Abstract
Burn patients who consumed alcohol prior to injury have worse clinical outcomes, including longer hospital stays, increased ventilator days, and more respiratory infections. Most alcohol consumers are binge drinkers and not chronic alcoholics and binge drinking patterns fluctuate over the week, with consecutive days of drinking over the weekend followed by relative abstinence during the week. We utilized a murine model simulating this drinking pattern in the context of burn injury. Mice were given ethanol for 3 days, rested for 4 days, given ethanol for 3 more days, followed by a sham or 15% total body surface area full-thickness burn. We previously demonstrated that mice exposed to the combined insult exhibited respiratory dysfunction and 50% mortality, with those that succumbed to injury dying between 24 and 72 hours, thus identifying a therapeutic intervention window. Our goal herein is to characterize inflammatory and respiratory parameters during this critical time frame. We saw that mice exposed to the combined insult had the highest circulating and pulmonary cytokine levels at 24 hours, which were normalized by 72 hours in survivors. Alveolar macrophage activation was observed at 24 hours in burned mice, regardless of intoxication (p<0.05). However, at 72 hours, alveolar macrophages from intoxicated burned mice had elevated CD206, relative to controls (p<0.05), indicative of an anti-inflammatory phenotype. Taken together, these findings suggest that while lung function and inflammation are normalized by 72 hours, the alterations in alveolar macrophage phenotype shed light on a potential mechanism underlying increased infection susceptibility in intoxicated burn patients.
Keywords: Cytokines, injury, neutrophils, alcohol, alternative activation, resolution
Introduction
In the United States, an estimated 485,000 people have burn injuries warranting medical attention, annually, with approximately 10% requiring hospital admission (1) Severe burn is arguably the most detrimental injury one can sustain, as profound physiological derangements extend beyond the cutaneous injury itself and often times lead to multiple organ failure (MOF) and an increased risk for infection and sepsis (2). Nearly half of all burn patients living in the US have a positive blood alcohol concentration (BAC) level at the time of hospital admission (3) Burn injury with recent alcohol consumption is associated with worsened adverse outcomes, including increased risk for nosocomial pneumonia infections, three times longer days of hospital admission, twice as many days requiring mechanical ventilation, and an increased rate of mortality, compared to patients who were not intoxicated prior to injury (4–6). Respiratory failure, acute respiratory distress syndrome (ARDS), and pulmonary infection are leading causes of post-burn morbidity and mortality (7). Clinical and experimental evidence provides clear links connecting excessive and prolonged lung inflammation and oxidative stress after severe burn with ARDS and MOF, although the contributing pathophysiologic mechanisms remain largely undefined (8, 9).
Almost immediately after burn injury, local and systemic release of pro-inflammatory molecules, referred to the “cytokine storm,” causes damage to the delicate alveolar architecture, which is compounded by excessive neutrophil infiltration and retention in the interstitium, and extracellular fluid accumulation, which is worsened when ethanol intoxication precedes injury (10, 11). The pulmonary inflammatory response to distal burn is characterized by the release of pro-inflammatory and chemotactic molecules, leukocyte infiltration, oxidative stress, and extracellular fluid accumulation, yet the underlying pathophysiological mechanisms remain largely undescribed, and even less is known regarding the mechanism(s) by which alcohol intoxication augments this response. Pre-clinical animal model studies demonstrated that when alcohol intoxication precedes burn, pulmonary inflammation is exacerbated and prolonged and lung congestion, characterized by neutrophil infiltration, alveolar wall thickening, and edema, is worsened (9, 12).
Binge alcohol consumption is the most common drinking pattern in the USA, with an estimated 38 million Americans engaging in this hazardous behavior (13). It is defined by either a BAC at or above 0.08% or by the amount of alcoholic beverages one consumes in a 2 hour time period (4 drinks or more for women, 5 or more for men), and is the drinking pattern the vast majority of trauma patients, including burn victims, engaged in prior to injury (3). Additionally, college student report a multiple day binge drinking pattern, with consecutive days of drinking over the weekend followed by relative abstinence during the week. (14). We previously demonstrated that mice subjected to our multi-day episodic binge ethanol treatment paradigm, modeling college student drinking behavior, had increased pulmonary congestion and neutrophil infiltration, elevated lung levels of neutrophil chemoattractants, and impaired respiratory function, when compared to burn injury alone (11). In addition, survival studies showed that multi-day ethanol treatment in the absence of injury was non-lethal, that mice exposed to multi-day binge ethanol prior to burn had 48% survival at 7-days post burn, whereas mice exposed to burn alone had 84% survival. We also observed that animals that succumbed to the combined insult died between 24 and 72 hours (11). Therefore, in this study we characterized this critical time frame by examining lung function and histology, measuring an array of immunomodulatory factors in both the blood and in lung tissue, quantifying pulmonary neutrophils, and characterizing alveolar macrophage phenotype. Our analyses suggest that multi-day binge ethanol consumption prior to burn worsens lung dysfunction, exacerbates circulating and pulmonary levels of pro-inflammatory molecules, and alters alveolar macrophage phenotype, when compared to burn injury or ethanol intoxication alone.
