Abstract
P2X purinergic receptors, activated by extracellular ATP, mediate a number of cardiac cellular effects and may be important under pathophysiological conditions. The objective of the present study was to characterize the P2X receptor-mediated ionic current and determine its role in heart failure using the calsequestrin (CSQ) model of cardiomyopathy. Membrane currents under voltage clamp were determined in myocytes from both wild-type (WT) and CSQ mice. The P2X agonist 2-methylthio-ATP (2-meSATP) induced an inward current that was greater in magnitude in CSQ than in WT ventricular cells. The novel agonist, MRS-2339, an N-methanocarba derivative of 2-chloro-AMP relatively resistant to nucleotidase, induced a current in the CSQ myocyte similar to that by 2-meSATP. When administered via a miniosmotic pump (Alzet), it significantly increased longevity compared with vehicle-injected mice (log rank test, P = 0.02). The improvement in survival was associated with decreases in the heart weight-to-body weight ratio and in cardiac myocyte cross-sectional area [MRS-2339-treated mice: 281 ± 15.4 (SE) μm2, n = 6 mice vs. vehicle-treated mice: 358 ± 27.8 μm2, n= 6 mice, P < 0.05]. MRS-2339 had no vasodilator effect in mouse aorta ring preparations, indicating that its salutary effect in heart failure is not because of any vascular unloading. The cardiac P2X current is upregulated in the CSQ heart failure myocytes. Chronic administration of a nucleotidase-resistant agonist confers a beneficial effect in the CSQ model of heart failure, apparently via an activation of the cardiac P2X receptor. Cardiac P2X receptors represent a novel and potentially important therapeutic target for the treatment of heart failure.
Keywords: ion channels, membrane current, mouse, heart
receptors for purine nucleotides, known as P2 purinergic receptors, mediate a number of potent and possibly important biological effects in the cardiovascular system (20, 22, 29). The P2X ion channels are receptor channels activated by extracellular ATP, whereas the P2Y receptors are G protein-coupled receptors. Together, they represent two subfamilies of the P2 nucleotide receptors. Previous studies have shown that extracellular ATP can cause an ionic current in murine (27), rat (26), and guinea pig (21) cardiac ventricular myocytes. The receptor that mediates this current appears to be a p2x receptor, of which the P2X4 receptor is an important subunit (27). Activation of P2X receptors leads to the opening of a nonse-lective cation channel permeable to Na+, K+, and Ca2+. The current is inward at negative membrane potentials, reverses near 0 mV, and becomes outward at positive potentials. The continuous activation of this receptor channel by endogenous extracellular ATP may assume an important biological function. This constant activation under the resting or negative membrane potentials would produce an inward current, whereas its activation during depolarized portions of the action potential should lead to an outward current. These currents represent a possible ionic mechanism by which the cardiac P2X channel achieves its biological effects.
A potential biologically important role of the cardiac P2X receptor was suggested by the finding that cardiac myocyte-specific overexpression of the P2X4 receptor can rescue the hypertrophic and heart failure phenotype of the calsequestrin (CSQ) model of cardiomyopathy (32). However, little is known regarding regulation of the cardiac P2X receptor in cardiac hypertrophy or failure. Furthermore, it is not clear whether an increased activation of the endogenous P2X receptor channel is beneficial or harmful in the progression of heart failure. Thus the principal objective of the present study was to investigate the regulation of the P2X receptor-mediated ionic current and its potential role in heart failure using several nucleotide agonists. The well-established CSQ model of cardiomyopathy was used. The P2X current was upregulated in cardiac ventricular myocytes of the CSQ hearts. Chronic administration of a novel nucleotidase-resistant P2 receptor agonist capable of inducing this ionic current and devoid of any vasodilator action reduced cardiac hypertrophy and increase lifespan. The data suggest that an important biological function of the cardiac P2X current is to favorably modulate the progression of cardiac hypertrophy and failure.
METHODS
Isolation of adult cardiac ventricular myocytes from wild-type and CSQ mice.
Mice displaying the CSQ model of severe cardiomyopathy and heart failure were bred and maintained according to a previously described method (32). The CSQ transgenic (TG) mice were originally provided by Dr. Larry Jones (Krannert Institute of Cardiology, Indiana University School of Medicine) and developed hypertrophy followed by a lethal heart failure phenotype with death near the age of 3 mo (12, 15). Ventricular myocytes were obtained from 3-mo-old wild-type (WT) and CSQ mice of either sex (26 WT, 47 TG) by an enzymatic dissociation procedure, as described previously (27, 32) Briefly, the hearts were rapidly excised from mice that had been anesthetized with pentobarbital sodium and treated with 1,000 units of heparin. The aorta was cannulated, and the heart was perfused in a Langendorff apparatus with oxygenated (95% O2-5% CO2) Ca2+-free solution (37°C) for 5 min at a rate of 2.5 ml/min. The solution composition was (in mM): 126 NaCl, 4.4 KCl, 1.0 MgCl2, 18 NaHCO3, 11 glucose, 4 HEPES, and 3 2,3-butanedione monoxime (BDM, pH 7.3 adjusted with NaOH). Thereafter, the perfusing solution was changed to that containing 25 μM CaCl2 and 70 μg/ml liberase (Roche Molecular Biochemicals) for 8–10 min. Cells were sedimented by gravity for 10–15 min, and the pellet was resuspended in 200 μM Ca2+-containing Tyrode solution (containing 3 mM BDM), allowed to settle for 30 min at room temperature, and suspended with Tyrode solution containing (in mM) 135 NaCl, 5.4 KCl, 1.0 CaCl2, 1.0 MgCl2, 10 HEPES, and 10 dextrose (pH 7.4 adjusted with NaOH). The experiments were carried out at room temperature (22–23°C) and were completed within 4–6 h after myocyte isolation. The use of animals was approved by the Institutional Animal Care and Use Committee at the University of Connecticut School of Medicine.
