Abstract
The transient receptor potential (TRP) channel TRPV4 participates in multiple biological processes, and numerous TRPV4 mutations underlie several distinct and devastating diseases. Here we present the structure of Xenopus tropicalis TRPV4 at 3.8 Å resolution by cryo-electron microscopy (cryo-EM). The ion conduction pore contains an intracellular gate formed by the inner helices, but lacks any extracellular gate in the selectivity filter, as is detected in other TRPV channels. Anomalous X-ray diffraction analyses identify a single ion-binding site in the selectivity filter, explaining non-selectivity. Structural comparison with other TRP channels and distantly related voltage-gated cation channels reveals an unprecedented, unique packing interface between the voltage sensor-like domain and the pore domain, suggesting distinct gating mechanisms. Moreover, our structure begins to provide mechanistic insights to the large set of pathogenic mutations and to offer new opportunities for drug development.
Keywords: Membrane proteins, Ion channels, TRP channels, TRPV4, cryo-electron microscopy, X-ray crystallography
Introduction
Transient receptor potential (TRP) ion channels are central to a vast array of physiological functions, responding to a wide spectrum of physical and chemical stimuli1–5. On the basis of amino acid sequence similarity, the mammalian TRP channel genes are grouped to six subfamilies, TRPA, TRPC, TRPM, TRPML, TRPP, and TRPV2. The TRP subfamily V member 4 (TRPV4) channel, a Ca2+-permeable, nonselective cation channel, is expressed in multiple tissues and is implicated in many biological processes, including osmoregulation6–10, bone homeostasis11, control of vascular tone12, regulation of adipose thermogenesis and inflammation13, and nociception14,15. The biological versatility of TRPV4 is enabled by polymodal gating behavior: the channel is activated by stimuli ranging from cell swelling8–10, shear stress16,17, and moderate heat18,19 to chemical agonists20,21. Over fifty TRPV4 mutations have been linked to a variety of inherited diseases, including osteoarthropathy, skeletal dysplasias (SD), and neurological disorders, such as congenital distal spinal muscular atrophy (CDSMA) and Charcot-Marie-Tooth disease type 2C (CMT2C)22,23. Therefore TRPV4 presents as a prominent potential drug target for the treatment of a broad range of diseases.
Recently, structures of the TRPV subfamily members including TRPV124–26, TRPV227,28, and TRPV629,30 determined at near-atomic resolution by single-particle cryo-EM or X-ray crystallography have revealed the overall architecture of these tetrameric channels. Each subunit consists of a transmembrane region resembling that of other tetrameric voltage-gated ion channels (VGIC) and a cytosolic portion characteristic for TRPV channels. Moreover, structures of TRPV1 in multiple functional states have illuminated two constrictions along the ion permeation pathway, an upper gate at the selectivity filter and a lower gate at the inner helix bundle-crossing region, as well as conformational changes at these two gates upon channel activation24,25. Interestingly, although the architecture of all TRPV channels may be similar, intriguing variations have been noted. In the TRPV1 and TRPV2 structures, like in many VGIC structures, the S1–S4 domain (termed the voltage-sensor domain in VGICs) packs against the pore domain S5–S6 from an adjacent subunit of the tetramer (domain-swapped) rather than from the same subunit (non-swapped). In contrast, in structures of TRPV6 and its variants, both domain-swapped and non-swapped transmembrane arrangements have been observed29,30. Recent studies have also revealed non-swapped transmembrane architecture in multiple VGICs including Eag131, Slo132 and HCN33. These differences and variations underscore structural divergence between closely related tetrameric cation channels, and potentially distinct gating mechanisms.
Functionally, TRPV4 has been one of the most intensively studied TRP channels owing to its essential physiological and pathological roles22,23. However, structural information has been limited to crystallographic studies of isolated cytoplasmic domains34,35 and a cryo-EM model at very low (35 Å) resolution36. To better understand the structural basis underlying its unique properties, we set out to determine high-resolution structures of TRPV4. Here we present the cryo-EM structure of TRPV4 determined at 3.8 Å resolution and X-ray crystallographic analyses of the ion-binding sites in the pore. These combined structural approaches illuminate previously unattainable features that are unique to TRPV4 and provide a molecular map for disease mutations. More broadly, we show how single-particle cryo-EM structure determination at near atomic resolution, together with anomalous X-ray diffraction even at limited resolution, illustrates a powerful duo for the investigation of ion channel structure and function.
Results
Structure determination
For structural analyses, we evaluated multiple TRPV4 orthologs and identified the Xenopus tropicalis channel, which shares 78% sequence identity with the human protein, as a promising candidate. We modified the full-length channel by deleting the unstructured N- and C- terminal regions and generated a truncation construct consisting of residues 133–797, which yielded crystals (Fig. 1a). This construct contains essentially all functional domains, including the proline-rich domain (PRD), the ankyrin-repeat domain (ARD), the linker domain, the transmembrane domain, and the TRP domain. To improve X-ray diffraction, we further eliminated a glycosylation site by introducing a point mutation N647Q. The resulting construct TRPV4cryst (133–797 N647Q) permitted crystallographic analyses of ion binding and cryo-EM structure determination at near atomic resolution (see below).
Fig. 1 |. A TRPV4 construct for structural analyses.
a, Domain organization of the Xenopus tropicalis TRPV4 channel. The crystallization and cryo-EM construct comprises residues 133–797 with mutation N647Q eliminating glycosylation.
b, 86Rb+ flux in transfected CosM6 cells. Activation of the full-length wild type channel (black squares, n = 3, mean ± SEM) and the truncated construct TRPV4cryst (gray squares, n = 3, mean ± SEM) by 1 μM GSK101 is evaluated by relative efflux of 86Rb+, in comparison with cells transfected with an empty vector (empty circles and squares, n = 3, mean ± SEM). Squares and circles indicate measurements with and without GSK101, respectively.
c–d, TRPV4 (c) and TRPV4cryst (d) currents in an inside-out membrane patch upon application of GSK101 (50 nM in C and 5 μM in D) from the cytoplasmic side. Unitary openings of ~ 8 pA at −30 mV are shown on the middle panels. The two first channel opening events are labeled as O1 and O2. All point histograms at the lower panels represent 5 seconds of traces. Wild type Xenopus TRPV4 has a unitary conductance of 252 ± 22 pS at −30 mV membrane in symmetric 150 mM KCl, while TRPV4cryst under the same conditions has a conductance of 264 ± 19 pS.
Like the full-length wild type channel, TRPV4cryst generates active channels when expressed in mammalian cells. The channels are robustly activated by the specific TRPV4 agonist GSK1016790A (GSK101) in intact cells, as detected by 86Rb+ efflux (Fig. 1b and Supplementary Fig. 1), and in excised patches (Figs. 1c and 1d and Supplementary Fig. 2). TRPV4cryst has essentially identical single-channel conductance (272 ± 13 pS in symmetrical 150 mM KCl) to that of the full-length channel (280 ± 11 pS in symmetrical 150 mM KCl) and maintains ion permeation and blockage properties (Figs. 1c and 1d and Supplementary Fig. 2). We observed less activity of TRPV4cryst constructs in CosM6 cells, both in 86Rb+ efflux experiments and in patch-clamp recordings, which appears to arise from impeded trafficking of expressed TRPV4cryst to the plasma membrane (PM) (Supplementary Fig. 2a). Consistently, previous studies have shown that the C-terminal tail region, which is absent in TRPV4cryst, is essential for channel localization to the PM37,38. Together these results indicate that TRPV4cryst retains similar pharmacological activation and inhibition and biophysical pore properties to wild type TRPV4, but does not traffic efficiently to the cell membrane.
