Abstract
Traffic-related air and noise pollution may increase the risk for cardiovascular disorders, especially among susceptible populations like pregnant women. The objective of this study was to evaluate the association of exposure to traffic-related air pollution and traffic noise with blood pressure in pregnant women. We extracted systolic blood pressure (SBP) and diastolic blood pressure (DBP) at ≥ 20 weeks gestation, as well as hypertensive disorders of pregnancy from medical records in the HOME Study, a prospective pregnancy and birth cohort from Cincinnati, OH (n = 370). We estimated exposure to elemental carbon attributable to traffic (ECAT)1, a marker of traffic-related air pollution, at women’s residences at ~20 weeks gestation using a validated land use regression model and traffic noise using a publicly available transportation noise model. We used linear mixed models and modified Poisson regression adjusted for covariates to examine associations of ECAT and traffic noise with blood pressure and hypertensive disorders of pregnancy risk, respectively. In adjusted models, we found a 1.6 (95% CI= 0.02, 3.3; p= 0.048) mm Hg increase in SBP associated with an interquartile range increase in ECAT concentration; the association was stronger after adjusting for traffic noise (1.9 mmHg, 95% = 0.1, 3.7; p = 0.035). ECAT concentrations were not significantly associated with DBP or hypertensive disorders of pregnancy, and traffic noise was not associated with SBP, DBP, or hypertensive disorders of pregnancy. There was no evidence of a joint effect of traffic noise and ECAT on any outcome. In this cohort, higher residential traffic-related air pollution exposure at ~20 weeks gestation was associated with higher SBP in late pregnancy. It is important for future studies of traffic-related air or noise pollution to jointly consider both exposures and neighborhood characteristics given their correlation and potential cumulative impact on cardiovascular health.
Keywords: traffic, air pollution, noise, maternal blood pressure, hypertension
1. INTRODUCTION
Hypertensive disorders of pregnancy, including gestational hypertension, pre-eclampsia, and eclampsia, are among the leading causes of maternal mortality worldwide (Lo et al., 2013; Say et al., 2014). The diagnosis of hypertensive disorders of pregnancy, which occurs in 2 – 6% of pregnancies, requires a measurement of at least two occurrences of systolic blood pressure (SBP) greater than or equal to 140 mm Hg or diastolic blood pressure (DBP) greater than or equal to 90 mm Hg after 20 weeks gestation (Wallis et al., 2008; Lo et al., 2013; Umesawa and Kobashi, 2017). Given the high maternal and fetal morbidity associated with hypertensive disorders of pregnancy and limited treatment options, identification of modifiable risk factors is of significant public health interest.
In urban settings, road traffic is an important source of ambient air and noise pollution, both of which have been associated with adverse birth outcomes and hypertension (Leon Bluhm et al., 2007; Fuks et al., 2011; van Kempen et al., 2012; Lee et al., 2013; e treault et al., 2013; Gehring et al., 2014; Hu et al., 2014; Pedersen et al., 2014; Lavigne et al., 2016; Pedersen et al., 2017; Zhu et al., 2017; Honda et al., 2017). Some researchers, using proximity to traffic as a measure of exposure, have not found an association between traffic and hypertensive disorders of pregnancy (van den Hooven et al., 2009); however, other researchers studying the relationship between constituents of traffic-related air pollution, such as elemental carbon, fine particulate matter (PM2.5), and nitrogen oxides, report that exposure to traffic-related air pollution is associated with higher risk for hypertensive disorders of pregnancy (Pedersen et al., 2017; Zhu et al., 2017; Pedersen et al., 2014). Additionally, the adverse health effects of traffic-related air pollution could be exacerbated by traffic noise pollution, which has also been linked to higher risk of hypertension (Leon Bluhm et al., 2007; van Kempen et al., 2012; Pedersen et al., 2017). Identifying the individual and joint effects of these two exposures on cardiovascular health among pregnant women may help guide regulatory interventions for susceptible populations (Te’treault et al., 2013).
Traffic-related air and noise pollution have both been independently associated with risk for hypertensive disorders of pregnancy, but few studies have specifically assessed the joint association between these exposures and maternal blood pressure (Hu et al., 2014; Pedersen et al., 2014; Pedersen et al., 2017; Zhu et al., 2017). Furthermore, we are unaware of any studies simultaneously evaluating the relationship of both road traffic air and noise pollution with maternal blood pressure in the United States. We are aware of only one other study, conducted by Pedersen et al. (2017), which assessed the association of both traffic air and noise pollution with risk for hypertensive disorders of pregnancy in a Danish cohort (Pedersen et al., 2017).
Accordingly, the objective of this study was to evaluate the independent and joint associations of traffic-related air and noise pollution with maternal blood pressure and hypertensive disorders of pregnancy. We hypothesized that higher exposure to traffic-related air pollution and traffic noise would be independently associated with higher maternal systolic and diastolic blood pressure in late pregnancy, as well as risk for hypertensive disorders of pregnancy.
2. METHODS
We used data from The Health Outcomes and Measures of the Environment (HOME) Study, a prospective pregnancy and birth cohort of women and children in greater Cincinnati, Ohio. Detailed information about recruitment and data collection has been published elsewhere (Braun et al., 2017). From nine area prenatal clinics associated with three delivery hospitals, we recruited pregnant women between 2003 and 2006 who were: 18 years of age or older; 16 ± 3 weeks gestation; HIV-negative; not taking medications for seizures or thyroid disorders; not diagnosed with diabetes, bipolar disorder, schizophrenia, or cancer; and living in homes built prior to 1978. Approval for the study was obtained from the institutional review boards of Cincinnati Children’s Hospital Medical Center and the participating delivery hospitals. Women provided written informed consent after study protocols were explained by trained research assistants.
Overall, 468 pregnant women were enrolled in the HOME Study. For this analysis, we included all women who delivered a live singleton between 2003 and 2006 (n = 389) with at least one blood pressure measurement during late pregnancy (≥ 20 weeks gestation), excluding women who delivered offspring with chromosomal or congenital abnormalities. Women with missing covariate (n = 10) and outcome (n = 9) data were also excluded from this analysis, resulting in a sample size of 370 pregnant women.