Cellular electrophysiological methods.
The whole cell patch-clamp technique was used for the experiments. Electrodes were prepared from borosilicate glass pipettes (1.2 mm ID) with a two-step pulling procedure and filled with pipette solution (see below). The pipette was connected via an Ag-AgCl wire to the head stage of an amplifier (model EPC-7; List Medical Systems, Greenvale, NY) controlled by a computer and Axon pClamp software. For voltage-clamp experiments, the electrodes were filled with a solution containing (in mM): 135 cesium aspartate, 5 NaCl, 5 Mg2ATP, 10 HEPES, and 10 EGTA (pH 7.3 adjusted with CsOH) with or without 2 mM guanosine 5′-O-(2-thiodiphosphate) (GDPβS). Electrode resistances were 2–4 MΩ. As soon as electrical contact was established, the superfusion medium was changed to a modified Tyrode solution (5.4 mM KCl was omitted and 10 mM CsCl and 5 μM ouabain were added to Tyrode solution to block K+ currents and the Na-K pump current, respectively). In quantifying the P2 agonist-induced current, the Tyrode solution contained the indicated concentration of each agent. In studying the effect of a P2 receptor antagonist, both agonist and antagonist were coapplied at the indicated concentrations. The voltage-clamp protocol used was a ramp voltage protocol from −100 mV holding potential to +50 mV as previously described (27). The ramp protocol was applied to cells at 20-s intervals for 1 min. Three current traces from −100 to 50 mV were averaged to construct the current-voltage (I-V) relationship.
Immunoblotting.
Hearts from 3-mo-old CSQ and WT mice were isolated, blotted dry, weighed, and homogenized in ice-cold buffer containing 0.25 M sucrose and 10 mM MOPS, pH = 7.2 (16 ml/g wt), using a tissue homogenizer (PowerGen model 125; Fisher Scientific, Pittsburgh, PA). After solublization in sample buffer, SDS-PAGE and immunoblotting were conducted, as recently described (32). Homogenate protein (25 μg) was electrophoresed per gel lane using 8% polyacrylamide and transferred to nitrocellulose. For detection of the P2X4 receptor, rabbit polyclonal antibody directed against a unique COOH-terminal sequence of the rat P2X4 receptor (Alomone, Jerusalem, Israel), which cross-reacted with both the human and mouse P2X4 receptors, was used (9). The membrane was incubated with peroxidase-coupled anti-rabbit Ig antibody (1:5,000) and developed with an ECL-Plus kit (Amersham). The level of the P2X4 receptor protein was quantified via a Bio-Rad Geldoc 2000 using the Discovery Series Quantity One version 4.5.2 (Bio-Rad, Hercules, CA). The quantity of the protein in each band was proportional to the sum of intensity of all pixels within the band boundary multiplied by the area of each pixel. Equal amounts of protein were loaded per gel lane, which was subsequently confirmed by Ponceau S staining of the blot (8, 34) and by probing with an affinity-purified goat polyclonal antibody against the COOH termini of a broad range of actin isoforms such as the β- and α-actins (Actin, 1–19: sc-1616; identical in human, rat, and mouse; see Ref. 2).
Quantification of myocyte cross-sectional area.
Cardiac myocyte cross-sectional area was quantified according to a previously described method (19, 24). Excised hearts were fixed in 4% paraformaldehyde and labeled with a fluorescein-conjugated wheat germ agglutinin. Photomicrographs were taken at the midleft ventricular wall as previously described. The images of cross-sectioned cells showing consistently round shapes were captured with Macrofire PictureFrame (Optronics, Goleta, CA), and the cross-sectional area was measured with ImageProPlus (MediaCybernetics, Silver Spring, MD). Typically, 50–100 cross-sectional areas were determined and averaged per heart.
MRS-2339 and vascular reactivity.
Thoracic aortas were quickly removed from 8- to 10-wk-old WT (C57 BL6) mice, cut into 3-mm rings, and studied as previously described (17). After preconstriction with phenylephrine (1 μM), increasing concentrations of ACh or MRS-2339 were added in a cumulative fashion to achieve a concentration-response curve. The percent relaxation was determined as the percent decrease in ring tension (gram) compared with the tension before the addition of ACh or MRS-2339. Data were shown as means ± SE.
Drugs and solutions.
2-Methylthio-ATP (2-meSATP), Brilliant Blue G (BBG), pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS), and 2-methylthio-ADP (2-meSADP) were obtained from Sigma Chemical (St. Louis, MO). NF-449 was from Tocris Bioscience (Ellisville, MO). Both 2-meSATP and 2-meSADP were dissolved just before each experiment. Stock solutions were prepared in PBS, pH = 7.4, and added to the Tyrode solution to obtain the desired concentrations. The N-methanocarba derivative of 2-chloro-AMP, MRS-2339, and the P2Y1 receptor antagonist MRS-2500 were synthesized as previously described (14, 23). These two P2 purinergic agents were characterized in detail previously. MRS-2339 was dissolved in PBS, pH = 7.4 at 3.3 μM (200 μl total volume), filtered for sterility for in vivo administration at 6 μl/day for 28 days via a miniosmotic pump (Alzet) implanted in the CSQ mice. The longevity of MRS-2339- or vehicle-administered animals was determined, and the survival difference was analyzed by log rank test as previously described (32).
Data.
Unless otherwise indicated, data were provided as means ± SE. Student’s t-test for paired or unpaired samples was used to evaluate the effects of experimental interventions; P < 0.05 was taken as statistically significant.
RESULTS
The extracellular 2-meSATP-stimulated current was greater in CSQ than in WT cardiac myocytes.