The cryo-EM structure of TRPV4cryst was determined at an overall resolution of 3.8 Å with C4 symmetry imposed (Supplementary Fig. 3, Table 1). Local resolution estimates indicate that the transmembrane, TRP, and linker domains were better resolved than the ARD. The crystal structure of the ARD (PDB ID: 4DX2) was docked into the density map and adjusted34, and the rest of the channel was built de novo using bulky side chains to register the amino acid sequence. Refinement of the atomic coordinates was performed in real and reciprocal space against one of the independently calculated half-maps (working) and validated against the other half-map (free) as well as the full map (Supplementary Fig. 4). The refined model has good geometry and fits well into the electron density map (Supplementary Fig. 4).
Table 1.
Cryo-EM data collection, refinement and validation statistics
| EMD-7075, PDB 6BBJ | |
|---|---|
| Data collection and processing | |
| Magnification | 22,500 |
| Voltage (kV) | 300 kV |
| Electron exposure (e–/Å2) | 32 |
| Defocus range (μm) | −1.0 to −2.5 |
| Pixel size (Å) | 1.088 |
| Symmetry imposed | C4 |
| Initial particle images (no.) | 85,253 |
| Final particle images (no.) | 72,684 |
| Map resolution (Å) | 3.84 |
| FSC threshold | FSC = 0.143 |
| Map resolution range (Å) | 400–3.84 |
| Refinement | |
| Initial model used | PDB code: 4DX2 |
| Model resolution (Å) | 4.1 |
| FSC threshold | FSC = 0.5 |
| Model resolution range (Å) | 3.80 |
| Map sharpening B factor (Å2) | −150 |
| Model composition | |
| Nonhydrogen atoms | 19,496 |
| Protein residues | 2,400 |
| Ligands | 0 |
| B factors (Å2) | |
| Protein | 97.9 |
| Ligand | |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.005 |
| Bond angles (°) | 1.1 |
| Validation | |
| MolProbity score | 1.44 |
| Clashscore | 3.59 |
| Poor rotamers (%) | 0.19 |
| Ramachandran plot | |
| Favored (%) | 95.8 |
| Allowed (%) | 4.2 |
| Disallowed (%) | 0.0 |
Overall architecture
The symmetric tetramer architecture of TRPV4 is similar to that of other TRPV channels, including TRPV1, TRPV2, and TRPV6 (Figs. 2a and 2b). The transmembrane domain resembles that of VGICs, comprising six membrane-spanning segments S1 to S6. The first four helices constitute a structural unit, the S1–S4 domain, analogous to the voltage-sensor domain in VGICs. Four S1–S4 domains surround the central ion conduction pore formed by S5 and S6 and the intervening pore loops from four identical subunits. Similar to TRPV1, TRPV2 and many VGICs, TRPV4 adopts a domain-swapped arrangement between the S1–S4 domain and the pore domain S5–S6. In contrast to TRPV1 and TRPV2, the connector between these two domains, the S4–S5 linker adopts an ordered loop structure rather than an α-helix (Fig. 2c). In VGICs, the S4–S5 linker usually forms an α-helical segment functioning as a mechanical lever to couple voltage sensor activation and pore opening39–41, and the absence of this α-helical coupler results in different gating behavior31,33. In addition, the relative position of the S1–S4 domain to the pore domain in TRPV4 is distinct from that of TRPV1 and TRPV2. As we will discuss later, the packing interface between the S1–S4 and pore domains in TRPV4 is unique among TRP channels and distantly related VGICs.
Fig. 2 |. Cryo-EM structure of TRPV4.
a, Cryo-EM reconstruction of the tetrameric channel. Each subunit is uniquely colored, and the membrane boundary is indicated as gray lines.
b, Orthogonal views of the overall structure. Subunits are colored as in (a).
c, Structure of a single subunit. Each domain is labeled and uniquely colored.
Like other TRPV channels, the intracellular domains of each subunit of the tetrameric TRPV4 channel contain an N-terminal ARD followed by a linker domain that precedes the S1–S4 domain (Fig. 2c). In the linker domain, two β-strands β1 and β2, in conjunction with a C-terminal β-strand β3, constitute a three-stranded β-sheet, which not only tethers the N-terminal ARD to the C-terminus within each subunit, but also interacts with the ARD from an adjacent subunit. These long-range tethering and packing interactions create an important subunit-subunit assembly interface24,27. Following the β-strands, a helix-turn-helix (HTH) motif and a pre-S1 helix snugly accommodate the C-terminal TRP helix (Supplementary Fig. 5), a conserved sequence motif among TRP channels that follows the inner helix S6 and regulates channel activity42. The amphipathic TRP helix runs parallel to the membrane and is in close contact with the ordered S4–S5 linker, which connects the S1–S4 and pore domains. Such arrangements strategically place the TRP helix between the cytosolic and transmembrane domains, where distinct physical or chemical stimuli exert their forces. The TRP helix is thus well positioned to orchestrate conformational changes between these two regions to influence the gate.
Ion permeation pathway
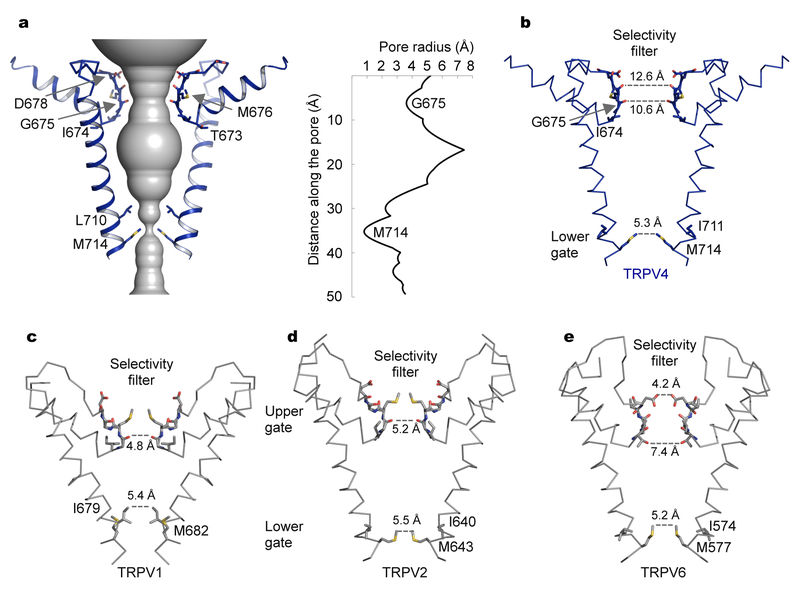
We calculated the pore radius along the ion permeation pathway using the program HOLE43, and observed a single constriction, the lower gate, where inner helices cross at the intracellular side. Side chains of M714 define the narrowest point measuring 5.3 Å in diameter, sufficient to prevent ion passage (Figs. 3a and 3b), indicating that the structure represents a closed conformation of the channel. This lower gate is a conserved structural feature among TRPV channels and the dimension of the TRPV4 gate is comparable to those of the closed lower gates in other TRPV channels (Figs. 3c–e). The corresponding methionine residues in TRPV2 (M643)27 and TRPV6 (M577)29 constitute the lower gates, whereas in TRPV1, side chains of the equivalent methionine (M682) point away from the central pathway and instead the nearby I679 defines the lower gate24,25. Interestingly, despite the high amino acid sequence conservation of S6 helices among TRPV channels, the lower gates are constructed by different residues (Figs 3b–e and Supplementary Fig. 5).
Fig. 3 |. Ion permeation pathway.
a, The ion conduction pore of TRPV4, shown as gray surface calculated with HOLE. Only two opposing subunits are shown for clarity. A single constriction formed by M714 residues defines the intracellular gate in S6. The pore radius along the permeation pathway is shown in the right panel.
b, Details of the TRPV4 pore. Comparison with other TRPV pore structures shown in (c–e) reveals a closed lower gate and the absence of an upper gate in TRPV4.
c–e, The pore structures of TRPV1 (PDB: 3J5P, c), TRPV2 (PDB: 5AN8, d), and TRPV6 (PDB: 5IWK, e), showing two constrictions: an upper gate in the selectivity filter and a lower gate in S6.
