Abstract
Infectious diarrheal diseases are the second leading cause of death in children under five, making vaccines against these diseases a high priority. It is known that certain vaccine adjuvants, chiefly bacterial ADP-ribosylating enterotoxins, can induce mucosal antibodies when delivered parenterally. Based on this, we reasoned vaccine-specific mucosal cellular immunity could be induced via parenteral immunization with these adjuvants. Here, we show that, in contrast to the TLR9 agonist CpG, intradermal immunization with non-toxic double-mutant heat-labile toxin from enterotoxigenic E. coli drives endogenous, antigen-specific CD4+ T cells to expand and upregulate the gut-homing integrin α4β7. This was followed by T cell migration into gut-draining lymph nodes and both small and large intestines. We also find dmLT produces a balanced Th1 and Th17 response whereas T cells from CpG immunized mice are predominantly Th1. Immunization with dmLT preferentially engages CD103+ dendritic cells compared to CpG, and mice deficient in CD103+ dendritic cells were unable to fully license antigen-specific T cell migration to the mucosae following parenteral immunization. This work has the potential to redirect the design of existing and next generation vaccines to elicit pathogen-specific immunity in the intestinal tract with non-mucosal immunization.
Keywords: CD4+ T cells, Dendritic cells, Vaccines, Adjuvant, Mucosal immunity
Introduction
Vaccines are one of the most successful medical interventions ever devised. Despite these successes, more children die every day from infectious diarrheal diseases than from malaria, HIV, and measles combined 1. While many vaccines are excellent at inducing systemic humoral immunity, vaccines that elicit cellular immunity, specifically in the mucosa, are rare. One way to circumvent this issue would be to deliver vaccines directly to the mucosa, and while some vaccines are delivered mucosally (predominantly orally) and are efficacious in developed countries, they often fail to protect children in developing countries, a phenomenon known as environmental enteropathy. An example of this is the live oral polio vaccine, which requires many more immunizations to achieve equivalent protective levels of immunity in children in developing countries compared to those where health care is more accessible and modernized 2. Multiple factors appear to be responsible for this; however, inadequate polio vaccine colonization of the intestinal mucosae due to ongoing diarrheal disease appears to play a meaningful role 3,4. This creates something of a paradox in that the very diseases requiring new vaccines may prevent oral immunization from being effective.
Avoiding the deficiencies of mucosally-delivered vaccines would be an important step in combatting enteric diseases in developing countries. One attractive possibility would be to deliver vaccines parenterally and use the vaccine formulation itself to dictate the nature and location of the immune response. Most previous studies were directed at assessing humoral immunity to parenterally delivered vaccines 5–7. How non-mucosal immunization affects antigen-specific T cell responses at the intestinal mucosae is poorly understood. It is also known that the correct parenteral route must be combined with an appropriate adjuvant to achieve mucosal immunity, yet the list of adjuvants that can promote mucosal responses is short, but includes bacterial ADP-ribosylating enterotoxins such as cholera toxin (CT) 7–9. In this study, we sought to determine how the choice of adjuvant, when delivered parenterally, could affect intestinal homing and phenotype of endogenous, antigen-specific CD4+ T cells and furthermore, what antigen presenting cells might dictate this type of response.
We use MHC class II tetramers to detect endogenous CD4+ T cells specific for a model antigen. We find that, when injected intradermally, the adjuvant double-mutant heat-labile toxin (dmLT), derived from enterotoxigenic E. coli, specifically upregulates the intestinal homing integrin α4β7 on antigen-specific T cells. This is in contrast to the well-studied TLR9 agonist adjuvant CpG, which does not increase α4β7 expression to the same degree. Moreover, dmLT induces preferential migration of these antigen-specific T cells into both the small and large intestine. Expectedly, CpG induced an almost exclusive Th1 T cell phenotype; however, dmLT produced T cells displaying a more balanced Th1/Th17 phenotype. Additionally, dmLT preferentially engaged CD103+ dendritic cells (DCs) compared to CpG. This is notable as CD103 expression on dendritic cells is linked to mucosal homing on primed CD4+ T cells 10–12. Lastly, gut homing phenotypes appeared to be independent of parenteral route, as intramuscular injection induced similar outcomes. These results demonstrate the importance of the choice of adjuvant in dictating the cellular immune responses in the intestine when vaccines are delivered parenterally and have the potential to change how future vaccines are designed.
Results
dmLT sustains a potent, endogenous antigen-specific CD4+ T cell response.
We first attempted to dissect the overall kinetics of the endogenous, antigen-specific CD4+ T cell response to immunization with dmLT compared to the well-described TLR9 agonist CpG. Mice were injected intradermally in the ear with either dmLT or CpG plus a protein antigen containing the well-characterized MHC class II (I-Ab) CD4+ T cell epitope 2W1S 13–16 fused to green fluorescent protein (hereafter 2W1S-GFP). After 4, 9, 14, 28, and 56 days, we used 2W1S:I-Ab tetramers to assess the number of 2W1S-specific T cells in the ear-draining cervical lymph nodes (CLN) and spleen. Throughout, the 2W1S-specific CD4+ T cell population showed dmLT induced a greater expansion compared to the CpG immunized group across all time points (Fig. S1A). Within the draining CLN, dmLT and CpG immunization induced peak expansion of 2W1S-specific T cells 9 days after intradermal immunization, with dmLT inducing greater overall numbers of Ag-specific CD4+ T cells. By 14 days, antigen-specific CD4+ T cell responses began to decline in the CLN in both groups, but there remained a higher number migrating to the spleen in the dmLT-immunized mice suggesting a greater overall peak response for 2W1S-specific CD4+ T cells in the dmLT immunized group. During the memory phase (days 28 to 56), systemic responses in the spleen were reduced compared to day 14, whereas the CLN response appeared to plateau. By day 56, the number of 2W1S-specific T cells were maintained in the spleen, and remained higher in dmLT-immunized mice, potentially highlighting the capacity for dmLT to induce a more sustained memory response. These results demonstrated that dmLT was the superior adjuvant compared to CpG, at least for expanding and maintaining endogenous, antigen-specific CD4+ T cells in systemic lymphoid tissues.
dmLT preferentially induces α4β7 integrin on antigen-specific CD4+ T cells and induces mucosal homing.
