Abstract
Cocaine addiction is a chronic relapsing disorder characterized by persistent perturbations to an organism’s homeostatic processes that result in maladaptive drug seeking. Although considerable attention has been directed at the consequences of neuronal changes following chronic cocaine taking, few studies have examined the role of microglia, the brain’s resident immune cells, following chronic cocaine administration. Toll-Like Receptor 4 (TLR4) is a molecular pattern receptor that recognizes pathogens, danger signals, and xenobiotics and induces proinflammatory signaling in the central nervous system. TLR4 is generally considered to be expressed primarily by microglia. Here, we used a rodent model of cocaine addiction to investigate the role of TLR4 in the ventral tegmental area (VTA) in cocaine seeking. Male Sprague-Dawley rats were trained to self-administer cocaine in daily 2-h sessions for 15 days. Following self-administration, rats underwent extinction training and were tested in a drug-primed reinstatement paradigm. Pharmacological antagonism of TLR4 in the VTA using lipopolysaccharide from the bacterium Rhodobacter sphaeroides (LPS-RS) significantly reduced cocaine-primed reinstatement of drug seeking but had no effect on sucrose seeking. TLR4 activation within the VTA using the TLR4 activator, lipopolysaccharide, was sufficient to moderately reinstate cocaine seeking. We also assessed changes in proinflammatory cytokine expression in the VTA following cocaine self-administration. Cocaine self-administration increased the expression of mRNA for the proinflammatory cytokine interleukin-1ß, but not tumor necrosis factor alpha, in the VTA. Pharmacological antagonism of the interleukin-1 receptor in the VTA reduced cocaine-primed drug seeking. These results are consistent with the hypothesis that chronic cocaine produces inflammatory signaling that contributes to cocaine seeking.
Keywords: Addiction, Cocaine, Neuroinflammation, Cytokines, Interleukin-1ß
1. Introduction
Cocaine is classically thought to exert its reinforcing effects by blocking the dopamine transporter and, subsequently, increasing dopamine (DA) to supraphysiological levels in the mesolimbocortical DA system (Thomsen et al., 2009; Chen et al., 2006). The ventral tegmental area (VTA)—a midbrain cluster of dopamine-, GABA-, and glutamate-containing cell bodies that forms part of the mesolimbocortical DA system—functions to update the motivational state of an organism, implement reward prediction error, and modulate value-based decision making through dynamic DA release into the nucleus accumbens (NAc) (Hamid et al., 2016; Steinberg et al., 2013; Saddoris et al., 2015). Chronic cocaine intake leads to progressive, allostatic neuroadaptations of the VTA and alters the plasticity of both afferent and efferent neural projections that contribute to cocaine seeking, effects that persist through periods of prolonged abstinence (Mameli et al., 2009). These neuroadaptations result in altered VTA dopamine neuron function hypothesized to contribute to binge patterns of cocaine use (Koob & Kreek, 2007; Siciliano et al., 2015). Additionally, VTA activity following acute re-exposure to cocaine is necessary for relapselike behavior as evidenced by pharmacological and optogenetic studies in which inhibition of the VTA prevents drug-primed cocaine seeking in the rodent self-administration model of cocaine relapse (McFarland & Kalivas, 2001; Shen et al., 2014; Stefanik et al., 2013). The development of effective treatments for Cocaine Use Disorder may be facilitated by understanding how VTA function is dysregulated following chronic cocaine exposure.
Emerging evidence suggests a role of neuroimmune signaling in the progression and maintenance of substance use disorders (Hutchinson & Watkins, 2014; Lacagnina et al., 2017). Several drugs of abuse including ethanol, opioids, methamphetamine and cocaine produce an acute inflammatory response within the brain (Cearley et al., 2011; Frank et al., 2016; Hutchinson et al., 2010; Wang et al., 2012; Zou & Crews, 2014). Additional evidence suggests that drugs of abuse also produce chronic neuroinflammation, defined as persistent and increased proinflammatory cytokine expression within the central nervous system (Little et al., 2009; Clark et al., 2013; Beardsley and Hauser, 2014). The functional implications of drug-induced neuroinflammation remain unclear. However, a recent study demonstrates that neuroinflammation induced by peripheral nerve injury dysregulates dopamine release in response to cocaine and morphine and may alter the rewarding properties of these drugs (Taylor et al., 2015). Non-selective reduction of neuroinflammation with the phosphodiesterase inhibitor ibudilast reduces methamphetamine, opioid, and cocaine seeking (Beardsley et al., 2010; Schwarz & Bilbo, 2013; Charntikov et al., 2015; Poland et al., 2016; Snider et al., 2013). These findings converge to provide evidence for a potential role of neuroimmune signaling in the pathophysiology of drug abuse.
Toll-like receptor 4/MD2 complex (TLR4), a pattern recognition receptor expressed primarily on microglia in the CNS, has recently become a target of interest for its potential role in drug abuse (Bachtell et al., 2015). TLR4 confers protective innate immunity by recognizing immunologic threats, such as xenobiotics, and inducing intracellular signaling that activates NFκB, a transcription factor responsible for the induction of inflammatory cytokines such as interleukin-1ß (IL-1ß), interleukin-6, and tumor necrosis factor alpha (TNF-α) (Kawai & Akira, 2010). Interestingly, inhibition of NFκB has been demonstrated to prevent cocaine conditioned place preference and cocaine induced synaptic plasticity in the NAc (Russo et al., 2009). Drugs of abuse are exogenous sub-stances that cross the blood brain barrier and may be recognized as xenobiotics by TLR4 (Hutchinson & Watkins, 2014). In fact, TLR4 signaling has been implicated in neuroinflammation induced by ethanol, methamphetamine, heroin, and cocaine (Alfonso-Loeches et al., 2010; Frank et al., 2016; Hutchinson et al., 2010; Northcutt et al., 2015). Furthermore, there is evidence that TLR4 signaling modulates the acute reinforcing and motivational effects of cocaine, opioids, and ethanol (Hutchinson et al., 2012; Northcutt et al., 2015; Montesinos et al., 2016; Theberge et al., 2013; Tanda et al., 2016 and Harris et al., 2017). In light of these findings, we hypothesized that TLR4 modulation may alter cocaine seeking.
Although previous work has examined the acute reinforcing effects of cocaine following TLR4 antagonism (Northcutt et al., 2015; Tanda et al., 2016), no studies exist demonstrating the necessity of TLR4 within the context of the drug-primed reinstatement model of cocaine relapse. Because the VTA is known to be an essential component of drug-primed relapse and cocaine induces proinflammatory cytokine expression in the VTA, this area was targeted as a region of interest. IL-1ß, a cytokine upregulated following TLR4 activation, is increased following acute cocaine exposure and is known to modulate neuronal activity (Cearley et al., 2011; Northcutt et al., 2015; Viviani et al., 2003). Hence, we examined whether IL-1ß is also regulated following cocaine selfadministration and whether IL-1ß signaling in the VTA contributes to cocaine seeking. Finally, to address conflicting reports regarding the non-specific, suppressive behavioral effects of TLR4 antagonism (Bajo et al., 2015; Northcutt et al., 2015; Tanda et al., 2016), reinstatement of sucrose seeking was assessed.