2.1. Traffic-Related Air Pollution Exposure
We assessed traffic-related air pollution exposure using a previously validated land use regression model developed for the greater Cincinnati area, as described elsewhere (Ryan et al., 2005; Hu et al., 2006; LeMasters et al., 2006; Brokamp et al., 2017). Briefly, from 2001–2006, we conducted 24-hour ambient air-sampling of PM2.5 on a rotating basis at 24 sites distributed throughout the area where participants lived (Supplemental Figure 1). After sample collection, gravimetric analysis was used to determine PM2.5 concentration. Teflon filters underwent X-ray fluorescence to quantify concentrations of 39 elements, and quartz filters were analyzed by thermal-optical transmittance to quantify elemental and organic carbon concentrations (Ryan et al., 2007). A multivariate receptor model, UNMIX, was used to determine traffic sources contributing to total PM2.5 concentration. A diesel-specific traffic signature, determined by elemental source profiles obtained from measurements at cluster sources, was used to quantify the contribution of diesel traffic to PM2.5 concentration (Hu et al., 2006). For each sampling site, the concentration of elemental carbon attributable to traffic (ECAT) (μg/m3), a measure of elemental carbon generated from traffic-related diesel combustion, was calculated (Hu et al., 2006; Sahu et al., 2011). Thus, ECAT serves as our marker for traffic-related air pollution. Spatial variation in ambient ECA concentration at each participant’s residential address around approximately 20 weeks gestation was estimated using a validated land use regression (LUR) model (R2=0.75). The LUR model provides estimates of the long-term spatial distribution of ECAT because we developed the model using the arithmetic mean of all 24-hour samples collected at each site (Ryan et al., 2007). We used the ECAT concentration at the residential address as an estimate of maternal exposure to traffic-related air pollution throughout pregnancy.
2.2. Noise Exposure
We obtained information on 2014 trends in average noise energy due to road traffic across the Cincinnati region from the U.S. Department of Transportation (DOT), National Transportation Noise Mapping Tool (U.S. Department of Transportation, 2017). Using ArcGIS, version 10.3, we used the CONUS Road Noise Image Service to estimate 24-hour average A-weighted road traffic noise levels (Leq) at participants’ geocoded addresses around 20 weeks gestation. The DOT road traffic noise model was based on acoustical algorithms and information about vehicle type, speed, and Average Annual Daily Traffic (AADT) from the Federal Highway Administration’s raffic Noise Model. Road traffic noise levels were estimated for 1.5 m above ground at a 30 m grid resolution where the sound pressure level from traffic was 35 dBA or greater (U.S. Department of Transportation, 2017). For the regression analyses, we assigned participants living in areas with less than 35 dBA of traffic noise values of 35 divided by the square root of two.
2.3. Maternal Blood Pressure and Hypertensive Disorders of Pregnancy
The primary outcomes in this analysis were the two highest maternal SBP and DBP measurements during late pregnancy (≥ 20 weeks gestation), which we extracted from medical charts. We used the two highest SBP and DBP measures because The American College of Obstetricians and Gynecologists (ACOG) diagnostic criteria for hypertension during pregnancy is based on two measurements collected after 20 weeks gestation (American College of Obstetricians and Gynecologists, 2013). For women using hypertension medication prior to pregnancy (n = 20), we added 10 mm Hg to SBP measurements and 5 mm Hg to DBP measurements to adjust for treatment effects (Balakrishnan et al., 2017; Tobin et al., 2005).
A secondary outcome in this analysis was risk of hypertensive disorders of pregnancy. Consistent with our prior study, a participant was considered to have a diagnosis of hypertensive disorders of pregnancy if her medical chart indicated that she had one or more of the following diagnoses: gestational hypertension (n = 13), pre-eclampsia (n = 22), eclampsia (n = 0), or HELLP (hemolysis, elevated liver enzyme levels, and low platelet levels) syndrome (n = 2) (Werner et al., 2015). In addition, participants were considered to have gestational hypertension if they had two blood pressure measurements collected at ≥ 20 weeks gestation and the SBP and DBP measurements met at least one of the following criteria: elevated SBP (≥ 140 mm Hg) at two measurements, elevated DBP (≥ 90 mm Hg) at two measurements, or one elevated SBP and one elevated DBP measurements for the same or different measurements during late pregnancy (Werner et al., 2015). We used these criteria, which are based on ACOG guidelines, in addition to diagnoses from medical records to assess hypertension because we found that some women did not have a diagnosis listed in their medical chart despite meeting the ACOG diagnostic criteria for hypertension (American College of Obstetricians and Gynecologists, 2013). Women using hypertension medication prior to pregnancy could have a hypertensive disorder of pregnancy if they developed eclampsia, pre-eclampsia, or HELLP.
2.4. Covariates
We considered the following covariates in this analysis: maternal age, race, education, marital status, tobacco smoke exposure, maternal body mass index (BMI), parity, household income, gestation weeks at time of blood pressure measurement, season of conception, and neighborhood socioeconomic status.
We assessed race (white, non-white), education (high school or less, some college, completed college), marital status (unmarried, married), and household income ( =< $20,000, > $20,000 to < $40,000, => $40,000 to < $80,000, $80,000 +) using standardized interviews during the HOME Study visits and analyzed these as categorical variables. We assessed maternal age at delivery as a continuous variable in regression models. Season of conception was determined based on date of delivery and gestational age. Maternal BMI at 16 weeks gestation (< 25, 25 to < 30, 30 +) and parity (0, 1, 2 or more) was obtained through medical chart review and analyzed as categorical variables. We measured maternal exposure to tobacco using serum cotinine (ng/mL) collected at 16 and 26 weeks gestation. The mean of these two serum cotinine concentrations, an indicator of either active smoking or secondhand tobacco smoke exposure, was assessed as a continuous variable in regression models.
We assessed neighborhood socioeconomic status using data from the 2000 U.S. Census (Diez Roux et al., 2001). For the census tract of each participant’s residence at 20 weeks gestation, a z-score was calculated for the log of median household income; percent of households with interest, dividend, or rental income; log of median value of housing unit; percent of residents who completed high school; percent of residents who completed college; and percent of residents employed with executive, managerial, or professional occupations. The z-scores for each measure were summed and categorized into terciles, with lower scores representing lower neighborhood socioeconomic status (Diez Roux et al., 2001).
2.5. Statistical Analysis
We examined the distribution of continuous variables and calculated univariate statistics for the exposures (ECAT concentration and traffic noise), outcomes (maternal SBP, DBP, and risk of hypertensive disorders of pregnancy), and covariates. The linearity of the dose-response relationship of ECAT concentration and traffic noise with each blood pressure measure was assessed using restricted cubic splines. We calculated the Spearman Rank correlation coefficient and p-value to evaluate the correlation between ECAT concentration and road traffic noise. We used the Cochran-Armitage Trend test to assess the trend in traffic noise (<35 dBA or ≥35 dBA) by neighborhood socioeconomic status. We used the Kruskal-Wallis test to compare median ECAT concentrations by neighborhood socioeconomic status categories.
