Abstract
Background:
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially cure for acute myeloid leukemia (AML). Patients who undergone HSCT are at increased risk of infection due to impaired immunity.
Objective:
To evaluate the rate of bacterial, viral and fungal infection and its relationship with 2-year overall survival of AML patients who had undergone HSCT.
Methods:
This was a retrospective cross-sectional study of 49 patients who underwent allogenic bone marrow transplantation (BMT) from full-matched donors at BMT Center, Imam Khomeini Hospital Complex, Tehran, Iran, from 2006 to 2013. All autologous transplantations and promyelocytic leukemia (PML) transplantations were excluded.
Results:
All patients, except for one, had fever for a mean of 7 days post-transplantation and received broad-spectrum antibiotic. The rate of severe sepsis was 6.1%. None of the patients developed fungal infection during admission. The rate of admission due to sepsis after discharge was 27% in the alive group (mean onset of 54 days), and 73% in the deceased group (mean onset of 52 days) (p<0.05). The most common site of infection was lung (70%). The rate of cytomegalovirus (CMV) antigenemia (positive PP65) was 20% during the 2-year period after HSCT.
Conclusion:
The rate of infection was a negative prognostic factor for 2-year overall survival. The rate of CMV antigenemia is less than similar studies (51%), which could be due to full-matched donor-recipients requiring less immunosuppression.
Key Words: Bone marrow transplantation; Infection; Acute myeloid leukemia
INTRODUCTION
Autologous or allogenic hematopoietic stem cell transplantation is currently used as a choice of treatment for patients with acute myeloid leukemia (AML), leading to long-term remission and cure [1-3]. Patients undergoing hematopoietic stem cell transplantation are at increased risk for bacterial and fungal infection due to invasive conditioning regimen, central venous line placement and prolonged immune suppression [4]. Infection is an important cause of morbidity and mortality after bone marrow transplantation (BMT) (e.g., up to 15% mortality rate) [5]. Therefore, early identification of high risk patients and applying more effective preventive and supportive strategies for reducing the rates of infection may potentially improve the survival rates [5-8]. Sepsis due to neutropenia is common during the first 30 days after BMT. Furthermore, factors related to acute graft versus host disease (GVHD) or its treatment may cause sepsis in the 30–100 days after transplantation [4].
There is a concern that higher rates of infection in developing countries may be associated with higher incidence of morbidity and mortality [4]. The present study aimed to analyze the pattern of post-transplantation infection in patients who underwent allogenic BMT at Imam Khomeini BMT Center during an 8-year period.
MATERIALS AND METHODS
This was a cross-sectional study of 49 patients diagnosed with AML. The study was performed at the BMT Center of Imam Khomeini Hospital Complex, Tehran, Iran. The inclusion criteria were all patients who had been diagnosed with AML and had received allo-hematopoietic stem cell transplantation (HSCT) from 2006 to 2013. The exclusion criteria were all patients with other malignancies and/or promyelocytic leukemia (PML) and those who had received autologous stem cell transplantation. All patients received allo-stem cells from fully HLA-matched donors.
The preparative regimen for all patients was cyclophosphamide 5 mg/kg/day on day 1 and 2 and busulphan 4 mg/kg/day on days 3 to 6.
Antimicrobial prophylaxis regimen was acyclovir 15 mg/kg/day, sulfamethoxazole/trimethoprim once daily and itraconazole 200 mg/day, which were all started on day +2, and continued for six months. The patient was considered to have fever if the measured core body temperature was above 38.5 °C once or above 38.3 °C twice.
Sepsis was defined according to the 2001 revised criteria, consisting of systemic inflammatory response syndrome (SIRS) plus some degrees of organ dysfunction (in severe sepsis) [9]. Diagnosis of cytomegalovirus (CMV) antigenemia was based on the PCR technique. The site of infection was determined by clinical evaluation, radiography (e.g., chest x-ray), or positive culture from blood, urine, sputum, abscess, or catheter samples. Broad-spectrum antibiotics were started when fever was detected or infection was documented.
Statistical Analysis
SPSS® ver 22.0 for Windows® (SPSS Inc, Chicago, IL, USA) was used for data analysis. Results are presented as mean±SD for quantitative variables and as absolute frequencies and percentages for categorical variables. Normal distribution of all data was checked using the Kolmogorov-Smirnoff test. Categorical variables were analyzed using χ2 test or Fisher’s exact test when more than 20% of cells with expected count of less than 5 were observed. Quantitative variables were analyzed using Student’s t test or Mann-Whitney U test. Survival rate was analyzed using the Kaplan-Meier curves and the log-rank test. A p value <0.05 was considered statistically significant.
