Abstract
Brown adipose tissue (BAT) has been identified as a potential target in the treatment and prevention of obesity and metabolic disease. The precise kinetics of BAT activation and the duration of stimulus required to recruit metabolically active BAT, and its subsequent deactivation, are not well-understood. In this clinical trial, 19 healthy adults (BMI: 23.7±0.7 kg/m2, Age: 31.2±2.8 y, 12 female) underwent three different cooling procedures to stimulate BAT glucose uptake, and active BAT volume was determined using 18F-Fluorodeoxyglucose (FDG) PET/CT imaging. We found that 20 minutes of pre-injection cooling produces activation similar to the standard 60 minutes (39.9 mL vs. 44.2 mL, p= 0.52), indicating that BAT activity approaches its peak function soon after the initiation of cooling. Furthermore, upon removal of cold exposure, active BAT volume declines (13.6 mL vs. 44.2 mL, p=0.002), but the deactivation process persists even hours following cessation of cooling. Thus, the kinetics of human BAT thermogenesis are characterized by a rapid increase soon after cold stimulation but a more gradual decline after rewarming. These characteristics reinforce the feasibility of developing mild, short-duration cold exposure to activate BAT and treat obesity and metabolic disease.
Introduction
Obesity and the resulting metabolic diseases are worldwide health concerns1 and are independently associated with all-cause mortality2. Obesity is primarily defined by an excess of white adipose tissue (WAT), whereas a greater presence of brown adipose tissue (BAT) is associated with lower body mass index (BMI)3,4,5 and improved glucose metabolism6. Due to BAT’s ability to consume glucose and free fatty acids in response to cold exposure7,8,9, cooling has been a common method for studying BAT’s potential for the prevention and treatment of obesity10. As cold exposure is widely explored and reproducibly activates BAT thermogenesis, there is a need to standardize cooling protocols to best compare results across the field11,12. Furthermore, determining ideal conditions with which to target BAT for its thermogenic capabilities can be challenging because BAT may need along-acting and persistent stimulus. On the other hand,a short-duration of activation may sufficiently activate BAT glucose uptake, similar to skeletal muscle13.The precise kinetics describing BAT activation in response to cold, when better understood, could lead to novel treatments for hyperglycemia and hypertriglyceridemia.
Two hours of cold exposure is the standard duration used to study cold-induced BAT metabolism11, yet it is unclear if the full exposure time is necessary to achieve the same amount of BAT thermogenesis. In rats, BAT thermogenesis increases substantially above baseline in less than 5 minutes14. Furthermore, it is unclear whether human BAT immediately suppresses its thermogenic processes following removal of a cold stimulus. When using mild cold to activate BAT to minimize subjects’ discomfort and maximize adherence to the cooling protocol, it is particularly important to determine the minimal effective duration of exposure.
While human BAT might achieve peak thermogenesis soon after initiation of cooling, its metabolic activity may decline much more slowly after a cold stimulus is removed. Evidence for this delayed process is suggested by the large retrospective studies of BAT prevalence using 18F-fluorodeoxyglucose positron emission tomographic/computed tomographic (18F-FDG PET/CT).In these studies, patients were not deliberately exposed to cold during the 60 minutes prior to FDG injection, yet 5-10% had detectable BAT glucose uptake15,16,3. These observations suggest several possibilities related to the nature of the cold-exposure experienced by these subjects including exposure well before FDG injection; cold-acclimation; and cold temperatures in the rooms where the patients waited for the FDG injection to distribute to metabolically active tissues. In all such scenarios, BAT glucose uptake was maintained during the scan acquisition. Therefore, we hypothesized that BAT can be activated using a duration of cold exposure shorter than two hours and that BAT remains activated even hours following removal of a cold stimulus. We tested these hypotheses in 19 healthy female and male volunteers who underwent three different cooling conditions: a “standard” condition of 60 minutes of pre-FDG cooling; 20 minutes of pre-FDG cooling; and also 120 minutes of cooling followed by 180 minutes of warming prior to 18F-FDG PET/CT imaging.
Materials and Methods
Study Population
Healthy volunteers (12 women, 9 men) were recruited through electronic advertisements (Table 1). Each subject participated in 2-3 separate in-patient study visits and wore standard hospital scrubs during each visit. This study followed institutional guidelines and was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (BIDMC) and Joslin Diabetes Center. Written informed consent was obtained from all volunteers.