The activation of a P2X receptor was studied by application of 2-meSATP (3 μM) for 3–5 min to cells obtained from CSQ TG mice and from WT mice using the whole cell voltage-clamp protocol. An extracellular 2-meSATP-stimulated current was observed in WT cardiac myocytes (Fig. 1), consistent with our previous finding (27). The same concentration of 2-meSATP caused a similar current that displayed a linear relation between −100 and +50 mV and reversed from inward to outward at ~0 mV in cardiac myocytes of CSQ hearts (Fig. 1), identical to that found in the WT cardiac myocyte. CSQ myocytes were more responsive than WT myocytes upon exposure to 2-meSATP in the induction of a current. Of cells that showed an induced current in response to 2-meSATP, the amplitude of the 2-meSATP-stimulated current was significantly greater in cells from CSQ than from WT hearts at −100 mV (Fig. 1, P < 0.05). At −100 mV, the 2-meSATP-stimulated current was −1.28 ± 0.15 (SE) pA/pF in CSQ myocytes (n = 15 cells from 7 mice) and −0.91 ± 0.09 pA/pF in WT myocytes (n = 18 cells from 10 mice; P < 0.05). Similarly, at −90 and −80 mV, the 2-meSATP-evoked current was also greater in CSQ than in WT myocytes (P < 0.05). That 2-meSATP-stimulated current showed similar voltage dependence and reversal potential in WT and CSQ cardiac myocytes is consistent with the presence of the same P2X current in both WT and CSQ myocytes.
Fig. 1.
Characterization of 2-methylthio-ATP (2-meSATP)-stimulated current in ventricular myocytes from calsequestrin (CSQ) hearts. Cardiac ventricular myocytes were prepared from wild-type (WT) and CSQ hearts, and currents were measured by voltage clamp in the whole cell configuration. The ramp voltage clamp was from −100 to +50 mV. A: 2-meSATP (3 μM) induced a steady inward current at negative potentials and an outward current at positive potentials with reversal potential near 0 mV in both WT (n = 18) and CSQ (n = 15) myocytes. The current-voltage (I-V) relationship was shown in current density (pA/pF) as means ± SE. B: effects on the 2-meSATP (3 μM)-evoked current of various P2X receptor antagonists PPADS (100 μM), NF-449 (300 nM), and Brilliant Blue G (5 μM) were determined. The ramp voltage clamp ranged from −100 to +50 mV. Data were means ± SE; PPADS: 4 cells (from 6 mice); NF-449: 5 cells (8 mice); Brilliant Blue G: 5 cells (6 mice).
Additional pharmacological characterization of the 2-meSATP-evoked current was carried out. Data summarized in Fig. 1B showed that PPADS was able to partially inhibit the 2-meSATP-induced current, whereas the P2X1 selective antagonist NF-449 or the P2X5 or P2X7-selective antagonist BBG had very little inhibitory effect on this current. The data are consistent with the notion that homomeric P2X1, P2X5, or P2X7 receptors are not involved in mediating the 2-meSATP-evoked current.
Function of the cardiac P2X current in heart failure: Salutary effect of a nucleotidase-resistant P2 receptor agonist in the CSQ mice.
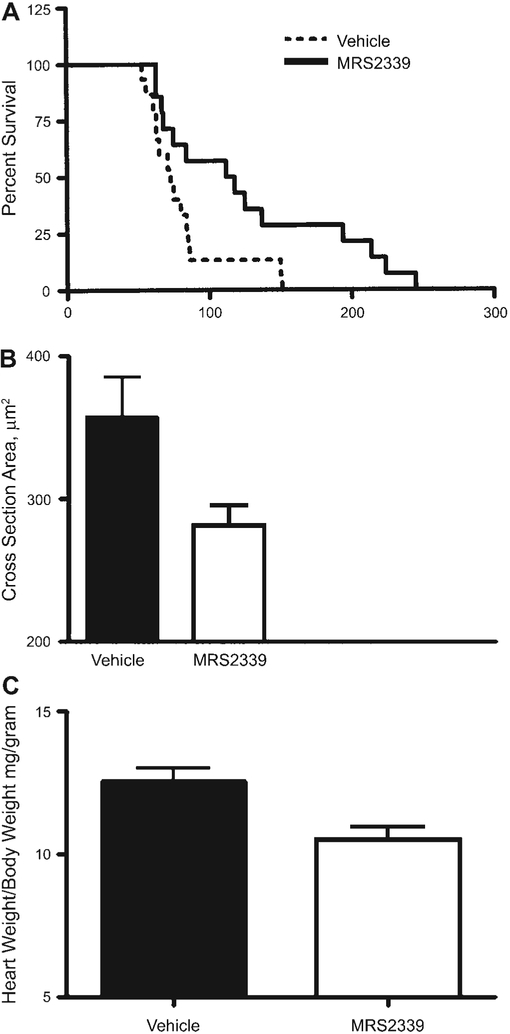
Although the cardiac P2X current is augmented in the failing myocytes, its function in modulating the progresssion of heart failure is unknown. To gain insights to this question, a nucleotidase-resistant P2 receptor agonist, MR-S2339, an N-methanocarba derivative of 2-chloro-AMP, was synthesized and administered to the CSQ animals via a minipump. Compared with vehicle-injected (14 mice) CSQ mice, MRS-2339-treated mice (15 mice) had a significantly longer lifespan (log rank test, P = 0.02; median lifespan was 115 days in MRS-2339-treated vs. 73 days in vehicle-treated animals; Fig. 2A). The improvement in survival was associated with a reduction in the cardiac myocyte hypertrophy, as reflected by a smaller cross-sectional area in MRS-2339-treated mice [281 ± 15 (SE) μm2, n = 6 mice] compared with that in vehicle-treated mice (358 ± 28 μm2, n = 6 mice; Fig. 2B; P < 0.05). Similarly, MRS-2339-treated animals exhibited a lower heart weight-to-body weight ratio (MRS-2339-treated: 10.5 ± 1.38, n = 9 hearts vs. controls: 12.56 ± 2.2, n = 23 hearts, P < 0.05; Fig. 2C). These data demonstrated that this nucleotide analog, when administered in vivo, can rescue the cardiac hypertrophic phenotype of the CSQ mouse.