2. Materials & methods
2.1. Animals
Male Sprague-Dawley rats (ENVIGO, Indianapolis, IN) weighing 340–360 g were singly housed in the animal vivarium at the University of Colorado Boulder. Rats were maintained on a 12 h light/12 h dark cycle and fed rat chow (ENVIGO, Indianapolis, IN) ad libitum unless otherwise noted. All experiments were conducted during the light cycle. Experiments were performed in accordance with the guidelines established by the Institutional Animal Care and Use Committee at the University of Colorado Boulder.
2.2. Drugs
Cocaine hydrochloride was obtained from Sigma-Aldrich (St. Louis, MO) and diluted with sterile saline (0.9% NaCl) to a concentration of 5 μg/ml. The TLR4 antagonist lippopolysaccharide from the species Rhodobacter sphaeroides (LPS-RS) was obtained from Invivogen (San Diego, CA) and administered at its stock concentration of 5 μg/μl. Lipopolysaccharide was obtained from Sigma-Aldrich (St. Louis, MO) and administered at concentrations of 10 ng/ml and 20 ng/ml. Interleukin-1 receptor antagonist (Aμgen, Thousand Oaks, CA) was diluted in sterile saline (0.9% NaCl) at con-centrations of 10 μg/μl and 20 μg/μl.
2.3. Intravenous and intracranial surgeries
Rats were allowed to habituate to their vivarium conditions for at least 7 days prior to surgery. Rats were anesthetized using 1–3% halothane gas and were implanted with intravenous catheters (7 cm silastic tubing) into the right jugular vein and 10 mm bilateral cannulas located 1 mm above the VTA (A/P = −5.6 mm, ML = +/0.8 mm, DV = 8.0 mm, in relation to bregma) using aseptic surgical technique.
2.4. Intracranial microinjection procedures
Rats received bilateral intracranial microinjections of 0.2 μl every 30 s for 2 min and 30 s (total volume 1 ml/side) into the VTA (A/P = −5.6 mm, ML = +/0.8 mm, DV = 8.0 mm, in relation to bregma). Microinjectors projected 1 mm below the cannula. Drugs were diluted in sterile saline and delivered manually via 5 ml Hamilton glass syringes (Sigma Aldrich, St. Louis, MO). Cannula placements were verified by post-mortem visual inspection with cresyl violet staining.
2.5. Cocaine self-administration, extinction, and reinstatement procedures
Self-administration procedures were conducted in operant chambers (Med Associates, St. Albans, VT) containing a house light illuminated throughout the session, two retractable levers (a drug-paired left lever and an inactive right lever), stimulus cue lights above each lever, and a sound-attenuating fan. Rats were initially trained to press the active lever on a fixed ratio 1 (FR1) schedule of reinforcement with a criterion of 100 sucrose-paired lever presses per session. Following sucrose training on the active-lever, rats were implanted with an intravenous catheter and intracranial cannula. Following surgical recovery, cocaine self-administration occurred over fifteen 2-h sessions using an FR1 schedule of reinforcement. Drug-paired lever presses resulted in the 5 s delivery of a 0.5 μg/kg infusion of cocaine in conjunction with the presentation of a stimulus cue light. Cocaine was delivered intravenously into the jugular vein via an automated glass syringe pump attached to Tygon tubing tethered to the rat’s catheter. Drug delivery was followed by a 20 s time-out from reinforcement period. During this period, the house-light was temporarily off and drug-paired lever responding resulted in no drug delivery. Following self-administration, rats received seven 2-h extinction sessions in which drug-paired lever presses no longer resulted in cocaine or stimulus cue light delivery.
After formerly drug-paired lever pressing was extinguished, rats were tested for reinstatement of drug-paired lever pressing in a separate 2-h session. Rats received an intra-VTA microinjection pretreatment followed 5 min later by a drug priming stimulus of 15 μg/kg of cocaine (i.p.) and were then immediately placed into the operant chamber for reinstatement testing. During the reinstatement test, neither cocaine nor drug-paired stimulus delivery were contingent on lever presses. Drug-paired and inactive lever presses were recorded and analyzed.
2.6. Sucrose self-administration, extinction, and reinstatement procedures
Rats were food restricted and maintained at a consistent weight (340–360 g) prior to the commencement of sucrose self-administration (Fig. 2A). Rats self-administered 45 μg banana-flavored sucrose pellets (Bio-Serv, Flemington, NJ) on a FR1 schedule of reinforcement in sound attenuated operant chambers for 2-h daily sessions over 15 days. Operant chambers contained two retractable levers and a food magazine that delivered sucrose pellets upon a sucrose-paired lever press. Similar to the cocaine self-administration procedures, sucrose-paired lever presses resulted in the delivery of a stimulus cue light and a 20 s time-out from reinforcement where sucrose-paired lever responding resulted in no sucrose pellet delivery. Following self-administration, rats received seven 2-h extinction sessions in which sucrose-paired lever presses no longer resulted in the delivery of a sucrose pellet or light stimulus.
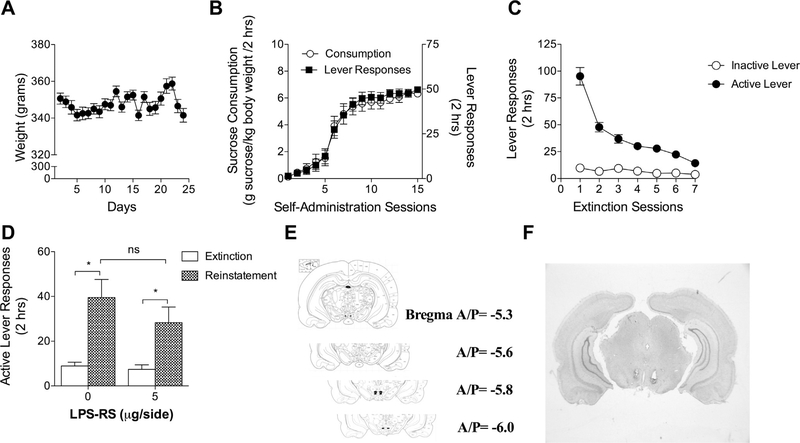
Fig. 2.
Intra-VTA TLR4 antagonism does not alter reinstatement of sucrose seeking. (A) Rats were food restricted and maintained at 340–360 g throughout the experiment. (B) Rats self-administered sucrose pellets during 2-h daily sessions over 15 days as measured by active lever responses and sucrose consumption. (C) Rats extinguished lever pressing on the formerly sucrose-paired lever during 2-h daily sessions over 7 days. (D) LPS-RS (5 μg/side) was administered bilaterally into the VTA 5 min prior to a sucrose reinstatement session. N = 11–12 rats per group. Sucrose seeking increased during the reinstatement session compared to the last extinction session in both the vehicle (*Bonferroni’s test, p < 0.001) and LPS-RS (*Bonferroni’s test, p < 0.05) treated groups. A significant effect of treatment was not found between the vehicle and LPS-RS treated groups (ns2-way ANOVA, p = 0.267).
