liu S, Molino BF. Chapter 2 Recent Developments in Monoamine Reuptake Inhibitors, Annual Reports in Medicinal Chemistry, Annual Reports in Medicinal Chemistry. Elsevier; 2007. [Google Scholar]
- McIlwain K, Merriweather M, Yuva-Paylor L, Paylor R. The use of behavioral test batteries: Effects of training history. Physiol Behav. 2001;73:705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- Mimura Y, Yasujima T, Ohta K, Inoue K, Yuasa H. Functional identification of plasma membrane monoamine transporter (PMAT/SLC29A4) as an atenolol transporter sensitive to flavonoids contained in apple juice. J Pharm Sci. 2017;106:2592–2598. doi: 10.1016/j.xphs.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Miura Y, Yoshikawa T, Naganuma F, Nakamura T, Iida T, Kárpáti A, Matsuzawa T, Mogi A, Harada R, Yanai K. Characterization of murine polyspecific monoamine transporters. Febs Open Bio. 2017;7:237–248. doi: 10.1002/2211-5463.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morón JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley R, Cidlowski J. The biology of the glucocorticoid receptor: New signaling mechanisms in health and disease. J Allergy Clin Immun. 2013;132:1033–1044. doi: 10.1016/j.jaci.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura T, Kato S, Takano Y, Sato T, Yamashita A, Morimoto R, Ohtsuki S, Terasaki T, Deguchi Y. Functional characterization of rat plasma membrane monoamine transporter in the blood–brain and blood–cerebrospinal fluid barriers. J Pharm Sci. 2011;100:3924–3938. doi: 10.1002/jps.22594. [DOI] [PubMed] [Google Scholar]
- Olivier JDA, Van Der Hart MGC, Van Swelm RPL, Dederen PJ, Homberg JR, Cremers T, Deen PMT, Cuppen E, Cools AR, Ellenbroek BA. A study in male and female 5-HT transporter knockout rats: an animal model for anxiety and depression disorders. Neuroscience. 2008;152:573–584. doi: 10.1016/j.neuroscience.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Patel M, Mandava N, Gokulgandhi M, Pal D, Mitra A. Amino acid prodrugs: An approach to improve the absorption of HIV-1 protease inhibitor, lopinavir. Pharm. 2014;7:433–452. doi: 10.3390/ph7040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perona M, Waters S, Hall F, Sora I, Lesch KP, Murphy D, Caron M, Uhl G. Animal models of depression in dopamine, serotonin, and norepinephrine transporter knockout mice: prominent effects of dopamine transporter deletions. Behav Pharmacol. 2008;19:566. doi: 10.1097/FBP.0b013e32830cd80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistell P, Gupta S, Knight A, Domingue M, Uranga R, Ingram D, Kheterpal I, Ruiz C, Keller J, Bruce-Keller A. Metabolic and neurologic consequences of chronic lopinavir/ritonavir administration to C57BL/6 mice. Antivir Res. 2010;88:334–342. doi: 10.1016/j.antiviral.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogorelov V, Rodriguiz R, Insco M, Caron M, Wetsel W. Novelty Seeking and Stereotypic Activation of Behavior in Mice with Disruption of the Dat1 Gene. Neuropsychopharmacol. 2005;30:1300724. doi: 10.1038/sj.npp.1300724. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJ. Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Be. 1995;52:297–303. doi: 10.1016/0091-3057(95)00138-m. [DOI] [PubMed] [Google Scholar]
- Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165:1260–1287. doi: 10.1111/j.1476-5381.2011.01724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo S, Nestler E. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, Mössner R, Gossmann A, Fischer IG, Gorboulev V, Murphy DL, Koepsell H, Lesch KP. Organic cation transporter capable of transporting serotonin is up‐regulated in serotonin transporter‐deficient mice. J Neurosci Res. 2003;71:701–709. doi: 10.1002/jnr.10521. [DOI] [PubMed] [Google Scholar]
- Schömig E, Babin-Ebell J, Russ H. 1,1“-diethyl-2,2-”cyanine (decynium22) potently inhibits the renal transport of organic cations. Naunyn-schmiedeberg’s Archives Pharmacol. 1993;347:379–383. doi: 10.1007/BF00165387. [DOI] [PubMed] [Google Scholar]