Material and Methods
Murine model of scald burn injury and multi-day binge ethanol intoxication
C57BL/6 male mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in sterile micro-isolator cages under pathogen-free conditions in the Loyola University Medical Center Comparative Medicine facility for a minimum of 1 week prior to experimentation. We utilized our well-established murine model of scald burn injury and episodic binge ethanol intoxication, as described (11). Briefly, 8–10 week-old male mice weighing 20–27g were injected intraperitoneally with ethanol (1.2 g/kg) or saline vehicle for 3 consecutive days, then unmanipulated for 4 days, and then injected with ethanol for an additional 3 consecutive days. This ethanol dosing strategy raises the blood alcohol concentration (BAC) to 150mg/dL, 30 minutes after injection. On the final day of treatment, 30 minutes after ethanol injection, mice were anesthetized (100 mg/kg ketamine and 10 mg/kg xylazine) (Webster Veterinary, Sterling, MA), dorsa were shaved, and then mice were subjected to a 15% total body surface area (TBSA) full thickness, insensate scald burn injury (15). This was achieved by placing each mouse in a plastic template that exposed the shaved dorsum to a 92–95°C (or room temperature for sham injury) water bath. All mice were treated with 1 ml of saline resuscitation fluid and cages were placed on warming pads during anesthesia recovery. Mice were euthanized using CO2 narcosis followed by exsanguination. Data presented herein are representative of two animal experiments, minimum. To avoid possible confounding factors caused by circadian rhythms, all animal experimentation was performed between 8 and 9 am. All protocols were approved by the Loyola University Chicago Institutional Animal Care and Use Committee.
Plethysmography
Lung function parameters were measured at 24, 48 and 72 hours using unrestrained whole body barometric plethysmography (Buxco Research Systems), as described (11). Briefly, mice were placed in the plethysmography chamber, allowed to acclimate, and then enhanced pause (Penh), minute volume (MVb), tidal volume (TVb), and breath frequency (f) measurements were recorded for 10 minutes, on a breath-by-breath basis. Two independent experiments were performed, n=3–13 per group.
Lung Histology
The upper right lobe was used for histopathologic analyses, as described (10). In summary, the lung lobe was gently inflated with 10% formalin, fixed overnight, paraffin-embedded, sectioned at 5μm, and then stained using hemoxylin and eosin (H&E). Stained images were photographed at 400x (EVOS; Thermo Fisher Scientific) and examined in a blinded fashion. Representative images from two independent animal experiments are shown, n = 3–6 per group.
Cytokine/Chemokine Quantification
The middle right lobe was flash frozen in liquid nitrogen, stored at −80ºC until use, and then homogenized in BioPlex cell lysis buffer (Bio-Rad, Hercules, CA), according to manufacturer guidelines. All samples were assayed in duplicate. Results were normalized to total protein, as determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA). In some instances, concentrations for cytokines/chemokines with low levels were extrapolated from the standard curve, though still within the BioPlex assay limit of detection. Experiments were performed a minimum of two times, n=12–22 per group, total. Data from one representative experiment is shown.
Enzymatic Lung Tissue Dissociations and FLOW cytometry
The upper left lung lobe was cut into small pieces, transferred to a C-tube (Miltenyi Biotec, Auburn, CA) containing digestion buffer (1mg/ml of Collagenase D and 0.1 mg/ml DNase I [Roche, Indianapolis, IN] in HBSS) and homogenized using a GentleMACS dissociator (Miltenyi Biotec), according to manufacturer guidelines. Single cell suspensions were obtained by passing homogenates through 70 um nylon cell strainers. Red blood cells were lysed in ACK lysis buffer (Life Technologies, Grand Island, NY) and remaining cells were counted using trypan blue exclusion of dead cells. Non-specific binding to the Fcy II/III receptor was prevented by incubating 1×106 lung cells with anti-CD16/32 (clone 93,eBioscience, San Diego, CA). Cells were immunostained with rat anti-mouse antibodies: CD45 e780 (clone30-F11, eBioscience), CD11b eFluor 450 (clone M1/70, eBioscience), Ly6G (Gr-1) PE Cy7-conjugated (clone RB6-8C5, eBioscience), CD206 PE, CD24 APC, CD64 PerCP, and MHC II v500. Antibody incubation was carried out for 30 minutes at 4°C. Cells were washed and fixed as described (16, 17). Flow experiments were performed using a BD Fortessa cytometer (BD Biosciences, San Jose, CA) and data analysed using Flow Jo FCS software (Tree Star Inc., Ashland, OR). Experiments were performed at minimum of two times, n=4–6 per group. Data from one representative experiment are shown.
Statistical Analyses
Statistical analyses comparing four treatment groups (sham vehicle, sham ethanol, burn vehicle, burn ethanol) were performed using One-way analysis of variance (ANOVA) with a Tukey’s multiple comparison test. Significant differences are reported when p<0.05. Data are presented as mean ± standard error of the mean (SEM) and graphs were generated using GraphPad Prism 5.02 software.