Following 7 days of extinction sessions, a sucrose reinstatement procedure was implemented. Rats received bilateral microinjections in the VTA in an identical manner to the cocaine reinstatement procedures. During the 1-h reinstatement session, noncontingent sucrose pellets and a light stimulus were delivered every 2 min, a procedure we have found to produce robust reinstatement of sucrose seeking. Sucrose-paired lever presses resulted in neither sucrose nor light delivery. Sucrose-paired lever presses were recorded and analyzed.
2.7. Collection of Tissue and mRNA detection by reverse transcriptase quantitative polymerase chain reaction (RT-qPCR)
A separate cohort of rats were trained to self-administer cocaine (or saline) and underwent extinction training as described in Section 2.5. To simulate the reinstatement session, rats were challenged with either saline or 15 μg/kg cocaine (i.p.), but did not undergo reinstatement testing in order to control for possible effects of behavioral responding and environmental cues on mRNA expression. Two hrs following the challenge, rats were euthanized with sodium pentobarbital (65 μg/kg) and trans-cardially perfused with ice-cold saline (0.9% wt./vol. NaCl) for approximately 5 min to ensure that brains were clear of blood. Brains were then removed, submerged in 2-methylbutane at −20 °C for 1 min, and stored at 80 °C. VTA circular tissue punches of 0.25 cm in length were taken from 30 μm sections that were obtained on a cryostat maintained at a temperature of −20 °C. Tissue punches were stored in 1.5 mL microcentrifuge tubes at −80 °C until RT-qPCR processing.
Tissue isolation, cDNA synthesis, and qPCR procedures were performed according to published procedures (Frank et al., 2006a, b). Briefly, total RNA was isolated from the tissue using a standard method phenol:chloroform extraction. Total RNA was reverse transcribed into cDNA using the SuperScript II First Strand Synthesis System for RT-PCR according to the manufacturer’s instructions (Invitrogen). Polymerase chain reaction (PCR) amplification of cDNA was performed in triplicate using the Quantitect SYBR Green PCR Kit (Qiagen, Valencia, CA, USA) with a MyiQ Single-Color Real Time PCR Detection System (BioRad, Hercules, CA, USA). The primer sequence for IL-1ß mRNA (Forward: 5’-CCT TGTGCAAGTGTCTGAAG-3’; Reverse: 5’-GGGCTTGGAAG CAATCCTTA-3’) TNF-α (Forward: 5’-CAAGGAGGAGAAGTTCCCA-3’; Reverse: 5’-TTGGTGGTTTGCTACGACG-3’), NFκBIA (Forward: 5’-C ACCAACTACAACGGCCACA-3’; Reverse: 5’- GCTCCTGAGCGTTGA CATGA-3’), CD11b (Forward: 5’-CTGGTACATCGAGACTTCTC; Reverse: 5’-TTGGTCTCTGTCTGAGCCTT-3’), and GFAP (Forward: 5’-AG ATCCGAGAAACCAGCCTG-3’; Reverse: 5’-CCTTAATGACCTCGC CATCC-3’) were obtained by using Genbank at the National Center for Biotechnology Information. Expression was normalized to the housekeeping gene GAPDH (Forward: 5’-TCTTCCAGGAGCGA GATCCC-3’; Reverse: 5’-TTCAGGTGAGCCCCAGCCTT-3’).
2.8. Data analysis
All statistical procedures were performed with GraphPad Prism 5 (San Diego, CA). The effect of TLR4 antagonism on cocaine-induced reinstatement of drug-paired lever pressing was analyzed using a 2 × 2 factorial ANOVA with lever (active inactive) and treatment (5 μg LPS-RS vehicle) as the factors. A 2 × 2 factorial ANOVA was used to determine the effect of session (extinction reinstatement) and treatment (vehicle 5 μg LPS-RS) on sucrose seeking. The effect of interleukin 1 receptor antagonist on cocaine-induced reinstatement of lever pressing was assessed using a 2 × 3 ANOVA analyzing lever (active × inactive) and treatment (vehicle 10 μg IL1-ra 20 μg IL1-ra) as the factors. The effect of cocaine self-administration (saline SA × cocaine SA) and acute cocaine (saline cocaine) on mRNA was examined with a 2 × 2 factorial ANOVA. Post-hoc Bonferroni’s tests controlling for multiple comparisons were performed to determine the interactive effects for all data analyses.
3. Results
3.1. Pharmacological antagonism of TLR4 in the VTA reduces cocaine seeking but not sucrose seeking
Rats self-administered cocaine for 15 days of 2-h daily sessions with a mean cocaine intake of 21.88 μg/kg/day during the last 5 days of self-administration (Fig. 1A). Drug-paired lever pressing was extinguished over 7 days (Fig. 1B). Cocaine seeking was induced by a cocaine prime (15 μg/kg, i.p.) preceded by a bilateral microinjection of 5 μg/side of the TLR4 antagonist LPS-RS or vehicle into the VTA. Intra-VTA TLR4 pharmacological antagonism decreased previously drug-paired lever pressing (Fig. 1C). A 2 × 2 ANOVA revealed a main effect of LPS-RS treatment (F2,62 = 22.83, p < 0.0001). A main effect of lever was also found such that subjects pressed the drug-paired lever more frequently than the inactive lever (F1,62 = 44.74, p < 0.0001). A significant interaction between lever and treatment was found (F2,62 = 21.33, p < 0.0001). A Bonferroni’s comparison revealed a significant reduction in drug-paired lever presses following intra-VTA LPS-RS compared to vehicle during reinstatement testing (t21 = 7.180, p < 0.001). A Bonferroni’s comparison also revealed a significant increase in drug-paired lever presses in the cocaine treated group compared to the saline treated group among rats receiving intraVTA vehicle (t22 = 9.773, p < 0.001).
Fig. 1.
Intra-VTA TLR4 antagonism reduces drug seeking in the cocaine primed reinstatement model of relapse. (A) Rats self-administered cocaine (0.5 μg/kg/infusion) during 2-h daily sessions over 15 days. (B) Rats extinguished formerly drug-paired lever pressing over 7 days of 2-h daily sessions. (C) The TLR4 antagonist LPS-RS (5 μg/side) or vehicle was administered bilaterally into the VTA 5 min prior to cocaine administration (15 μg/kg, i.p.) or saline (0.9% NaCl wt./vol., i.p.). N = 11–13 rats per group. Drug seeking at the previously drug-paired lever increased in the cocaine/vehicle group compared to the vehicle/vehicle group (*Bonferroni’s test, p < 0.001). Intra-VTA LPS-RS decreased drug seeking compared to the cocaine/vehicle group (#Bonferroni’s test, p < 0.001). (D) Cannula placements were verified by post-mortem visual inspection.