Fig. 2.
Effects on lifespan and cardiac hypertrophy from chronic administration of MRS-2339 in CSQ mice. A: Kaplan-Meier analysis was used to determine the survival probability in CSQ animals receiving vehicle or MRS-2339 via Alzet minipump. MRS-2339 was infused as a 3-μM sterile solution at a rate of ~6 μl/day for 28 days. Log rank test method was used to analyze the survival curves (P = 0.02). B: myocyte cross-sectional areas were determined in vehicle- and MRS-2339-treated mice, as described in METHODS. Chronic treatment with MRS-2339 caused a significant decrease in the cell cross-sectional area (P < 0.05). C: MRS-2339 treatment decreased the heart weight-to-body weight ratio in CSQ mice (P < 0.05). All data were provided as means ± SD.
N-methanocarba derivative of 2-chloro-AMP can induce a P2X-like current in the CSQ cardiac myocyte.
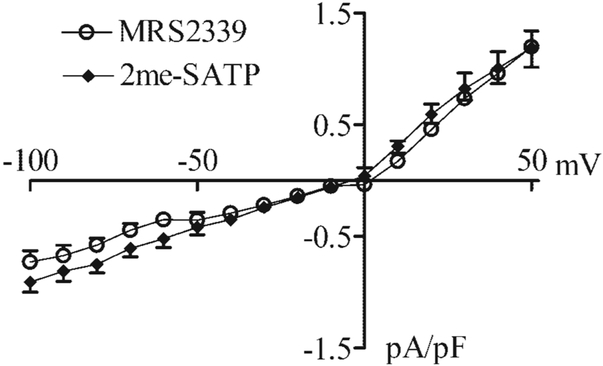
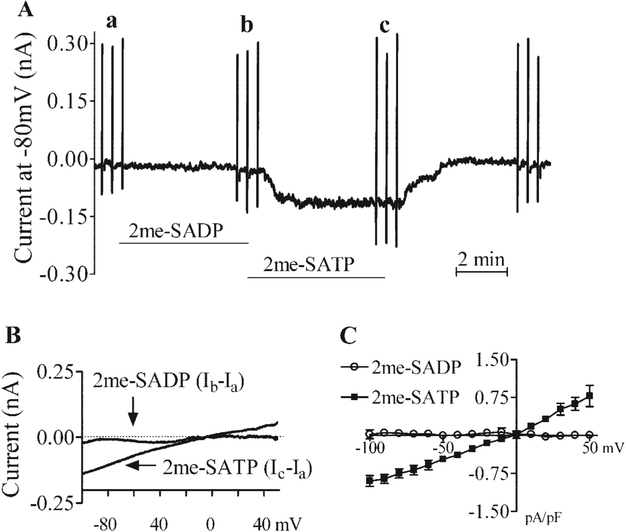
Because cardiac myocyte-specific overexpression of the P2X4 receptor in the CSQ mice also reduced the heart weight-to-body weight ratio and cardiac myocyte hypertrophy (27, 31), one possible mechanism by which MRS-2339 might rescue the hypertrophic phenotype of the CSQ mice is its activation of the P2X receptor on the cardiac myocytes of these animals. In fact, this nucleotidase-resistant P2 receptor agonist can evoke a current with a similar I-V relationship and reversal potential as the P2X agonist 2-meSATP in both WT (Fig. 3) and CSQ (Fig. 4) cardiac myocytes. MRS-2339 was initially characterized as a weak P2Y1 receptor agonist (23). However, the potent P2Y1 receptor-selective antagonist MRS-2500 could not block the current evoked by 2-meSATP (Fig. 5) or by MRS-2339 (data not shown), suggesting against a role of the P2Y1 receptor in mediating the cardiac current effect of 2-meSATP or MRS-2339. That 2-meSATP is in some systems an antagonist at the P2Y1 receptor (1) but was a potent agonist at inducing this current further argues against a role for the P2Y1 receptor in mediating this current. It is possible that 2-meSATP could be degraded to 2-meSADP, which in turn might evoke a current via its activation of the P2Y1, P2Y12, or P2Y13 receptors (1), if these P2Y receptors were indeed present on the murine cardiac myocyte. However, 3 μM 2-meSADP failed to induce any current in myocytes that showed a full response to 3 μM 2-meSATP (Fig. 6), further arguing against a role of the P2Y receptor in mediating the current effect of 2-meSATP. Finally, the presence of GDPβS in the pipette solution did not affect the ability of 2-meSATP to evoke the current (current without GDPβS: −0.908 ± 0.09 and 1.1 ± 0.175 pA/pF at −100 and +50 mV, respectively, n= 18 myocytes from 22 mice; current with GDPβS: −0.877 ± 0.198 and 0.85 ± 0.193 pA/pF at −100 and +50 mV, respectively, n= 5 myocytes from 7 mice; P > 0.05). Similarly, the addition of GDPβS in the pipette also did not decrease the MRS-2339-evoked current. At −100 mV, current density was −0.73 ± 0.15 pA/pF in the absence of GDPβS (n = 7 cells); with GDPβS, the current density was −0.66 ± 0.10 pA/pF (n = 3 cells, P > 0.1 vs. no GDPβS). At +50 mV, there was also no difference in the current density whether or not GDPβS was added. The data argue against a role of G protein or G protein-coupled P2Y receptors in the induction of this current.
Fig. 3.