Results
Lung Function after Burn and Intoxication
Whole body unrestrained plethysmography was used to examine breathing patters and respiratory function in mice subjected to multi-day binge ethanol intoxication, burn injury, or the combined insult. In our previous study, we observed respiratory dysfunction, characterized by shallow breaths and a slower breathing rate, at 24 hours post-injury in mice subjected burn alone, which was exacerbated when intoxication preceded injury (11). Herein, we evaluated whether defective breathing parameters persisted over time, by collecting respiratory measurements at 24, 48 and 72 hours after treatment. Mice subjected to multi-day ethanol intoxication alone had no signs of impaired respiration at later time points. However, at 24 hours, control (vehicle-treated, sham injured) mice had a mean breath rate of 473.51 ± 21.5 breaths per minute, which was reduced by 49% to 239.9 ± 42.5 breaths per minute (p<0.05) by burn alone, and by 60% to 190.8 ± 9.1 breaths per minute (p<0.05) when ethanol preceded injury (Figure 1A). Additionally, enhanced pause (Penh), a measure of airway resistance and bronchoconstriction, was increased nearly 7-fold to 5.5 ± 1.8 by burn injury, compared to a Penh of 0.7 ± 0.2 observed in controls. Mice treated with ethanol prior to burn had more than a 2-fold increase in Penh compared to those with burn injury alone (10.5± 0.5) which was an 11-fold increase compared to controls (p<0.05, compared to sham vehicle and sham ethanol groups) (Figure 1B). Tidal volume, indicative of inspiration/expiration volumes, was decreased 47% below control (0.08 ± 0.01 ml) (p<0.05) in burn injured mice and by 50% (0.08 ± 0.01 ml) in intoxicated, burned mice (p<0.05) (Figure 1C). Minute volume, the amount of air inhaled/exhaled per minute (Figure 1D), showed similar patterns as both were reduced by burn injury, though statistical significance was only reached when comparing sham-injured, intoxicated mice to intoxicated, burned mice. (p<0.05), Sham injured, vehicle-treated controls had a mean minute volume of 74.1 ± 15.8 ml/min which was reduced by 72% to 20.8 ± 6.6 ml/min by burn injury and by 80% to 15.1 ± 1.8 ml/min, when ethanol intoxication preceded burn (p<0.05, compared to sham, ethanol-treated mice). At 48 hours, control mice had a mean breath rate of 464.8 ± 14.0 breaths per minute, which was reduced by 37% to 289.6 ± 43.1 breaths per minute (p<0.05) by burn alone, and by 58% to 196.8 ± 9.8 breaths per minute (p<0.05) when ethanol preceded injury (Figure 1E). Additionally, enhanced pause (Penh) was increased by 3-fold to 3.0 ± 0.9 by burn injury, compared to a Penh of 0.8 ± 0.1 observed in controls (p<0.05). Mice treated with ethanol prior to burn had a 2-fold increase in Penh compared to those with burn injury alone (7.9 ± 0.5) (p<0.05) which was a 9-fold increase compared to controls (p<0.05) (Figure 1F). Tidal volume (Figure 1G) and minute volume, (Figure 1H), showed similar patterns, as both were reduced by burn alone (p<0.05 compared to sham controls), and were further decreased in those with the combined insult (p<0.05 compared to sham controls). Sham injured, vehicle-treated controls had a mean tidal volume of 0.17 ± 0.02 ml which was reduced by 42% to 0.10 ± 0.02 ml by burn injury (p<0.05) and by 53% to 0.08 ± 0.01, when ethanol intoxication preceded burn (p<0.05). Similarly, sham-injured, non-intoxicated control mice had a mean minute volume of 79.0 ± 10.2 ml which was reduced by 59% to 32.1 ± 9.7 ml by burn injury (p<0.05) and by 80% to 63.4 ± 1.6 in intoxicated, injured mice (p<0.05). Mice surviving until 72 hours had similar lung function across all groups (Figure 1I–1L). Overall, these data indicate that burn injury alone causes respiratory dysfunction at 48 hours post-injury, which is exacerbated when ethanol intoxication precedes burn. While only 48% of the mice survived to 72 hours (11), those that did had respiratory parameters similar to controls.
Figure 1. Serum cytokine and chemokine levels 24 hours.
Serum levels of A) IL-6, B) IL-10, C) G-CSF, D) KC, and E) MCP-1 were measure by Bioplex assay. *p<0.05 compared to sham vehicle, # p<0.05 compared to sham ethanol, @ p<0.05 compared to all other groups by One-way ANOVA with Tukey’s multiple comparison test. Data from two independent experiments were combined, n=5–10 mice total, and are presented as mean values per treatment group ± SEM.
Lung Histology
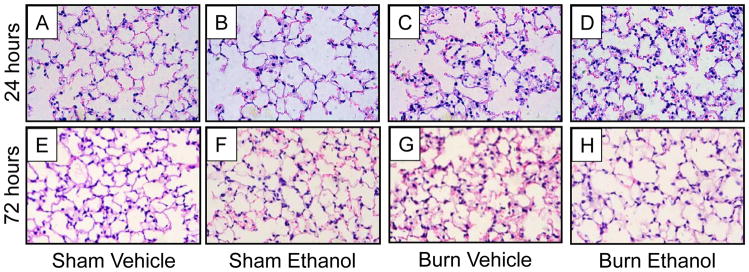
Using our model of multi-day binge ethanol intoxication followed by burn injury, our lab previously reported that 24 hours after injury, lungs from mice exposed to the combined insult had pulmonary edema and congestion, primarily due to an increase in neutrophil sequestration in the interstitium. There was a 10-fold increase in the number of neutrophils in intoxicated, burned mice compared to sham which was 1.5-fold above burn alone (11). Here, we report that this histopathology persisted over time. Groups of mice were euthanized at either 24 or 72 hours after insult to examine lung histology. Similar to our previous findings, lung histology from animals treated with ethanol alone looked similar to vehicle treated mice (11) (Figure 2A, 2B). Increased cellularity and alveolar wall thickening was observed in burned mice which was amplified in mice treated with multiday binge ethanol intoxication prior to burn, at 24 hours after injury (Figure 2C, 2D). At 72 hours post-insult, lungs from ethanol treated animals appeared similar to controls (Figure 2E, 2F). Interestingly, the increased cellularity and pulmonary congestion observed in burned animals persisted to 72 hours (Figure 2G) but this was not observed in mice treated with ethanol prior to burn (Figure 2H).
Figure 2. Lung inflammatory immunomodulator levels at 24 hours.
Lung tissue homogenate levels of A) IL-1β, B) TNF-α, C) IL-10, D) IL-6, E) MCP-1, and F) LIF were measure by Bioplex assay. *p<0.05 compared to sham vehicle, @ p<0.05 compared to all other groups by One-way ANOVA with Tukey’s multiple comparison test. Data are representative of two independent experiments, n=8–10 mice total, and are presented as mean values per treatment group ± SEM.