RESULTS
A total of 49 patients with AML were registered to have allogeneic-HSCT during 2006-2013 at the BMT Center of Imam Khomeini Hospital Complex. Baseline characteristics of the study sample are summarized in Table 1.
Table 1.
Baseline characteristics of the study sample
| Variable | n (%) |
|---|---|
| Age (yrs) | |
| ≤40 | 36 (74) |
| >40 | 13 (27) |
| Sex | |
| Female | 27 (55) |
| Male | 22 (45) |
| Recipient-donor sex | |
| Matched | 18 (37) |
| Unmatched | 31 (63) |
| Blood group | |
| Matched | 28 (58) |
| Unmatched | 20 (42) |
| Donor type | |
| Sibling | 48 (98) |
| Father | 1 (2) |
| Underlying disease | |
| Lymphoma | 3 (6) |
| CML | 2 (4) |
| Autoimmune hemolytic anemia | 1 (2) |
| Diabetes | 4 (8) |
| Tuberculosis | 1 (2) |
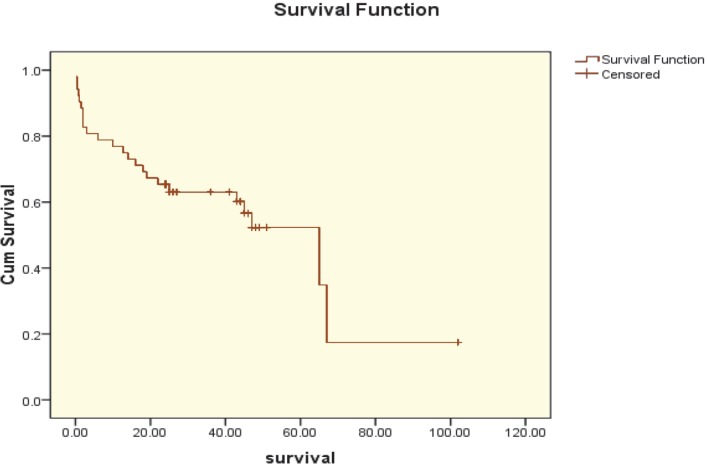
The overall two-year survival rate of patients who underwent transplantation was 65.4%±6.6% (Fig 1). As assessed by Cox proportional hazard analysis, the main predictor for reduced 2-year survival after transplantation was post-transplantation infection (p=0.021).
Figure 1.
The Kaplan-Mayer survival curve in AML patients treated with allogeneic stem cell transplantation
All patients, but one, had fever for a mean of seven days after transplantation and received broad-spectrum antibiotics. The rate of severe sepsis during the admission for transplantation was 6.1%. No fungal infection was detected. None of the patients had viral hepatitis (Table 2).
Table 2.
Complications during the admission for transplantation
| Complication | n (%) |
|---|---|
| Fever | 48 (98) |
| Severe sepsis | 3 (6) |
| Fungal infection | 0 (0) |
| Viral hepatitis | 0 (0) |
The rate of admission after discharge due to sepsis was 27% in the alive group (mean onset of 54 days) and 73% in the deceased group (mean onset of 52 days) (p<0.05, Table 3). The most common site of infection was lung (47%) (Table 4). The rate of CMV antigenemia after transplantation (positive PP65) was 20% during a 2-year period after HSCT.
Table 3.
The rate of sepsis after transplantation
| Group | Post-BMT sepsis, n (%) | Post-BMT sepsis onset (days), Mean±SD |
|---|---|---|
| Alive (two years post-BMT) | 4 (27) | 54.1±18 |
| Deceased (two years post-BMT) | 11 (73) | 52.3±20 |
| Total | 15 (100) | 52.9±20 |
Table 4.
Sites of post-BMT infection
| Site of post-BMT infection | n (%) |
|---|---|
| Lung | 7 (47) |
| Skin | 2 (13) |
| Central nervous system | 1 (6) |
| Unknown origin | 5 (34) |
| Total | 15 (100) |
DISCUSSION
The present study was an overview of post-BMT infection rate in patients with AML. The 2-year overall survival was 65.4%±6%. Other studies have reported different 2-year overall survival according to the patients’ characteristics and comorbid diseases. In a study by Hamidieh, et. al., on HSCT in Iranian children, the overall survival during two years after allogenic transplantation in leukemic patients was 60%–70% [10]. Similar results were reported in another study from an Italian center [11].
