Abstract
Objectives
Avascular necrosis (AVN) is associated with significant morbidity potentially causing severe pain and debility; patients with inflammatory bowel diseases (IBD) have a higher prevalence of AVN compared to non-IBD populations. The purpose of our study was to determine the prevalence of AVN in our IBD population and to evaluate these subjects for the presence of clinical characteristics associated with AVN on CT imaging.
Methods
In 1313 IBD patients with abdomen/pelvis CT scans we identified 27 patients (2.1%) with CT findings consistent with AVN. Through historical chart review we confirmed that most patients had prior exposure to steroids, although 2 patients had no documented steroid exposure at all.
Results
We found that 59% of the concurrent radiology reports did not comment on the presence of AVN, suggesting that incidental CT findings of AVN among IBD patients is likely under-reported. Notably, we found that 63% of these cases had documented complaints of low back and/or hip pain. Using logistic regression, we found an association between ANCA positive status across IBD (p=0.007) and a smoking history in CD (p=0.03) with the presence of AVN.
Conclusions
In conclusion, we found that a significant proportion of IBD patients with AVN are reported in their records as not having hip or low back pain, and review of CT imaging under dedicated bone windows may identify AVN among this population. Our findings also suggest that additional etiological factors, beyond corticosteroids, contribute to the development of AVN in IBD. Further investigation is warranted regarding the mechanisms associated with AVN in IBD.
Keywords: Avascular necrosis, inflammatory bowel disease
Background and Aims
Avascular necrosis (or osteonecrosis) is a condition of bone destruction through infarction of its cellular components that is most commonly but not exclusively observed in the femoral head.(1) The presumed mechanism is an abrupt loss of blood supply as a result of vascular compromise commonly associated with an underlying disease including a variety of factors including hematologic disorders, hemoglobinopathies, and systemic lupus erythematosus (SLE).
Avascular necrosis (AVN) has also been associated with high dose corticosteroid disease management as this intervention is also believed to contribute to bone destruction.(2,3) It is estimated that AVN develops in 9–40% of patients receiving long-term corticosteroid therapy and this risk appears to be increased with higher doses and prolonged courses.(4–6) The adverse impact of chronic corticosteroid utilization on bone are multifactorial; there are direct effects on osteoclasts and osteoblasts and apoptosis of osteoblasts are accompanied by prolongation of the lifespan of osteoclasts. Further, increased apoptosis of osteocytes also occurs with concomitant decreases of bone strength.(7) Patients with inflammatory bowel diseases (IBD) can be exposed to prolonged and/or high doses of corticosteroids, and this relationship has been suggested as the cause of a low but increased incidence of AVN in this patient population.(2) However, as observed with SLE, it has been suggested that IBD itself may also predispose to osteonecrosis with or without concomitant use of corticosteroid therapies.(8)
In a systematic review of CT scans by an experienced, musculoskeletal fellowship-trained radiologist (TJL) that had been previously obtained for gastrointestinal clinical indications in patients with IBD, we observed a cohort of patients with CT evidence of AVN of the hip. This finding enabled us to identify the prevalence of AVN in our IBD study population as well as to evaluate IBD-related risk factors, steroid exposure, and whether CT reports detected AVN on initial reads. We also observed whether or not there was a clinical correlation between the radiologic findings and the patient’s signs and symptoms. The results of these assessments are reported herein.
Methods
The MIRIAD (Mucosal Immunology Repository for Inflammatory and Digestive Diseases) biorepository at the IBD Center at Cedars-Sinai Medical Center houses bio-specimens from IBD subjects and contains demographic, longitudinal clinical, serological, and genetic data generated from these subjects. Using the database, we identified 3176 patients with IBD and available genomic data (GWAS).(9,10) Among this cohort, 1313 patients with CT scans of the abdomen and pelvis that were obtained for IBD-related clinical indications were available for re-review by a fellowship-trained musculoskeletal radiologist. From these 1313 subjects, 27 patients were identified as having clear cut CT findings consistent with AVN. We used standard CT descriptive criteria for AVN; these criteria were applied to axial CT scans, that were also reformatted into coronal and sagittal images. Imaging criteria include loss of normal trabecular pattern in the femoral head, sclerosis of femoral head, crescentic subchondral lucency, geographic lesion of the femoral head with serpentine or crescentic border, and collapse of superior femoral head surface. Some of the cases had confirmation from magnetic resonance imaging (MRI), which is a more sensitive modality to evaluate for presence of AVN.