Serum Cytokine/Chemokine Levels at 24 Hours
Circulating levels of the pro-inflammatory cytokine, interleukin (IL)-6, anti-inflammatory cytokine, IL-10, as well as chemokines granulocyte-colony stimulating factor (G-CSF), KC (CXCL-1), and monocyte-chemoattractant protein (MCP)-1 (CCL2), were measured in serum collected from animals euthanized 24 hours after intoxication and injury. Multi-day binge ethanol intoxication alone had no effect on levels of any molecule measured. However, burn injury resulted in a non-statistically significant 16-fold increase in serum levels of IL-6, raising the levels from 7.4 ± 1.1 pg/ml observed in sham-injured, vehicle-treated controls to 124.8 ± 26.5 pg/ml in burned mice. When mice were given ethanol prior to burn, IL-6 levels were increased to 351.9 pg/ml, which is 47-fold higher than controls and 14-fold higher than burn alone (p<0.05 compared to all other groups) (Figure 3A). Similar responses were observed with other immunomodulatory molecules, where burn-injured mice had heightened circulating levels of IL-10, G-CSF, KC, and MCP-1 which was further elevated in mice exposed to the combined insult. Compared to control levels of IL-10 (8.3 ± 3.9 pg/ml), burned animals had a non-statistically significant 26-fold increase to 485.0 ±150.6 pg/ml, and those given multi-day binge ethanol prior to burn had a 37-fold increase to 670.7 ± 232.1 pg/ml (p<0.05 compared to uninjured mice) (Figure 3B). Although not statistically significant, burn injury dramatically increased the circulating level of G-CSF 86-fold to 28752.0 ± 7002.8 pg/ml, compared to 332.5 ± 60.7 pg/ml in controls. G-CSF levels were further increased by 301-fold to 100318.7 pg/ml ± 28284.2 pg/ml in mice exposed to the combined insult, when compared to controls (p<0.05 compared to all other groups) (Figure 3C). KC levels were 186.1 ± 90.0 pg/ml in control mice, which increased 4-fold to 878.0 ± 84.1 pg/ml in burned mice (p<0.05), and was further elevated 5-fold higher than controls to 1046.2 ± 177.6 pg/ml in mice where ethanol intoxication preceded burn (Figure 3D). Lastly, levels of MCP-1 were elevated from 899.3 ± 212.1 pg/ml in sham-injured, vehicle-treated mice to 2078.6 ± 599.0 pg/ml in mice exposed to the combined insult (p<0.05 compared to sham-injured, ethanol-intoxicated mice) (Figure 3E).
Figure 3. Lung function assessment.
Mice were individually placed in unrestrained whole body barometric plethysmography chamber, and breathing parameters were measured for 10 minutes at 24, 48 and 72 hours post-injury. *p<0.05 compared to sham vehicle, #p<0.05 compared to sham ethanol, @p<0.05 compared to all other groups by One-way ANOVA with Tukey’s multiple comparison test. Data points represent measurements obtained from individual mice. n=3–13 per treatment group.
Lung Cytokine/Chemokine Levels at 24 Hours
We previously demonstrated that 24 hours after intoxication and injury, mice with the combined insult had higher levels of the neutrophil chemoattractants, KC and macrophage inflammatory protein (MIP-2, CXCL2) (11). To further investigate the lung inflammatory milieu at this time point, multiplex analyses were performed on lung tissue homogenates. Levels of the pro-inflammatory cytokines, IL-1β and TNF-α, and the anti-inflammatory cytokine, IL-10 were similar between groups (Figure 4A–C). However, a non-statistically significant trend towards increased levels of IL-6 in lungs from burned mice was observed, raising levels from 2.39 ± 0.38 pg/mg protein in controls to 9.63 ± 5.5 pg/mg protein in injured mice. When mice were treated with ethanol prior to burn, IL-6 levels were 9-fold higher than controls, at 23.2 ± 4.7 pg/mg total protein (p<0.05 compared to sham-injured, vehicle treated mice) (Figure 4D). Lung MCP-1 levels were more than doubled by burn injury, raising levels from 151.9 ± 18.7 pg/mg protein in controls to 400.7 ± 92.6 pg/mg total protein in burned mice, which was similar to the levels observed in mice given the combined insult, 433.9 ± 109.1 pg/mg total protein, though not statistically significant (Figure 4E). Leukemia inhibitory factor (LIF) was elevated 2-fold from 2.33 ± 0.1 pg/mg total protein in control mice to 4.51 ± 0.4 pg/mg total protein by burn injury alone (p<0.05), and further increased to 8.38 ± 1.3 pg/mg total protein when ethanol intoxication preceded burn (p<0.05 compared to all other groups) (Figure 4F).
Figure 4. Time course of lung histology after intoxication and burn injury.
Lungs were sectioned and stained with hematoxylin and eosin at 24 and 72 hours post-intoxication and injury. Light micrographs are shown at 400x magnification. Representative images from two independent animal experiments are shown, n = 3–6 per group.
Lung Cytokine Levels at 72 Hours and 7 Days
Since we observed increased cellularity and pulmonary congestion that persisted out to 72 hours in burned mice, we characterized the inflammatory milieu at this time point and in lungs from mice surviving to 7 days post insult using lung tissue homogenates in multiplex bead arrays. We observed no statistically significant differences in IL-1β, TNF-α, IL-10, IL-6, MCP-1, and KC at 72 hours (Figure 5A–F) or at 7 days (Supplemental Table 1).
Figure 5. Lung cytokine levels 72 hours after injury.
Lung tissue homogenate levels of A) IL-1β, B) TNF-α, C) IL-10, D) IL-6, E) MCP-1, and F) KC were measure by Bioplex assay. Statistical analyses were performed using One-way ANOVA. Data are representative of two independent experiments, n=3–6 per group, and are presented as mean values per treatment group ± SEM.