While the previous results showed that dmLT excelled at inducing antigen-specific CD4+ T cell responses in local draining lymph nodes, it was unclear if these responses were confined to this site. It is known that ADP-ribosylating adjuvants such as dmLT or CT induce intestinal humoral immunity 7,17,18 so we investigated whether they could induce antigen-specific T cell migration to the gut when administered parenterally. Initially we assessed the absolute number of 2W1S-specific CD4+ T cells and demonstrated that dmLT was a more potent adjuvant compared to CpG (Fig. S1A). We next examined whether dmLT preferentially upregulated the intestinal homing integrin α4β7 in comparison to CpG. From day 4 to day 28, dmLT induced a greater total number of Ag-specific CD4+ T cells migrating systemically (in spleen) that expressed α4β7 (Fig. S1B). This observation regarding α4β7+ CD4+ T cell numbers could simply have been a function of the greater total magnitude of dmLT-induced 2W1S-specific T cells. To examine this, mice immunized with either dmLT or CpG plus 2W1S-GFP were assayed for lymph node responses using 2W1S:I-Ab tetramer enrichment and the percentage of 2W1S-specific T cells and α4β7 expression in this 2W1S-specific fraction was determined 19. Local responses in the skin-draining CLN were much higher after dmLT injection at day 4, having nearly three times the percentage of CD4+ T cells specific for 2W1S compared with CpG (Fig. 1A). Importantly, the proportion of 2W1S-specific cells expressing α4β7 was also significantly higher in the case of dmLT (Fig. 2A). At the peak of the response (day 9) the 2W1S-specific responses remained higher in the dmLT immunized group in the CLN, and more 2W1S-specific T cells were expressing more α4β7 when dmLT was administered, both in total number and as a percent of 2W1S-specific T cells (Fig. 1C&D. Fig. 2C&D and Fig. S2A&B). It is known that α4β7+ cells migrate to the gut mucosa and intestine-draining mesenteric lymph nodes (MLN) 20–22. This led us to speculate that the α4β7+ cells were migrating out of the CLN and into these mucosal tissues over time. To address this, we examined the (MLN) for the presence of 2W1S-specific, α4β7+ CD4+ T cells. While there was no difference at day 4, by day 9 a significantly greater proportion and total number of 2W1S-specific CD4+ T cells were found in the MLN with dmLT immunization compared to CpG (Fig. 1 and Fig. S2); however, there was less difference between immunization groups in α4β7 expression on Ag-specific T cells in the MLN (Fig. 2). This is perhaps unsurprising and may be reflective of the fact that many cells entering the MLN should already express α4β7. Similar patterns were observed in the spleen, which functions as a surrogate of the systemic compartment (Fig. 1 & 2). Interestingly, the gut-homing chemokine receptor CCR9 did not appear to be upregulated on 2W1S-specific CD4 T cells (data not shown). This shows that while both adjuvants can induce a potent, systemic antigen-specific CD4+ T cell response, dmLT was significantly better at upregulating α4β7 on antigen-specific endogenous CD4+ T cells; however it is clear that CpG also had the capacity to induce α4β7 to some degree.
Figure 1. Both dmLT and CpG induce potent, endogenous antigen-specific local and systemic CD4+ T cell responses following intradermal immunization.
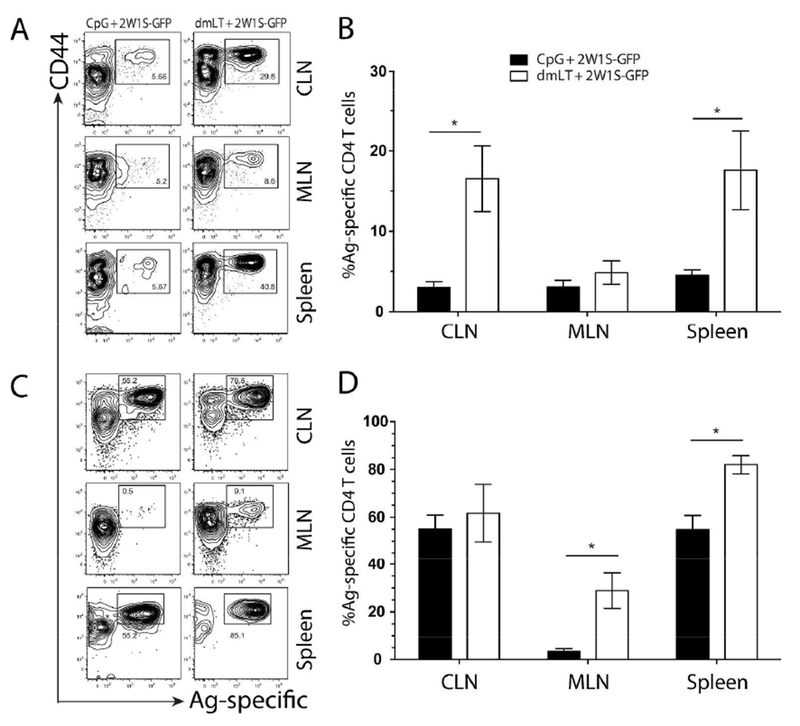
C57BL/6 mice were immunized intradermally in each ear pinna with CpG (left panes) or dmLT (right panes) plus 2W1S-GFP. Draining CLN, MLN, and spleens were harvested at 4 days (A and B) or 9 days (C and D) post immunization. Harvested tissues were dissociated and labeled with I-Ab:2W1S tetramer followed by magnetic bead enrichment of 2W1S-specific CD4+ T cells. Enriched fractions were labeled with T cell lineage negative surface antibodies (CD19, CD11c, CD11b, and F4/80) along with CD4+ T cell markers (CD3, CD4+, and CD44). Contour plots are representative of greater than six independent experiments with two to three mice per group. Numbers on each plot represent the percentage of cells in boxed gate. Graphs represent the pooled results of two experiments from more than six independent experiments with two to four mice per experiment. Significance was determined by student’s two-tailed t-test with Holm-Sidak correction for multiple comparisons. Statistical significance is defined as follows: *, P<0.05; **, P<0.01; ***, P<0.005. Error bars represent SEM.