The recruitment of study subjects is described in Figure 1. In cases where multiple CT scans were available on a single subject, the most recent CT scans were reviewed. This study was approved as exempt by the institutional review board at Cedars-Sinai Medical Center. Historical chart review was performed to obtain demographic and clinical data for this subset of IBD patients with AVN including age, gender, type of IBD, disease duration and extent, data on steroid use, and documentation in the medical records of hip or low back pain.
Figure 1.
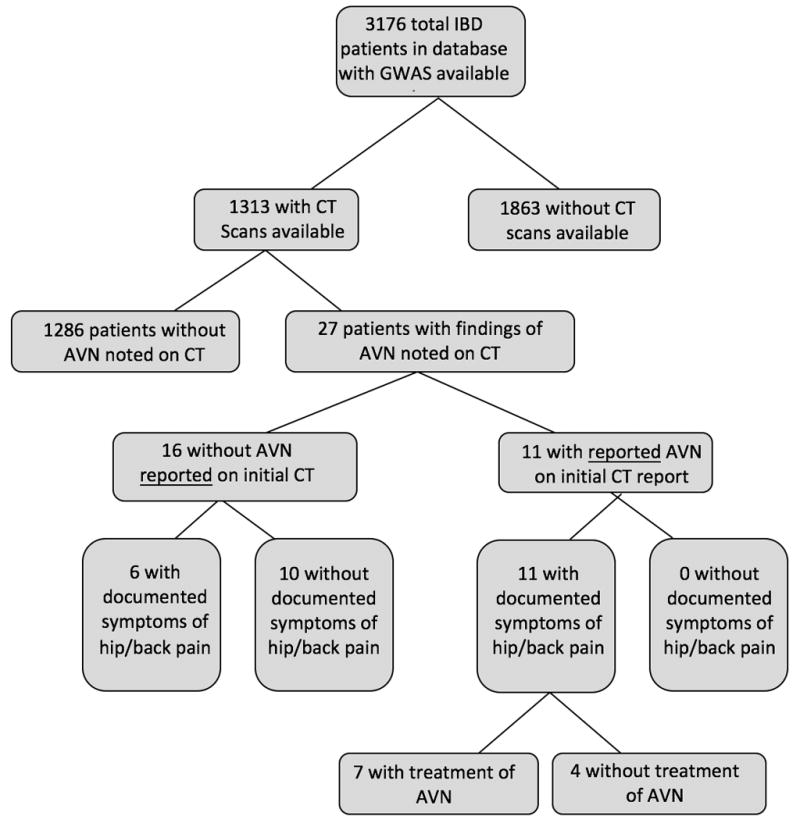
Study Subject Flow Chart
Logistic regression was used to examine the association of AVN with serologic markers, IBD clinical phenotypes, as well as smoking behaviors. Current age and gender were included as covariates to control for potential confounding. P <0.05 was considered to be statistically significant.
Results
Prevalence of AVN
Twenty-seven of 1313 IBD patients (~2.1%) were found to have CT evidence of AVN (Figure 1). Of these 27 patients, 16 did not have AVN reported on the initial CT read. Within this sub-group of 16, 6 patients had documented complaints of hip or low back pain, while 10 did not. Among the 11 patients with AVN reported on the initial CT read, all had documented symptoms of hip or low back pain, while 7 had undergone treatment of AVN. None of the 16 patients that did not have a report AVN on the initial CT read had undergone treatment of AVN.
Characteristics of the Study Population
Table 1 provides demographic characteristics of these 27 patients. The majority had Crohn’s disease (70%), and were male (63%). The mean age at time of diagnosis of IBD among our cohort with AVN was 29 years old (range 11 to 68 years old). Among those with Crohn’s, most had more severe disease (68% with either stricturing and/or internal penetrating disease (B2 or B3 according to Montreal Classification) and the majority had ileo-colonic disease (58%) (L3).(11) Among the 8 patients with Ulcerative Colitis or inflammatory bowel disease unclassified (IBDU) all but one had pan-colonic disease (Table 1).
Table 1.