We used linear mixed models with an unstructured covariance matrix and random intercept to estimate the association of SBP and DBP in late pregnancy with ECAT concentrations and traffic noise. We adjusted all models for maternal age, serum cotinine concentration, maternal BMI, gestational age at time of blood pressure measurement, income, education, season, race, and neighborhood socioeconomic status. Directed acyclic graphs (DAGs) and stepwise procedures were used to identify and select covariates to include in all adjusted regression models (Supplemental Figure 2). Covariates that were statistically significant in the model (p <0.05), improved the fit of the model (based on AIC), or changed the ECAT or traffic noise effect estimates (>10%) were retained. For each blood pressure measure outcome, we constructed three models to assess the fixed effect of 1) ECAT only, 2) traffic noise only, and 3) ECAT and traffic noise mutually adjusted for each other. We also constructed these three models with blood pressure measures in early pregnancy (<20 weeks) as the outcome variables. We used modified Poisson regression to estimate the risk ratio for hypertensive disorders of pregnancy by ECAT concentration and traffic noise (Spiegelman et al., 2005).
Based on methods outlined by Rothman (2012), we assessed the joint effects of ECAT concentration and traffic noise for both outcome measures (Rothman, 2012). We added an ECAT and traffic noise product term to assess the relative excess SBP or DBP due to additive interaction in linear mixed models. For the modified Poisson regression model, we calculated the relative excess risk due to interaction (RERI) to assess the joint effect (additive interaction) of an interquartile range increase in ECAT and traffic noise, and used bootstrapping to approximate 95% confidence intervals (Knol, 2007; Richardson and Kaufman, 2009). Relative excess difference in blood pressure or hypertensive disorders of pregnancy risk greater than 0 indicate the presence of an additive interaction between ECAT and noise exposure (Rothman, 2012).
We conducted several sensitivity analyses to evaluate the robustness of our results. First, we adjusted for the year participant’s residence was built. Second, we used measured blood pressure estimates for women taking hypertension medication pre-pregnancy. Third, we adjusted for the mean maternal blood lead level measured at 16 and 26 weeks gestation. Statistical analyses were conducted using SAS v.9.4 (SAS Institutes, Inc.; Cary, NC) and R (v. 3.5.1).
3. RESULTS
At delivery, the 370 women in the HOME Study had a mean (SD) age of 29 (6) years (Table 1). The majority of women were white (62%), married (66%), and well-educated (51% completed college). Approximately 10% of women moved during pregnancy and 9% of women had hypertensive disorders of pregnancy. From the study sample, there were 737 repeated blood pressure measurements. Three participants only had one blood pressure measurement at ≥ 20 weeks gestation. The median (25th, 75th) gestational age for the two highest blood pressure measurements collected was 31 (26, 35) weeks.
Table 1.
Summary statistics of hypertensive disorders during pregnancy, road traffic noise (dBA), ECAT concentration (ug/m3), SBP and DBP (mmHg) at ≥ 20 weeks gestation according to covariates (The HOME Study, 2003–2006).
| Demographic Characteristic | Women with HDP | Total | ECAT | Traffic Noise | SBP | DBP | |
|---|---|---|---|---|---|---|---|
| n (%)a | N (%) | Median (25th, 75th) | < 35 dBA n (%) | Medianb (25th, 75th) | Mean (SD) | Mean (SD) | |
| Overall | 35 (9) | 370 (100) | 0.37 (0.30, 0.46) | 226 (61) | 47 (39, 54) | 115 (13) | 69 (9) |
| Maternal age | |||||||
| < 25 years | 13 (37) | 88 (24) | 0.41 (0.32, 0.50) | 42 (19) | 48 (40, 53) | 116 (13) | 69 (9) |
| 25 to < 35 years | 18 (51) | 221 (60) | 0.35 (0.30, 0.44) | 147 (65) | 45 (39, 54) | 116 (14) | 70 (9) |
| 35 + years | 4 (11) | 61 (16) | 0.39 (0.33, 0.48) | 37 (16) | 50 (43, 57) | 114 (11) | 68 (8) |
| Race | |||||||
| White | 14 (40) | 231 (62) | 0.35 (0.29, 0.44) | 157 (69) | 45 (39, 52) | 115 (13) | 69 (8) |
| Non-White | 21 (60) | 139 (38) | 0.41 (0.33, 0.50) | 69 (31) | 48 (41, 56) | 117 (13) | 70 (10) |
| Education | |||||||
| High School or less | 12 (34) | 87 (24) | 0.38 (0.30, 0.49) | 41 (18) | 48 (42, 55) | 118 (13) | 70 (10) |
| Some College | 9 (26) | 95 (26) | 0.36 (0.30, 0.47) | 57 (25) | 47 (38, 52) | 114 (12) | 69 (9) |
| Completed College | 14 (40) | 188 (51) | 0.36 (0.30, 0.45) | 128 (57) | 45 (39, 56) | 115 (14) | 69 (7) |
| Marital Status | |||||||
| Unmarried | 17 (49) | 126 (34) | 0.40 (0.32, 0.50) | 64 (28) | 48 (41, 55) | 116 (13) | 70 (9) |
| Married | 18 (51) | 244 (66) | 0.36 (0.30, 0.45) | 162 (72) | 45 (39, 53) | 115 (14) | 69 (9) |
| Household Income | |||||||
| $80,000+ | 4 (11) | 102 (28) | 0.37 (0.32, 0.46) | 68 (30) | 44 (39, 57) | 113 (13) | 68 (8) |
| => $40,000 to < 80,000 | 14 (40) | 126 (34) | 0.33 (0.29, 0.41) | 92 (41) | 48 (41, 54) | 117 (14) | 70 (9) |
| > $20,000 to < 40,000 | 4 (11) | 63 (17) | 0.36 (0.30, 0.47) | 33 (15) | 45 (38, 52) | 115 (12) | 68 (9) |