Sucrose seeking was assessed in a separate cohort of rats that were food restricted and maintained at a consistent weight (340– 360 g) prior to the commencement of sucrose self-administration (Fig. 2A). Self-administration occurred over 15 days of 2-h daily sessions with a maximum of 50 pellets per session (Fig. 2B). Sucrose-paired lever pressing was extinguished over 7 days of training (Fig. 2C). Rats then underwent a reinstatement session consisting of non-contingent delivery of sucrose pellets and pre-sentation of a previously conditioned sucrose-paired discrete stim- sucrose seeking (Fig. 2D). A main effect of session was found such ulus (i.e., a light). In contrast to the effects of VTA TLR4 antagonism that rats pressed the sucrose-paired lever more during the reinon cocaine seeking, TLR4 antagonism in the VTA did not reduce statement session than the prior extinction session (F1,48 = 23.44, p < 0.0001). Post-hoc Bonferroni tests revealed significant differences between the reinstatement session and the extinction session in both the vehicle group (t12 = 4.271, p < 0.001) and the LPS-RS group (t12 = 2.515, p < 0.05) (Fig. 2D). Statistical significant changes were not observed with either the main effect of LPS-RS treatment (F1,48 = 1.369, p = 0.248) or the interaction between session and treatment (F1,48 = 2.014, p = 0.162).
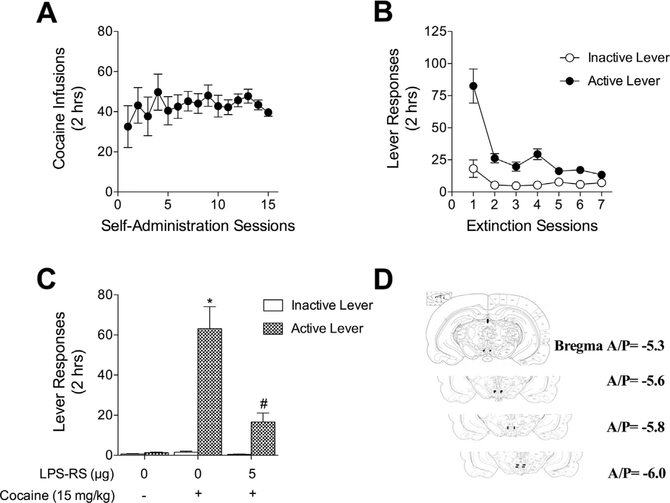
3.2. Pharmacological activation of TLR4 in the VTA induces a moderate increase in drug seeking
The sufficiency of TLR4 activation to induce cocaine seeking was assessed by administering the TLR4 agonist lipopolysaccharide (LPS) intracranially into the VTA. Rats self-administered cocaine (0.5 μg/kg/infusion) for 15 days, averaging 20.5 μg/kg/day over the last 5 days of self-administration (Fig. 3A). Lever pressing was extinguished over 7 days (Fig. 3B). Rats received bilateral intracranial infusions of vehicle or LPS (10 ng/side or 20 ng/side) into the VTA prior to the reinstatement session and lever responding was recorded (Fig. 3C). A two-way ANOVA revealed a significant main effect of LPS treatment (F2,42 = 5.71, p < 0.01) and lever (F1,42 = 19.04, p < 0.0001). A significant interaction between treatment and lever was also found (F2,42 = 6.12, p < 0.01). A post hoc test with a Bonferroni correction revealed a significant increase in cocaine seeking in the group receiving 20 ng/side LPS and the vehicle group (t14 = 4.514, p < 0.001).
Fig. 3.
Intra-VTA TL4 activation moderately induces drug-seeking following extinction of cocaine self-administration. (A) Rats self-administered cocaine (0.5 μg/kg/infusion) during 2-h daily sessions over 15 days. (B) Rats extinguished formerly drug-paired lever pressing over 7 days of 2-h daily sessions. (C) Bilateral intra-VTA LPS (20 ng/side) increased cocaine seeking at the active lever compared to the vehicle treated group. N = 8 rats per group. (*Bonferroni’s test, p < 0.001). (D) Cannula placements were verified by post-mortem visual inspection.
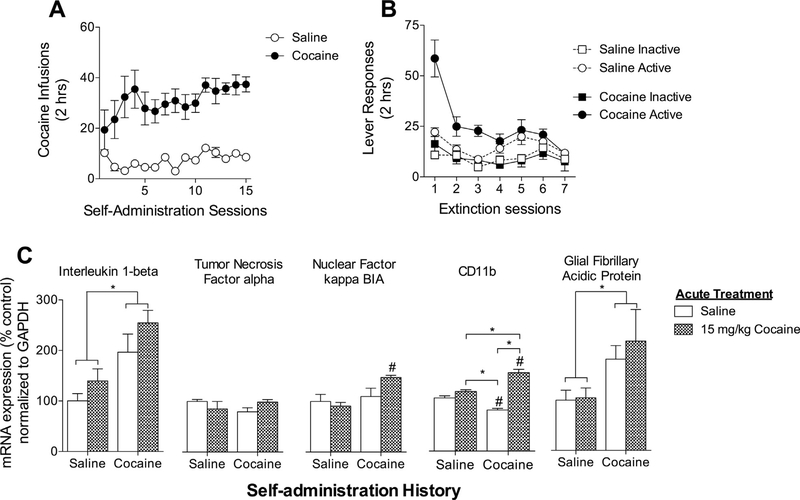
3.3. Cocaine self-administration increases IL-1ß mRNA expression in the ventral tegmental area
Pattern recognition receptors such as TLR4 are primarily expressed on innate immune cells, such as microglia, raising questions about how TLR4 may contribute to cocaine seeking. We therefore investigated the expression of proinflammatory cytokines following cocaine self-administration, extinction and subsequent cocaine challenge that may communicate with neurons within the VTA. Rats self-administered cocaine or saline for 15 days with an average of 18.21 μg/kg/day during the last 5 days of self-administration (Fig. 4A). Following a 2-day abstinence period, rats underwent extinction training and decreased drug-paired lever pressing across 7 days (Fig. 4B). Rats were then challenged with saline or cocaine (15 μg/kg, i.p.) and sacrificed 2 h later. Tissue was extracted and processed for RT-qPCR detection of IL-1ß, TNF-a, GFAP, and NFκBIA, and CD11 b mRNA (Fig. 4C). A 2 × 2 ANOVA did not reveal a significant main effect of acute cocaine administration (15 μg/kg, i.p.) on RT-qPCR measurement of IL1-ß mRNA (Fig. 4C; F1,13 = 3.63, p = 0.07). A main effect of cocaine self-administration history was found such that rats undergoing cocaine self-administration exhibited elevated levels of IL1-ß mRNA expression in the VTA (F1,13 = 16.75, p = 0.0013). A statistically significant interaction between acute cocaine and cocaine self-administration was not found (F1,13 = 0.13, p = 0.726).
Fig. 4.
Cocaine self-administration increases the expression of IL-1ß and GFAP mRNA in the VTA. (A) Rats self-administered cocaine (0.5 μg/kg/infusion) during 2-h daily sessions over 15 days. (B) Rats extinguished formerly drug-paired lever pressing over 7 days of 2-h daily sessions. (C) Cocaine self-administration history increased IL-1ß, NFκB, and GFAP mRNA expression, but had no effect on TNFa mRNA expression in the VTA. NFκB mRNA expression was increased by a cocaine challenge only in rats having a history of cocaine self-administration. N = 3–6 rats per group. CD11 b mRNA expression was greatest in the cocaine self-administering group that received an acute cocaine challenge. (*p < 0.05; #Bonferroni’s test, p < 0.05 compared with saline control group.)