IBD Characteristics
| Characteristics | n (%) |
|---|---|
| Male | 17 (63) |
| Mean Age at IBD Diagnosis (years) | 29 (11-68) |
| IBD Disease Duration at time of CT (years) | 13 (0.5-49) |
| Crohn’s Disease | 19 (70) |
| Location* | |
| L1 (ileal disease) | 3 (16) |
| L2 (colonic) | 5 (26) |
| L3 (ileocolonic) | 11 (58) |
| L4 (upper GI involvement) | 3 (16) |
| Behavior* | |
| B1 (non-stricturing, non-penetrating) | 6 (32) |
| B2 (stricturing disease) | 5 (26) |
| B3 (penetrating disease) | 8 (42) |
| Ulcerative Colitis/IBDU | 8 (30) |
| E2 (Left sided UC) | 1 (13) |
| E3 (extensive UC or pancolitis) | 7 (88) |
AVN and Steroid exposure
We defined high steroid exposure as being exposed to 20mg of prednisone (or equivalent formulation) daily for at least 6 months, as previously described.(12–14) While most AVN patients had documentation of prior exposure to some level of steroids (93%), less than half had been on high doses of steroids by our definition and 2 (7%) had no documented steroid exposure at all.
Radiologic reporting and symptoms suggestive of AVN
In our population only 11 (41%) of the initial radiology reports commented on the presence of AVN. The remaining 59% were identified retrospectively by musculoskeletal radiologist review, with emphasis on use of bone windows. An example of the detection of AVN that is more prominent on bone windows but not on soft tissue windows is demonstrated in Figure 2. 63% of the IBD cases with AVN had documented complaints of low back pain and/or hip pain in the record. However, because these studies were referred for abdominal pelvic pathology, these symptoms were unlikely to have been communicated with the radiologist. Notably, of those with negative initial CT reports, only 6 (38%) had documented complaints of hip/low back pain.
Figure 2.
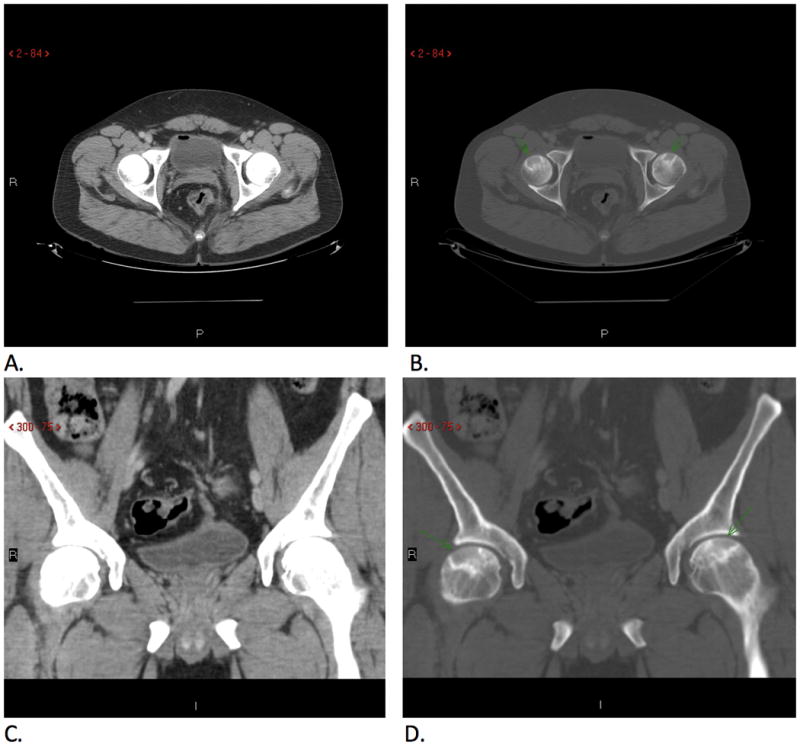
Images Comparing Bone Windows and Soft Tissue Windows for Detecting AVN
Axial (A, B) and coronal images (C, D) demonstrate bilateral femoral head bone infarcts (arrows) seen on bone windows (B, D) but not on soft tissue windows (A, C).
Association between demographic, clinical, and serological characteristics and AVN
Logistic regression was performed to assess demographic and clinical parameters associated with AVN in our patients when compared with the 1286 AVN-negative patients confirmed by CT scan. In CD, smokers were more likely to develop AVN than non-smokers (OR = 2.9, p = 0.03). Using the Montreal classification, we observed no differences between the presence of AVN regarding disease behavior and disease location in either CD or UC. We did observe an association between ANCA positive status and AVN (OR = 3.05, p = 0.007) in all IBD patients. We also performed a multivariate analysis with all the variables with P <0.05 in univariate analysis included in a joint model. Similar results were observed (data not shown).