In addition to the lower intracellular gate in S6, other TRPV channels have a second constriction in the selectivity filter, the so-called upper gate, close to the extracellular side. Structures of TRPV1 in closed and open states have indicated that both the upper and lower gates undergo conformational changes upon channel activation, suggesting a dual gating mechanism24,25. Indeed, the selectivity-filter gate in TRPV1 responds to activation by heat and chemical ligands44–46. In both TRPV1 and TRPV2, side chains of methionine residues form a narrow, hydrophobic constriction and the carbonyl atoms of glycine residues define the narrowest point in the selectivity filter (4.8 Å in TRPV1 and 5.2 Å in TRPV2) (Figs. 3c and 3d). The selectivity-filter sequences (TIGMGD/E) are well conserved among TRPV1, TRPV2, and TRPV4 channels (Supplementary Fig. 5). However, the selectivity filter is exceptionally wide in TRPV4 (Figs. 3b–3e). In our TRPV4 structure, the narrowest point in this region, defined by the carbonyl atoms of the corresponding glycine residues G675, is 10.6 Å in diameter. This distance is larger than the equivalent distance in the open, activated TRPV1 structure (7.6 Å) and is sufficiently large to conduct even hydrated cations such as Na+, K+, and Ca2+, which have effective diameters in the range of 6–10 Å. Interestingly, the cryo-EM structure of a full-length TRPV2 channel28, albeit at low resolution, showed wider openings at both the upper and lower gates compared with a truncated construct27, suggesting a potential dual gating mechanism for TRPV2. In marked contrast, the wide-open selectivity filter of TRPV4 in a closed state suggests the absence of any upper gate. Consistent with this notion, no physiological stimuli have been identified that work through the selectivity filter of TRPV4 to activate the channel.
Ion binding in the pore
TRPV4cryst crystals grew in the absence of agonists or antagonists and belonged to space group P4212, with each asymmetric unit containing a single subunit of the tetrameric channel. Despite extensive optimization, native crystals diffracted X-rays to a limited resolution of ~5 Å, which precluded atomic structure determination by X-ray crystallography. However, using a single subunit from the cryo-EM structure as the search model, we obtained molecular replacement solutions for our collected X-ray diffraction datasets. The tetrameric channel structure generated by a crystallographic four-fold symmetry is essentially the same as the cryo-EM structure, strengthening our interpretation of the cryo-EM reconstruction. Moreover, anomalous X-ray diffraction allows unambiguous determination of the locations of certain atoms in crystal structures, owing to the characteristic anomalous dispersion near their X-ray absorption edges. Analogous methods in single-particle cryo-EM currently do not exist. Therefore we used X-ray crystallography to identify ion-binding sites along the permeation pathway.
TRPV4 is a nonselective cation channel with higher permeability for divalent ions such as Ca2+, Ba2+ and Mg2+ than for monovalent ions such as Na+, Cs+ and K+47. To analyze the ion binding properties of the pore, we selected Cs+ and Ba2+ as representative monovalent and divalent permeant ions (Supplementary Fig. 2), because these ions have strong signals in anomalous X-ray diffraction. We also examined gadolinium ions (Gd3+), which provide strong anomalous X-ray diffraction signals and block many TRP channels including TRPV4 (Supplementary Fig. 2). We co-crystallized TRPV4cryst with Cs+ and Ba2+, and soaked apo crystals with Gd3+, and then measured X-ray diffraction at wavelengths close to the respective absorption edges to determine ion-binding sites in the crystals (Table 2). Even at limited resolutions, the anomalous difference maps for Cs+, Ba2+ and Gd3+ clearly showed single peak representing the cation-binding site, located on the central pore axis in the vicinity of the carbonyl oxygen atoms of G675 (Figs. 4a–d). The distance between diagonal oxygen atoms is ~10.6 Å, as in the cryo-EM structure, and appears to be large enough to accommodate hydrated ions at this site.
Table 2.
Data collection and refinement statistics (molecular replacement)
| Cs+ (PDB 6C8F) |
Ba2+ (PDB 6C8G) |
Gd3+ (PDB 6C8H) |
|
|---|---|---|---|
| Data collection | |||
| Space group | P4212 | P4212 | P4212 |
| Cell dimensions | |||
| a, b, c (Å) | 164.4, 164.4, 101.8 | 165.5, 165.5, 102.7 | 164.8, 164.8, 101.5 |
| α, β, γ (°) | 90, 90, 90 | 90, 90, 90 | 90, 90, 90 |
| Resolution (Å) | 6.5 (6.73–6.50)a | 6.3 (6.52–6.30) | 6.5 (6.73–6.50) |
| Rmerge | 0.072 (0.948) | 0.085 (1.349) | 0.068 (1.354) |
| I/σ(I) | 19.6 (2.0) | 30.7 (2.1) | 23.3 (1.6) |
| CC1/2 | 0.999 (0.733) | 0.999 (0.667) | 0.998 (0.665) |
| Completeness (%) | 99.6 (100) | 99.8 (100) | 99.8 (100) |
| Redundancy | 7.7 (7.0) | 9.9 (10.7) | 7.8 (7.2) |
| Refinement | |||
| Resolution (Å) | 20 – 6.5 | 20 – 6.3 | 20 – 6.5 |
| No. reflections | 2589 | 2909 | 2594 |
| Rwork / Rfree | 0.364 / 0.373 | 0.370 / 0.365 | 0.373 / 0.373 |
| No. atoms | |||
| Protein | 4874 | 4874 | 4874 |
| Ligand/ion | 1 (Cs+) | 1 (Ba2+) | 1 (Gd3+) |
| Water | 0 | 0 | 0 |
| B factors | |||
| Protein | 100.0 | 100.0 | 100.0 |
| Ligand/ion | 100.0 | 100.0 | 100.0 |
| Water | |||
| R.m.s. deviations | |||
| Bond lengths (Å) | 0.005 | 0.005 | 0.005 |
| Bond angles (°) | 1.1 | 1.1 | 1.1 |
A single crystal was used for each structure.
Values in parentheses are for highest-resolution shell.
Fig. 4 |. Ion binding in the pore.
a–c, Side views of the TRPV4 pore with a bound Cs+ (magenta, a), Ba2+ (Orange, b), and Gd3+ (Cyan, c) respectively. Only two opposing subunits are shown for clarity. Ions are shown as spheres. The anomalous difference electron densities for Cs+ (magenta, a), Ba2+ (Orange, b), and Gd3+ (Cyan, c) are shown as meshes, contoured at 5.0 σ, 7.0 σ, and 6.0 σ respectively.
d, Top view of the pore with a bound Gd3+. All four subunits are shown and the contour level is the same as in (c).
e, Top view of the pore showing strong densities potentially corresponding to tightly bound lipid molecules.
f, Side view of the densities as in (e).
The locations are only slightly different for these ions with various charges and permeation and blockage properties. No additional anomalous difference electron densities are observed in the outer vestibule region. Thus, in our TRPV4 structures, the wide-open selectivity filter coordinates a single hydrated cation at nearly the same location, regardless of ion valency. This clearly explains the non-selectivity of the channel. For a filter with a single ion-binding site, any entering ion, be it Ca2+, K+ or Na+, can spontaneously exit to either side of the ion-binding site. In other words, it can permeate. However, for a filter with multi-ion binding sites, an entering ion with lower affinity would be stopped by a previously bound ion with higher affinity along the permeation path, and therefore it would exit to the same side as it enters. That is, it cannot permeate. Multi-ion occupancy appears essential for selectivity, elegantly demonstrated in the NaK channel and its variants48–51. The exception we observed for Gd3+, which blocked the channel at millimolar concentration (Supplementary Fig. 2e), is likely explained by its abnormally high affinity for the filter owing to its carried charge.