A 2 × 2 ANOVA did not reveal an effect of acute cocaine (F1,18 = 0.05, p = 0.815) or cocaine self-administration history (F1,18 = 0.13, p = 0.724) on TNF-α (Fig. 4C ). A significant interaction was also not found (F1,13 = 3.11, p = 0.095).
Cocaine self-administration increased expression of NFκBIA mRNA (Fig. 4C; F1,16 = 8.172, p = 0.011), which is induced by and inhibits NFκB. An effect of acute cocaine was not found, however (F1,16 = 1.511, p = 0.237). A moderate interaction between cocaine self-administration and acute cocaine on NFκBIA mRNA was also found (F1,16 = 4.049, p = 0.061). Post-hoc analysis revealed a significant increase in NFκBIA mRNA expression in the cocaine treated rats with a cocaine self-administration history compared to the saline treated rats with a saline self-administration history (t8 = 3.23, p < 0.05).
Fig. 4C also demonstrates a significant main effect of acute cocaine for the microglia activation marker CD11b (F1,14 = 16.79, p = 0.001). A main effect of cocaine self-administration was not found (F1,14 = 0.431, p = 0.522). An interaction between acute cocaine and cocaine self-administration was found (F1,14 = 8.408, p = 0.012). Post-hoc Bonferroni’s tests revealed that rats with a cocaine self-administration history that received acute cocaine had significantly greater expression of CD11b mRNA than rats witha saline self-administration history that received acute saline (t8 = 7.604, p < 0.001), rats with a saline self-administration history that received acute cocaine (t9 = 5.910, p < 0.001), and rats with a cocaine self-administration history that received acute saline (t6 = 9.829, p < 0.001). Rats with a cocaine self-administration history that received acute saline had a significant reduction in CD11b mRNA expression compared to saline self-administering rats that received acute saline (t7 = 3.295, p < 0.05) and saline selfadministering rats that received acute cocaine (t8 = 5.221). No difference was found between rats with a saline self-administration history that received acute saline compared to acute cocaine (t7 = 2.123).
Glial fibrillary acidic protein (GFAP), an intermediate filament protein specific to astrocytes that is upregulated during reactive gliosis, was found to be increased in the VTA following cocaine self-administration (Fig. 4C; F1,14 = 5.75, p = 0.03) an effect that further implicates chronic cocaine abuse in the alteration of glia phenotype from an inactive to a reactive, inflammatory state. An effect of acute cocaine (F1,14 = 0.25, p = 0.62) was not found. An interaction between acute cocaine and cocaine self-administration was also not found (F1,14 = 0.15, p = 0.71).
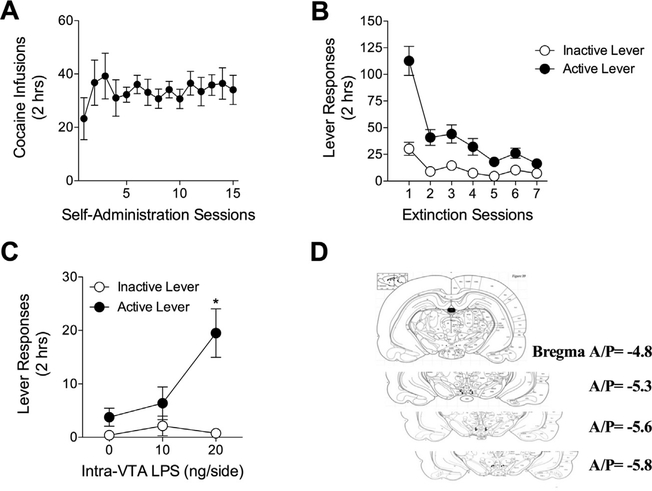
3.4. Administration of the interleukin-1 receptor antagonist into the VTA attenuates cocaine seeking
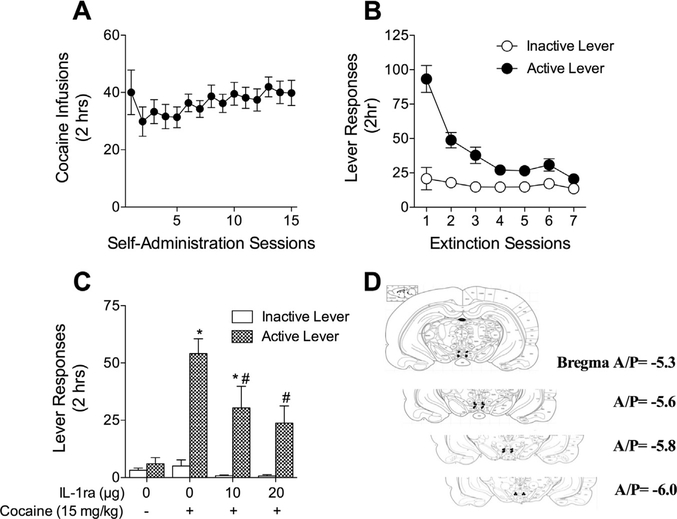
To test the necessity of IL-1ß signaling in cocaine seeking, rats self-administered cocaine for 15 days with a mean cocaine intake of 22.49 μg/kg/day over the last 5 days of self-administration (Fig. 5A). Drug-paired lever presses decreased across 7 days of extinction training (Fig. 5B). Intra-VTA administration of IL-1ra attenuated cocaine seeking at the previously drug-paired lever (Fig. 5C). A 2 × 2 ANOVA revealed a significant main effect of IL1ra treatment (F3,48 = 5.10, p < 0.01). A main effect of lever was found such that the formerly drug-paired lever was pressed more frequently than the inactive lever (F1,48 = 25.11, p < 0.0001). A significant interaction between treatment and lever was also found (F3,48 = 4.36, p < 0.01). Bonferroni’s comparisons revealed a significant increase in formerly drug-paired lever presses only among rats in the cocaine/vehicle group compared to those in the vehicle/vehicle groups (t17 = 5.581, p < 0.001). Furthermore, a significant reduction in previously drug-paired lever presses between the 10 μg IL-1ra dose and the vehicle/cocaine group (t18 = 2.787, p < 0.05) and the 20 μg IL1-ra dose and the vehicle/cocaine group (t16 = 3.238, p < 0.01) was found. Rats receiving the 10 μg IL-1ra dose pressed the active lever significantly more than those in the vehicle/vehicle group (t9 = 2.341, p < 0.05). However, there was no difference in active lever presses between rats receiving the IL-1ra 20 μg dose and the vehicle/vehicle treated rats (t7 = 1.594, p > 0.05).
Fig. 5.