| =< $20,000 | 13 (37) | 79 (21) | 0.42 (0.33, 0.55) | 33 (15) | 47 (41, 55) | 116 (13) | 70 (10) |
| Maternal Tobacco Smoke Exposurec | |||||||
| None: < 0.015 | 9 (26) | 138 (37) | 0.36 (0.30, 0.43) | 97 (43) | 45 (39, 54) | 116 (15) | 70 (9) |
| Secondhand: 0.015–3 | 22 (63) | 195 (53) | 0.37 (0.30, 0.46) | 107 (47) | 47 (39, 54) | 115 (12) | 69 (9) |
| Active >3 | 4 (11) | 37 (10) | 0.40 (0.32, 0.49) | 22 (10) | 46 (40, 55) | 115 (14) | 70 (9) |
| Maternal BMI at 16 weeks (kg/m2) | |||||||
| < 25 | 6 (17) | 158 (43) | 0.37 (0.31, 0.46) | 95 (42) | 46 (39, 57) | 112 (13) | 66 (8) |
| 25 to < 30 | 12 (34) | 121 (33) | 0.36 (0.30, 0.46) | 76 (34) | 47 (42, 52) | 115 (13) | 70 (9) |
| 30 + | 17 (49) | 91 (25) | 0.37 (0.30, 0.47) | 55 (24) | 47 (40, 53) | 121 (12) | 73 (9) |
| Parity | |||||||
| 0 | 21 (60) | 168 (45) | 0.37 (0.30, 0.46) | 103 (46) | 46 (39, 57) | 116 (14) | 70 (9) |
| 1 | 5 (14) | 114 (31) | 0.36 (0.29, 0.46) | 69 (31) | 46 (39, 52) | 114 (12) | 68 (9) |
| 2 + | 9 (26) | 88 (24) | 0.37 (0.31, 0.46) | 54 (24) | 47 (42, 55) | 116 (12) | 70 (9) |
| Season of conception | |||||||
| Spring (March-May) | 11 (31) | 112 (30) | 0.36 (0.30, 0.45) | 70 (31) | 47 (40, 52) | 115 (13) | 69 (8) |
| Summer (Jun- Aug) | 7 (20) | 89 (24) | 0.36 (0.30, 0.43) | 63 (28) | 46 (39, 52) | 116 (13) | 69 (8) |
| Fall (Sept-Nov) | 7 (20) | 73 (20) | 0.39 (0.30, 0.53) | 35 (15) | 46 (39, 57) | 115 (12) | 71 (8) |
| Winter (Dec-Feb) | 10 (29) | 96 (26) | 0.37 (0.32, 0.46) | 58 (26) | 48 (40, 56) | 116 (15) | 70 (10) |
| Neighborhood socioeconomic status | |||||||
| First (Low) | 18 (51) | 122 (33) | 0.40 (0.33, 0.50) | 62 (27) | 48 (41, 55) | 116 (13) | 70 (9) |
| Second (Medium) | 8 (23) | 125 (34) | 0.35 (0.29, 0.44) | 78 (35) | 45 (39, 53) | 115 (12) | 69 (8) |
| Third (High) | 9 (26) | 123 (33) | 0.36 (0.30, 0.44) | 86 (38) | 45 (38, 52) | 115 (14) | 69 (10) |
| Year residence was built | |||||||
| 1924 or before | 11 (31) | 122 (33) | 0.43 (0.37, 0.52) | 64 (28) | 47 (39, 56) | 117 (14) | 70 (9) |
| 1925 to 1955 | 9 (26) | 126 (34) | 0.35 (0.30, 0.45) | 80 (35) | 46 (40, 53) | 113 (12) | 69 (8) |
| 1956 to 1978 | 15 (43) | 122 (33) | 0.33 (0.29, 0.43) | 82 (36) | 47 (41, 53) | 116 (13) | 70 (9) |
Average SBP and DBP of first highest blood pressure measure collected at ≥ 20 weeks gestation.
Abbreviations: SBP = Systolic blood pressure, DBP = Diastolic blood pressure, HDP = Hypertensive disorders of pregnancy, ECAT = Elemental carbon attributable to traffic. ECAT exposure during pregnancy estimated at residences ~20 weeks gestation. Approximately 10% of women moved residence during pregnancy.
Proportion of women with HDP
Median (25th, 75th) dBA among traffic noise estimates ≥ 35 Dba
Maternal tobacco smoke exposure was estimated by the average of serum cotinine (ng/mL) collected at 16 and 26 weeks gestation.
The median ECAT concentration was 0.37 μg/m3 (25th,75th: 0.30, 0.46). There was not a statistically significant difference in ECAT concentration between the two highest neighborhood socioeconomic status terciles (median ECAT (25th,75th): middle neighborhood socioeconomic status tercile = 0.35 (0.29, 0.44) vs. high neighborhood socioeconomic status tercile = 0.36 (0.30, 0.44 ); Kruskal-Wallis test (1 df) = 0.43; p = 0.51). Compared with women who lived in census tracts in the two highest neighborhood socioeconomic status terciles combined, women living in census tracts in the lowest neighborhood socioeconomic status tercile had higher concentrations of ECAT (median ECAT (25th,75th): 0.35 (0.29, 0.44) vs 0.40 (0.33,0.50), respectively; Kruskal-Wallis test (1 df) = 17.9; p < 0.0001).
The majority (61%) of women lived in areas with < 35 dBA of noise from road traffic. Among women living in areas with traffic noise of ≥ 35 dBA, the median noise levels were 47 dBA (25th, 75th: 39, 54). The proportion of women exposed to traffic noise ≥ 35 dBA decreased with increasing neighborhood socioeconomic status, from 42% in the lowest neighborhood socioeconomic status tercile to 26% in the highest neighborhood socioeconomic status tercile (Cochran-Armitage Trend test = 3.1; p = 0.002).
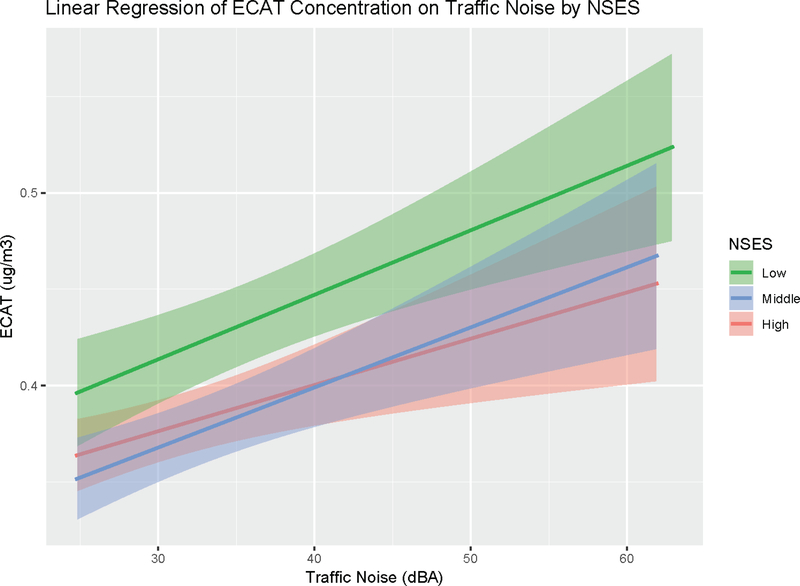
We found a modest correlation between ECAT concentrations and traffic noise (Spearman Rank correlation coefficient = 0.32; p < 0.0001). Women exposed to ≥ 35 vs < 35 dBA of traffic noise were exposed to higher concentrations of ECAT (median ECAT (25th, 75th): 0.43 (0.34, 0.54) vs 0.35 (0.29, 0.43), respectively; Kruskal-Wallis test (1 df) = 37.5; p = <0.0001). Women living in neighborhoods of lower socioeconomic status and exposed to higher levels of road traffic noise, were also exposed to higher ECAT concentrations compared to women in the middle and high neighborhood socioeconomic groups (Figure 1).
Figure 1.
Linear regression of ECAT concentration (ug/m3) on traffic noise (dBA) stratified by neighborhood socioeconomic status tercile. Abbreviations: NSES = neighborhood socioeconomic status, ECAT = elemental carbon attributable to traffic. Shaded bands represent 95% confidence intervals.