Conclusions
AVN is a diagnosis that carries significant morbidity, potentially causing severe pain and debility. It is known that patients with IBD have a higher prevalence of AVN compared to the general population, however little is known about the risk factors associated with the development of AVN, the true prevalence among patients with IBD, nor measures to prevent susceptibility or progression of this disease.(2) We have confirmed that the prevalence of AVN is higher in IBD populations compared to non-IBD patients with AVN found in approximately 1 in 50 IBD subjects in our study. This is consistent with prior literature that demonstrates a prevalence of 0.5-4.3% among patients with IBD, compared to the <0.1% in populations without immune-mediated diseases or steroid use.(2,15)
Our study has several novel and/or important findings. We found a significant proportion of IBD patients that are asymptomatic from hip or low back pain having CT evidence of AVN. We found that by re-examining CT images we could detect findings of AVN, suggesting that careful evaluation of bone windows would allow for improved sensitivity and detection of MSK disease, not necessarily limited to patients with reported hip or low back pain. Further, and consistent with prior literature, our data suggests there are other factors that contribute to development of AVN beyond steroid exposure in IBD patients. Lastly, by utilizing existing serological and demographic data we found an association between both ANCA positive status across IBD and a smoking history in CD, and the presence of AVN within our population.
Our study was notable for the finding of patients with CT evidence of AVN despite the lack of documented symptoms of low back or hip pain. Lack of symptomatology from AVN has been assessed in other disease entities including patients with systemic lupus erythematous (SLE) and in patients that have undergone organ transplantation, where significant proportions of these patients have been found to have CT findings of AVN, without symptoms of hip pain.(16–18) These findings and our results suggest occult disease exists among asymptomatic, high-risk patient populations.
This is the first study to our knowledge that examines the prevalence of incidental findings of AVN among patients with IBD. We found that among IBD patients with CT evidence of AVN, many had radiologic reports that did not comment on the presence of AVN. One study which reviewed prior CT enterography images of 357 patients with IBD identified 1 patient with undocumented AVN.(19) This study together with our findings suggests that incidental radiologic findings of AVN among IBD patients is likely under-reported. This is an area of concern, given the severe debility, morbidity and pain associated with progression of AVN. This is especially pertinent among patients with IBD, as previous literature on IBD patients with AVN may have higher revision and complication rates following hip arthroplasty compared to non-IBD patients.(20) Early recognition of AVN may allow for early interventions including use of arthroscopic injections, bone grafts, arthroplasty, utilization of stem cells, or surgical interventions to potentially prevent later complications of untreated AVN and to prevent progression of this disease. In addition, knowledge of underlying AVN may affect treatment decisions by gastroenterologists, including decreased use of corticosteroids. While abdominal CT scans are most often obtained to evaluate for acute abdominal pathology, it may be beneficial to review imaging using bone window settings with an MSK radiologist in patients with IBD, whether they report symptoms of hip/low back pain or not.
Consistent with prior literature, the increased prevalence of AVN among patients with IBD is not adequately explained by steroid exposure.(2,21–23) Our study suggests that less than half of our cohort had documented exposure to high dose steroids and 2 subjects had no steroid exposure at all. In addition, it has also been demonstrated that IBD patients are susceptible to osteonecrosis at lower steroid doses than non-IBD patients.(8) A series cohort study by Klingenstein et al in 2005 examined cumulative steroid doses among IBD patients who developed AVN, and were unable to establish a threshold level of corticosteroids associated with the development of osteonecrosis, in keeping with our results.(2)
While steroid exposure is unlikely to be the sole etiologic factor, the exact mechanism responsible for AVN associations with IBD are not known. Inherent IBD-associated coagulopathy, small-vessel vasculitis, and immunologic mechanisms (bone formation and modeling may be suppressed by the presence of inflammatory cytokines including IL-1 and TNF-alpha) have all been suggested as etiological factors in the development of osteonecrosis in IBD.(2,21) While the focus of our study did not include evaluation of these factors, our findings of AVN in patients without exposure to high-dose steroids emphasizes the need for further research into these mechanisms. Finally, our findings of an association between smoking history in CD as well as ANCA positive disease and the presence of AVN, point to the possibility of disease severity as an etiologic factor; however, our numbers were too small to draw any firm conclusions. Furthermore, both the smoking and ANCA associations also potentially implicate a vasculitis contribution but additional work in this under-researched phenomenon will be necessary to confirm this. Our study is observational in nature, limited by the case selection and convenient sample of patients studied, and our imaging findings were read and confirmed by only one (yet highly skilled and well-published) musculoskeletal radiologist.