N-methanocarba derivative of 2-chloro-AMP, MRS-2339, can evoke an ionic current similar to that induced by 2-meSATP in WT murine cardiac myocytes. Cardiac ventricular myocytes were prepared from WT hearts, and the current was measured by voltage clamp in the whole cell configuration. The ramp voltage clamp was from −100 to +50 mV. MRS-2339 (10 μM) induced a current similar to that evoked by 2-meSATP (3 μM) with identical I-V relationships and reversal potentials. Data were shown as current density in pA/pF. Data were means ± SE of 18 myocytes from 10 mice for 2-meSATP-induced current and 7 myocytes from 4 mice for the current induced by MRS-2339.
Fig. 4.
Effects of MRS-2339 and 2-meSATP on membrane currents in CSQ myocytes. Cardiac ventricular myocytes were prepared from CSQ hearts, and the effects of MRS-2339 and 2-meSATP on the current were measured by voltage clamp in the whole cell configuration. The ramp voltage clamp was from −100 to +50 mV. Cells were held at −80 mV with the ramp voltage-clamp protocol applied, as described in methods. Addition of either MRS-2339 or 2-meSATP induced an inward current that dissipated upon washout of agonist. MRS-2339 (10 μM) induced a current similar to that evoked by 2-meSATP (3 μM) with identical I-V relationships and reversal potentials in the CSQ cardiac myocyte. Data were shown as current density in pA/pF. Data were means ± SE of 15 myocytes from 7 mice for the 2-meSATP-induced current and 8 myocytes from 5 mice for the current evoked by MRS-2339.
Fig. 5.
P2Y1 receptor does not mediate the current evoked by 2-meSATP. Cardiac ventricular myocytes were prepared from WT hearts, and the effect of MRS-2500 (1 μM) on 2-meSATP-evoked current was measured by voltage clamp in the whole cell configuration. 2-meSATP was present at 3 μM. The ramp voltage clamp was from −100 to +50 mV, as described in methods. The 2-meSATP-evoked current was similar whether or not MRS-2500 was present. Data were shown as current density in pA/pF and were means ± SE of 4 myocytes from 4 mice.
Fig. 6.
P2Y receptor agonist 2-methylthio-ADP (2-meSADP) does not evoke any current in adult WT mouse cardiac myocytes. A: 2-meSATP, but not 2-meSADP, was able to induce a steady inward current in an adult (3-mo-old) mouse cardiac myocyte held at − 80 mV. Both agonists were present at 3 μM. The current induced by 2-meSATP reversed upon washout of the agonist. The vertical marks on the current trace are ramp voltage clamps from −100 to +50 mV. B: The I-V relations taken at a and b (for 2-meSADP) and at a and c (for 2-meSATP) in A were subtracted, and the differences were plotted as a function of the voltage in pA/pF. Data were shown as means ± SE from 8 WT cardiac myocytes from 14 mice.
MRS-2339 lacks vasodilator effect in mouse aorta preparation.
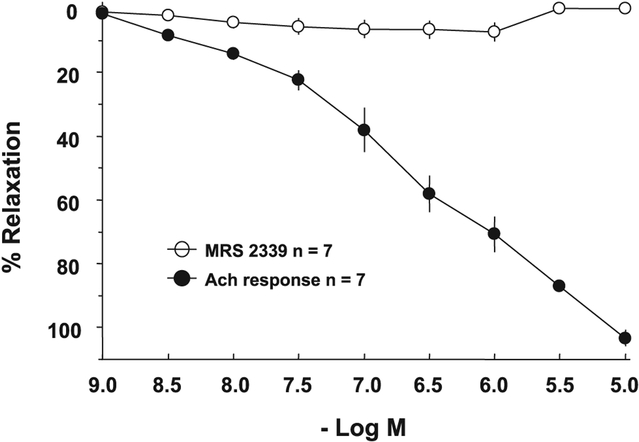
Because MRS-2339 is a nucleotide analog, it may in theory have a vascular effect via activation of either vascular P2Y or P2X receptors. An arterial vasodilator effect of MRS-2339 would unload the failing heart and potentially explain the decrease in heart hypertrophy and the increase in lifespan of the CSQ animals. With the use of an adult mouse thoracic aorta ring preparation preconstricted with phenylephrine, ACh was able to cause a dose-dependent relaxation of the ring segments (Fig. 7). In the same vessel rings, MRS-2339 did not induce relaxation over the same range of concentrations (10−9 to 10−5 M). These data suggest against a vasodilator effect of MRS-2339 and make it unlikely that vascular unloading is a beneficial mechanism here.
Fig. 7.
MRS-2339 lacks vasodilator effect in adult mouse aorta ring. Adult (2- to 3-mo-old) WT mouse thoracic aorta ring segments were studied in organ chambers as described in methods. Relaxation in response to various indicated concentrations of ACh and MRS-2339 was determined. Seven ring segments from 3 mice were used. Data were shown as means ± SE. ACh caused a significant and pronounced relaxation, whereas MRS-2339 did not elicit relaxation.
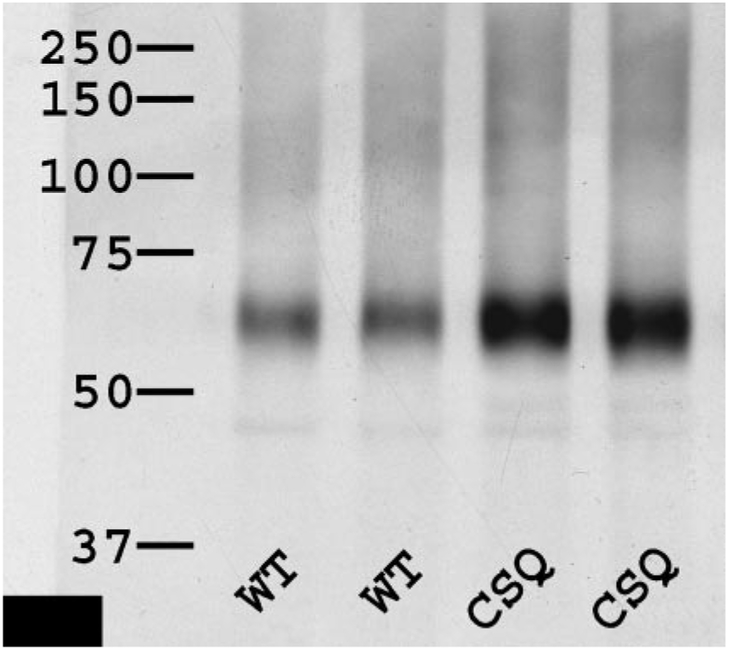
Increased expression of endogenous cardiac P2X4 receptors in the CSQ mice.