In our cryo-EM reconstruction, we noticed additional robust densities in the selectivity-filter region that could not be attributed to polypeptide (Figs. 4e and 4f). X-ray diffraction analyses eliminate the possibility of bound ions accounting for these densities. Therefore we attribute these large densities to additional tightly-bound molecules that co-purify with the channel protein. We suggest that these additional molecules are likely to be lipids, consistent with our observation that additionally supplemented lipids are necessary to maintain protein stability and prevent aggregation during purification. Intriguingly the additional densities are intercalated between the selectivity-filter loops and appear to be an integral part of the selectivity filter, filling the otherwise empty space created by the wide-open selectivity filter (Figs. 4e and 4f). Although lipids are well-recognized modulators of ion channel activity and direct interaction of lipids and the ion-conduction pore may not be uncommon52–54, putative lipid binding in the pore represents an unprecedented feature in TRPV4, which, to our knowledge, has not been noted in other tetrameric cation channels. The identity and functional role of these co-purified molecules must await further characterization.
Unique arrangement of the S1–S4 domain
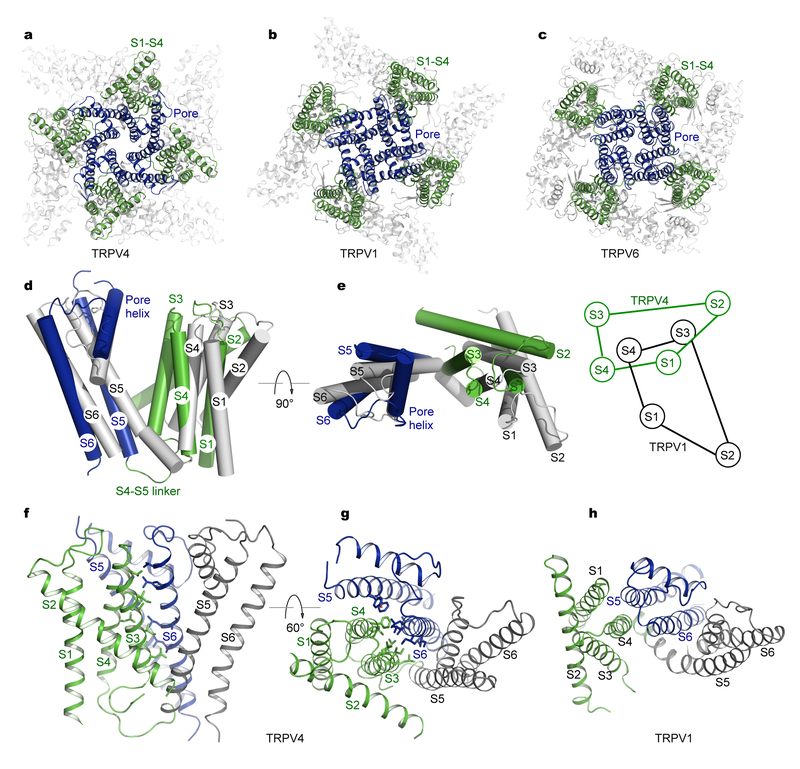
In TRP channels and in VGICs, the peripheral S1–S4 domains and the central pore domains S5–S6 can be arranged in a domain-swapped or non-swapped manner, leading to distinct activation mechanisms31,33. Among available TRPV channel structures, TRPV1 and TRPV2 are domain-swapped24,27 whereas TRPV6 can be domain-swapped or non-swapped29,30. Nonetheless, the overall location and orientation of the S1–S4 and S5–S6 domains are similar and these domains align well among TRPV1, TRPV2, and TRPV6 channels. Like TRPV1 and TRPV2, TRPV4 adopts a domain-swapped configuration but shows striking differences in the packing of the S1–S4 against the S5–S6 transmembrane domains (Figs. 5a–c).
Fig. 5 |. Structural comparisons of the transmembrane domains of TRPV channels.
a–c, Structures of TRPV4 (a), TRPV1 (b) (PDB: 3J5P), and TRPV6 (c) (PDB: 5IWK). The S1–S4 domains and the pore domains are highlighted in green and blue respectively.
d and e, Orthogonal views of TRPV4 (green and blue) and TRPV1 (gray) transmembrane domains aligned by their pore domains (all four S5 and S6 helices). Also shown in (e) is a schematic diagram illustrating the re-orientation of the S1–S4 domain in TRPV4.
f and g, Two views showing extensive packing interactions between helices S3 and S4 from one subunit and helices S5 and S6 from an adjacent subunit in TRPV4. Side chains of tightly packed residues are shown as sticks.
h, A similar view as in (g) for TRPV1, showing that only S4 makes considerable contacts with the pore domain from an adjacent subunit.
An objective comparison of structural differences between TRPV4 and other TRPV channels, including TRPV1, TRPV2, and TRPV6, is achieved by superimposing Cα atoms of the tetrameric pore domains using all four S5 and S6 helices. From the superposition, it becomes apparent that the S1–S4 domains in TRPV4 are organized in a completely novel manner (Figs. 5d and 5e). While the S1–S4 domain has a similar four-helix bundle structure as in other TRPV channels (Supplementary Fig. 6), it rotates ~90° counterclockwise around the S4 helix, as viewed from the extracellular side (Figs. 5d and 5e), in comparison with the S1–S4 domain in TRPV1. This domain reorientation generates a unique packing interface between the S1–S4 and the pore domains (Figs. 5f–5h). S3 moves towards the central pore and, together with S4, creates an extensive packing interface with S5–S6 of the adjacent subunit. Remarkably, S3 intimately contacts S6 for virtually its entire length and bulky hydrophobic side chains from S3 and S6 interdigitate like a zipper (Figs. 5f and 5g). This new feature, together with the absence of an α-helical coupling element in the S4–S5 linker, raises the possibility that gating stimuli acting on S1–S4 could act through S3 directly exerting force on S6 to open the intracellular bundle-crossing gate in S6, a novel mechanism of transducing the gating signal. Consistent with this idea, previous studies have suggested that agonist 4αPDD activates TRPV4 through binding to a pocket formed by S3 and S455. Conceptually, structural perturbation in S3 (e.g. helix bending) could directly propagate to S6 and thus pull open the intracellular gate.
Structural insights into channelopathies
Human TRPV4 mutations result in a wide spectrum of autosomal dominant diseases ranging from disability to lethality, which can be divided into three distinct groups: osteoarthropathy, skeletal dysplasias, and peripheral neuropathies22 (Fig. 6a). We mapped known pathogenic human mutations onto our Xenopus TRPV4 channel cryo-EM structure. Most of these disease mutations are conserved between the human and frog orthologs (Supplementary Fig. 5). To be consistent with the literature and for ease of understanding, residues discussed hereafter are numbered according to the human TRPV4 sequence.
Fig. 6 |. A map for TRPV4 channelopathies.
a, Disease mutations are mapped onto the structure of a single subunit (gray ribbon). Mutations causing skeletal dysplasias (SD), peripheral neuropathy, and osteoarthropathy are shown as red, blue, and green spheres, respectively. For consistency, labeled residues are numbered according to the human TRPV4 sequence.
b, Osteoarthropathy mutations in finger 3. F273 (shown as green sticks) interacts with a network of aromatic residues (shown as sticks) both within the same subunit and from the β sheet (shown in cyan) of an adjacent subunit.
c, SD mutations in the S4–S5 linker and its surrounding area.
d, SD mutations in the pore helices S5 and S6.
e, Locations of SD (red) and neuropathy mutations (blue) in the tetrameric channel.