Alveolar Macrophage Phenotype
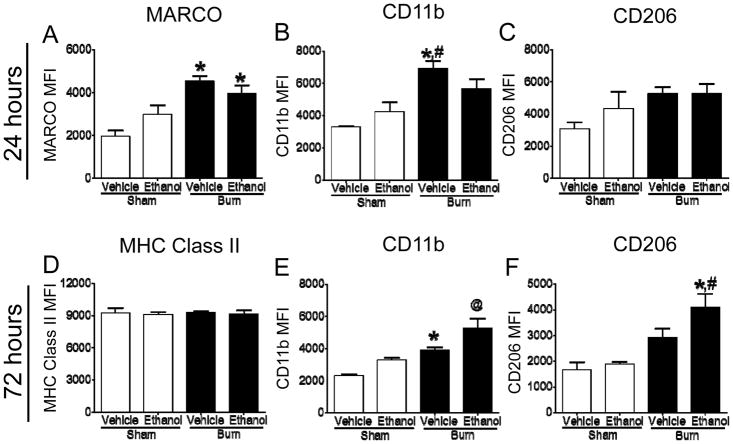
Alveolar macrophages play a critical role in innate immune responses by first initiating inflammation and then switching phenotype to promote resolution through efferocytosis of dead cells and the production of anti-inflammatory, pro-resolving molecules. Flow cytometry was utilized to assess alveolar macrophage phenotype by characterizing cell surface receptor levels. Using enzymatically-dissociated lung tissue, which contains a heterogeneous population of lung cell types, including leukocytes, the mean fluorescent intensity (MFI) of pro-inflammatory, classical activation markers, macrophage receptor with collagenous structure (MARCO), CD11b, and MHC Class II, and the anti-inflammatory marker, CD206, was determined in CD45+CD11c+CD24−CD64+F4/80+ alveolar macrophages. Consistent with our previous findings using a single binge ethanol intoxication prior to burn injury model (12) when compared to vehicle-treated, sham-injured mice, MARCO MFI was two times higher in alveolar macrophages from burned mice 24 hours after injury, regardless of prior multi-day binge ethanol intoxication (p<0.05) (Figure 6A). However, the MFI of CD11b increased by 110% in burn-injured mice (p<0.05) (Figure 6B) and minor increases of CD206 MFI were observed in all treatment groups, though no statistically significant differences were observed at this time point (Figure 6C). At 72 hours after injury, MHC Class II MFI, was nearly identical across groups (Figure 6D). In contrast, the MFI of CD11b and CD206 were increased by 70% and 130%, respectively, on alveolar macrophages from intoxicated burn mice compared to uninjured controls (p<0.05), indicating an alternatively activated phenotype (Figure 6E, 6F).
Figure 6. Flow cytometry analyses of alveolar macrophage cell surface receptors.
Alveolar macrophages (CD45+CD11c+CD24−CD64+F4/80+) were harvested and stained for A) MARCO and B) CD11b, and C) CD206 at 24 hours after injury, and for D) MHC Class II and E) CD11b, and F) CD206 at 72 hours. Data are presented as mean MFI ± SEM. *p<0.05 compared to sham vehicle, #p<0.05 compared to sham ethanol, @p<0.05 compared to all other groups by One-way ANOVA with Tukey’s multiple comparison test. Experiments were performed a minimum of two times, n=4–6 per group. Data from one representative experiment are shown.
Discussion
Uncontrolled inflammation underlies sepsis, multiple organ failure, and death after burn injury. Inflammatory mediators are also key contributors to ARDS development, regardless of etiology. Cytokine levels can serve as biomarkers of injury severity and predictors of mortality. For example, serum IL-6 and MCP-1 levels are elevated in patients with severe burn injury and high levels are correlated with mortality (18). Consistent with these findings, at 24 hours after injury, we observed elevated circulating levels of IL-6 and MCP-1 in burned mice, and even higher levels when multiple days of ethanol intoxication preceded injury (Figure 3). In addition, the concentration of G-CSF in burn patient serum is elevated and is positively correlated with burn size (19). In this study, increased G-CSF levels observed in burned mice were exacerbated when intoxication preceded injury. Increased circulating levels of the chemokine IL-8 in septic/systemic inflammatory response syndrome (SIRS) patients is also predictive of morbidity and mortality (20). As described herein, KC, the murine IL-8 homologue, was increased in serum from burned mice. The additional insult of multi-day intoxication prior to burn raised levels slightly higher than isolated burn injury alone. Recently, the serum TNF-α/IL-10 ratio was inversely correlated with injury severity, burn size, and predictive of hyper-susceptibility to repeated infection in burn patients (21), further demonstrating the potential of inflammatory mediators as predictive biomarkers. Lastly, here we report elevated serum IL-10 concentrations in burn-injured mice, which was further escalated by multi-day binge ethanol intoxication, highlighting the deleterious consequences of ethanol consumption on post-burn inflammation and resolution.