P2X4 receptors are an important subunit of the native cardiac P2X receptors (27). The protein expression level of this receptor in the WT and CSQ hearts was determined by immunoblotting. Figure 8 showed that the level of cardiac P2X4 receptors is greater in the CSQ than in the WT animals (P < 0.05). The data are consistent with a greater level of the native P2X receptor in the CSQ heart and may provide an explanation for the greater 2-meSATP-stimulated current in these failing cardiac myocytes.
Fig. 8.
P2X4 receptor level is increased in the failing CSQ hearts. Hearts of 3-mo-old WT and CSQ mice were homogenized, solublized, and immunoblotted, and the relative level of P2X4 receptor was quantified as described in methods. Identical amounts of protein were loaded per lane for each antibody used. Antibodies were specific for P2X4 receptor. The autoradiograph was typical of 4 similar experiments. The level of P2X4 receptor was higher in the CSQ than in the WT hearts (P < 0.05, t-test).
DISCUSSION
Acting via cell surface receptors, extracellular ATP can exert potentially important biological effects in the heart. Extracellular ATP can induce increases in ionic current and contractility in cardiac myocytes of a number of species (5, 9, 20–22, 25–27, 29). The receptor that mediates the effect of ATP on ionic current was recently characterized and appears to be a heteromer of several members of the P2X family (20, 27). Although the extracellular ATP-induced, P2X receptor-mediated current is present in normal adult cardiac myocytes, its regulation under pathological conditions is not known. TG cardiac myocyte-specific overexpression of the human P2X4 receptor showed an increased extracellular ATP-evoked current and reversed the hypertrophic and failing phenotype of the CSQ model of cardiomyopathy (32). These data suggest that an increased expression or activation of the cardiac P2X receptor may exert a salutary role in cardiac hypertrophy and failure. With the use of the CSQ model of hypertrophy and severe heart failure, this study characterized the regulation of cardiac P2X current in failing cardiac myocytes and determined whether chronic in vivo administration of a nucleotidase-resistant P2X agonist can confer a beneficial effect in these heart failure animals.
We found that the P2X agonist 2-meSATP induced a membrane current in ventricular myocytes isolated from both WT mice and CSQ mice. The I-V relationship and the reversal potential were similar in both kinds of cardiac myocytes. However, the density of the 2-meSATP-evoked current was greater in CSQ than in WT myocytes. Although the CSQ myocytes are hypertrophic and larger, the current was normalized against the larger capacitance of the CSQ myocytes as current density in picoampere per picofarads. These data suggest that the P2X current is upregulated in the cardiac myocytes of the CSQ mice. Although it is not known whether the cardiac P2X receptor protein is increased and accounts for the larger current density in the CSQ myocytes, the level of one of the known subunits, that of the P2X4 receptor, was increased in these myocytes. Because the exact identity of the other P2X subunits of this native cardiac P2X receptor is not known, the level of these other subunits may also be upregulated in the failing CSQ hearts. Nevertheless, the larger current density mediated via the endogenous P2X receptor and higher levels of its P2X4 subunit suggest an upregulation of this receptor in the failing cardiac myocyte.
Further characterization of the 2-meSATP-evoked current in the WT murine cardiac myocyte demonstrated a lack of inhibition of the 2-meSATP current by GDPβS that was present at a concentration known to block GDP/GTP exchange (28, 33), suggesting against a role of the G protein in mediating this current. This finding, along with the observations that the P2Y agonist 2-meSADP could not evoke any current and that P2Y1 selective antagonist MRS-2500 could not block the current induced by either 2-meSATP or MRS-2339, strongly suggests against a role of the G protein-coupled P2Y receptor in the induction of this current. Three additional P2X receptor-selective antagonists were used. NF-449 is selective for native rat P2X1 receptors vs. native guinea pig P2X3 and P2Y1 receptors, or vs. native human P2Y2 receptors in HEK293 cells (4). NF-449 is also selective for recombinant human P2X1 vs. P2X7 receptors expressed in Xenopus oocytes (10). Although the selectivity of NF-449 for native murine P2 receptors is not known, one may infer from the data using NF-449 that homomeric P2X1 receptor is unlikely part of the native cardiac P2 receptor mediating the 2-meSATP-induced current. At 5 μM, BBG can block the rat and human P2X7 receptor but will not block rat P2X3, P2X2/3, P2X4, or P2X1/5 receptors. It may partially inhibit the rat P2X2 or the human P2X4 receptor (11). At this concentration, BBG can nearly abrogate human P2X5 receptor-mediated current (3). Because there are no data regarding BBG’s effect on any of the murine P2X receptor, one cannot definitively exclude a role of homomeric P2X5 and P2X7 receptors in mediating the 2-meSATP current in the murine myocyte. That PPADS can block most of the 2-meSATP-evoked current is consistent with a role of the P2X4 receptor as a subunit of the native P2X receptor that mediates this current. This conclusion is also consistent with our previous findings that showed a partial sensitivity of the current to antagonism by suramin (27).