The osteoarthropathy mutations G270V, R271P, and F273L (green spheres), located at the finger 3 loop in the ARD, reduce channel activity56. The mutant channels expressed in mammalian cells showed impaired membrane localization, reduced activation by agonists, and lack of activation by hypotonic stress56. Structurally, finger 3 is strategically positioned at the cytoplasmic assembly interface between subunits (Fig. 6b). This is a shared structural feature among TRPV channels, in which finger 3 may affect channel gating by enabling cooperativity between subunits24,27. In TRPV4, the side chain of F273 interacts with side chains of a network of aromatic residues both within the same subunit and from an adjacent subunit (Fig. 6b). Our structure immediately offers a plausible explanation for how the F273L substitution might weaken inter-subunit interactions, thus impairing gating transitions. G270V and R271P in finger 3 may introduce a similar destabilizing effect.
The second group of mutations causes skeletal dysplasias characterized by irregularities in bone and cartilage growth, resulting in an abnormal skeleton with a short trunk, with disease conditions ranging from mild to lethal phenotypes57–61. These mutations (red spheres), which increase channel activity, occur throughout the protein sequence but concentrate in three prominent regions. One location is in the S4–S5 linker and surrounding region that are in close contact with the highly conserved TRP helix (Fig. 6c), which immediately follows the C-terminal end of the S6 intracellular gate. Conceptually, conformational perturbations in the S4–S5 linker and hence the TRP helix could directly influence the S6 gate. This is consistent with the observation that this region harbors many pathological mutations, including S542Y, F592L, R594H, L596P, G600W, Y602C, I604M, and T740I (Fig. 6c). The second location is in the pore domain S5–S6 and these mutations might directly alter the S6 gate conformation (Fig. 6d). The third location includes mutations that are scattered in amino acid sequence but cluster near the cytoplasmic assembly interface between subunits, reinforcing the importance of this interface in channel gating (Fig. 6e).
The third group of mutations (blue spheres) results in peripheral neuropathies associated with degeneration of motor neurons and peripheral nerves62–65. In the primary sequence, most of these mutations, except for T701I in S6, are in the cytosolic N-terminal domains interspersed with mutations causing other diseases. Structurally, these mutations all segregate at the outer perimeter of the tetrameric channel (Fig. 6e). This leads us to speculate that, in addition to altering channel activity, these mutations might affect channel interactions with other protein partners or signaling molecules, which in turn could contribute to the broad spectrum of disease phenotypes.
Discussion
Crystals of eukaryotic membrane proteins with multiple domains often diffract X-rays to limited resolution owing to their intrinsic complexity, thus preventing atomic structure determination. Single-particle cryo-EM has proven that many membrane protein structures, apparently unsolvable by X-ray crystallography, can be resolved to near atomic resolution. By additionally measuring anomalous X-ray diffraction even at relatively low resolution, we can access otherwise unattainable information such as conclusively determined ion-binding sites. Such combination of anomalous X-ray diffraction and single-particle cryo-EM, as illustrated in our study, may offer significant advantages for the illumination of structure and function of other ion channels and transporters.
Motivated by the physiological complexity of TRPV4 channels, and the wide spectrum of disease pathologies caused by TRPV4 mutations, we carried out cryo-EM and X-ray structural analyses to illuminate the molecular basis of TRPV4 function. Despite the recent structure determination of several closely-related TRPV channels, our TRPV4 structure illustrates multiple unusual structural features of this unique channel.
First, the transmembrane domains are arranged in an unconventional fashion. The S1–S4 voltage sensor-like domain engages both S3 and S4 to form extensive interactions with the pore domain S5–S6 (Fig. 5). In particular, S3 uses almost its entire length to tightly pack against S6 (Figs. 5f and 5g), an aspect that has not been previously observed in other TRP channels or related VGICs. Structures of TRPV1 determined in distinct functional states suggest that upper and lower gates along the ion permeation pathway undergo conformational changes while the S1–S4 domains remain static during channel activation24,25. However, in TRPV4, the distinct S1–S4 domain arrangement suggests an alternative gating mechanism, in which structural perturbations in S3 would directly exert force on S6 to open the intracellular gate, given the tight packing interactions between S3 and S6. It is also important to note the possibility that this unique S1–S4 arrangement originates from the truncation construct TRPV4cryst. Certainly, understanding the functional and biophysical consequences of this unexpected arrangement needs further investigation.
Second, the ion conduction pore of TRPV4 also possesses features that differ from other closely related TRPV channels. The selectivity filter is in such an expanded conformation that additional molecules occupy the otherwise empty space between selectivity-filter loops (Figs. 4e and 4f). These molecules, which we tentatively identify as lipids, and their functional relevance are important questions to pursue further, and might reveal new roles of lipids in ion channel regulation beyond our current understanding. Anomalous X-ray diffraction analyses unambiguously identify a single ion-binding site in the selectivity filter regardless of the charges carried by the bound ion. The wide selectivity filter accommodates a single hydrated ion at nearly the same location, thus providing a straightforward explanation for the nonselective nature of the pore.
The intriguingly wide spectrum of disease phenotypes and surprisingly large set of mutations associated with a single channel protein TRPV4 underscore its complex cellular functions and essential physiological roles. Elucidating the molecular and cellular mechanisms underlying these pathogenic mutations is paramount to understanding channel function and designing effective therapies. Our atomic model represents a fundamental step in this direction and establishes a framework for better understanding the structural and functional consequences of these mutations. Indeed, we observe that different types of disease mutations predominantly concentrate and segregate into distinct regions in the three-dimensional structure, opening the possibility for disease-specific drug targeting to these areas. Naturally, our structure provides a molecular blueprint for such drug design approaches, however, to further facilitate drug development, it would also be important to achieve atomic structures of the channel in complex with known agonists and antagonists.
Methods
Protein expression and purification
The DNA encoding the Xenopus tropicalis TRPV4 channel (Gene ID: 100496204) was codon optimized for eukaryotic expression systems, synthesized (Genewiz, Inc.), and served as the template for subcloning. DNA fragments were ligated into a modified pPICZ-B vector (Invitrogen) with a PreScission protease cleavage site and a C-terminal GFP-His10 tag. For flux assay and electrophysiology, the corresponding gene fragments were transferred into a modified pCEH vector with a C-terminal GFP-His8 tag. The mutant N647Q was generated by site directed mutagenesis.
For large-scale expression, the construct including residues 133–797 with a point mutation N647Q was expressed in Pichia pastoris. Cells were disrupted by milling (Retsch MM400) and resuspended in lysis buffer containing 50 mM Tris pH 8.0 and 500 mM NaCl. Lysate was extracted with 2% (w/v) n-dodecyl-β-D-maltopyranoside (DDM, Anatrace) for 2 hours with stirring at 4°C and then centrifuged for 1 hour at 30,000g. Supernatant was added to cobalt-charged resin (G-Biosciences), and suspension was mixed by inversion for 3 hours. Resin was then washed with 10 column volumes of buffer containing 20 mM Tris pH 8.0, 500 mM NaCl, 10 mM imidazole, 4 mM DDM, and 0.1 mg/ml lipid - 3:1:1 POPC : POPE : POPG [1-Palmitoyl-2-Oleoyl-sn-Glycero-3-Phosphocholine, 1-Palmitoyl-2-Oleoyl-sn-Glycero-3-Phosphoethanolamine, and 1-Palmitoyl-2-Oleoyl-sn-Glycero-3-Phospho-(1’-rac-glycerol) (Avanti Polar Lipids, Inc.). Protein was digested on resin with PreScission protease at 4°C overnight to remove the C-terminal GFP-His10 tag. Flow-through was collected, concentrated and further purified by gel filtration using a Superose 6 column (GE Healthcare) pre-equilibrated with buffer containing 20 mM Tris pH 8.0, 150 mM NaCl, 5 mM dithiothreitol (DTT), 1 mM DDM and 0.1 mg/ml lipid. Peak fractions corresponding to the tetrameric TRPV4 channel were collected and concentrated to ~6 mg/ml for crystallization experiments. Protein samples for cryo-EM grid preparation were prepared following the same procedure except for the gel-filtration buffer, which contains 20 mM Tris pH 8.0, 150 mM NaCl, 5 mM DTT, 0.5 mM DDM, and 0.05 mg/ml lipid.