- Shen HWW, Hagino Y, Kobayashi H, Shinohara-Tanaka K, Ikeda K, Yamamoto H, Yamamoto T, Lesch KPP, Murphy DL, Hall FS, Uhl GR, Sora I. Regional differences in extracellular dopamine and serotonin assessed by in vivo microdialysis in mice lacking dopamine and/or serotonin transporters. Neuropsychopharmacol. 2004;29:1790–1799. doi: 10.1038/sj.npp.1300476. [DOI] [PubMed] [Google Scholar]
- Shirasaka Y, Lee N, Duan H, Ho H, Pak J, Wang J. Interspecies comparison of the functional characteristics of plasma membrane monoamine transporter (PMAT) between human, rat and mouse. J Chem Neuroanat. 2017;83–84:99–106. doi: 10.1016/j.jchemneu.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solich J, Faron-Gorecka A, Kusmider M, Palach P, Gaska M, Dziedzicka-Wasylewska M. Norepinephrine transporter (NET) knock-out upregulates dopamine and serotonin transporters in the mouse brain. Neurochem Int. 2011;59:185–191. doi: 10.1016/j.neuint.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui T, Nakazawa A, Okura T, Deguchi Y, Akanuma SII, Kubo Y, Hosoya KII. Histamine elimination from the cerebrospinal fluid across the blood-cerebrospinal fluid barrier: involvement of plasma membrane monoamine transporter (PMAT/SLC29A4) J Neurochem. 2016;139:408–418. doi: 10.1111/jnc.13758. [DOI] [PubMed] [Google Scholar]
- Vialou V, Balasse L, Callebert J, Launay JM, Giros B, Gautron S. Altered aminergic neurotransmission in the brain of organic cation transporter 3-deficient mice. J Neurochem. 2008;106:1471–1482. doi: 10.1111/j.1471-4159.2008.05506.x. [DOI] [PubMed] [Google Scholar]
- Wagner DJ, Hu T, Wang J. Polyspecific organic cation transporters and their impact on drug intracellular levels and pharmacodynamics. Pharmacol Res. 2016;111:237–246. doi: 10.1016/j.phrs.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Tzavara E, Davis R, Nomikos G, McIntosh J, Giros B, Martres MP. Functional alterations of nicotinic neurotransmission in dopamine transporter knock-out mice. Neuropharmacology. 2007;52:1496–1508. doi: 10.1016/j.neuropharm.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Wellman C, Izquierdo A, Garrett J, Martin K, Carroll J, Millstein R, Lesch KP, Murphy D, Holmes A. Impaired Stress-Coping and Fear Extinction and Abnormal Corticolimbic Morphology in Serotonin Transporter Knock-Out Mice. J Neurosci. 2007;27:684–691. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KC, Lu YH, Peng YH, Hsu LC, Lin CJ. Effects of lipopolysaccharide on the expression of plasma membrane monoamine transporter (PMAT) at the blood-brain barrier and its implications to the transport of neurotoxins. J Neurochem. 2015;135:1178–1188. doi: 10.1111/jnc.13363. [DOI] [PubMed] [Google Scholar]
- Wu X, Kekuda R, Huang W, Fei YJ, Leibach FH, Chen J, Conway SJ, Ganapathy V. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem. 1998;273:32776–32786. doi: 10.1074/jbc.273.49.32776. [DOI] [PubMed] [Google Scholar]
- Wultsch T, Grimberg G, Schmitt A, Painsipp E, Wetzstein H, Breitenkamp AFS, Gründemann D, Schömig E, Lesch KP, Gerlach M, Reif A. Decreased anxiety in mice lacking the organic cation transporter 3. J Neural Transm (Vienna) 2009;116:689–697. doi: 10.1007/s00702-009-0205-1. [DOI] [PubMed] [Google Scholar]
- Zhou F, Lesch KP, Murphy D. Serotonin uptake into dopamine neurons via dopamine transporters: a compensatory alternative. Brain Res. 2002;942:109–119. doi: 10.1016/s0006-8993(02)02709-9. [DOI] [PubMed] [Google Scholar]
- Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos. 2007;35:1956–1962. doi: 10.1124/dmd.107.015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Xia L, Engel K, Wang J. Molecular determinants of substrate selectivity of a novel organic cation transporter (PMAT) in the SLC29 family. J Biol Chem. 2007;282:3188–3195. doi: 10.1074/jbc.M609421200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Verhaagh S, Buitelaar M, Popp-Snijders C, Barlow DP. Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Mol Cell Biol. 2001;21:4188–4196. doi: 10.1128/MCB.21.13.4188-4196.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel L, Fadok J, Argilli E, Garelick M, Jones G, Dickerson T, Allen J, Mizumori S, Bonci A, Palmiter R. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 2011;14:620–626. doi: 10.1038/nn.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