To investigate the possible importance of the native cardiac P2X receptor in heart failure, a nucleotidase-resistant P2 receptor agonist, MRS-2339, was synthesized. Previous studies have shown that structurally constraining the methanocarba ring in the N conformation confers relative resistance to 5′-nucleotidase-mediated hydrolysis of AMP analogs (23). The rate of hydrolysis by rat 5′-ectonucleotidase of N-methanocarba-AMP was only 0.14% of the rate of hydrolysis of AMP. When administered chronically in vivo to the failing CSQ mice, MRS-2339 reduced cardiac hypertrophy, as shown by decreases in myocyte size and heart weight-to-body weight ratios, and by prolonged survival. The exact mechanism by which this beneficial effect is achieved and the cause-effect importance of P2X receptor stimulation are not clear. However, a number of lines of evidence point to activation of the cardiac P2X receptor as likely important. First, the antihypertrophic effect of myocyte-specific overexpression of the P2X4 receptor is similar to that of the in vivo administration of MRS-2339. The salutary effects of MRS-2339 and myocyte-specific overexpression of the P2X4 receptor in the same animal model of heart failure are nearly identical with reductions in myocyte size and heart weight-to-body weight ratios as well as prolongation of lifespan. Second, this P2 agonist was capable of inducing a current similar to that evoked by the P2X agonist 2-meSATP in not only the WT but also the CSQ cardiac myocytes. During administration of this 5′-nucleotidase-resistant agonist, the cardiac P2X receptor was likely activated to some degree in vivo. Third, this agonist is devoid of any vasodilator effect at concentration as high as 10 μM, whereas the vasodilator action of ACh was striking and readily demonstrated in the same vascular ring preparation. The lack of any in vitro vasodilator effect suggests against any vascular unloading as a cause of the beneficial effect of this agonist in heart failure animals. However, because blood pressure in the CSQ animals is not known, a possible MRS-2339-induced decrease in blood pressure may have contributed to the salutary effect observed.
The endogenous free plasma ATP concentration has been measured as 93 ± 27 nM in rats (6). Using a dialysate measurement, the basal interstitial fluid ATP concentration has been estimated to be 38 ± 8 nM in the in situ rat heart (16). It is not clear to what extent the basal extracellular ATP can activate this nonselective current in the heart. Nevertheless, the chronic administration of MRS-2339 may evoke a continued activation of this current and thus cause a salutary effect in the failing heart. The CSQ model is a heart failure model that arises from abnormal calcium handling and homeostasis. It is believed that an abnormal calcium handling plays an important contributing if not a pathogenic role in the development of heart failure. Therefore, it is quite possible that the beneficial effect of MRS-2339 and its evoked current may also be generalized to other models of heart failure. The mechanism by which the cardiac P2X current achieves its salutary effect in heart failure is unknown. The inward current at negative potentials may increase the sarcoplasmic reticulum calcium loading and enhance the performance of the failing heart. The outward current at positive potentials may enhance repolarization during phase 1 or 2, and thereby shorten the action potential duration with resultant decreased calcium influx and reduced stimulus for cardiac hypertrophy. The duration of action potential and its manipulation have been implicated in the development or modulation of cardiac hypertrophy (7, 13, 18, 30). These possibilities await and deserve further investigation.
Overall, the cardiac myocyte P2X receptor is upregulated in the CSQ model of hypertrophy and heart failure. Chronic in vivo administration of the P2X agonist MRS-2339 can rescue the hypertrophic phenotype of the CSQ animals and prolong their longevity. This salutary effect appears to be mediated by activation of the upregulated cardiac P2X receptor. The data imply that augmentation of the cardiac myocyte P2X current can reverse or attenuate cardiac hypertrophy and failure and suggest that agonists at this nucleotide-gated receptor channel represent a new therapeutic target.
Acknowledgments
GRANTS
This work was supported in part by National Heart, Lung, and Blood Institute Grant RO1-HL-48225 and a Ray Neag Distinguished Professorship (to B. T. Liang). B. V. Joshi and K. A. Jacobson acknowledge support from the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
REFERENCES
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology. Update of the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacolog Rev 58: 281–341, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barkalow K, Hartwig JH. Actin cytoskeleton: setting the pace of cell movement. Curr Biol 5: 1000–1002, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Bo X, Jiang LH, Wilson HL, Kim M, Burnstock G, Surprenant A, North RA. Pharmacological and biophysical properties of the human P2X5 receptor. Mol Pharmacol 63: 1407–1416, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Braun K, Rettinger J, Ganso M, Kassack M, Hildebrandt C, Ullmann H, Nickel P, Schmalzing G, Lambrecht G. NF449: a subnanomolar potency antagonist at recombinant rat P2X1 receptors. Naunyn-Schmiedeberg’s Arch Pharmacol 364: 285–290, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Danziger R, Raffaeli S, Moreno-Sanchez R, Sakai M, Capogrossi MC, Spurgeon HA, Hansford RG, and Lakatta EG. Extracellular ATP has a potent effect to enhance cytosolic calcium and contractility in single ventricular myocytes. Cell Cal 9: 193–199, 1988. [DOI] [PubMed] [Google Scholar]