In addition to measuring cytokine/chemokine levels in mouse serum, we also examined levels in lung tissue homogenates, 24 and 72 hours after intoxication and injury (Figures 4 and 5). Levels of the pro-inflammatory mediators, IL-1β and TNFα, as well as the anti-inflammatory molecule, IL-10 were similar across groups, over time. Conversely, IL-6 and MCP-1 levels were both elevated lungs of intoxicated, burned mice, 24 hours after injury, demonstrating that in addition to elevated circulating pro-inflammatory molecules, the local pulmonary inflammatory milieu in mice exposed to the combined insult was more severe. Moreover, burned mice had increased leukemia inhibitory factor (LIF) levels and mice exposed to the combined insult had levels nearly two times higher than isolated injury. LIF, an acute phase protein and member of the IL-6 family of cytokines, acts to promote tissue homeostasis and limit pneumonic lung injury in mouse models (22), yet increased levels have been correlated with poor outcomes, as LIF levels are elevated in BAL fluid from ARDS patients (23) and in the serum of patients with significant burns (TBSA >20%), with the highest levels observed in those who did not survive (24). These observations further highlight the complexity of maintaining pulmonary homeostasis after burn injury, requiring initial dampening of inflammation to prevent tissue damage while not suppressing the ability of the immune system to respond to pathogen attack. Our observation that lung levels of IL-1β, TNFα, IL-10, KC, MCP-1, and IL-6 are not different from controls 72 hours post-injury, could be interpreted such that early inflammation has been resolved by this time point, regardless of treatment. However, given that nearly 50% of the mice treated with multiple days of ethanol intoxication prior to burn died within the first 72 hours, it is highly likely that uncontrolled/excessive inflammation contributed to mortality. Our results from a small pilot study are in support of this notion, as mice euthanized 48 hours after injury and intoxication have dramatic alveolar wall thickening, congestion, and leukocyte infiltrates (Curtis and Kovacs, unpublished observation). In addition, the plethysmography results presented herein show lung dysfunction 48 hours post-intoxication and injury, with increased Penh values indicative of bronchoconstriction and airway resistance, and fewer, shallow breaths per minute (Figure 1). Furthermore, we previously demonstrated that impaired lung function was correlated with an increased number of neutrophils (11). Ineffective neutrophil clearance prolongs inflammation, contributing to increased capillary endothelial cell permeability and pulmonary edema, likely contributing to the 50% mortality previously observed in mice treated with multiple days of ethanol prior to burn injury (11).
Alveolar macrophages are a complex cell type able to differentiate between inhaled particulates, innocuous antigens, and commensal microbes, preventing inappropriate inflammatory responses. However, they can also be activated by a variety of stimuli, triggering a pro-inflammatory immune response to pathogenic microorganisms. As such, alveolar macrophages are key regulators of lung homeostasis after injury. Involved in neutrophil recruitment and efferocytosis as well as the production of reparative and pro-resolving molecules, understanding alveolar macrophage fate is a principal component to deciphering the complex mechanisms contributing to the excessive and prolonged pulmonary response seen after intoxication and burn. Both ethanol intoxication and burn injury are known to independently alter alveolar macrophage phenotype. Ethanol intoxication alone causes immunosuppression through a variety of mechanisms, including impaired immune cell phagocytic activity, reduced ability to respond to chemotactic stimuli, and diminished capacity to present antigen (25, 26). Alveolar macrophages isolated from mice exposed to a chronic ethanol consumption treatment paradigm undergo oxidative stress (25) and exhibit impaired phagocytosis of Pseudomonas aeruginosa after a single in vivo binge ethanol exposure or when treated with ethanol in vitro (26, 27). Conflicting results regarding alveolar macrophage responsiveness to infectious stimuli after burn have been reported. Alveolar macrophages from burn patients with inhalation injury have increased chemotaxis towards casein and zymosan-activated serum in vitro (28) and are primed for an over-exuberant pro-inflammatory response following TLR2 or TLR4 activation (29, 30). In contrast, alveolar macrophage hyporesponsiveness to endotoxin was correlated with mortality in burn victims with inhalation injury (31). In a mouse model of burn injury and pulmonary infection, alveolar macrophage phagocytic activity was diminished after burn rendering cells incapable of phagocytosing and clearing P. aeruginosa, leading to sepsis and mortality (32). Since nearly 50% of burn patients are intoxicated at the time of injury (14), it is imperative to understand not only how intoxication or injury alone alter alveolar macrophage phenotype but also to study the combined effect.
Alveolar macrophage homeostatic maintenance of the lung inflammatory milieu is facilitated in part through cell surface receptor expression. The scavenger receptor, MARCO, binds unopsonized bacteria and particulate matter and its activation promotes the clearance of apoptotic cells via efferocytosis (33). Hence, MARCO activation mediates clearance of lung pathogens, yet at the same time it can block inflammatory responses through efferocytosis. Additionally, CD11b contributes to complement receptor-3 mediated efferocytosis of iC3b-opsonized apoptotic cells (34). Conversely, the mannose receptor, CD206, selectively binds to glycosylated lipids and proteins found on pathogen surfaces as well as to unopsonized bacteria, suppressing a pro-inflammatory response to commensals (35), and promotes tissue repair and resolution. In this study, we observed increased MARCO activation at 24 hours post-burn, regardless of ethanol intoxication, indicative of macrophage activation Furthermore, at 24 hours post-injury, CD11b levels were increased in burn-injured mice, but not in those treated with ethanol prior to burn. However, at 72 hours, CD11b levels remained elevated after burn, but were highest in intoxicated, burned mice. CD206 levels were also significantly elevated in mice treated with ethanol prior to burn. Speculatively, these findings could indicate that alveolar macrophages from intoxicated, burned mice have a pro-inflammatory phenotype at 24 hours, yet their ability to efferocytose apoptotic cells is diminished due to reduced or delayed CD11b upregulation. Moreover, this response has been mounted by the 72 hour time point. Our observation that CD206 is only upregulated at 72 hours in intoxicated, burned mice suggests that 1) the lung damage in mice exposed to the combined insult triggers a more profound reparative, anti-inflammatory response and 2) the anti-inflammatory nature of alternatively activated macrophages may render them less capable of mounting an effective immune response to infectious pathogens.
In summary, our results demonstrate that multi-day ethanol intoxication prior to burn contributes to increased mortality through additive effects of two insults. The observed pulmonary edema, leukocyte congestion, and impaired respiration are likely caused by exacerbated inflammatory responses early after injury which may be due, in part, to the ineffectiveness of alveolar macrophages to resolve inflammation. Our finding that alveolar macrophages from intoxicated, burned mice have elevated levels of CD206 at 72 hours, evidence of a heightened anti-inflammatory phenotype, may represent an underlying mechanism driving the increased susceptibility to infection observed in intoxicated burn patients.
Supplementary Material
Acknowledgments
Research funding: NIH R01 GM115257 (EJK), R21 AA023193 (EJK) and R01 AG018859 (EJK). The authors declare no conflicts of interest.