Based on linearity tests using restricted cubic splines, we determined that ECAT and traffic noise had linear relationships with both blood pressure measurements (all non-linearity p-values > 0.17). We found evidence of a positive monotonic association between ECAT and SBP in late pregnancy, adjusted for covariates (Table 2). This dose-response relationship remained in models further adjusted for traffic noise and an interquartile range increase in ECAT was associated with a 1.9 mm Hg increase in SBP (95% CI= 0.1, 3.7). In addition, we found a similar association between ECAT concentrations and SBP in early pregnancy (Supplemental Table 1). In contrast, we found little evidence of an association between traffic noise and SBP, or a joint (additive) effect for ECAT and traffic noise on SBP (relative excess SBP difference = −1.0, 95% CI = −3.2, 1.3). In early pregnancy, we found a positive relationship between traffic noise and SBP; however, this association was not statistically significant (Supplemental Table 1).
Table 2.
Adjusted differences in SBP and DBP (mm Hg) at ≥ 20 weeks gestation and risk ratio of HDP across categories of residential ECAT concentrations and traffic noise levels.
| DBP | SBP | HDP | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Difference (mmHg) | 95% CI | p-value | Difference (mmHg) | 95% CI | p-value | RR | 95% CI | p-value | |
| ECAT | 0.3 | (−0.9, 1.4) | 0.63 | 1.6 | (0.02, 3.3) | 0.048 | 0.8 | (0.5, 1.3) | 0.35 |
| ECAT, adjusted | 0.4 | (−0.9, 1.6) | 0.56 | 1.9 | (0.1, 3.7) | 0.035 | 0.7 | (0.5, 1.2) | 0.23 |
| Traffic Noise | −0.2 | (−1.4, 0.9) | 0.72 | −0.4 | (−2.1, 1.2) | 0.60 | 1.2 | (0.7, 1.8) | 0.52 |
| Traffic Noise, adjusted | −0.3 | (−1.6, 0.9) | 0.62 | −1.0 | (−2.8, 0.8) | 0.28 | 1.3 | (0.8, 2.0) | 0.32 |
All models adjusted for maternal age, cotinine level, BMI, gestational age, income, education, season, race, neighborhood socioeconomic status. Change in blood pressure measurements and HDP risk calculated for an interquartile range (25th to 75th percentile) increase in ECAT concentration (ug/m3 ) or Traffic noise (dBA).
ECAT, adjusted = effect estimate for ECAT concentration when regression model is also adjusted for Traffic Noise. Traffic Noise, adjusted = effect estimate for Traffic Noise when regression model is also adjusted for ECAT concentration.
SBP and DBP repeated observations n = 737.
Abbreviations: SBP = Systolic blood pressure, DBP = Diastolic blood pressure, HDP = Hypertensive disorders of pregnancy, ECAT = Elemental carbon attributable to traffic.
We evaluated the magnitude of potential confounding of the association of SBP with ECAT or traffic noise by comparing linear mixed models only adjusted for gestational age to our fully adjusted models (Supplemental Table 2). The association between ECAT and SBP became more pronounced after adjustment for confounders, while the association between traffic noise and SBP changed little in the fully versus minimally adjusted models. We also assessed the possibility of overfitting of the models by comparing effect estimates and standard errors in the fully adjusted model to a less adjusted model without neighborhood socioeconomic status and race. We found no substantial difference in the effect estimates or 95% confidence intervals from the fully adjusted model and the less adjusted model. The results were not materially different in sensitivity analyses for the SBP outcome, which additionally adjusted for age of residence and maternal blood lead level, or used measured SBP and DBP values for women taking hypertension medications pre-pregnancy (Supplemental Table 3).
Neither traffic noise nor ECAT were associated with DBP in late pregnancy. There was also no evidence of a joint effect of traffic noise and ECAT on DBP (relative excess DBP difference = −1.2, 95% CI = −2.8, 0.3). Furthermore, there was no significant association of either ECAT or traffic noise with hypertensive disorders of pregnancy. In the joint effect analysis, there was no relative excess risk of hypertensive disorders of pregnancy due to the combined effect of an interquartile range increase in ECAT and traffic noise (RERI= −0.16; 95% CI= (−0.7,2.6)).
4. DISCUSSION
In this cohort, we found a statistically significant association between residential ECAT concentrations assessed during pregnancy and SBP in late pregnancy. This association persisted when we also adjusted for traffic noise. Furthermore, in sensitivity analyses where we considered lead exposure, a risk factor for hypertensive disorders of pregnancy, the association between ECAT and SBP was not attenuated (Poropat et al., 2018). While ECAT and traffic noise were correlated, contrary to our hypothesis, we found no statistically significant association between traffic noise and either measure of blood pressure. There was no statistically significant relationship of ECAT or traffic noise with risk for hypertensive disorders of pregnancy.
During pregnancy, women may be especially susceptible to the cardiovascular effects of air pollution due to the rapid physiological changes in respiration, sympathetic nervous system activity, circulation, and cardiac output that occur in the first and second trimester (reviewed by: Pedersen et al., 2014; Sanghavi and Rutherford, 2014). Exposure to air pollution may impact blood pressure by interacting with the sympathetic nervous system, promoting oxidative stress and circulating cytokines, or altering the vascular endothelium (Hu et al., 2014; Pedersen et al., 2014). In addition to air pollution, traffic noise may impact cardiovascular health by increasing stress hormone concentrations and disrupting sleep patterns (van Kempen et al., 2012; Te treault et al., 2013; Pedersen et al, 2017).
In our study, we used ECAT as a marker of exposure to traffic-related air pollution. Other studies assessing traffic-related air pollutants have led to similar results. Lee et al. (2012) reported a 1.2 mm Hg (95%CI = 0.1, 2.3) higher SBP in late pregnancy associated with an IQR increase in PM10 exposure in the first trimester (Lee et al., 2012). Previous research has also found a significant relationship between traffic-related air pollution and gestational hypertension risk. In an analysis of medical record data from the Consortium on Safe Labor/Air Quality and Reproductive Health Study, each IQR increase in elemental carbon, PM2.5, and nitrogen oxides exposure during weeks 1–20 of gestation was associated with 8% - 17% higher risk of gestational hypertension (Zhu et al., 2017). Relative risk estimates from this analysis are similar to the hypertensive disorders of pregnancy risk ratio estimates from our analysis. Findings from the Zhu et al. (2017) study, as well as the Mannisto et al. (2015) study, also suggest that other windows of susceptibility to hypertensive disorders of pregnancy, ranging from three months preconception to 4 hours prior to hospital admission for delivery, may be important to assess (Zhu et al., 2017; Mannisto et al., 2015).