Figure 2. Intradermal immunization with dmLT induces greater levels of α4β7 on 2W1S-specific CD4+ T cells compared to CpG.
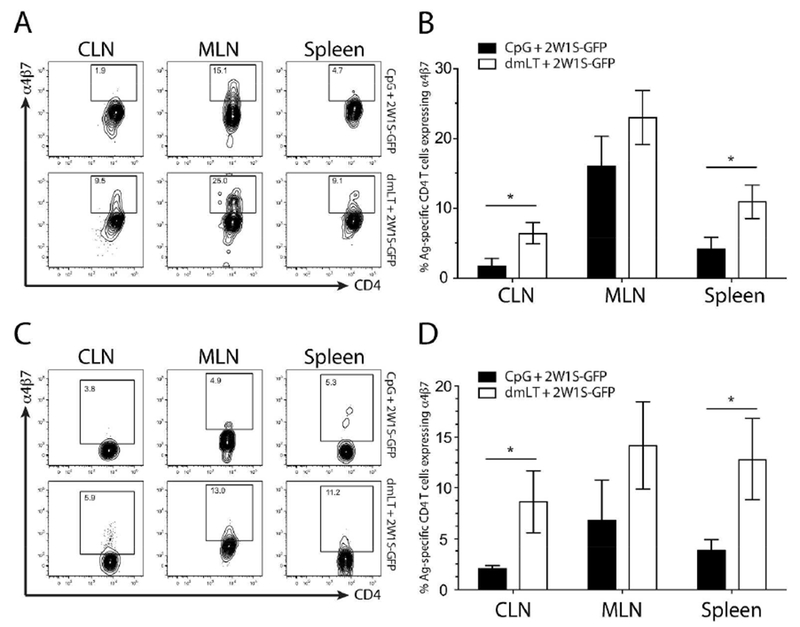
Cell suspensions isolated from the CLN, MLN and spleens of mice at day 4 (A and B) and day 9 (C and D) after intradermal immunization with either dmLT of CpG plus 2W1S-GFP were surface stained with I-Ab:2W1S tetramer and enriched using magnetic beads then stained for CD4+ T cell markers and α4β7 expression. Contour plots shown are representative examples from six independent experiments with two to three mice per group. Numbers on each plot represent the percentage of cells in boxed gate. Top plots in A and C are representative of CpG + 2W1S-GFP immunized mice and the bottom plots represent dmLT + 2W1S-GFP from each tissue assayed. Graphs represent the pooled results of two experiments from more than six independent experiments with two to four mice per experiment. Significance was determined by student’s two-tailed t-test with Holm-Sidak correction for multiple comparisons. Statistical significance is defined as follows: *, P<0.05; **, P<0.01; ***, P<0.005. Error bars represent SEM.
Because α4β7+ lymphocytes are known to traffic to the intestinal mucosae 21,23, we next assessed whether dmLT was specifically capable of routing antigen-specific CD4+ T cells into the gut tissues following intradermal immunization. Mice were immunized intradermally with a single dose of dmLT or CpG plus 2W1S-GFP and small and large intestines were assayed 9 and 14 days later for 2W1S-specific CD4+ T cell responses. We initially found there were little to no 2W1S-specific T cells detectable in the Peyer’s Patches or epithelial tissue (data not shown) so we focused on the intestinal lamina propria. At day 9, 2W1S-specific CD4+ T cells could be detected in the lamina propria of the small intestine (Fig. 3A), and were significantly higher in the dmLT immunized groups compared to CpG. There was also a substantial increase in 2W1S-specific CD4+ T cells in the large intestine lamina propria that was reflected in the total number of 2W1S-specific T cells (Fig. S2C). Mice immunized with dmLT plus 2W1S-GFP had an approximately ten-fold increase in the fraction of 2W1S-specific CD4+ T cells when compared to mice immunized with CpG (Fig. 3).
Figure 3. Antigen-specific T cells preferentially migrate into the small and large intestine lamina propria following intradermal dmLT immunization.