Infection is one of the most common causes of mortality in patients who undergo transplantation [12]. In the study conducted by Hamidieh, et. al., infections accounted for 18.5% of all deaths [10]. In our study, 11 patients in the deceased group got infection (Table 3), which accounted for 22% of all deaths.
Fever and neutropenia after transplantation are common and require broad-spectrum antibiotics. Almost all of our patients (98%) had fever by a mean onset of seven days after transplantation and received empiric antibiotic therapy. This rate of fever is higher than similar BMT centers; an Indian study reports fever in 82.5% of patients post-HSCT [4], while in western studies, this rate varies between 60% and 90% [6]. In a study conducted by Luznik, et. al., the rate of fever with neutropenia was 51% during the first 60 days after transplantation, and infections without neutropenia accounted for 22% of post-BMT admissions. The Luznik article does not mention the mean day of fever onset [13]. Hayatshahi and colleagues, report a 63.2% rate of neutropenic fever in adult BMT Department of Shariati Hospital, Tehran, Iran, consisting of both autologous and allogenic transplantations with different underlying diseases [14]. In our study, neutropenic vs. non-neutropenic fever was not specified.
One of the limitations of our study was lack of enough data to report the frequency of bacterial species in febrile and septic patients. We did not detect any fungal infection during admission. However, the incidence of fungal infection varies from 4% to 30% in different transplant centers [15, 16]; studies from Asian countries like India and Israel have typically observed higher rates (19%) [4, 17].
One of the patients had pulmonary tuberculosis before transplantation, which was completely cured and did not reactivate. Surprisingly, none of the transplant recipients in our study was infected by tuberculosis. The incidence rate of the disease, however, varies between 1% and 12% in developing countries [18, 19].
Viral pathogens including herpes simplex and herpes zoster were not detected in this study. The worldwide incidence of viral infections, however, is about 8%–10% in similar studies [20, 21]. The incidence of CMV infection was not evaluated in our study, but the rate of CMV antigenemia during the 2-year period after transplantation was 20.4%, which was lesser than that reported in similar studies (51%) [22]. This difference could be due to full-match donor-recipient profile that requires less immunosuppressive therapy.
In conclusion, we found that the overall 2-year survival rate was similar to other BMT centers, but the rate of fever after transplantation was higher, and the incidence of viral, fungal, and tuberculosis infections was lesser than that reported in other centers.
ACKNOWLEDGMENTS
We would like to thank the staff of the BMT Center of Imam Khomeini Hospital Complex, Tehran University of Medical Sciences, Tehran, Iran.
CONFLICTS OF INTEREST:
None declared.
References
- 1.Ghavamzadeh A, Alimoghaddam K, Ghaffari F, et al. Twenty Years of Experience on Stem Cell Transplantation in Iran. Iran Red Crescent Med J. 2013;15:93–100. doi: 10.5812/ircmj.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sánchez-Guijo FM, Orfao A, Cañizo MC. Bone Marrow Transplantation Extends Its Scope. Stem Cell Transplantation . 2012:121–34. doi: 10.1007/978-1-4614-2098-9_9. [DOI] [PubMed] [Google Scholar]
- 3.Petersen SL. Alloreactivity as therapeutic principle in the treatment of hematologic malignancies Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning. Dan Med Bull. 2007;54:112–39. [PubMed] [Google Scholar]
- 4.George B, Mathews V, Srivastava A, Chandy M. Infections among allogeneic bone marrow transplant recipients in India. Bone Marrow Transplantation. 2004;33:311–15. doi: 10.1038/sj.bmt.1704347. [DOI] [PubMed] [Google Scholar]
- 5.Baddley J, Stroud T, Salzman D, Pappas P. Invasive Mold Infections in Allogeneic Bone Marrow Transplant Recipients, Presented in part. 39th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 1999; San Francisco. pp. 26–29. [Google Scholar]