Research in this publication was supported by NIH R01 GM115257 (EJK), R21 AA023193 (EJK) and R01 AG018859 (EJK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of abbreviations
- ARDS
acute respiratory distress syndrome
- BAC
blood alcohol concentration
- BAL
bronchoalveolar lavage
- CD
cluster of differentiation
- H&E
hemoxylin and eosin
- IL
interleukin
- IF
immunofluorescence
- KC
CXCL1
- LIF
leukemia inhibitory protein
- LPS
lipopolysaccharide
- MIP-2
macrophage inflammatory protein-2
- MCP-1
macrophage chemoattractant protein-1
- MFI
mean fluorescence intensity
- MOF
multiple organ failure
- Penh
enhanced pause
- SIRs
systemic inflammatory response syndrome
- TBSA
total body surface area
- TNF-α
tumor necrosis factor-alpha
References
- 1.ABA, A. B. A. Burn Incidence Fact Sheet, Burn Incidence and Treatment in the United States: 2016 2016 [Google Scholar]
- 2.Aikawa N, Shinozawa Y, Ishibiki K, Abe O, Yamamoto S, Motegi M, Yoshii H, Sudoh M. Clinical analysis of multiple organ failure in burned patients. Burns, including thermal injury. 1987;13:103–109. doi: 10.1016/0305-4179(87)90097-0. [DOI] [PubMed] [Google Scholar]
- 3.Howland J, Hingson R. Alcohol as a risk factor for injuries or death due to fires and burns: review of the literature. Public health reports. 1987;102:475–483. [PMC free article] [PubMed] [Google Scholar]
- 4.Silver GM, Albright JM, Schermer CR, Halerz M, Conrad P, Ackerman PD, Lau L, Emanuele MA, Kovacs EJ, Gamelli RL. Adverse clinical outcomes associated with elevated blood alcohol levels at the time of burn injury. Journal of burn care & research: official publication of the American Burn Association. 2008;29:784–789. doi: 10.1097/BCR.0b013e31818481bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin R, Poe AM, Cross JM, Rue LW, 3rd, McGwin G., Jr The association between blood alcohol level and infectious complications among burn patients. Journal of burn care & research: official publication of the American Burn Association. 2009;30:395–399. doi: 10.1097/BCR.0b013e3181a28966. [DOI] [PubMed] [Google Scholar]
- 6.Grobmyer SR, Maniscalco SP, Purdue GF, Hunt JL. Alcohol, drug intoxication, or both at the time of burn injury as a predictor of complications and mortality in hospitalized patients with burns. The Journal of burn care & rehabilitation. 1996;17:532–539. doi: 10.1097/00004630-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Steinvall I, Bak Z, Sjoberg F. Acute respiratory distress syndrome is as important as inhalation injury for the development of respiratory dysfunction in major burns. Burns: journal of the International Society for Burn Injuries. 2008;34:441–451. doi: 10.1016/j.burns.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Turnage RH, Nwariaku F, Murphy J, Schulman C, Wright K, Yin H. Mechanisms of pulmonary microvascular dysfunction during severe burn injury. World journal of surgery. 2002;26:848–853. doi: 10.1007/s00268-002-4063-3. [DOI] [PubMed] [Google Scholar]
- 9.Bird MD, Kovacs EJ. Organ-specific inflammation following acute ethanol and burn injury. Journal of leukocyte biology. 2008;84:607–613. doi: 10.1189/jlb.1107766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel PJ, Faunce DE, Gregory MS, Duffner LA, Kovacs EJ. Elevation in pulmonary neutrophils and prolonged production of pulmonary macrophage inflammatory protein-2 after burn injury with prior alcohol exposure. American journal of respiratory cell and molecular biology. 1999;20:1229–1237. doi: 10.1165/ajrcmb.20.6.3491. [DOI] [PubMed] [Google Scholar]
- 11.Shults JA, Curtis BJ, Chen MM, O'Halloran EB, Ramirez L, Kovacs EJ. Impaired respiratory function and heightened pulmonary inflammation in episodic binge ethanol intoxication and burn injury. Alcohol. 2015;49:713–720. doi: 10.1016/j.alcohol.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shults JA, Curtis BJ, Boe DM, Ramirez L, Kovacs EJ. Ethanol intoxication prolongs post-burn pulmonary inflammation: role of alveolar macrophages. Journal of leukocyte biology. 2016;100:1037–1045. doi: 10.1189/jlb.3MA0316-111R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease and Prevention. Vital signs: binge drinking prevalence, frequency, and intensity among adults - United States, 2010. MMWR. Morbidity and mortality weekly report. 2012;61:14–19. [PubMed] [Google Scholar]
- 14.Hoeppner BB, Barnett NP, Jackson KM, Colby SM, Kahler CW, Monti PM, Read J, Tevyaw T, Wood M, Corriveau D, Fingeret A. Daily college student drinking patterns across the first year of college. Journal of studies on alcohol and drugs. 2012;73:613–624. doi: 10.15288/jsad.2012.73.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faunce DE, Llanas JN, Patel PJ, Gregory MS, Duffner LA, Kovacs EJ. Neutrophil chemokine production in the skin following scald injury. Burns: journal of the International Society for Burn Injuries. 1999;25:403–410. doi: 10.1016/s0305-4179(99)00014-5. [DOI] [PubMed] [Google Scholar]
- 16.Boehmer ED, Goral J, Faunce DE, Kovacs EJ. Age-dependent decrease in Toll-like receptor 4-mediated proinflammatory cytokine production and mitogen-activated protein kinase expression. Journal of leukocyte biology. 2004;75:342–349. doi: 10.1189/jlb.0803389. [DOI] [PubMed] [Google Scholar]