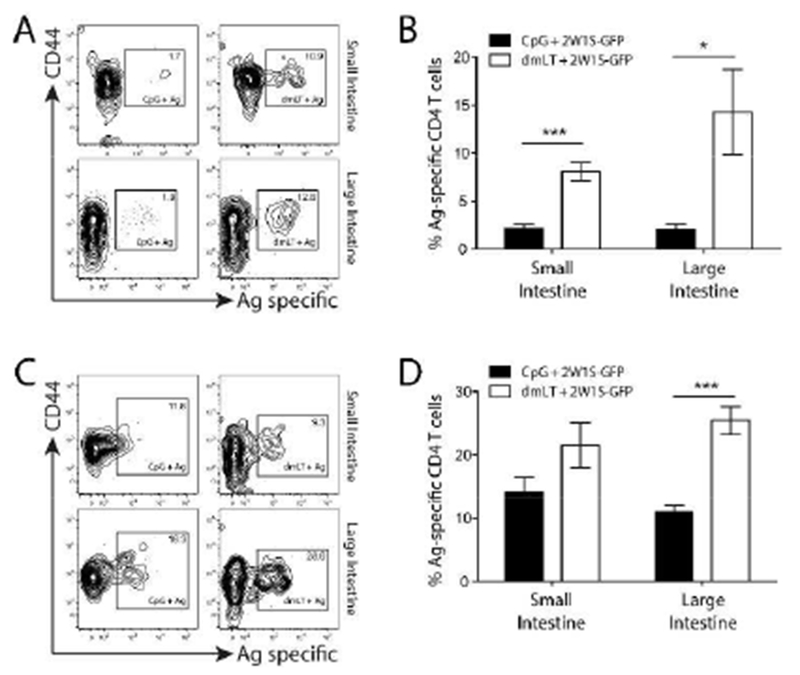
CpG or dmLT plus 2W1S-GFP immunized mice were assayed at day 9 (A and B) or day 14 (C and D) for 2W1S-specific CD4+ T cell responses in the intestinal lamina propria. (A and C) Top contour plots are representative of the small intestine and the bottom plots are representative of the large intestine from three to four experiments for each tissue using two to three mice per experiment. Numbers on each plot represent the percentage of cells in boxed gate. (B and D) Graphs represent the pooled results of two experiments from four independent experiments with two to four mice per experiment. Significance was determined by student’s two-tailed t-test with Holm-Sidak correction for multiple comparisons. Statistical significance is defined as follows: *, P<0.05; **, P<0.01; ***, P<0.005. Error bars represent SEM.
Since we observed a greater overall response following dmLT immunization, it is possible this intestinal migration was simply a function of this increased number migrating into any non-lymphoid tissues and not actually a function of preferential movement. To address this possibility, we assessed lung responses to CpG versus dmLT; at day 9 post-immunization there was no significant difference in the 2W1S-specfic CD4+ T cell responses (Fig. S3), supporting the hypothesis that dmLT induces specific migration to the intestinal mucosae. One other issue that arose is whether CpG is simply a poor adjuvant comparator for dmLT in terms of intestinal migration. To address this possibility, we substituted CpG with the TLR1/2 agonist, Pam3CSK(4). Similar to our data using CpG, Pam3CSK(4) had a limited capacity to induce α4β7 on antigen-specific T cells compared to dmLT (Fig. S4). Another possibility was that the immunization route itself was important for inducing a mucosal homing phenotype. Because the tissue we chose to inject initially (the ear) drains to the same lymph node as the nasal associated lymphoid tissue (NALT) 24, this may factor into the mucosal homing observation in dmLT immunization, particularly because nasal immunization is well-known to induce mucosal homing 11,25,26. Modifying the route of administration could potentially eliminate the increased mucosal homing observed with dmLT immunization. We tested this by immunizing mice with dmLT by either intradermal immunization of the flank (where the associated lymph nodes do not also drain a mucosal tissue) or via more traditional intramuscular immunization. Notably with either injection, α4β7 expression on 2W1S-specific T cells was comparable to intradermal ear immunizations (Fig. S5). These results suggest adjuvant choice, regardless of route, dictates T cell intestinal migration characteristics and that dmLT specifically drives this migration to a greater degree compared to other adjuvants.
dmLT induces a balanced Th1/Th17 phenotype on antigen-specific CD4+ T cells.
In addition to the observation that dmLT can induce mucosal immune responses, it has also been shown dmLT and related toxins are uniquely capable of driving balanced, or even “unpolarized” T helper (Th) immune responses17,26–28. While those studies characterized the phenotype imparted globally on bulk CD4+ T cells or adoptively transferred monoclonal CD4+ T cells, it is unknown how enterotoxins, like dmLT, impact endogenous CD4+ phenotypes against companion antigens. To address this, mice were immunized intradermally in the ear with CpG or dmLT plus 2W1S-GFP and cytokine release from 2W1S-specific CD4+ T cells was assessed following in vitro stimulation with 2W1S peptide. At nine days post immunization, mice immunized with CpG predominantly made IFN-γ (Fig. 4 A & B), which was expected as CpG is known to polarize the Th1 phenotype in CD4+ T cells 29. Mice immunized with antigen plus dmLT also produced large amounts of IFN-γ; however, a portion of 2W1S-specific T cells also released significantly more IL-17A when compared to CpG (Fig. 4 A & B). These results suggest a balanced Th1/Th17 cytokine response when using dmLT as an adjuvant. While the IFN-γ and IL-17 production was prominent, we also noted that Il-5 appeared to be produced at very low levels (data not shown). We also assessed whether the canonical Th1 and Th17 transcription factors (T-bet and Rorγt, respectively) were differentially expressed in dmLT versus CpG immunized mice. We found that expression of these transcription factors tracked closely with the cytokine data (Fig. 5). Additionally, similar cytokine results were found in the spleen using an intravenous in vivo 2W1S peptide injection confirming that the results were not merely attributable to the effects of the in vitro stimulus (Fig. S6). This demonstrates CpG drives a dominant Th1 T cell response while dmLT appears to be capable of a more balanced Th1/Th17 response in endogenous, antigen-specific CD4 T cells.