Cryo-EM sample preparation and imaging
3.5 μl of purified channel concentrated to ~4 mg/ml was pipetted onto glow-discharged gold Quantifoil R1.2/1.3 grids (Quantifoil). Grids were blotted for 2 seconds at ~100% humidity and flash frozen in liquid ethane using an FEI Vitrobot Mark IV (FEI). Grids were then transferred to an FEI Titan Krios (FEI) electron microscope operating at an acceleration voltage of 300 kV. Images were recorded in an automated fashion on a Gatan K2 Summit (Gatan) detector in super-resolution counting mode with a super-resolution pixel size of 0.544 Å (physical pixel size of 1.088 Å) using SerialEM66. Dose-fractionated images were recorded for 8 seconds with a per-frame exposure time of 200 ms and a dose of ~8.4 electrons per Å2 per second (~1.7 electrons per Å2 per frame) at the specimen level and ~10 electrons per pixel per second at the detector level. Total accumulated dose was ~68 electrons per Å2.
Image processing and map calculation
Dose-fractionated super-resolution images were 2 × 2 down sampled by Fourier cropping (final pixel size of 1.088 Å) for whole-frame and patch-based motion correction and dose filtration with MotionCor267. ~2,000 particles were manually selected using RELION to generate templates for automated particle selection68,69. Following automated particle selection in RELION, false positives were manually eliminated, resulting in 85,253 particles from 1,282 images. 256 × 256 pixel particle images were extracted from the motion-corrected and dose-filtered images in RELION. The parameters of the contrast transfer function were estimated by Ctffind470.
Class averages were calculated from the initial set of 85,253 particles in RELION and used for initial model generation using EMAN271. All 85,253 particles were included for RELION 3D auto-refine to generate an improved model for RELION 3D classification. Four of the five 3D classes appeared similar and were grouped together, resulting in 72,684 particles. The 72,684 particles were polished using RELION particle polishing, keeping only frames 2–19, corresponding to an accumulated dose of 32 electrons per Å2. Rotational and translational parameters for the polished particles were determined using two iterations of FREALIGN global search followed by 20 iterations of FREALIGN local search, resulting in a map that achieved a resolution of 3.8 Å as assessed by Fourier shell correlation (FSC) using the 0.143 cut-off criterion72,73. Reference maps were low-pass filtered to 6 Å for all FREALIGN refinement iterations.
Local resolution estimates were calculated using ResMap with the two FREALIGN half-maps as the inputs74. The FREALIGN map was sharpened using an isotropic b-factor of −150 Å2 prior to model building and coordinate refinement. The final map was sharpened to best fit the molecular transform of the refined atomic model by Diffmap (grigoriefflab.janelia.org/diffmap).
Model building and coordinate refinement
The structure of the isolated human TRPV4 ankryin repeat domain (PDB ID: 4DX2)34 was docked into the cryo-EM density map using UCSF CHIMERA75 and then manually rebuilt in COOT76 to fit the density. The remainder of the protein was de novo built into the map using bulky side chains to register the sequence. For coordinate refinement in real and reciprocal space, the cryo-EM density map of one of the half-maps was extracted in a new smaller unit cell that extended 5 Å from the model in all directions. Real space refinement using phenix.real_space_refine77 and reciprocal space refinement using REFMAC78 were alternated with manual model rebuilding in COOT. Geometric and secondary structure restraints were maintained throughout refinement to minimize over fitting. To monitor the effects of over fitting during refinement, the Fourier shell correlations were calculated for the half map used during refinement and the refined model (FSC work) as well as for the half map that was not used at any point during refinement and the refined model (FSC free). The final model contained residues 144–530, 536–635, 657–762 and 770–784.
Crystallization and X-ray data collection and analyses
Native crystals were grown at 4°C using hanging-drop vapor diffusion by mixing 1 μl protein with 1 μl reservoir solution containing 50 mM MES pH6.0, 100 mM NaCl, 5.5% PEG4000, and 10% glycerol. Crystals appeared overnight and grew to full size within three days. To identify ion-binding sites, crystals were grown in the presence of CsCl or BaCl2, or were soaked with buffer containing GdCl3. For Cs+-bound crystals, in the crystallization solution, 100 mM NaCl was replaced by 100 mM CsCl. To obtain Ba2+-bound crystals, protein was incubated with 10 mM BaCl2 at 4°C for 30 minutes prior to crystallization experiments. Gd3+-bound crystals were obtained by soaking native crystals in buffer containing 2 mM GdCl3, 50 mM MES pH6.0, 100 mM NaCl, 5.7% PEG4000, 10% glycerol, 1 mM DDM, and 0.1 mg/ml lipid for 1 hour. Crystals were transferred to reservoir solution supplemented with 100 mM NaCl, 0.5% PEG 4000, 1 mM DDM, 0.1 mg/ml lipid, and 20% glycerol and were immediately frozen in liquid nitrogen.
X-ray diffraction data were measured at the Advanced Photon Source beamline 24-ID-C with the wavelength at 1.71 Å for Cs+, Ba2+, and Gd3+ (Table 2), and were processed with the HKL2000 program suite79. Molecular replacement was performed using PHASER80 and a single subunit of the cryo-EM structure was used as the search model. One round of rigid-body refinement was carried out in REFMAC81 prior to calculation of the anomalous difference Fourier maps. All structural figures were prepared in PYMOL (www.pymol.org) and CHIMERA75. Sequences of TRPV channels were aligned using Clustal Omega82, and adjusted manually. The sequence alignment figure was prepared using ALINE83.
Electrophysiological recordings
CosM6 cells were transfected with 1–2 μg of wild type or truncated constructs using FuGENE6 (Promega) and used for patching within 1–2 days after transfection. Symmetric internal potassium (Kint, 140 mM KCl, 1 mM EGTA, 1 mM K2EDTA, 4 mM K2HPO4, pH 7.38) or cesium (Csint, 150 mM CsCl, 1 mM EGTA, 1 mM K2EDTA, 5 mM Hepes, pH 7.38) buffers were used for single-channel recordings. Upon patch excision in the inside-out configuration, the bath was perfused with Kint or Csint containing GSK101 (10 nM to 5μM), so that the drug was applied from the cytoplasmic side of the membrane. For experiments with divalent ions (Ca2+ and Ba2+), cells were patched in symmetric buffer (75 mM CaCl2 or BaCl2 respectively with 5 mM Hepes, pH 7.3). Upon patch excision the bath was perfused with Kint including 10 nM of GSK101. Recordings were made and digitized with the Axopatch 1D patch-clamp amplifier and the Digidata 1200 digitizer (Molecular Devices). Data were collected at 10 kHz, low-pass filtered at 5 kHz and analyzed with the pClamp software suite (Molecular Devices). The pipettes with bubble number (BN)84 4.5 – 5.0 (~ 2 M Ohm in symmetric Kint) were fabricated from the Kimble Chase soda lime glass with a Sutter P-86 puller (Sutter Instruments). All measurements were carried out at −30 mV membrane potential or as specified in the text.