Intra-VTA interleukin-1 receptor antagonist reduces drug-seeking behavior in the cocaine-primed model of relapse. (A) Rats self-administered cocaine (0.5 μg/kg/ infusion) during 2-h daily sessions over 15 days. (B) Rats extinguished formerly drug-paired lever pressing over 7 days. (C) IL-1ra (10 ng/side and 20 ng/side) or vehicle was administered bilaterally into the VTA 5 min prior to non-contingent injection of cocaine (15 μg/kg, i.p.) or vehicle (NaCl 0.9%, wt./vol.) N = 4–12 rats per group. Vehicle/cocaine treated rats increased cocaine seeking compared to the vehicle/vehicle treated group (*Bonferroni’s test, p < 0.001). Intra-VTA IL-1ra reduced drug-seeking behavior at both the 10 ng/side (#Bonferroni’s test, p < 0.05) and 20 ng/side (#Bonferroni’s test, p < 0.01) doses, although responding following 10 ng/side remained elevated compared with the vehicle/vehicle control group (*Bonferroni’s test, p < 0.05). (D) Placements were verified by post-mortem visual inspection.
4. Discussion
Effective pharmacological targets for the treatment of drug abuse must reduce drug craving while leaving adaptive, goal-directed behavior intact. TLR4 has become a target of interest for the treatment of addiction because of its possible involvement in modulating the acute reinforcing effects of cocaine and opioids by altering activity of the mesolimbocortical DA system (Hutchinson et al., 2012; Northcutt et al., 2015).
The specific role of the TLR4 system in psychostimulant abuse has come under question in light of a study by Tanda et al. (2016) in which a wide range of doses of the TLR4 antagonists (+) naltrexone and (+) naloxone reduced cocaine selfadministration only at the highest tested dose, a dose that also reduced sucrose self-administration. Furthermore, it was also found that chronic delivery of (+) naltrexone did not have an effect on the incubation of cue-induced methamphetamine seeking (Theberge et al., 2013). Here, potential non-specific effects of TLR4 antagonism were addressed by using a site-specific approach where the TLR4 antagonist, LPS-RS, was directed at the VTA, a brain region necessary for drug-primed cocaine seeking. Intra-VTA pharmacological antagonism of TLR4 with LPS-RS reduced drug-primed reinstatement of cocaine seeking but did not alter the reinstatement of sucrose seeking. These findings are consistent with the hypothesis that the effects of TLR4 antagonism are specific to cocaine-seeking in this model of relapse-like behavior. Furthermore, the data suggest that there may be differences in the role of TLR4 signaling in modulating cue-induced psychostimulant craving compared to drug-primed psychostimulant seeking where drug re-exposure may reactivate TLR4.
Activation of TLR4 with LPS moderately increased cocaine seeking, an effect that corroborates findings from a previous study demonstrating that TLR4 stimulation in the VTA significantly increases NAc shell dopamine release, albeit to levels that are much lower than those found with drugs of abuse (Northcutt et al., 2015). These results are consistent with the hypothesis that cocaine, but not natural reinforcers, interacts with TLR4 to modulate VTA dopamine releasing efferent projections and contribute to cocaine seeking.
In addition to pathogen recognition, the TLR4 complex binds chemical patterns of foreign substances, so-called xenobiotic associated molecular patterns (XAMPs) (Kawai & Akira, 2010). Cocaine could be considered a xenobiotic agent. Indeed, cocaine competitively binds to MD2 in vitro, and isolated microglia exposed to cocaine increase the expression of proinflammatory cytokines through toll-receptor family intracellular signaling proteins (Liao et al., 2016; Northcutt et al., 2016). In vivo cocaine administration in rats increases the expression of IL-1ß mRNA within the brain, an effect that is blocked by TLR4 antagonism(Cearley et al., 2011; Northcutt et al., 2014). Chronic administration of cocaine at doses similar to those self-administered by rats causes brain capillary lesions and extravasation of toxins into the brain, an effect that may exacerbate the neuroinflammatory state caused by interactions of cocaine with the TLR4 complex (Barroso-Moguel et al., 1997). Moreover, cocaine results in upregulation of cell adhesion molecules that traffic peripheral monocytes across the BBB (Yao et al., 2011). Thus, cocaine induced neuroinflammation may result from multiple sources. It is, nevertheless, likely that acute cocaine increases in proinflammatory cytokine expression through stimulation of TLR4.
Repeated TLR4 stimulation can lead to a state of chronic proinflammatory signaling characterized by a reactive, ramified microglia phenotype and increased levels of cytokines, a phenomenon that has been implicated in a range of neuropsychiatric disorders(Njjar et al., 2013). Cocaine alters microglia from an inactive, ramified phenotype to a reactive, amoeboid phenotype, a process that involves the endoplasmic reticulum stress pathway (Guo et al., 2015). We show that chronic cocaine self-administration increases the expression of IL-1ß within the VTA, an effect that persists into abstinence. Our results corroborate a relationship between chronic inflammation and cocaine abuse found in clinical studies. For instance, post-mortem analysis of human brains from individuals meeting the criteria for cocaine dependence reveals microglial and peripheral macrophage activation correlated with decreased numbers of dopaminergic neurons (Little et al., 2009). Furthermore, plasma levels of IL-1ß and IL-6 are positively correlated with cocaine use disorder symptom severity; whereas, plasma levels of crack cocaine addicts in acute withdrawal show a positive relationship between TFN-alpha, IL-6, and IL-1ß and withdrawal symptom severity (Araos et al., 2015; Levandowski et al., 2016; Moreira et al., 2016). Interestingly, the anti-inflammatory cytokine IL-10 is reduced in the serum of individuals meeting cocaine abuse criteria (Moreira et al., 2016).
Transcription of IL-1ß is enhanced by TLR4 activation and increased in the VTA after a history of cocaine self-administration. Therefore, we hypothesized that cocaine-induced TLR4 activation may enhance IL-1ß production and release, subsequently activating IL-1 receptor to modulate cocaine seeking. We found that reducing the availability of IL-1ß to bind to and activate its receptor within the VTA reduced cocaine seeking, an effect that indicates IL-1ß may act as a proximate mediator between microglia-TLR4 signaling and neuronal activity in cocaine seeking. IL-1ß is a neuromodulator in the CNS (Long-Smith et al., 2010). For instance, IL-1ß increases neuronal excitability by facilitating calcium permeability of glutamatergic NMDA receptors and modulates GABAergic signaling in the central amygdala (Bajo et al., 2015; Viviani et al., 2003). Deviation from homeostatic levels of IL-1ß disrupts hippocampal long-term potentiation (Avital et al., 2003; Goshen et al., 2007; Matsumoto et al., 2004). Importantly for reward related behaviors, intra-VTA IL-1ra also attenuates cocaine-induced elevation of DA in the NAc shell, a result that suggests IL-1ß may increase the excitability of VTA dopamine neurons and perhaps contributes to cocaine seeking (Northcutt et al., 2015).
Here, we have presented for the first time data implicating TLR4 activation and IL-1ß signaling in drug-primed reinstatement model of cocaine relapse. Cocaine self-administration increases basal levels IL-1ß in the VTA, an effect indicative of reactive, ramified microglia. Intra-VTA blockade of either TLR4 or the IL-1 receptor attenuates cocaine seeking induced by a cocaine challenge. IL-1ß is a neuromodulator that alters dopamine signaling in the VTA and appears to contribute to cocaine seeking. These results remain consistent with the hypothesis that innate immune signaling in the CNS contributes to the underlying mechanisms of cocaine seeking.