In conclusion, our study demonstrated that approximately 1 in 50 IBD subjects may be at risk of avascular necrosis, and that a significant proportion of findings of AVN are not reported on routine CT scan radiologist reads. The majority of patients with incidental findings of AVN had reported hip/low back pain to their gastroenterologist, and sharing these symptoms with radiologists – or reviewing imaging with MSK radiologists – offer an opportunity to detect early AVN. Further investigation is warranted regarding the etio-pathogenetic mechanisms predisposing this population to AVN, and to determine preventative strategies in patients with IBD to avoid the potentially debilitating and painful outcomes of this complication.
Table 2.
AVN Characteristics
| Characteristics | n (%) |
|---|---|
| Cumulative Steroid Exposure | |
| None | 2 (7) |
| Low* | 13 (48) |
| High** | 12 (44) |
| AVN noted on initial radiology read | 11 (41) |
| Documented Symptoms of hip/back pain at or prior to initial read | 17 (63) |
| Reported symptoms with negative initial CT read | 6 (38) |
Low: less than 20mg daily of prednisone (or equivalent formulation and less than 6 months consecutively.
High: at least 20mg of prednisone (or equivalent formulation) daily for at least 6 months
Acknowledgments
The MIRIAD IBD Biobank is supported by the F. Widjaja Foundation Inflammatory Bowel and Immunobiology Research Institute, NIH/NIDDK grants P01DK046763, DK062413, and U54 DK102557, and The Leona M. and Harry B. Helmsley Charitable
Abbreviations
- AVN
avascular necrosis
- IBD
inflammatory bowel disease
- CT
computed tomography
- MSK
musculoskeletal radiologist
Footnotes
Conflict of Interest: none
Author Contributions:
Vineet S Rolston M.D.: Data collection, literature search, data interpretation, writing, figures
Anish V. Patel M.D.: Data interpretation, literature search, writing
Thomas J. Learch M.D.: Data collection, imaging review
Dalin Li PhD: Data analysis, writing
Dmitry Karayev M.D.: study design, data collection
Chadwick Williams M.D.: Study design
Madhavi L. Siddanthi M.D.: data collection, study design
Stephan R. Targan: study design, data interpretation
Michael H. Weisman M.D.: study design, data analysis, writing
Dermot P.B. McGovern M.D. Ph.D.: study design, data analysis, data interpretation, writing
References
- 1.Mont MA, Hungerford DS. Non-traumatic avascular necrosis of the femoral head. J Bone Joint Surg Am. 1995;77:459–74. doi: 10.2106/00004623-199503000-00018. [DOI] [PubMed] [Google Scholar]
- 2.Klingenstein G, Levy RN, Kornbluth A, Shah AK, Present DH. Inflammatory bowel disease related osteonecrosis: report of a large series with a review of the literature. Aliment Pharmacol Ther. 2005;21:243–9. doi: 10.1111/j.1365-2036.2005.02231.x. [DOI] [PubMed] [Google Scholar]
- 3.Cruess RL. Steroid-induced osteonecrosis: a review. Can J Surg J Can Chir. 1981;24:567–71. [PubMed] [Google Scholar]
- 4.Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. N Engl J Med. 2011;365:62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 5.Felson DT, Anderson JJ. Across-study evaluation of association between steroid dose and bolus steroids and avascular necrosis of bone. Lancet Lond Engl. 1987;1:902–6. doi: 10.1016/s0140-6736(87)92870-4. [DOI] [PubMed] [Google Scholar]
- 6.McAvoy S, Baker KS, Mulrooney D, Blaes A, Arora M, Burns LJ, et al. Corticosteroid dose as a risk factor for avascular necrosis of the bone after hematopoietic cell transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant. 2010;16:1231–6. doi: 10.1016/j.bbmt.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol Metab Clin North Am. 2012;41:595–611. doi: 10.1016/j.ecl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vakil N, Sparberg M. Steroid-related osteonecrosis in inflammatory bowel disease. Gastroenterology. 1989;96:62–7. doi: 10.1016/0016-5085(89)90764-6. [DOI] [PubMed] [Google Scholar]