- 6.Hoyle C, Goldman JM. Life-threatening infections occurring more than 3 months after BMT 18 UK Bone Marrow Transplant Teams. Bone Marrow Transplantation. 1994;14:247–52. [PubMed] [Google Scholar]
- 7.Lee S, Aljurf M, Atsuta Y, et al. Recommended Screening and Preventive Practices for Long-Term Survivors after Hematopoietic Cell Transplantation. Hematol Oncol Stem Cell Ther. 2012;5:1–30. doi: 10.5144/1658-3876.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marr K, Carter R, Crippa F, et al. Epidemiology and Outcome of Mould Infections in Hematopoietic Stem Cell Transplant Recipients. Clin Infect Dis. 2002;34:909–17. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 9.Singer M, Deutschman C, Seymour C. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamidieh AA, Behfar M, Babaki AES, et al. Hematopoietic SCT in Iranian children 1991–2012. Bone Marrow Transplantation. 2015;50:517–22. doi: 10.1038/bmt.2014.299. [DOI] [PubMed] [Google Scholar]
- 11.Aristei C, Santucci A, Corvo R, et al. In haematopoietic SCT for acute leukemia TBI impacts on relapse but not survival: results of a multicentre observational study. Bone Marrow Transplant. 2013;48:908–14. doi: 10.1038/bmt.2013.66. [DOI] [PubMed] [Google Scholar]
- 12.Young H, Logan B, Wu J, et al. Infections After Transplantation Of bone marrow or peripheral blood stem cells. Biology of blood and marrow transplantation. 2016;22:359–70. doi: 10.1016/j.bbmt.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luznik L, Donnell P, Symons H, et al. HLA-Haploidentical Bone Marrow Transplantation for Hematologic Malignancies Using Nonmyeloablative Conditioning and High-Dose, Posttransplantation Cyclophosphamide. Biology of Blood and Marrow Transplantation. 2008;14:641–50. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayatshahi A, Javadi M, Torkamandi H, et al. drug utilization review of vancomycin in febrile neutropenic patients hospitalized at a BMT center. IJHOSCR. 2010;11:10–13. [Google Scholar]
- 15.Ninin E, Milpied N, Moreau P, et al. Study of bacterial, viral and fungal infections in adult recipients of bone marrow transplants. Clin Infect Dis. 2001;33:41–7. doi: 10.1086/320871. [DOI] [PubMed] [Google Scholar]
- 16.Hovi L, Saarinen-Pihkala UM, Vettenranta K, et al. Invasive fungal infections in pediatric bone marrow transplant recipients: single center experience of 10 years. Bone Marrow Transplant. 2000;26:999–1004. doi: 10.1038/sj.bmt.1702654. [DOI] [PubMed] [Google Scholar]
- 17.Weinberger M, Sacks T, Sulkes J, et al. Increasing fungal isolation from clinical specimens: experience in a university hospital over a decade. J Hosp Infect. 1997;35:185–95. doi: 10.1016/s0195-6701(97)90206-1. [DOI] [PubMed] [Google Scholar]
- 18.Budak-Alpdogan T, Tangun Y, Kalayoglu-Besisik S, et al. The frequency of tuberculosis in adult allogeneic stem cell transplant recipients in Turkey. Biol Blood Marrow Transplant. 2000;6:370–4. doi: 10.1016/s1083-8791(00)70013-9. [DOI] [PubMed] [Google Scholar]
- 19.Mary S, Ip M, Yuen KY, et al. Risk factors for pulmonary tuberculosis in bone marrow transplant recipients. Am J Respir Crit Care Med. 1998;158:1173–7. doi: 10.1164/ajrccm.158.4.9712072. [DOI] [PubMed] [Google Scholar]
- 20.Wingard JR. Viral infections in leukemia and bone marrow transplant patients. Leuk Lymphoma. 1993;11:115–25. doi: 10.3109/10428199309064271. [DOI] [PubMed] [Google Scholar]
- 21.Kawasaki H, Takayama J, Ohira M. Herpes zoster infection after bone marrow transplantation in children. J Pediatr. 1996;128:353–6. doi: 10.1016/s0022-3476(96)70280-9. [DOI] [PubMed] [Google Scholar]
- 22.Bonon S, Rossi C, Souza C, et al. Comparison of serology, antigenemia assay and the polymerase chain reaction for monitoring active cytomegalovirus infections in hematopoietic stem cell transplantation patients. Rev Inst Med Trop Sao Paulo. 2006;48:275–8. doi: 10.1590/s0036-46652006000500007. [DOI] [PubMed] [Google Scholar]