Table 1.
Subject Characteristics; Mean ± Standard Deviation shown. A Student’s t-test was used to compare Women vs. Men.
| Characteristic (units) | Combined | Women | Men | Women vs. Men (p) |
|---|---|---|---|---|
|
| ||||
| Sex | 12W / 7M | 12 | 7 | |
| Age (y) | 31.2 ± 2.8 | 31.8 ± 3.5 | 30.1 ± 5.0 | 0.78 |
| Height (cm) | 169.3 ± 2.6 | 163.7 ± 2.5 | 179.0 ± 3.5 | < 0.01 |
| Weight (kg) | 68.4 ± 3.3 | 62.8 ± 3.6 | 77.9 ± 4.9 | 0.02 |
| Body mass index (kg/m2) | 23.7 ± 0.7 | 23.3 ± 0.9 | 24.2 ± 1.0 | 0.52 |
| Body surface area (m2) | 1.8 ± 0.1 | 1.7 ± 0.1 | 2.0 ± 0.1 | < 0.01 |
| Waist-hip ratio | 0.79 ± 0.02 | 0.75 ± 0.01 | 0.85 ± 0.02 | < 0.01 |
| Body fat (%) | 23.6 ± 1.7 | 27.0 ± 6.6 | 17.8 ± 1.8 | < 0.01 |
| Percent Lean (%) | 73.0 ± 1.7 | 69.6 ± 1.8 | 78.8 ± 1.7 | < 0.01 |
Cold Exposure Design
Cooling was performed with a cooling vest with circulating water at 12.8-16.1°C in a room at 20 °C17. Three cooling designs were used (Fig 1A): Short Cooling, Standard Cooling, and Rewarming. For the Short Cooling day, FDG was administered after 20 minutes of cold exposure, and subjects remained in the cooling vest for 60 more minutes. On the Standard Cooling day, FDG was injected after 60 minutes of cold exposure and the volunteer wore the cooling vest for another 60 minutes17. On the Rewarming day, volunteers wore the cooling vest for 120 minutes, the vest was removed for 120 minutes of resting at 23°C (re-warming), the FDG was injected, then the subjects rested at 23°C for 60 more minutes.
Figure 1.
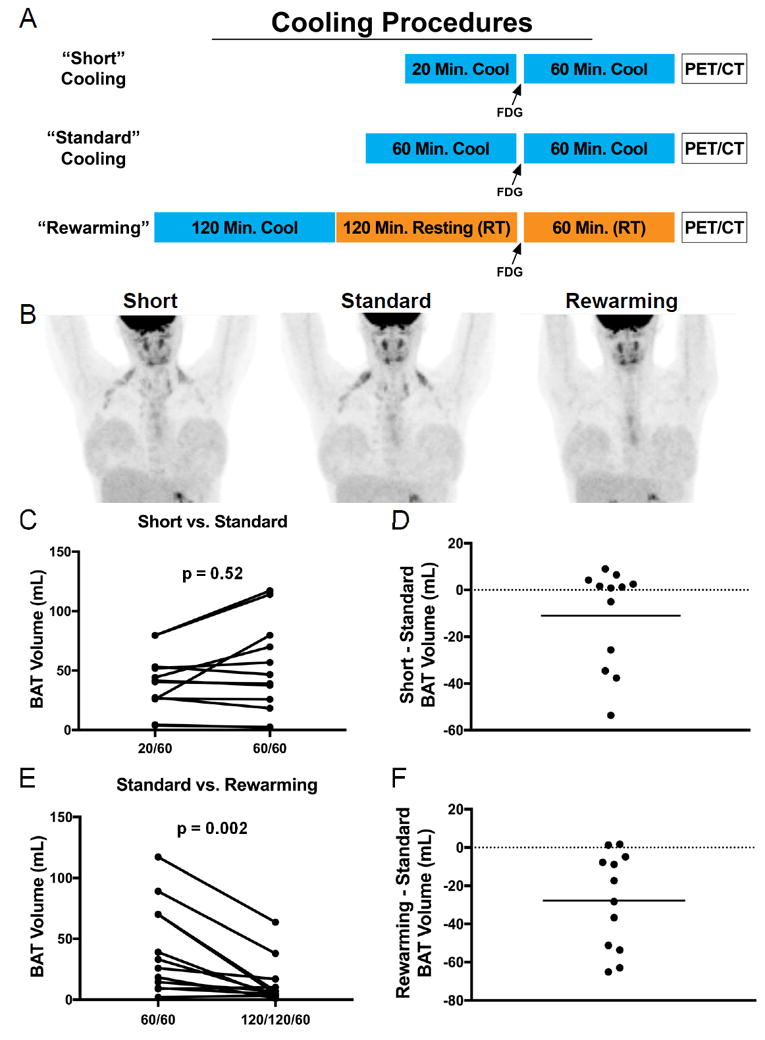
Procedures and results for cold-induced PET/CT imaging studies. Three different cooling protocols are shown, with cooling in blue, room temperature (RT) in orange, and PET/CT acquisition in clear box (A). Representative image of one subject on three different imaging days (B). Results for BAT Volumes in Standard vs. Short Cooling Days (C, D) and Standard vs. Rewarming Days (E, F). Probability values were determined by Wilcoxon Signed Rank Tests.