Figure 4. dmLT promotes a balanced Th1/Th17 cytokine phenotype in antigen-specific CD4+ T cells following intradermal immunization.
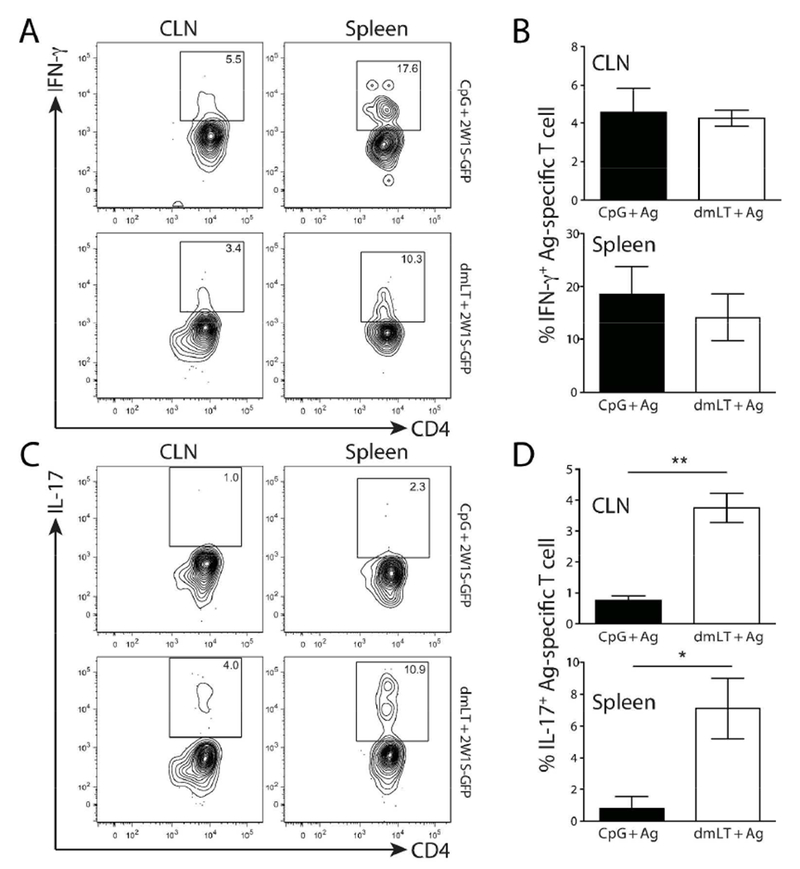
Mice were intradermally immunized with CpG or dmLT plus 2W1S-GFP and the 2W1S-specific CD4+ T cell cytokine response was assessed on day nine. CLN and spleen were harvested, dissociated, and plated into tissue culture dishes. Tissue explants were restimulated with 2W1S peptide for six hours (the last four with Brefeldin A) then stained for CD4+ T cell markers and 2W1S-specificity with tetramer followed by intracellular cytokine staining for IFN-γ (A & B) and IL-17A. (C & D). Contour plots are representative of cytokine staining from each immunization. Numbers on each plot represent the percentage of cells in boxed gate. Graphs represent one experiment with three to four mice per group and is representative of four independent experiments. Significance was determined by student’s two-tailed t-test. Statistical significance is defined as follows: *, P<0.05; **, P<0.01; ***, P<0.005. Error bars represent SEM.
Figure 5. dmLT promotes a balanced Th1/Th17 transcriptional phenotype in antigen-specific CD4+ T cells following intradermal immunization.
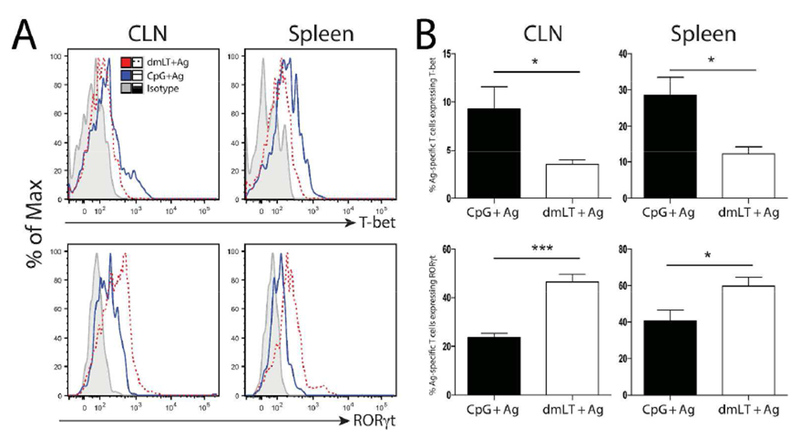
Mice were intradermally immunized with CpG or dmLT plus 2W1S-GFP and the 2W1S-specific CD4+ T cell transcription factor expression was assessed on day nine. CLN and spleen were harvested, dissociated, and immediately stained for the intracellular transcription factors T-bet and Rorγt. A) representative histograms of each transcription factor are shown including the isotype control. B) Graphs represent the percent of 2W1S-specific T cells expressing each transcription factor in immunized groups using 5 mice per group. Results are representative of four independent experiments. Significance was determined by student’s two-tailed t-test. Statistical significance is defined as follows: *, P<0.05; **, P<0.01; ***, P<0.005. Error bars represent SEM.
CD103+ dendritic cells present antigen and are required for dmLT induced mucosal trafficking.