Acknowledgments
This project was funded by the Department of Defense (PR110146) and the National Institute of Health (DA033358).
References
- Alfonso-Loeches S, Pascual-Lucas M, Blanco AM, Sanchez-Vera I, Guerri C, 2010. Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci 3, 8285–8295. http://dx.doi.org/10.1523/JNEUROSCI.0976-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araos P, Pedraz M, Serrano A, Lucena M, Barrios V, García-Marchena N, Campos-Cloute R, Ruiz JJ, Romero P, Suárez J, Baixeras E, de la Torre R, Montesinos J, Guerri C, Rodríguez-Arias M, Miñarro J, Martínez-Riera R, Torrens M, Chowen JA, Argente J, Mason BJ, Pavón FJ, Rodríguez de Fonseca F, 2015. Plasma profile of pro-inflammatory cytokines and chemokines in cocaine users under outpatient treatment: influence of cocaine symptom severity and psychiatric co-morbidity: Inflammation and cocaine addiction. Addict. Biol 20, 756–772. http://dx.doi.org/10.1111/adb.12156. [DOI] [PubMed] [Google Scholar]
- Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R, 2003. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus 13,826–834. http://dx.doi.org/10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Hutchinson MR, Wang X, Rice KC, Maier SF, Watkins LR, 2015. Targeting the toll of drug abuse: The translational potential of toll-like receptor 4. CNS Neurol. Disorders-Drug Targets 14, 692–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajo M, Varodayan FP, Madamba SG, Robert AJ, Casal LM, Oleata CS, Siggins GR, Roberto M, 2015. IL-1 interacts with ethanol effects on GABAergic transmission in the mouse central amygdala. Front. Pharmacol 6 http://dx.doi.org/10.3389/fphar.2015.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso-Moguel R, Villeda-Hernández J, Méndez-Armenta M, Ríos C, 1997. Brain capillary lesions produced by cocaine in rats. Toxicol. Lett 92, 9–14. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Shelton KL, Hendrick E, Johnson KW, 2010. The glial cell modulator and phosphodiesterase inhibitor, AV411 (ibudilast), attenuates prime- and stress-induced methamphetamine relapse. Eur. J. Pharmacol 637, 102–108. http://dx.doi.org/10.1016/j.ejphar.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Hauser KF, 2014. Glial modulators as potential treatments of psychostimulant abuse. In: Adv. Pharmacol. Elsevier, pp. 1–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley CN, Blindheim K, Sorg BA, Krueger JM, Churchill L, 2011. Acute cocaine increases interleukin-1b mRNA and immunoreactive cells in the cortex and nucleus accumbens.Neurochem. Res 36, 686–692. http://dx.doi.org/10.1007/s11064-011-0410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Pittenger ST, Thapa I, Bastola DR, Bevins RA, Pendyala G, 2015. Ibudilast reverses the decrease in the synaptic signaling protein phosphatidylethanolamine-binding protein 1 (PEBP1) produced by chronic methamphetamine intake in rats. Drug Alcohol Depend. 152, 15–23. http://dx.doi.org/10.1016/j.drugalcdep.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, et al. , 2006. Abolished cocaine reward in mice with a cocaineinsensitive dopamine transporter. Proc. Natl. Acad. Sci 103, 9333–9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KH, Wiley CA, Bradberry CW, 2013. Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotox. Res 23, 174–188. http://dx.doi.org/10.1007/s12640-012-9334-7. [DOI] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF, 2006a. (a). Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J. Neurosci. Methods 151, 121–13. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF, 2006b. (b). mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol. Aging 27, 717–722. [DOI] [PubMed] [Google Scholar]
- Frank MG, Adhikary S, Sobesky JL, Weber MD, Watkins LR, Maier SF, 2016. The danger-associated molecular pattern HΜGB1 mediates the neuroinflammatory effects of methamphetamine. Brain Behav. Immun 51, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R, 2007. A dual role for interleukin-1 in hippocampaldependent memory processes. Psychoneuroendocrinology 32, 1106–1115. http://dx.doi.org/10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Guo ML, Liao K, Periyasamy P, Yang L, Cai Y, Callen SE, Buch S, 2015. Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy 11, 995–1009. http://dx.doi.org/10.1080/15548627.2015.1052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, Berke JD, 2016. Mesolimbic dopamine signals the value of work. Nat. Neurosci 19, 117–126. http://dx.doi.org/10.1038/nn.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Bajo M, Bell RL, Blednov YA, Varodayan FP, Truitt JM, de Guglielmo G, Lasek AW, Logrip ML, Vendruscolo LF, Roberts AJ, Roberts E, George O, Mayfield J, Billiar TR, Hackam DJ, Mayfield RD, Koob GF, Roberto M, Homanics GE, 2017. Genetic and pharmacologic manipulation of TLR4 has minimal impact on ethanol consumption in rodents. J. Neurosci 37, 1139 http://dx.doi.org/10.1523/JNEUROSCI.2002-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Northcutt AL, Hiranita T, Wang X, Lewis SS, Thomas J, van Steeg K, Kopajtic TA, Loram LC, Sfregola C, Galer E, Miles NE, Bland ST, Amat J, Rozeske RR, Maslanik T, Chapman TR, Strand KA, Fleshner M, Bachtell RK, Somogyi AA, Yin H, Katz JL, Rice KC, Maier SF, Watkins LR, 2012. Opioid activation of toll-like receptor 4 contributes to drug reinforcement. J. Neurosci 32, 11187–11200. http://dx.doi.org/10.1523/JNEUROSCI.0684-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Watkins LR, 2014. Why is neuroimmunopharmacology crucial for the future of addiction research? Neuropharmacology 76, 218–227. http://dx.doi.org/10.1016/j.neuropharm.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR, 2010. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav. Immun 24, 83–95. http://dx.doi.org/10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S, 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol 11, 373–384. http://dx.doi.org/10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ, 2007. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am. J. Psychiatry 164, 1149–1159. http://dx.doi.org/10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacagnina MJ, Rivera PD, Bilbo SD, 2017. Glial and neuroimmune mechanisms as critical modulators of drug use and abuse. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandowski ML, Viola TW, Prado CH, Wieck A, Bauer ME, Brietzke E, Grassi-Oliveira R, 2016. Distinct behavioral and immunoendocrine parameters during crack cocaine abstinence in women reporting childhood abuse and neglect. Drug Alcohol Depend. 167, 140–148. http://dx.doi.org/10.1016/j.drugalcdep.2016.08.010. [DOI] [PubMed] [Google Scholar]
- Liao K, Guo M, Niu F, Yang L, Callen SE, Buch S, 2016. Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J. Neuroinflamm 13 http://dx.doi.org/10.1186/s12974-016-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little KY, Ramssen E, Welchko R, Volberg V, Roland CJ, Cassin B, 2009. Decreased brain dopamine cell numbers in human cocaine users. Psychiatry Res. 168, 173–180. http://dx.doi.org/10.1016/j.psychres.2008.10.034. [DOI] [PubMed] [Google Scholar]