Imaging Protocol and Quantification of FDG Uptake
PET/CT images were obtained from the base of the skull to the kidneys with an i.v. bolus administration of 444 MBq (12 mCi) of 18F-FDG and image acquisition using a Discovery LS multidetector helical PET/CT scanner (GE Medical Systems). Areas of 18F-FDG uptake on PET co-localizing with regions of fat were identified on CT (-250 to -10 Hounsfield Units) were quantified by their Standard Uptake Value (SUV): average activity per unit volume within the region of interest divided by the injected dose per body mass in kg. The SUV threshold used was >1.0 g/mL. We then applied a post-hoc individualized adjustment for lean body mass (LBM) applied to BAT volume: ((1.5 g/mL) * (0.73))/ (individualized LBM); where 0.73 is the average LBM proportion of this cohort. Regions of interest were drawn axially on each image slice around metabolically active adipose tissue within the scanning window.18
Statistical Analysis
The goal of this pilot study was to detect the difference in BAT activation between Standard cooling and Rewarming. We calculated that we would need to study 12 subjects to reject the null hypothesis that this response difference is 0, with a power of 0.80 and an α of 0.05. Prism software (version 7; GraphPad, La Jolla, CA, USA) was used for statistical analysis. All the data are expressed as the mean ± standard deviation. Significant differences between means were identified using Wilcoxon Signed Rank tests. Differences were considered significant at P < 0.05.A Student’s t-test was used to compare male vs. female demographics.
Results
BAT Kinetics
The standard two hours of cold exposure (60 minutes pre-injection) induced the highest detectable volume of activated BAT (44.2 ± 36.9 mL). Twenty minutes of pre-FDG cold exposure yielded a similar amount of BAT volume compared to the Standard Cooling (39.9 ± 24.2 mL, p=0.52) (Fig 1C). In fact, the majority of subjects had more detectable BAT with the shorter duration of pre-injection cold exposure (Fig 1D).
When subjects were re-warmed for three hours following two hours of cold exposure, a substantially lower volume of BAT remained metabolically active (13.6 ± 18.7 mL) when compared to Standard Cooling (p = 0.002) (Fig 1E). Under conditions of re-warming following cold exposure, nearly every subject had less activated BAT than without re-warming, yet several subjects still had detectable BAT (Fig 1F). The tissue SUVmean, another measure of BAT metabolic activity, was highest under standard cooling (1.68 ± 0.52 g/mL), followed by 20 minutes of pre-injection cooling (1.55 ± 0.43 g/mL, P=0.27), while the Rewarming condition had the lowest SUVmean of 1.21 ± 0.18 g/mL (P=0.02 compared with Standard cooling).
Discussion
The elucidation of the kinetics of BAT activation is another important step in understanding its regulation under standard physiological conditions. Our results demonstrate that 80 minutes of cold exposure elicits similar BAT activation as 120 minutes, indicating that brown adipocytes are recruited soon after cold exposure to maintain heat production. During the re-warming period, those subjects with the highest amount of BAT volume demonstrated the greatest reductions in detectable BAT, whereas among those with little to moderate amounts, the amounts of detectable BAT were not substantially different during different cold exposure conditions. This distinction suggests that subjects with large volumes of BAT have more flexibility in the activation and de-activation in response to temperature challenges.