An analysis by Pedersen et al. (2017) of data from the Danish National Birth Cohort reported that a 10dB increase in traffic noise was associated with a small increase in the risk of hypertensive disorders of pregnancy (OR= 1.08, 95%CI=1.02, 1.15) (Pedersen et al., 2017). However, this relationship was attenuated when they adjusted for ambient nitrogen dioxide levels, frequently considered a marker of traffic-related air pollution. While our results for the association between traffic noise and hypertensive disorders of pregnancy were not statistically significant, the point estimates were similar to those of Pedersen et al. (2017).
Distinguishing maternal health implications of traffic noise from other traffic-related air pollutants may require more precise consideration of the frequency, duration, and timing of noise exposure, as well as an individual’s sensitivity and perception of the noise (Jakovljevic et al., 2009; Okokon et al., 2015; Casey et al., 2017; Kim et al., 2017). More accurately characterizing noise exposure and individual response is important given recent research showing disparities in exposure to noise pollution across the U.S., as well as evidence implicating noise pollution with hypertension, obesity, arterial stiffness, and diabetes (van Kempen et al., 2012; Casey et al., 2017; Clark et al., 2017;Foraster et al., 2017; Pyko et al., 2017).
Traffic-related air pollution, traffic noise, and neighborhood socioeconomic status are frequently correlated and are all documented risk factors for cardiovascular health (Diez Roux et al., 2001; Dubowitz et al., 2012). However, few prior studies have considered the joint effects of all three exposures. In particular, few studies have considered neighborhood socioeconomic characteristics when evaluating the association of traffic-related air pollution and hypertensive disorders of pregnancy (reviewed by: Hu et al., 2014; Pedersen et al., 2014). In our study, women who lived in census tracts with lower neighborhood socioeconomic status were on average exposed to higher concentrations of ECAT and higher levels of traffic noise. Similar relationships of neighborhood socioeconomic status and traffic-related pollution have been found in several countries (Casey et al., 2017; Havard et al., 2009; reviewed by: Hajat et al., 2015). Given this collective evidence, we hypothesize that women living in neighborhoods of low socioeconomic status and higher traffic-related air and noise pollution will be at particularly high risk of hypertensive disorders of pregnancy. Additional studies estimating the joint effects of socioeconomic factors, traffic noise, and traffic-related air pollution on pregnancy outcomes are needed to confirm or refute this hypothesis.
Some limitations of this analysis need to be considered. First, blood pressure measurements were collected by medical chart review rather than standardized research protocol at specified points during the pregnancy. Differences in the collection procedures are likely to have led to measurement error of the outcome, potentially decreasing the precision of our estimates. Second, ECAT was only estimated at one residential address to represent residential exposure around 20 weeks gestation, although women may have subsequently moved. Furthermore, because the LUR model only represents the long-term spatial distribution of ECAT, we are unable to differentiate ECAT exposure estimates for early and late pregnancy. Geocoding errors, housing characteristics and individual time-activity patterns could also contribute to misclassification of exposure, which on average is expected to bias our results towards the null. Third, other air pollutants, like nitrogen dioxide, ozone, and carbon monoxide were not considered in this analysis. These pollutants have been found to be associated with hypertensive disorders of pregnancy and some could also be correlated with ECAT concentration and traffic noise exposure (Hu et al., 2014). Fourth, our ability to detect an association between traffic-related air pollution and risk of hypertensive disorders of pregnancy was likely limited by the relatively few women with hypertensive disorders of pregnancy in the HOME Study. Furthermore, due to the few number of women with more severe hypertensive disorders of pregnancy, such as eclampsia and HELLP, we were unable to assess those outcomes separately. These outcomes may differ in terms of etiology and risk factors from the other less severe hypertensive disorders.
We note that the DOT traffic noise model is based on data from 2014, but the HOME cohort was established between 2003 and 2006. While the location of major roadways has not materially changed over this time period, differences in vehicle design, vehicle fleet, road usage, and speed limits could lead to misclassification of average road traffic relative to the early 2000’s. We expect this misclassification to be non-differential with respect to the outcomes and thus likely to bias our results, on average, towards the null. However, other features of the DOT road traffic noise model may have led to less predictable bias. For example, the noise model assumes acoustically soft ground and does not account for use of highway noise barriers, potentially leading to either under or overestimates of traffic noise levels. (U.S. Department of Transportation, 2017). If these factors are also associated with socioeconomic factors or other determinants of maternal health, the bias implied by the measurement error in traffic noise could have biased our results either towards or away from the null. If the traffic noise model does have more measurement error than the ECAT model, it is expected that the association between ECAT and blood pressure would appear stronger. The DOT road traffic noise model provides a preliminary model for identifying populations that may be more exposed to traffic-related noise pollution across the U.S., but clearly our results for traffic noise require further study with improved noise estimates.
On the other hand, our analysis also had several strengths. First, the outcome was based on multiple blood pressure measures throughout pregnancy and made clinically relevant by focusing the analysis on the two highest measurements ≥ 20 weeks gestation. Second, diagnosis of hypertensive disorders of pregnancy was confirmed by medial chart review. Third, we were able to leverage detailed information on a wide array of covariates collected as part of the HOME Study using standardized procedures. Of note, we were able to assess tobacco smoke exposure by cotinine levels instead of relying on self-reported tobacco use, minimizing the potential for residual confounding by this factor. Lastly, ECAT concentration, a robust marker of diesel exhaust particulates, was estimated at women’s residential addresses using a validated model that has been used in other health studies (Ryan et al., 2007; Sahu et al., 2011).
5. CONCLUSIONS
In this study, we found that residential exposure to traffic-related air pollution during pregnancy was associated with higher SBP during late pregnancy. While traffic-related air and noise pollution exposures were correlated, traffic noise was not associated with blood pressure. Women who lived in census tracts with lower neighborhood socioeconomic status were exposed to higher concentrations of traffic-related air pollution and traffic noise compared to women in census tracts with higher neighborhood socioeconomic status. Future research should assess time points during pregnancy when women may be more susceptible to increases in SBP related to traffic-related air pollution exposure. In addition, better characterization of traffic noise exposure across the U.S. as well as identification of socioeconomic factors that are related to exposure and maternal health outcomes are warranted.
Supplementary Material
Highlights for The association of traffic-related air and noise pollution with maternal blood pressure and hypertensive disorders of pregnancy in the HOME Cohort.
We evaluated the association of traffic-related air and noise pollution with maternal blood pressure and risk of hypertensive disorders of pregnancy.
Traffic-related air pollution exposure was associated with higher SBP in late pregnancy.
While traffic-related air and noise pollution exposures were correlated, traffic noise was not associated with maternal blood pressure.
Women in neighborhoods of low socioeconomic status were exposed to higher traffic-related air pollution concentrations and were more likely to be exposed to traffic noise.
Acknowledgements:
The authors would like to acknowledge Melissa N. Eliot and Lynn Carlson for their technical assistance with the statistical analysis.