To assess potential differences in each adjuvants’ capacity to engage different populations of APCs, we intradermally immunized mice with either dmLT or CpG plus 2W1S-GFP. One day later, when it is known that antigen has drained fully into lymph nodes 30, CLNs were harvested and single cell preparations were stained with the newly described W6 antibody that preferentially detects 2W1S:I-Ab antigen presenting complexes. This allowed us to quantify and phenotype cells specifically presenting the 2W1S peptide 31. We selected markers to account for the major DC subsets located in the dermis: CD11c+ CD11b+; CD11c+ CD11b−; CD11c+ CD103+, and Langerhans cells (EpCam+ DEC-205+) 32–34. Figure 6A shows CpG and dmLT both induced comparable overall levels of 2W1S peptide antigen presentation, which is quantified in Fig. 6B; however, there were clear differences in the phenotype of cells presenting antigen in each immunization group. There was a five-fold greater proportion of CD11c+ CD11b− DCs presenting 2W1S antigen in response to dmLT compared to CpG (Fig. 6 A&C). Upon further examination of these CD11c+ CD11b− DCs, we found there were tenfold more CD103+ dermal DCs presenting 2W1S in the dmLT immunized group. In fact, CD103+ DCs accounted for roughly one-third of the CD11c+ CD11b− DCs in the dmLT immunized group. Additionally, we observed that CpG plus 2W1S immunization induced more CD11b+ CD11c+ DCs to present 2W1S antigen in the CLN compared to dmLT. Lastly, we detected a slightly greater fraction of CD11b+ CD11c+ DEC-205+ EpCam+ DCs presented antigen in the dmLT immunization. It is likely these are Langerhans cells as they are the only DCs located in the epidermis known to express surface EpCam in conjunction with CD11c and DEC-205 33.
Figure 6. Intradermal immunization with dmLT preferentially engages CD103+ dermal dendritic cells and Langerhans cells compared to CpG.
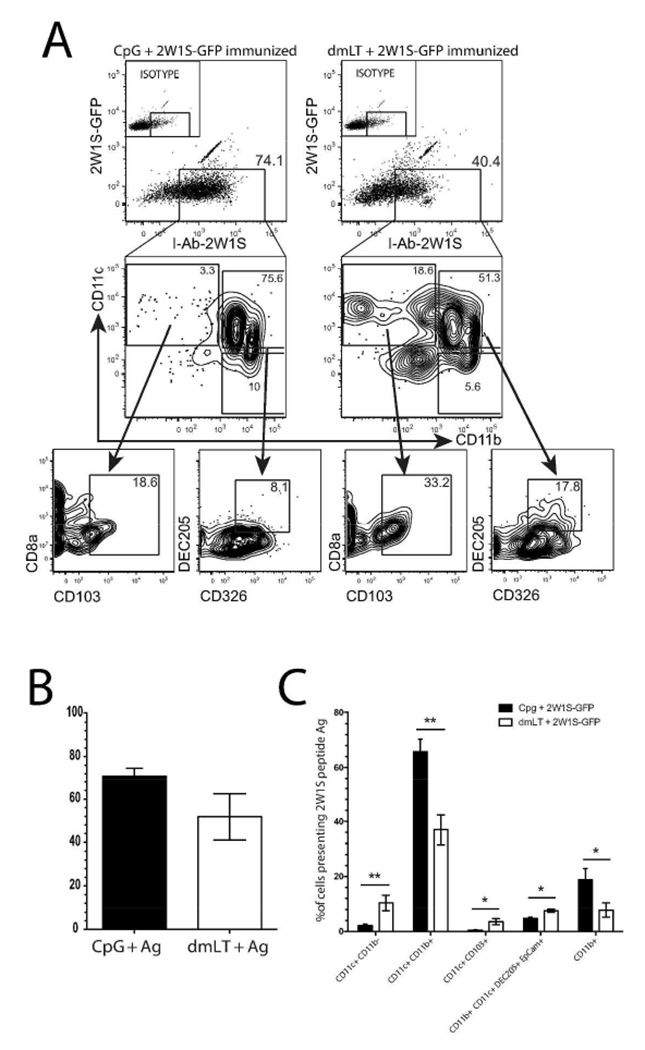
A) C57BL/6 mice were immunized intradermally with either CpG (left panes) or dmLT (right panes) plus 2W1S-GFP. Twenty-four hours after immunization, draining CLN were harvested and DCs were isolated. Digested lymph nodes were labeled with anti-I:Ab:2W1S antibody and subjected to magnetic bead enrichment on cells presenting 2W1S. Cells were gated on B cell lineage negative and for dendritic cell/monocytes on CD11b and CD11c. Downstream gates show the level of expression of CD103 (on CD11c+ CD11b−) or DEC-205 and CD326 (on CD11b+ CD11c+) or Langerhans cells. Numbers on each plot represent the percentage of cells in boxed gate. B) Quantification of total antigen presentation of 2W1S antigen in each immunized group. From one independent experiment using three mice per group and representative of three independent experiments. C) Graph represents differential antigen presentation by distinct DC subsets. Shown are two pooled independent experiments with three mice per group from a total of four independent experiments with two to four mice per group. Significance was determined by student’s twotailed t-test with Holm-Sidak correction for multiple comparisons. Statistical significance is defined as follows: *, P<0.05; **, P<0.01; ***, P<0.005. Error bars represent SEM.