The evidence here indicates the novel finding that the thermogenic effects of cooling are not immediately suppressed in the absence of a cold stimulus, suggesting that BAT continues to utilize glucose for hours after initial recruitment. These findings are corroborated by another study demonstrating that heat production remains elevated for at least one hour during re-warming following cold exposure19. In fact, longer cold challenges (up to 24-hours) have shown that human core and skin temperatures level out after 6 hours20.
There are several methodological considerations that impact the interpretation of our data. First, we focused the BAT quantification on the principal cervical-supraclavicular-axillary BAT depots rather than including other, smaller depots; however, this approach leads to volumes that correlate well with total-body BAT metabolic activity18. We also could not distinguish beige/brite from brown adipocytes21,22 with FDG PET/CT alone, so the different contributions from these cell types23 to glucose uptake could not be determined. In addition, we did not collect skin or core temperature measures or calculate energy expenditure in order to assess the intensity of the cold stimulus, so it is not clear if a stronger or weaker cold challenge would yield the same findings. The FDG dose administered in this study limited the number of testable conditions to three, so we could not also measure baseline BAT activity in the absence of cold. However, a parallel study of ours that employed this negative control showed that there was a median volume of 0 mL detectable BAT17. Therefore, the detectable BAT volumes shown here should be considered absolute changes above a baseline of 0 mL. Finally, 18F-FDG uptake may not consistently correlate with oxidative phosphorylation of glucose, but rather glucose may be taken into BAT to serve as a substrate for anaplerosis, fatty acid synthesis, or other metabolic pathways24. The use of dynamic 18F-FDG imaging, and other metabolic tracers such as 15O-H2O,15O-O2or 11C-acetate may help better characterize the thermogenesis within metabolically active adipocytes. The purpose of persistent BAT glucose uptake requires further investigation in this context.
Taken together, our data demonstrate that BAT is robustly activated immediately following just 20 minutes of pre-injection cold exposure, and BAT activation declines, but may persist, even hours following removal of a thermogenesis-provoking stimulus. These observations have implications for protocols in understanding BAT physiology, therapeutic provocations of BAT in obesity prevention and/or treatment, and also for diagnostic 18F-FDG PET/CT assessments in a variety of clinical settings. In designing treatments that maximize efficacy while minimizing patient discomfort, the kinetics of BAT activation and deactivation highlight a unique window into the use of BAT thermogenesis for the treatment of obesity and associated metabolic diseases.
Acknowledgments
We thank the Beth Israel Deaconess Medical Center (BIDMC) Clinical Research Center nursing team, Bionutrition Core, research pharmacy, and nuclear medicine technologists for the excellent support they provided; C. Ronald Kahn for his advice in experimental design; and our volunteers for their commitment to the studies. This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), DK075112 and DK075116; National Institutes of Health (NIH) Grants DK081604 and DK046200 (to A.M.C.); RR025757; Grant P30 DK036836 from the NIDDK; Clinical Translational Science Award UL1RR025758 to Harvard University and the BIDMC from the National Center for Research Resources, Harvard Catalyst/The Harvard Clinical and Translational Science Center (NIH Award UL1 RR 025758 and financial contributions from Harvard University and its affiliated academic health care centers); and the Eli Lilly Foundation.