- Long-Smith CM, Collins L, Toulouse A, Sullivan AM, Nolan YM, 2010. Interleukin-1b contributes to dopaminergic neuronal death induced by lipopolysaccharide-stimulated rat glia in vitro. J. Neuroimmunol 226, 20–26. http://dx.doi.org/10.1016/j.jneuroim.2010.05.03. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Lüscher C, 2009. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat. Neurosci 12, 1036–1041. http://dx.doi.org/10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Yamaguchi T, Watanabe S, Yamamoto T, 2004. Involvement of arachidonic acid cascade in working memory impairment induced by interleukin-1 beta. Neuropharmacology 46, 1195–1200. http://dx.doi.org/10.1016/j.neuropharm.2004.02.012. [DOI] [PubMed] [Google Scholar]
- McFarland K, Kalivas PW, 2001. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci 21, 8655–8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos J, Pascual M, Rodríguez-Arias M, Miñarro J, Guerri C, 2016. Involvement of TLR4 in the long-term epigenetic changes, rewarding and anxiety effects induced by intermittent ethanol treatment in adolescence. Brain Behav. Immun 53, 159–171. http://dx.doi.org/10.1016/j.bbi.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Moreira FP, Medeiros JRC, Lhullier AC, de M Souza LD, Jansen K, Portela LV, Lara DR, da Silva RA, Wiener CD, Oses JP, 2016. Cocaine abuse and effects in the serum levels of cytokines IL-6 and IL-10. Drug Alcohol Depend. 158, 181–185. http://dx.doi.org/10.1016/j.drugalcdep.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Njjar S, Pearlman DM, Alper K, Njjar A, Devinsky A, 2013. Neuroinflammation and psychiatric illness. J. Neuroinflamm 10, 10–43. http://dx.doi.org/10.1186/1742-2094-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt AL, Hutchinson MR, Wang X, Baratta MV, Hiranita T, Cochran TA, Pomrenze MB, Galer EL, Kopajtic TA, Li CM, Amat J, Larson G, Cooper DC, Huang Y, O’Neill CE, Yin H, Zahniser NR, Katz JL, Rice KC, Maier SF, Bachtell RK, Watkins LR, 2015. DAT isn’t all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol. Psychiatry 20, 1525–1537. http://dx.doi.org/10.1038/mp.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland RS, Hahn YK, Knapp PE, Beardsley PM, Bowers MS, 2016. Ibudilast attenuates expression of behavioral sensitization to cocaine in male and female rats. Neuropharmacology 109, 281–292. http://dx.doi.org/10.1016/j.neuropharm.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Wilkinson MB, Mazei-Robison MS, Dietz DM, Maze I, Krishnan V, Renthal W, Graham A, Birnbaum SG, Green TA, Robison B, Lesselyong A, Perrotti LI, Bolanos CA, Kumar A, Clark MS, Neumaier JF, Neve RL, Bhakar AL, Barker PA, Nestler EJ, 2009. Nuclear factor B signaling regulates neuronal morphology and cocaine reward. J. Neurosci 29, 3529–3537. http://dx.doi.org/10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Stuber GD, Witten IB, Deisseroth K, Carelli RM, 2015. Mesolimbic dopamine dynamically tracks, and is causally linked to, discrete aspects of value-based decision making. Biol. Psychiatry 77, 903–911. http://dx.doi.org/10.1016/j.biopsych.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD, 2013. Adolescent morphine exposure affects long-term microglial function and later-life relapse liability in a model of addiction. J. Neurosci 33, 961–971. http://dx.doi.org/10.1523/JNEUROSCI.2516-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Gipson CD, Huits M, Kalivas PW, 2014. Prelimbic cortex and ventral tegmental area modulate synaptic plasticity differentially in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology 39, 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Ferris MJ, Jones SR, 2015. Cocaine self-administration disrupts mesolimbic dopamine circuit function and attenuates dopaminergic responsiveness to cocaine. Eur. J. Neurosci 42, 2091–2096. http://dx.doi.org/10.1111/ejn.12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Hendrick ES, Beardsley PM, 2013. Glial cell modulators attenuate methamphetamine self-administration in the rat. Eur. J. Pharmacol 701, 124–13. http://dx.doi.org/10.1016/j.ejphar.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Kupchik YM, Brown RM, Kalivas PW, 2013. Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. J. Neurosci 33, 13654–13662. http://dx.doi.org/10.1523/JNEUROSCI.1570-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH, 2013. A causal link between prediction errors, dopamine neurons and learning. Nat. Neurosci 16, 966–973. http://dx.doi.org/10.1038/nn.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda G, Mereu M, Hiranita T, Quarterman JC, Coggiano M, Katz JL, 2016. Lack of specific involvement of (+)-naloxone and (+)-naltrexone on the reinforcing and neurochemical effects of cocaine and opioids. Neuropsychopharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AMW, Castonguay A, Taylor AJ, Murphy NP, Ghogha A, Cook C, Xue L, Olmstead MC, De Koninck Y, Evans CJ, Cahill CM, 2015. Microglia disrupt mesolimbic reward circuitry in chronic pain. J. Neurosci 35, 8442–845. http://dx.doi.org/10.1523/JNEUROSCI.4036-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theberge FR, Li X, Kambhampati S, Pickens CL, St. Laurent R, Bossert JM, Baumann MH, Hutchinson MR, Rice KC, Watkins LR, Shaham Y, 2013. Effect of chronic delivery of the toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol. Psychiatry 73, 729–737. http://dx.doi.org/10.1016/j.biopsych.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Han DD, Gu HH, Caine SB, 2009. Lack of cocaine selfadministration in mice expressing a cocaine-insensitive dopamine transporter. J. Pharmacol. Exp. Ther 331, 204–211. http://dx.doi.org/10.1124/jpet.109.156265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, et al. , 2003. Interleukin-1b enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci 23, 8692–8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Loram LC, Ramos K, de Jesus AJ, Thomas J, Cheng K, Reddy A, Somogyi AA, Hutchinson MR, Watkins LR, Yin H, 2012. Morphine activates neuroinflammation in a manner parallel to endotoxin. Proc. Natl. Acad. Sci 109, 6325–633. http://dx.doi.org/10.1073/pnas.1200130109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao H, Kim K, Duan M, Hayashi T, Guo M, Morgello S, Prat A, Wang J, Su TP, Buch S, 2011. Cocaine hijacks 1 receptor to initiate induction of activated leukocyte cell adhesion molecule: implication for increased monocyte adhesion and migration in the CNS. J. Neurosci 31, 5942–5955. http://dx.doi.org/10.1523/JNEUROSCI.5618-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou JY, Crews FT, 2014. Release of neuronal HΜGB1 by ethanol through decreased HDAC activity activates brain neuroimmune signaling. PLoS ONE 9, e87915 http://dx.doi.org/10.1371/journal.pone.0087915. [DOI] [PMC free article] [PubMed] [Google Scholar]