Funding: This work was supported by grants R01-ES025214, P01-ES011261, R21-ES023073, R01-ES011170 and R01-ES019890 from NIEHS, NIH, as well as grant RD-83544201 from the US Environmental Protection Agency. C.G. Sears is partially supported by the Institute at Brown for Environment and Society and NIEHS Superfund Research Program Grant P42 ES013660. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the sponsoring organizations.
Footnotes
Declarations of interest: none
ECAT = elemental carbon attributable to traffic
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- American College of Obstetricians and Gynecologists, 2013. Task force on Hypertension in Pregnancy. "Hypertension in pregnancy." Retrieved on July 9th, 2018 from https://www.acog.org/Clinical-Guidance-and-Publications/Task-Force-and-Work-Group-Reports/Hypertension-in-Pregnancy. [DOI] [PubMed]
- Balakrishnan P, Beaty T, Young JH, Colantuoni E and Matsushita K, 2017. Methods to estimate underlying blood pressure: The atherosclerosis risk in communities (aric) study. PLoS One 12(7),e0179234. doi: 10.1371/journal.pone.0179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokamp C, Jandarov R, Rao MB, LeMasters G and Ryan P, 2017. Exposure assessment models for elemental components of particulate matter in an urban environment: A comparison of regression and random forest approaches. Atmos Environ 151,1–11. doi: 10.1016/j.atmosenv.2016.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, Xu Y, Yolton K and Lanphear BP, 2017. Cohort profile: The health outcomes and measures of the environment (home) study. Int J Epidemiol 46(1),24. doi: 10.1093/ije/dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JA, Morello-Frosch R, Mennitt DJ, Fristrup K, Ogburn EL and James P, 2017. Race/ethnicity, socioeconomic status, residential segregation, and spatial variation in noise exposure in the contiguous united states. Environ Health Perspect 125(7),077017. doi: 10.1289/EHP898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C, Sbihi H, Tamburic L, Brauer M, Frank LD and Davies HW, 2017. Association of long-term exposure to transportation noise and traffic-related air pollution with the incidence of diabetes: A prospective cohort study. Environ Health Perspect 125(8),087025. doi: 10.1289/EHP1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto J, Sorlie P, Szklo M, Tyroler HA, Watson RL, 2001. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 345(2),99–106. [DOI] [PubMed] [Google Scholar]
- Dubowitz T, Ghosh-Dastidar B, Eibner C, Slaughter ME, Fernandes M, Whitsel EA, Bird CE, Jewell A, Margolis KL, Li W, Michael YL, Shih RA, Manson JE, Escarce JJ, 2012. The women's health initiative: The food environment, neighborhood socioeconomic status, bmi, and blood pressure. Obesity (Silver Spring) 20(4),862–871. doi: 10.1038/oby.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foraster M, Eze IC, Schaffner E, Vienneau D, Heritier H, Endes S, Rudzik F, Thiesse L, Pieren R, Schindler C, Schmidt-Trucksass A, Brink M, Cajochen C, Marc Wunderli J, Roosli M and Probst-Hensch N, 2017. Exposure to road, railway, and aircraft noise and arterial stiffness in the sapaldia study: Annual average noise levels and temporal noise characteristics. Environ Health Perspect 125(9),097004. doi: 10.1289/EHP1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuks K, Moebus S , Hertel S , iehmann A , Nonnemacher M , Dragano N , M hlen amp S, Jakobs H, Kessler C, Erbel R, Hoffman B, 2011. Long-term urban particulate air pollution, traffic noise, and arterial blood pressure. Environ Health Perspect 119(12),1706–1711. doi: 10.1289/ehp.1103564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring U, Tamburic L, Sbihi H, Davies HW, Brauer M, 2014. Impact of noise and air pollution on pregnancy outcomes. Epidemiology 25(3),351–358. doi: 10.1097/eDe.0000000000000073. [DOI] [PubMed] [Google Scholar]
- Hajat A , Hsia C and O’Neill M S., 2015. Socioeconomic disparities and air pollution exposure: A global review. Curr Environ Health Rep 2(4),440–450. doi: 10.1007/s40572-015-0069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havard S, Deguen S, Zmirou-Navier D, Schillinger C, Bard D, 2009. Traffic-related air pollution and socioeconomic status: A spatial autocorrelation study to assess environmental equity on a small-area scale. Epidemiology 20(2),223–230. doi: 10.1097/EDE.0b013e31819464e1. [DOI] [PubMed] [Google Scholar]
- Honda T, Eliot MN, Eaton CB, Whitsel E, Stewart JD, Mu L, Suh H, Szpiro A, Kaufman JD, Vedal S, Wellenius GA, 2017. Long-term exposure to residential ambient fine and coarse particulate matter and incident hypertension in post-menopausal women. Environ Int 105,79–85. doi: 10.1016/j.envint.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Ha S, Roth J, Kearney G, Talbott EO, Xu X, 2014. Ambient air pollution and hypertensive disorders of pregnancy: A systematic review and meta-analysis. Atmos Environ 97, 336–345. doi: 10.1016/j.atmosenv.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, McDonald R, Martuzevicius D, Biswas P, Grinshpun SA, Kelley A, Reponen T, Lockey J and Lemasters G, 2006. Unmix modeling of ambient pm(2.5) near an interstate highway in Cincinnati, OH, USA. Atmos Environ 40(S2),378–395. doi: 10.1016/j.atmosenv.2006.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovljevic B, Paunovic K, Belojevic G, 2009. Road-traffic noise and factors influencing noise annoyance in an urban population. Environ Int 35(3),552–556. doi: 10.1016/j.envint.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Kim A, Sung JH, Bang JH, Cho SW, Lee J, Sim CS, 2017. Effects of self-reported sensitivity and road-traffic noise levels on the immune system. PLoS One 12(10),e0187084. doi: 10.1371/journal.pone.0187084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knol MJ, van der Tweel I, Grobee DE, Numans ME, Geerlings MI, 2007. Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int J Epidemiol 36,1111–1118. doi: 0.1093/ije/dym157. [DOI] [PubMed] [Google Scholar]
- Lavigne E, Yasseen AS, Stieb DM, Hystad P, van Donkelaar A, Martin RV, Brook JR, Crouse DL, Burnett RT, Chen H, Weichenthal S, Johnson M, Villeneuve PJ, Walker M, 2016. Ambient air pollution and adverse birth outcomes: Differences by maternal comorbidities. Environ Res 148,457–466. doi: 10.1016/j.envres.2016.04.026. [DOI] [PubMed] [Google Scholar]