- 9.McGovern DPB, Jones MR, Taylor KD, Marciante K, Yan X, Dubinsky M, et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn’s disease. Hum Mol Genet. 2010;19:3468–76. doi: 10.1093/hmg/ddq248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGovern DPB, Gardet A, Törkvist L, Goyette P, Essers J, Taylor KD, et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet. 2010;42:332–7. doi: 10.1038/ng.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spekhorst LM, Visschedijk MC, Alberts R, Festen EA, van der Wouden E-J, Dijkstra G, et al. Performance of the Montreal classification for inflammatory bowel diseases. World J Gastroenterol. 2014;20:15374–81. doi: 10.3748/wjg.v20.i41.15374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aaron RK, Voisinet A, Racine J, Ali Y, Feller ER. Corticosteroid-associated avascular necrosis: dose relationships and early diagnosis. Ann N Y Acad Sci. 2011;1240:38–46. doi: 10.1111/j.1749-6632.2011.06218.x. [DOI] [PubMed] [Google Scholar]
- 13.Atsumi T, Kuroki Y. Role of impairment of blood supply of the femoral head in the pathogenesis of idiopathic osteonecrosis. Clin Orthop. 1992:22–30. [PubMed] [Google Scholar]
- 14.Bradbury G, Benjamin J, Thompson J, Klees E, Copeland J. Avascular necrosis of bone after cardiac transplantation. Prevalence and relationship to administration and dosage of steroids. J Bone Joint Surg Am. 1994;76:1385–8. doi: 10.2106/00004623-199409000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Dilisio MF. Osteonecrosis following short-term, low-dose oral corticosteroids: a population-based study of 24 million patients. Orthopedics. 2014;37:e631–636. doi: 10.3928/01477447-20140626-54. [DOI] [PubMed] [Google Scholar]
- 16.Caramaschi P, Biasi D, Dal Forno I, Adami S. Osteonecrosis in systemic lupus erythematosus: an early, frequent, and not always symptomatic complication. Autoimmune Dis. 2012;2012:725249. doi: 10.1155/2012/725249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klippel JH, Gerber LH, Pollak L, Decker JL. Avascular necrosis in systemic lupus erythematosus: Silent symmetric osteonecroses. Am J Med. 1979;67:83–7. doi: 10.1016/0002-9343(79)90077-9. [DOI] [PubMed] [Google Scholar]
- 18.Tervonen O, Mueller DM, Matteson EL, Velosa JA, Ginsburg WW, Ehman RL. Clinically occult avascular necrosis of the hip: prevalence in an asymptomatic population at risk. Radiology. 1992;182:845–7. doi: 10.1148/radiology.182.3.1535906. [DOI] [PubMed] [Google Scholar]
- 19.Bruining DH, Siddiki HA, Fletcher JG, Tremaine WJ, Sandborn WJ, Loftus EV. Prevalence of penetrating disease and extraintestinal manifestations of Crohn’s disease detected with CT enterography. Inflamm Bowel Dis. 2008;14:1701–6. doi: 10.1002/ibd.20529. [DOI] [PubMed] [Google Scholar]
- 20.Kapadia BH, Issa K, Nagrare N, Pivec R, Banerjee S, Mont MA. Higher revision and complication rates following total hip arthroplasty in patients with inflammatory bowel disease. J Arthroplasty. 2014;29:596–600. doi: 10.1016/j.arth.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Khan A, Illiffe G, Houston DS, Bernstein CN. Osteonecrosis in a patient with Crohn’s disease unrelated to corticosteroid use. Can J Gastroenterol J Can Gastroenterol. 2001;15:765–8. doi: 10.1155/2001/293059. [DOI] [PubMed] [Google Scholar]
- 22.Freeman HJ. Osteomyelitis and osteonecrosis in inflammatory bowel disease. Can J Gastroenterol J Can Gastroenterol. 1997;11:601–6. doi: 10.1155/1997/953252. [DOI] [PubMed] [Google Scholar]
- 23.Freeman HJ, Freeman KJ. Prevalence rates and an evaluation of reported risk factors for osteonecrosis (avascular necrosis) in Crohn’s disease. Can J Gastroenterol J Can Gastroenterol. 2000;14:138–43. doi: 10.1155/2000/958086. [DOI] [PubMed] [Google Scholar]