- 17.Murdoch EL, Karavitis J, Deburghgraeve C, Ramirez L, Kovacs EJ. Prolonged chemokine expression and excessive neutrophil infiltration in the lungs of burn-injured mice exposed to ethanol and pulmonary infection. Shock. 2011;35:403–410. doi: 10.1097/SHK.0b013e31820217c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hur J, Yang HT, Chun W, Kim JH, Shin SH, Kang HJ, Kim HS. Inflammatory cytokines and their prognostic ability in cases of major burn injury. Annals of laboratory medicine. 2015;35:105–110. doi: 10.3343/alm.2015.35.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HS, Kim JH, Yim H, Kim D. Changes in the levels of interleukins 6, 8, and 10, tumor necrosis factor alpha, and granulocyte-colony stimulating factor in Korean burn patients: relation to burn size and postburn time. Annals of laboratory medicine. 2012;32:339–344. doi: 10.3343/alm.2012.32.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hack CE, Hart M, van Schijndel RJ, Eerenberg AJ, Nuijens JH, Thijs LG, Aarden LA. Interleukin-8 in sepsis: relation to shock and inflammatory mediators. Infection and immunity. 1992;60:2835–2842. doi: 10.1128/iai.60.7.2835-2842.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsurumi A, Que YA, Ryan CM, Tompkins RG, Rahme LG. TNF-alpha/IL-10 Ratio Correlates with Burn Severity and May Serve as a Risk Predictor of Increased Susceptibility to Infections. Frontiers in public health. 2016;4:216. doi: 10.3389/fpubh.2016.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Traber KE, Symer EM, Allen E, Kim Y, Hilliard KL, Wasserman GA, Stewart CL, Jones MR, Mizgerd JP, Quinton LJ. Myeloid-epithelial cross talk coordinates synthesis of the tissue-protective cytokine leukemia inhibitory factor during pneumonia. American journal of physiology. Lung cellular and molecular physiology. 2017;313:L548–L558. doi: 10.1152/ajplung.00482.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jorens PG, De Jongh R, Bossaert LL, De Backer W, Herman AG, Pollet H, Bosmans E, Taupin JL, Moreau JF. High levels of leukaemia inhibitory factor in ARDS. Cytokine. 1996;8:873–876. doi: 10.1006/cyto.1996.9999. [DOI] [PubMed] [Google Scholar]
- 24.Akita S, Akino K, Ren SG, Melmed S, Imaizumi T, Hirano A. Elevated circulating leukemia inhibitory factor in patients with extensive burns. Journal of burn care & research: official publication of the American Burn Association. 2006;27:221–225. doi: 10.1097/01.BCR.0000197679.08671.A5. [DOI] [PubMed] [Google Scholar]
- 25.Yeligar SM, Harris FL, Hart CM, Brown LA. Ethanol induces oxidative stress in alveolar macrophages via upregulation of NADPH oxidases. Journal of immunology. 2012;188:3648–3657. doi: 10.4049/jimmunol.1101278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karavitis J, Murdoch EL, Gomez CR, Ramirez L, Kovacs EJ. Acute ethanol exposure attenuates pattern recognition receptor activated macrophage functions. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2008;28:413–422. doi: 10.1089/jir.2007.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karavitis J, Murdoch EL, Deburghgraeve C, Ramirez L, Kovacs EJ. Ethanol suppresses phagosomal adhesion maturation, Rac activation, and subsequent actin polymerization during FcgammaR-mediated phagocytosis. Cellular immunology. 2012;274:61–71. doi: 10.1016/j.cellimm.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riyami BM, Kinsella J, Pollok AJ, Clark C, Stevenson RD, Reid WH, Campbell D, Gemmell CG. Alveolar macrophage chemotaxis in fire victims with smoke inhalation and burns injury. European journal of clinical investigation. 1991;21:485–489. doi: 10.1111/j.1365-2362.1991.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 29.Oppeltz RF, Rani M, Zhang Q, Schwacha MG. Burn-induced alterations in toll-like receptor-mediated responses by bronchoalveolar lavage cells. Cytokine. 2011;55:396–401. doi: 10.1016/j.cyto.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright MJ, Murphy JT. Smoke inhalation enhances early alveolar leukocyte responsiveness to endotoxin. The Journal of trauma. 2005;59:64–70. doi: 10.1097/01.ta.0000171588.25618.87. [DOI] [PubMed] [Google Scholar]
- 31.Davis CS, Albright JM, Carter SR, Ramirez L, Kim H, Gamelli RL, Kovacs EJ. Early pulmonary immune hyporesponsiveness is associated with mortality after burn and smoke inhalation injury. Journal of burn care & research: official publication of the American Burn Association. 2012;33:26–35. doi: 10.1097/BCR.0b013e318234d903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis KA, Santaniello JM, He LK, Muthu K, Sen S, Jones SB, Gamelli RL, Shankar R. Burn injury and pulmonary sepsis: development of a clinically relevant model. The Journal of trauma. 2004;56:272–278. doi: 10.1097/01.TA.0000108995.64133.90. [DOI] [PubMed] [Google Scholar]
- 33.Palecanda A, Paulauskis J, Al-Mutairi E, Imrich A, Qin G, Suzuki H, Kodama T, Tryggvason K, Koziel H, Kobzik L. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. The Journal of experimental medicine. 1999;189:1497–1506. doi: 10.1084/jem.189.9.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mevorach D, Mascarenhas JO, Gershov D, Elkon KB. Complement-dependent clearance of apoptotic cells by human macrophages. The Journal of experimental medicine. 1998;188:2313–2320. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Tachado SD, Patel N, Zhu J, Imrich A, Manfruelli P, Cushion M, Kinane TB, Koziel H. Negative regulatory role of mannose receptors on human alveolar macrophage proinflammatory cytokine release in vitro. Journal of leukocyte biology. 2005;78:665–674. doi: 10.1189/jlb.1204699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.