- Le Masters GK, Wilson K, Levin L, Biagini J, Ryan P, Lockey JE, Stanforth S, Maier S, Yang J, Burkle J, Villareal M, Khurana Hershey GK, Bernstein DI, 2006. High prevalence of aeroallergen sensitization among infants of atopic parents. J Pediatr 149(9),505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PC, Roberts JM, Catov JM, Talbott EO, Ritz B, 2013. First trimester exposure to ambient air pollution, pregnancy complications and adverse birth outcomes in allegheny county, pa. Matern Child Health J 17(3),545–555. doi: 10.1007/s10995-012-1028-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon Bluhm G, Berglind N, Nordling E, Rosenlund M, 2007. Road traffic noise and hypertension. Occup Environ Med 64(2),122–126. doi: 10.1136/oem.2005.025866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JO, Mission JF, Caughey AB, 2013. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol 25(2),124–132. doi: 10.1097/GCO.0b013e32835e0ef5. [DOI] [PubMed] [Google Scholar]
- Männistö T, Mendola P, Liu D, Leishear K, Sherman S, Laughon SK, 2015. Acute air pollution exposure and blood pressure at delivery among women with and without hypertension. Am J Hypertens 28(1),58–72. doi: 10.1093/ajh/hpu077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okokon EO, Turunen AW, Ung-Lanki S, Vartiainen AK, Tiittanen P, Lanki T, 2015. Road-traffic noise: Annoyance, risk perception, and noise sensitivity in the finnish adult population. Int J Environ Res Public Health 12(6),5712–5734. doi: 10.3390/ijerph120605712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen M, Halldorsson TI, Olsen SF, Hjortebjerg D, Ketzel M, Grandstr m C, Raaschou-Nielsen OR, Sørensen M, 2017. Impact of road traffic pollution on pre-eclampsia and pregnancy-induced hypertensive disorders. Epidemiology 28(1),99–106. doi: 10.1097/eDe.0000000000000555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen M, Stayner L, Slama R, Sørensen M, Figueras F, Nieuwenhuijsen MJ, Raaschou-Nielsen O, Dadvand P, 2014. Ambient air pollution and pregnancy-induces hypertensive disorders. Hypertension 64,494–500. doi: 10.1161/HYPERTENSIONAHA0.1161/HYPERTENSIONAHA.114.03545. [DOI] [PubMed] [Google Scholar]
- Poropat AE, Laidlaw MAS, Lanphear B, Ball A, Mielke HW, 2018. Blood lead and preeclampsia: A meta-analysis and review of implications. Environ Res 160,12–19. doi: 10.1016/j.envres.2017.09.014. [DOI] [PubMed] [Google Scholar]
- Pyko A, Eriksson C, Lind T, Mitkovskaya N, Wallas A, Ogren M, Ostenson CG, Pershagen G, 2017. Long-term exposure to transportation noise in relation to development of obesity-a cohort study. Environ Health Perspect 125(11),117005. doi: 10.1289/EHP1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DB, Kaufman JS, 2009. Estimation of the relative excess risk due to interaction and associated confidence bounds. Am J Epidemiol 169(6),756–760. doi: 10.1093/aje/kwn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, 2012. Measuring Interactions, In: Epidemiology, an introduction, 2nd ed, 198–210. Oxford University Press: New York, New York. [Google Scholar]
- Ryan PH, LeMasters G, Biagini J, Bernstein D, Grinshpun SA, Shukla R, Wilson K, Villareal M, Burkle J, Lockey J, 2005. Is it traffic type, volume, or distance? Wheezing in infants living near truck and bus traffic. J Allergy Clin Immunol 116(2),279–284. doi: 10.1016/j.jaci.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Ryan PH, Lemasters GK, Biswas P, Levin L, Hu S, Lindsey M, Bernstein DI, Lockey J, Villareal M, Khurana Hershey GK and Grinshpun SA, 2007. A comparison of proximity and land use regression traffic exposure models and wheezing in infants. Environ Health Perspect 115(2),278–284. doi: 10.1289/ehp.9480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu M, Hu S, Ryan PH, Le Masters G, Grinshpun SA, Chow JC and Biswas P, 2011. Chemical compositions and source identification of pm(2.5) aerosols for estimation of a diesel source surrogate. Sci Total Environ 409(13),2642–2651. doi: 10.1016/j.scitotenv.2011.03.032. [DOI] [PubMed] [Google Scholar]
- Sanghavi M and Rutherford JD, 2014. Cardiovascular physiology of pregnancy. Circulation 130(12),1003–1008. doi: 10.1161/CIRCULATIONAHA.114.009029. [DOI] [PubMed] [Google Scholar]
- Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, Gülmezoglu AM, Temmerman M, Alkema L, 2014. Global causes of maternal death: A who systematic analysis. The Lancet Global Health 2(6),e323–e333. doi: 10.1016/s2214-109x(14)70227-x. [DOI] [PubMed] [Google Scholar]
- Spiegelman D, Hertzmark E, 2005. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol 162 (3),199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- Te’treault L, Perron S, Smargiassi A, 2013. Cardiovascular health, traffic-related air pollution and noise: Are associations mutually confounded? A systematic review. Int J Public Health 58, 649–666. doi: 0.1007/s00038-013-0489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin MD, Sheehan NA, Scurrah KJ and Burton PR, 2005. Adjusting for treatment effects in studies of quantitative traits: Antihypertensive therapy and systolic blood pressure. Stat Med 24(19),2911–2935. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Transportation, 2017. The National Transportation Noise Map. Accessed last Febuary 2, 2018 from: http://osavusdot.opendata.arcgis.com/datasets/6b5fd2f7b10c4ffc85b2a6f4ccb1118c.
- Umesawa M, Kobashi G, 2017. Epidemiology of hypertensive disorders in pregnancy: Prevalence, risk factors, predictors and prognosis. Hypertens Res 40(3),213–220. doi: 10.1038/hr.2016.126. [DOI] [PubMed] [Google Scholar]
- van den Hooven EH, Jaddoe VW, de Kluizenaar Y, Hofman A, Mackenbach JP, Steegers EA, Miedema HM, Pierik FH, 2009. Residential traffic exposure and pregnancy-related outcomes: A prospective birth cohort study. Environ Health 8(59),1–11. doi: 10.1186/1476-069X-8-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kempen E, Babisch W, 2012. The quantitative relationship between road traffic noise and hypertension: A meta-analysis. Journal of Hypertension 30(6),1075–1086. doi: 0.1097/HJH.0b013e328352ac54. [DOI] [PubMed] [Google Scholar]
- Wallis AB, Saftlas AF, Hsia J, Atrash HK, 2008. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens 21(5),521–526. doi: 10.1038/ajh.2008.20. [DOI] [PubMed] [Google Scholar]
- Werner EF, Braun JM, Yolton K, Khoury JC, Lanphear BP, 2015. The association between maternal urinary phthalate concentrations and blood pressure in pregnancy: The home study. Environ Health 14(75),1–9. doi: 10.1186/s12940-015-0062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang C, Liu D, Ha S, Kim SS, Pollack A, Mendola P, 2017. Ambient air pollution and risk of gestational hypertension. Am J Epidemiol 186(3),334–343. doi: 10.1093/aje/kwx097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.