It is known CD103 expression in a variety of tissue DCs correlates with intestinal homing 10–12. Based on this and our data showing increased numbers of CD103+ DCs presenting 2W1S antigen in the dmLT immunized group, we hypothesized these DCs at least partly accounted for the higher expression of α4β7 and increased mucosal homing following immunization with dmLT. To test this, we immunized WT mice and mice deficient in the basic leucine zipper transcriptional factor ATF-like 3 (Batf3), which are unable to develop CD103-expressing DCs, including those found in the dermis (Fig. S7 and 35). Mice were immunized with dmLT plus 2W1S-GFP as before, and nine days post immunization, we analyzed 2W1S-specific T cells in the CLN, MLN, Spleen, as well as the small and large intestine lamina propria. Local draining CLN and systemic splenic responses were equivalent, indicating there was no defect in the induction of 2W1S-specific T cell responses (Fig. 7A); however, there was a five-fold greater proportion of 2W1S-specific CD4+ T cells in WT immunized MLNs compared to Batf3−/− mice. Interestingly, the large intestine lamina propria was the only mucosal tissue affected by this deficiency (Fig. 7C). Additionally, α4β7-expression was significantly higher in the spleens of WT mice compared to Batf3−/− mice (Fig. 7B). Notably, neither IL-17 nor IFN-γ production was affected in Batf3−/− mice suggesting APCs distinct from CD103+ DCs might be inducing these cytokines (data not shown). These results imply CD103+ dDCs preferentially present antigen in response to dmLT intradermal immunization. While these cells are important for mucosal homing in response to dmLT immunization, they do not appear to account for the presence of all 2W1S-specific T cells in the mucosa.
Figure 7. Batf3−/− mice have reduced mucosal phenotypes and homing in response to dmLT immunization.
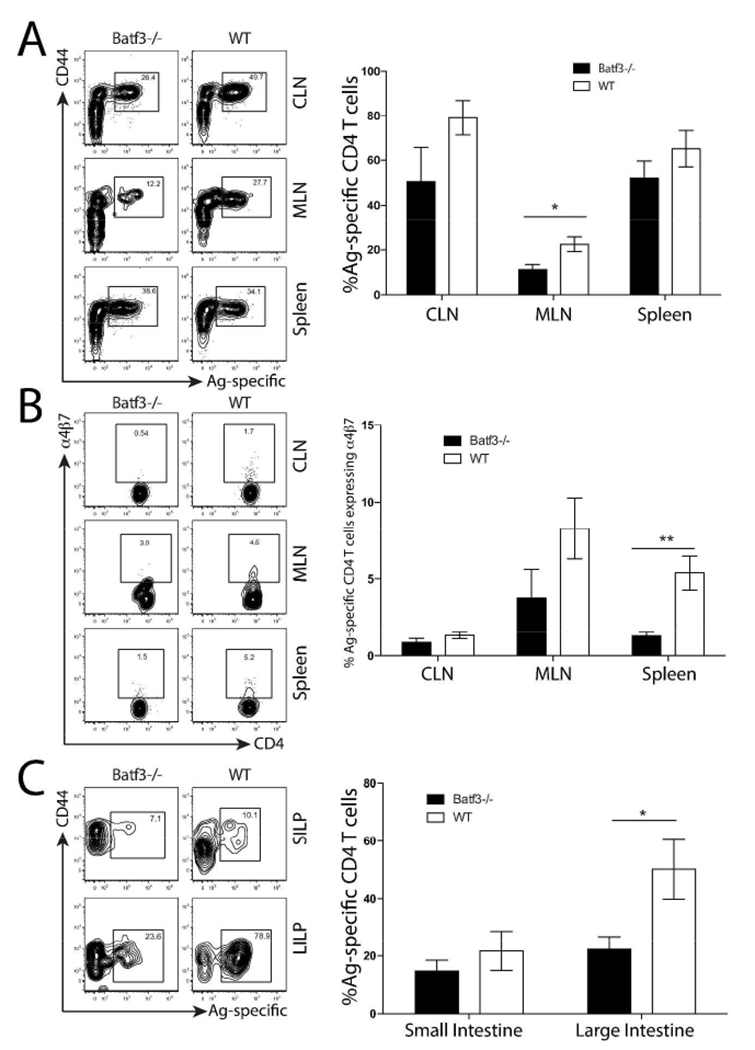
Batf3 −/− and WT mice were intradermally immunized with dmLT plus 2W1S-GFP. Nine days after immunization, CLN, MLN, spleen, and lamina propria were assayed for 2W1S-specific CD4+ T cells and α4β7 expression using 2W1S:MHCII tetramers. Contour plots are representative of staining from each immunization. Numbers on each plot represent the percentage of cells in boxed gate. A) Proportion of 2W1S-specific CD4+4+ CD4+ T cells isolated from CLN, MLN and spleens. B) Proportion of 2W1S-specific cells expressing α4β7 isolated from lymphoid tissue. C) Proportion of 2W1S-specific CD4+4+ CD4+ T cells isolated from small and large intestine. Data represent two pooled independent experiments from a total of three experiments with two to three mice per group. Significance was determined by student’s two-tailed t-test with Holm-Sidak correction for multiple comparisons. Statistical significance is defined as follows: *, P<0.05; **, P<0.01; ***, P<0.005. Error bars represent SEM.
Discussion
It is well accepted that adjuvants are essential mediators of vaccine induced immunity, yet it is not clear how, or even if, different adjuvants might be used to manipulate an immune response tailored to certain pathogen classes (i.e. enteric bacteria). Here, we examined how adjuvant choice might determine the magnitude, homing, and phenotype of the endogenous, antigen-specific CD4+ T cell immune response with parenteral vaccination. While TLR agonists were excellent at inducing potent, antigen-specific CD4+ T cell responses, the type of response was one dimensional (predominantly Th1) and confined primarily to the local draining lymph nodes and spleen. In contrast, the bacterial enterotoxin-derived adjuvant dmLT induced a balanced cytokine response and stimulated robust migration of these cells to both the small and large intestinal mucosae. Induction of the gut homing integrin α4β7 was dependent on the presence of dermal CD103+ DCs and mice lacking these cells were also deficient in inducing homing to the gut and gut-draining lymph nodes.