- 6.Douillet CD, Suy S, Zarzaur BL, Robinson IIIWP, Milano PM, Boucher RC, Rich PB. Measurement of free and bound fractions of extracellular ATP in biological solutions using bioluminescence. Luminescence 20: 435–441, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad and the ugly. Annu Rev Physiol 65: 45–79, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Gotzmann J, Gerner C. A method to produce Ponceau replicas from blots: Application for Western analysis. Electrophoresis 21: 523–525, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Hu B, Mei Q, Smith E, Barry WH, Liang BT. A novel cardiac inotropic phenotype with cardiac transgenic expression of human P2X4 receptor transgenic mouse. FASEB J 15: 2739–2741, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Hulsmann M, Nickel P, Kassack M, Schmalzing G, Lambrecht G, Markwardt F. NF449, a novel picomolar potency antagonist at human P2X1 receptors. Eur J Pharmacol 470: 1–7, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Jiang LH, Mackenzie AB, North RA, Surprenant A. Brilliant Blue G selectively blocks ATP-gated rat P2X7 receptors. Mol Pharmacol 58: 82–88, 2000. [PubMed] [Google Scholar]
- 12.Jones LR, Suzuki YJ, Wang W, Kobayashi YM, Ramesh V, Franzini-Armstrong Cleemann L C, Morad M. Regulation of Ca2+ signaling in transgenic mouse cardiac myocytes overexpressing calsequestrin. J Clin Invest 101: 1385–1393, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kassiri Z, Zobel C, Nguyen T, Molkentin JD, Backx PH. Reduction of Ito causes hypertrophy in neonatal rat ventricular myocytes. Circ Res 90: 578–585, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, Maddileti S, Marquez VE, Harden TK, Jacobson KA. 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.10]hexane ring system locked in a Northern conformation: enhanced potency as P2Y1 receptor antagonists. J Med Chem 46: 4974–4987, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knollman BC, Knollmann-Ritschel BEC, Weismann NJ, Jones LR, Morad M. Remodeling of ionic currents in hypertrophied and failing hearts of transgenic mice overexpressing calsequestrin. J Physiol 525.2: 483–498, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuzmin AI, Lakomkin VL, Kapelko VI, Vassort G. Interstitial ATP level and degradation in control and postmyocardial infracted rats. Am J Physiol Cell Physiol 275: C766–C771, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Laursen JB, Somers M, Kurz S, McCann L, Warnholz A, Freeman BA, Tarpey M, Fukai T, Harrison DG. Endothelial regulation of vasomotion in ApoE-deficient mice. Implications for the interactions between peroxynitrite and tetrahydrobiopterin. Circulation 103: 1282–1307, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Lebeche D, Kaprielian R, del Monte F, Tomaselli G, Gwathmey JK, Schwartz A, Hajjar RJ. In vivo cardiac gene transfer of Kv4.3 abrogates the hypertrophic response in rats after aortic stenosis. Circulation 110: 3435–3443, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Milano CA, Dolber PC, Rockman HA, Bond RA, Venable ME, Allen LF, Lefkowitz RJ. Myocardial expression of a constitutively active α1B-adrenergic receptor in transgenic mice induced cardiac hypertrophy. Proc Natl Acad Sci USA 91: 10109–10113, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.North RA. Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Parker KE, Scarpa A. An ATP-activated nonselective cation channel in guinea pig ventricular myocytes. Am J Physiol Heart Circ Physiol 269: H789–H797, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 50: 413–492, 1998. [PubMed] [Google Scholar]
- 23.Ravi RG, Kim HS, Servos J, Zimmermann H, Lee K, Maddileti S, Boyer JL, Harden T, Jacobson KA. Adenine nucleotides analogues locked in a Northern methanocarba conformation: Enhanced stability and potency as P2Y1 receptor agonists. J Med Chem 45: 2090–2100, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarma T, Voyno-Yasenetskaya T, Hope TJ, Rasenick MM. Heterotrimeric G-proteins associate with microtubules during differentiation in PC12 pheochromocytoma cells. FASEB J 17: 848–859, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Scamps F, Legssyer A, Mayoux E, Vassort G. The mechanism of positive inotropy induced by adenosine triphosphate in rat heart. Circ Res 67: 1007–1016, 1990. [DOI] [PubMed] [Google Scholar]
- 26.Scamps F, Vassort G. Pharmacological profile of the ATP-mediated increase in L-type calcium current amplitude and activation of a non-specific cation current in rat ventricular cells. Br J Pharmacol 113: 982–986, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen JB, Pappano A, Liang BT. Extracellular ATP-stimulated current in wild type and P2X4 receptor transgenic mouse ventricular myocytes: implication for a cardiac physiologic role of P2X4 receptors. FASEB J 20: 277–284, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Shintaro YM, Wang GX, Hume JR. P2Y purinergic receptor regulation of CFTR chloride channels in mouse cardiac myocytes. J Physiol 556.3: 727–737, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adenosine Vassort G. 5′-triphosphate: a P2-Purinergic agonist in the myocardium. Physiol Rev 81: 767–806, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Wickenden AD, Kaprielian Kassiri Z R, Tsoporis JN, Tsushima R, Fishman GI, Backx PH. The role of action potential prolongation and altered intracellular calcium handling in the pathogenesis of heart failure. Cardiovasc Res 37: 312–323, 1998. [DOI] [PubMed] [Google Scholar]
- 31.Wu J, Shen JB, Werner B, Yang A, Pappano A, Liang BT. Cardiac overexpression of the human P2X4 receptor exhibits an anti-hypertrophic phenotype (Abstract). FASEB J 20: 164.16, 2006. [Google Scholar]
- 32.Yang A, Sonin D, Jones L, Liang BT. A beneficial role of cardiac P2X4 receptors in heart failure: Rescuing the calsequestrin-overexpression model of cardiomyopathy. Am J Physiol Heart Circ Physiol 287: H1096–H1103, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Zankov DP, Omatsu-Kanbe M, Isono T, Toyoda F, Ding WG, Matsuura H, Horie M. Angiotensin II potentiates the slow component of delayed rectifier K+ current via the AT1 receptor in guinea pig atrial myocytes. Circulation 113: 1278–1286, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Zhu MY, Klimek V, Haycock JW, Ordway GA. Quantitation of tyrosine hydroxylase protein in the locus coeruleus from postmortem human brain. J Neurosci Methods 99: 37–44, 2000. [DOI] [PubMed] [Google Scholar]