Rb flux assay
CosM6 cells were transfected with 1μg of wild type or truncated constructs per well in a 12-well dish using FuGENE6 (Promega) and were used for experiments in 24 hours. Radioactive 86Rb+ flux assay was performed as described earlier85. Briefly, the transfected cells were loaded with radioactive rubidium in DMEM media (1 μCi/ml 86RbCl, PerkinElmer) for 6 hours. After incubation, DMEM in the wells was replaced with buffer containing 105 mM NaCl, 6 mM CsCl, 1 mM MgCl2, 5 mM CaCl2, 10 mM Glucose, 90 mM of D-mannitol, 10 mM Hepes pH 7.4. For measuring activation, 40 nM, 200 nM, or 1 μM GSK101 were additionally added. For measuring blockage, 1 μM GSK101 with 1 mM GdCl3 or 40 nM GSK101 with 10 μM GSK219 were added. At time points of 2.5, 5, 7.5, 15, 25 and 40 minutes, media in the wells were collected for radioactivity measurements, and were replaced with equal volumes of fresh media with drugs. Finally, cells were lysed with 2% SDS and lysate was collected. Radioactivity measurements were performed on a TRI-CARB 2900TR liquid scintillation analyzer (Packard Bioscience Company). Rubidium efflux was calculated as a fraction of the cumulative amount.
The normalized data from the GSK101 dose-response experiments (GSK101 at 0, 40, 200 and 1000 nM) were used to estimate the apparent steady-state GSK101 activation constants for wild type Xenopus TRPV4 and TRPV4cryst. The nonspecific leak at zero GSK101 was fitted with function f(t) = 1 - e−kL*t between 0 and ∞. The derived constant kL was then applied in fitting the GSK101-induced signals using function f(t) = A×(1 - e(-kL+k1)*t) + (1-A)×(1 - e−kL*t), where k1 is the activation constant and A is a parameter which accounts for a fraction of radioactive 86Rb+ that cannot be transported outside the cells through TRPV4 channels (e.g., being trapped in cellular compartments or due to channel inactivation during the course of experiments). The activation constants for wild type and TRPV4cryst were then normalized to the maximum values at 1000 nM GSK101 (KRelative) and the relative constant as a function of GSK101 concentration was fitted with a steady-state Hill function.
Data availability
The Cryo-EM map of TRPV4 has been deposited to Electron Microscopy Data Bank with accession code EMD-7075. Atomic coordinates for TRPV4 cryo-EM structure have been deposited to the Protein Data Bank (PDB) with accession code 6BBJ. X-ray structures of Cs+, Ba2+, and Gd3+-bound TRPV4 have been deposited to PDB with accession codes 6C8F, 6C8G and 6C8H, respectively. Other source data are available from the corresponding authors upon request.
Supplementary Material
Acknowledgments
We thank staff at APS beamlines 24-ID C/E, especially Kanagalaghatta Rajashankar, Kay Perry, and Narayanasami Sukumar for assistance at the synchrotron. This work used NE-CAT beamlines (GM103403), a Pilatus detector (RR029205), an Eiger detector (OD021527) at the APS (DE-AC02–06CH11357). We thank the staff of the Sloan Kettering cryo-EM facility and Subangstrom LLC for assistance with cryo-EM data collection. This work was supported by start-up funds from Washington University School of Medicine, the Mallinckrodt Foundation grant, American Heart Association Award 17SDG33400229, National Institutes of Health Grant R01NS099341–01A1 (all to P.Y.) and by start-up funds from Memorial Sloan Kettering Cancer Center (to R.K.H.).
Footnotes
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Clapham DE TRP channels as cellular sensors. Nature 426, 517–524 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Ramsey IS, Delling M & Clapham DE An introduction to TRP channels. Annu. Rev. Physiol 68, 619–647 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Venkatachalam K & Montell C TRP channels. Annu. Rev. Biochem 76, 387–417 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nilius B & Owsianik G The transient receptor potential family of ion channels. Genome Biol. 12, 218 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Julius D TRP channels and pain. Annu. Rev. Cell Dev. Biol 29, 355–384 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Mizuno A, Matsumoto N, Imai M & Suzuki M Impaired osmotic sensation in mice lacking TRPV4. Am. J. Physiol. Cell Physiol 285, C96–C101 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Liedtke W & Friedman JM Abnormal osmotic regulation in trpv4−/− mice. Proc. Natl. Acad. Sci. U. S. A 100, 13698–13703 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G & Plant TD OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat. Cell Biol 2, 695–702 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Liedtke W et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 103, 525–535 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wissenbach U, Bödding M, Freichel M & Flockerzi V Trp12, a novel Trp related protein from kidney. FEBS Lett. 485, 127–134 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Masuyama R et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 8, 257–265 (2008). [DOI] [PubMed] [Google Scholar]
- 12.Sonkusare SK et al. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 336, 597–601 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye L et al. TRPV4 is a regulator of adipose oxidative metabolism, inflammation, and energy homeostasis. Cell 151, 96–110 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alessandri-Haber N et al. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 39, 497–511 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Alessandri-Haber N et al. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J. Neurosci 24, 4444–4452 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao X, Wu L & O’Neil RG Temperature-modulated diversity of TRPV4 channel gating: Activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J. Biol. Chem 278, 27129–27137 (2003). [DOI] [PubMed] [Google Scholar]
- 17.Köhler R et al. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler. Thromb. Vasc. Biol 26, 1495–1502 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Güler AD et al. Heat-evoked activation of the ion channel, TRPV4. J. Neurosci 22, 6408–6414 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe H et al. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J. Biol. Chem 277, 47044–47051 (2002). [DOI] [PubMed] [Google Scholar]
- 20.Watanabe H et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J. Biol. Chem 277, 13569–13577 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Watanabe H et al. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424, 434–438 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Nilius B & Voets T The puzzle of TRPV4 channelopathies. EMBO Rep. 14, 152–163 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White JPM et al. TRPV4: molecular conductor of a diverse orchestra. Physiol. Rev 96, 911–973 (2016). [DOI] [PubMed] [Google Scholar]
- 24.Liao M, Cao E, Julius D & Cheng Y Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao E, Liao M, Cheng Y & Julius D TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504, 113–118 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Y, Cao E, Julius D & Cheng Y TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 534, 347–351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zubcevic L et al. Cryo-electron microscopy structure of the TRPV2 ion channel. Nat. Struct. Mol. Biol 23, 180–186 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huynh KW et al. Structure of the full-length TRPV2 channel by cryo-EM. Nat. Commun 7, 11130 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saotome K, Singh AK, Yelshanskaya MV & Sobolevsky AI Crystal structure of the epithelial calcium channel TRPV6. Nature 534, 506–511 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh AK, Saotome K & Sobolevsky AI Swapping of transmembrane domains in the epithelial calcium channel TRPV6. Sci. Rep 7, 10669 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whicher JR & MacKinnon R Structure of the voltage-gated K+ channel Eag1 reveals an alternative voltage sensing mechanism. Science 353, 664–669 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao X, Hite RK & MacKinnon R Cryo-EM structure of the open high-conductance Ca2+-activated K+ channel. Nature 541, 46–51 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee CH & MacKinnon R Structures of the Human HCN1 Hyperpolarization-Activated Channel. Cell 168, 111–120 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inada H, Procko E, Sotomayor M & Gaudet R Structural and biochemical consequences of disease-causing mutations in the ankyrin repeat domain of the human TRPV4 channel. Biochemistry 51, 6195–6206 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi N et al. TRPV4 channel activity is modulated by direct interaction of the ankyrin domain to PI(4,5)P(2). Nat. Commun 5, 4994 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Shigematsu H, Sokabe T, Danev R, Tominaga M & Nagayama KA 3.5-nm structure of rat TRPV4 cation channel revealed by zernike phase-contrast cryoelectron microscopy. J. Biol. Chem 285, 11210–11218 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becker D, Müller M, Leuner K & Jendrach M The C-terminal domain of TRPV4 is essential for plasma membrane localization. Mol. Membr. Biol 25, 139–151 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Lei L et al. A TRPV4 channel C-terminal folding recognition domain critical for trafficking and function. J. Biol. Chem 288, 10427–10439 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long SB, Campbell EB & MacKinnon R Crystal structure of a mammalian voltage-dependent shaker family K+ channel. Science 309, 897–903 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Long SB, Campbell EB & Mackinnon R Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science 32, 903–908 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Long SB, Tao X, Campbell EB & MacKinnon R Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature 450, 376–382 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Gregorio-Teruel L et al. The Integrity of the TRP Domain Is Pivotal for Correct TRPV1 Channel Gating. Biophys. J 109, 529–541 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smart OS, Neduvelil JG, Wang X, Wallace BA & Sansom MS HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph 14, 354–360, 376 (1996). [DOI] [PubMed] [Google Scholar]