Footnotes
Conflict of Interest
No conflicts of interest are reported
References
- 1.Collaborators TG 2015 O. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. New England Journal of Medicine. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of All-Cause Mortality With Overweight and Obesity Using Standard Body Mass Index Categories: A Systematic Review and Meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, et al. Identification and Importance of Brown Adipose Tissue in Adult Humans. New England Journal of Medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JMAFL, Kemerink GJ, Bouvy ND, et al. Cold-Activated Brown Adipose Tissue in Healthy Men. New England Journal of Medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 5.Brendle C, Werner MK, Schmadl M, la Fougère C, Nikolaou K, Stefan N, et al. Correlation of Brown Adipose Tissue with Other Body Fat Compartments and Patient Characteristics: A Retrospective Analysis in a Large Patient Cohort Using PET/CT. Academic Radiology. 2017 doi: 10.1016/j.acra.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Matsushita M, Yoneshiro T, Aita S, Kameya T, Sugie H, Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes. 2014;38:812–817. doi: 10.1038/ijo.2013.206. [DOI] [PubMed] [Google Scholar]
- 7.Orava J, Nuutila P, Lidell ME, Oikonen V, Noponen T, Viljanen T, et al. Different Metabolic Responses of Human Brown Adipose Tissue to Activation by Cold and Insulin. Cell Metabolism. 2011;14:272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 8.Chen KY, Brychta RJ, Linderman JD, Smith S, Courville A, Dieckmann W, et al. Brown Fat Activation Mediates Cold-Induced Thermogenesis in Adult Humans in Response to a Mild Decrease in Ambient Temperature. The Journal of Clinical Endocrinology & Metabolism. 2013;98:E1218–E1223. doi: 10.1210/jc.2012-4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. Journal of Clinical Investigation. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanssen MJW, Hoeks J, Brans B, van der Lans AAJJ, Schaart G, van den Driessche JJ, et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat Med. 2015;21:863–865. doi: 10.1038/nm.3891. [DOI] [PubMed] [Google Scholar]
- 11.van der Lans AAJJ, Wierts R, Vosselman MJ, Schrauwen P, Brans B, van M Lichtenbelt WD. Cold-activated brown adipose tissue in human adults: methodological issues. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 2014;307:R103–R113. doi: 10.1152/ajpregu.00021.2014. [DOI] [PubMed] [Google Scholar]
- 12.Chen KY, Cypess AM, Laughlin MR, Haft CR, Hu HH, Bredella MA, et al. Brown Adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): Recommendations for Standardized FDG-PET/CT Experiments in Humans. Cell Metabolism. 2016;24:210–222. doi: 10.1016/j.cmet.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sylow L, Kleinert M, Richter EA, Jensen TE. Exercise-stimulated glucose uptake — regulation and implications for glycaemic control. Nat Rev Endocrinol. 2017;13:133–148. doi: 10.1038/nrendo.2016.162. [DOI] [PubMed] [Google Scholar]
- 14.Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
- 15.Hany TF, Gharehpapagh E, Kamel EM, Buck A, Himms-Hagen J, von Schulthess GK. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur J Nucl Med. 2002;29:1393–1398. doi: 10.1007/s00259-002-0902-6. [DOI] [PubMed] [Google Scholar]
- 16.Cohade C, Osman M, Pannu HK, Wahl RL. Uptake in Supraclavicular Area Fat (“USA-Fat”): Description on 18F-FDG PET/CT. J Nucl Med. 2003;44:170–176. [PubMed] [Google Scholar]
- 17.Cypess AM, Chen Y-C, Sze C, Wang K, English J, Chan O, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A. 2012;109:10001–10005. doi: 10.1073/pnas.1207911109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, McGehee S, et al. Mapping of human brown adipose tissue in lean and obese young men. PNAS. 2017 doi: 10.1073/pnas.1705287114. 201705287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ooijen AMJC, Westerterp KR, Wouters L, Schoffelen PFM, van Steenhoven AA, van Lichtenbelt WDM. Heat Production and Body Temperature During Cooling and Rewarming in Overweight and Lean Men. Obesity. 2006;14:1914–1920. doi: 10.1038/oby.2006.223. [DOI] [PubMed] [Google Scholar]
- 20.Haman F, Mantha OL, Cheung SS, DuCharme MB, Taber M, Blondin DP, et al. Oxidative fuel selection and shivering thermogenesis during a 12- and 24-h cold-survival simulation. Journal of Applied Physiology. 2016;120:640–648. doi: 10.1152/japplphysiol.00540.2015. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang A-H, et al. Beige Adipocytes are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic Peroxisome Proliferator-activated Receptor γ (PPARγ) Activation of Epididymally Derived White Adipocyte Cultures Reveals a Population of Thermogenically Competent, UCP1-containing Adipocytes Molecularly Distinct from Classic Brown Adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, et al. A Creatine-Driven Substrate Cycle Enhances Energy Expenditure and Thermogenesis in Beige Fat. Cell. 2015;163:643–655. doi: 10.1016/j.cell.2015.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blondin DP, Labbé SM, Tingelstad HC, Noll C, Kunach M, Phoenix S, et al. Increased Brown Adipose Tissue Oxidative Capacity in Cold-Acclimated Humans. The Journal of Clinical Endocrinology & Metabolism. 2014;99:E438–E446. doi: 10.1210/jc.2013-3901. [DOI] [PMC free article] [PubMed] [Google Scholar]