- 44.Jordt S-E, Tominaga M & Julius D Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci 97, 8134–8139 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui Y et al. Selective disruption of high sensitivity heat activation but not capsaicin activation of TRPV1 channels by pore turret mutations. J. Gen. Physiol 139, 273–283 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryu S, Liu B, Yao J, Fu Q & Qin F Uncoupling proton activation of vanilloid receptor TRPV1. J. Neurosci 27, 12797–12807 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Voets T et al. Molecular determinants of permeation through the cation channel TRPV4. J. Biol. Chem 277, 33704–33710 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Shi N, Ye S, Alam A, Chen L & Jiang Y Atomic structure of a Na+- and K+-conducting channel. Nature 440, 570–574 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Derebe MG et al. Tuning the ion selectivity of tetrameric cation channels by changing the number of ion binding sites. Proc. Natl. Acad. Sci. U. S. A 108, 598–602 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sauer DB, Zeng W, Canty J, Lam Y & Jiang Y Sodium and potassium competition in potassium-selective and non-selective channels. Nat. Commun 4, 2721 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lockless SW Determinants of cation transport selectivity: Equilibrium binding and transport kinetics. J. Gen. Physiol 146, 3–13 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Cruijsen EAW et al. Importance of lipid-pore loop interface for potassium channel structure and function. Proc. Natl. Acad. Sci. U. S. A 110, 13008–13013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brohawn SG, Campbell EB & MacKinnon R Physical mechanism for gating and mechanosensitivity of the human TRAAK K+ channel. Nature 516, 126–130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang H et al. Pore architecture of TRIC channels and insights into their gating mechanism. Nature 538, 537–541 (2016). [DOI] [PubMed] [Google Scholar]
- 55.Vriens J, Owsianik G, Janssens A, Voets T & Nilius B Determinants of 4 alpha-phorbol sensitivity in transmembrane domains 3 and 4 of the cation channel TRPV4. J Biol Chem 282, 12796–12803 (2007). [DOI] [PubMed] [Google Scholar]
- 56.Lamandé SR et al. Mutations in TRPV4 cause an inherited arthropathy of hands and feet. Nat. Genet 43, 1142–1146 (2011). [DOI] [PubMed] [Google Scholar]
- 57.Nishimura G et al. TRPV4-associated skeletal dysplasias. Am. J. Med. Genet. Part C Semin. Med. Genet 160 C, 190–204 (2012). [DOI] [PubMed] [Google Scholar]
- 58.Krakow D et al. Mutations in the gene encoding the calcium-permeable ion channel TRPV4 produce spondylometaphyseal dysplasia, Kozlowski type and metatropic dysplasia. Am. J. Hum. Genet 84, 307–315 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Camacho N et al. Dominant TRPV4 mutations in nonlethal and lethal metatropic dysplasia. Am. J. Med. Genet. Part A 152, 1169–1177 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dai J et al. Novel and recurrent TRPV4 mutations and their association with distinct phenotypes within the TRPV4 dysplasia family. J. Med. Genet 47, 704–709 (2010). [DOI] [PubMed] [Google Scholar]
- 61.Nishimura G et al. Spondylo-epiphyseal dysplasia, Maroteaux type (pseudo-Morquio syndrome type 2), and parastremmatic dysplasia are caused by TRPV4 mutations. Am. J. Med. Genet. Part A 152, 1443–1449 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Landouré G et al. Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat. Genet 42, 170–174 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Auer-Grumbach M et al. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat. Genet 42, 160–164 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fiorillo C et al. TRPV4 mutations in children with congenital distal spinal muscular atrophy. Neurogenetics 13, 195–203 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Deng H-X et al. Scapuloperoneal spinal muscular atrophy and CMT2C are allelic disorders caused by alterations in TRPV4. Nat. Genet 42, 165–169 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mastronarde DN Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol 152, 36–51 (2005). [DOI] [PubMed] [Google Scholar]
- 67.Zheng SQ et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scheres SHW RELION: Implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol 180, 519–530 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scheres SHW Semi-automated selection of cryo-EM particles in RELION-1.3. J. Struct. Biol 189, 114–122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rohou A & Grigorieff N CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol 192, 216–221 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ludtke SJ Single-Particle Refinement and Variability Analysis in EMAN2.1. Methods Enzymol. 579, 159–189 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lyumkis D, Brilot AF, Theobald DL & Grigorieff N Likelihood-based classification of cryo-EM images using FREALIGN. J. Struct. Biol 183, 377–388 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rosenthal PB & Henderson R Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol 333, 721–745 (2003). [DOI] [PubMed] [Google Scholar]
- 74.Kucukelbir A, Sigworth FJ & Tagare HD Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pettersen EF et al. UCSF Chimera--A visualization system for exploratory research and analysis. J. Comput. Chem 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 76.Emsley P, Lohkamp B, Scott WG & Cowtan K Features and development of Coot. Acta Crystallogr. D. Biol. Crystallogr 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adams PD et al. PHENIX : a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. Biol. Crystallogr 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown A et al. Tools for macromolecular model building and refinement into electron cryo-microscopy reconstructions. Acta Crystallogr. D. Biol. Crystallogr 71, 136–153 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Otwinowski Z & Minor W Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997). [DOI] [PubMed] [Google Scholar]
- 80.McCoy AJ Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr. D. Biol. Crystallogr 63, 32–41 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Murshudov GN, Vagin AA & Dodson EJ Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D. Biol. Crystallogr 53, 240–255 (1997). [DOI] [PubMed] [Google Scholar]
- 82.Sievers F et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol 7, 539 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bond CS & Schüttelkopf AW ALINE: a WYSIWYG protein-sequence alignment editor for publication-quality alignments. Acta Crystallogr. D. Biol. Crystallogr 65, 510–512 (2009). [DOI] [PubMed] [Google Scholar]
- 84.Schnorf M, Potrykus I & Neuhaus G Microinjection technique: routine system for characterization of microcapillaries by bubble pressure measurement. Exp. Cell Res 210, 260–267 (1994). [DOI] [PubMed] [Google Scholar]
- 85.Cooper PE, Sala-Rabanal M, Lee SJ & Nichols CG Differential mechanisms of Cantú syndrome–associated gain of function mutations in the ABCC9 (SUR2) subunit of the K ATP channel. J. Gen. Physiol 146, 527–540 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Cryo-EM map of TRPV4 has been deposited to Electron Microscopy Data Bank with accession code EMD-7075. Atomic coordinates for TRPV4 cryo-EM structure have been deposited to the Protein Data Bank (PDB) with accession code 6BBJ. X-ray structures of Cs+, Ba2+, and Gd3+-bound TRPV4 have been deposited to PDB with accession codes 6C8F, 6C8G and 6C8H, respectively. Other source data are available from the corresponding authors upon request.