In this study, we administered immunizations parenterally, which is how most vaccines are currently delivered. This is the route of choice since it is technically easier, potentially less expensive, and not likely subject to the environmental enteropathy that affects orally administered vaccines. Additionally, it circumvents potential side effects, such as Bell’s Palsy or diarrhea, attributed to some mucosally-delivered vaccines 36,37. While many of the findings presented here used intradermal injections, it was notable this route did not appear to be essential as intramuscular injections induced gut homing markers with equal efficiency. The implication of this result is that the adjuvant, not the route, is dictating the final phenotype and localization of the T cell response.
These results confirm earlier observations that ADP-ribosylating toxins engage CD103+ DCs after cutaneous administration 38,39, however this DC subset has never been previously demonstrated to affect CD4+ T cells. This is the first direct evidence specifically demonstrating adjuvant choice guiding differential antigen presentation by distinctive DC subsets. This observation was achieved through the use of a recently described anti-peptide MHC II antibody, which can precisely allow visualization of specific peptide:MHCII complexes 31. We also show epidermal Langerhans cells are preferentially engaged in the presence of dmLT compared to CpG. Combined, these are notable findings as it is likely this differential antigen presentation largely contributes to imparting a gut homing phenotype in addition to the diverse cytokine production seen in antigen-specific CD4+ T cells from dmLT immunized mice. Supporting this contention, the gut resident CD103+ DC subset is known to impart α4β7 expression on CD4+ T cells following intestinal antigen exposure 10,12. Our evidence that Batf3−/− mice lacking CD103 DCs fail to induce the full expression of α4β7 and are deficient in large intestine homing supports the concept that CD103+ skin DCs act similarly to those in the gut. With both adjuvants, there was a strong Th1 bias in 2W1S-specific CD4+ T cells; however, dmLT also promoted a Th17 phenotype. It is notable Batf3−/− mice did not demonstrate significant defects in IL-17, implying other antigen presenting cells are possibly participating in priming Th17 cells in the presence of dmLT. The most likely candidate is Langerhans cells which were recently described as a prominent inducer of skin Th17 responses and in our experiments are also preferentially presenting 2W1S antigen in response to dmLT immunization compared to CpG 40.
While it is clear from this work Batf3−/− mice, and the resulting CD103 DC deficiency, are defective in targeting antigen-specific CD4+ T cells to the large intestine, it appears these cells may be dispensable for migration to the small intestine. This is surprising considering expression of α4β7 is attenuated in the spleen and MLN in the absence of this DC subset. While it has been shown T cells can migrate into the small intestine in the absence of α4β7 41, it is also possible that this migration is more dependent on other homing molecules such as G protein coupled receptor 18 (GPR18) or CCR9, although in our hands, CCR9 was not significantly upregulated and may not contribute as much as other homing markers 42,43. Additionally, while our data shows a significant decrease in antigen-specific T cell migration into the large intestine in Batf3−/− mice, this migration is not entirely abrogated, implying other DCs participate in the induction of mucosal homing. Langerhans cells, again, are possible candidates as they are a source of TGF-β1 44, a cytokine important for inducing a mucosal homing phenotype 45. Additionally, other potential homing markers such as the recently described GPR15 may be playing a role in homing despite the downregulation of α4β7 in our study 46,47. Notably, Hammerschmidt et al showed that subcutaneously administered retinoic acid (RA) preferentially induced migration of antigen-specific T and B cells into the intestines 48. Interestingly, RA also induced production of retinal dehydrogenase (RALDH) in skin-draining DCs which could impart a gut-homing phenotype on lymphocytes. It is possible that dmLT similarly affects DC function by inducing RALDH, leading to downstream T cell migration into the gut. Future studies could potentially elucidate these mechanisms.
This work has important implications for vaccine design, particularly with respect to provoking specific immune responses tailored to certain pathogen classes. For example, it is known CD4+ T cells are required for optimal immunity against the enteric pathogen Salmonella 49 so supporting migration of Salmonella-specific CD4+ T cells to the site of pathogen entry in the gut has the potential to prevent infection before the onset of severe pathology. It is possible this is true for other enteric pathogens such as Shigella or Enterotoxigenic E. coli. One conclusion that can be drawn from this work is that adjuvant choice can have a significant impact on the outcome of vaccine-mediated immunity, both in terms of localization and cytokine phenotype of immune cells. It may be possible to use these findings to explore the possibility of targeting other mucosal surfaces such as the respiratory or urogenital tract. Further, the possibility exists that combining adjuvants may have the potential to dramatically alter the type of immune response (humoral, Th1, Th17, Th2) while retaining the capacity for migration to a relevant site. Alternatively, strict cytokine polarization observed in a particular formulation may impede the capacity to properly limit infection, and as such a balanced cytokine profile may be ideal in certain infections. Lastly, while it is well-appreciated adjuvants such as dmLT or CT can induce antigen-specific IgA responses at the intestinal mucosae 7,17, it is not clear whether these adjuvants directly affect migration of vaccine-specific IgA producing B cells to these sites. Our future studies will explore both possibilities and have the potential to shift the paradigm for how the next generation of vaccines are devised.
Methods
Mice
C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) or from the NCI Mouse Repository via Charles River (Wilmington, MA). All mice used were female and approximately 8-12 weeks of age. Mice were housed under SPF conditions and food and water were given ad libitum. BATF3−/− were purchased from Jackson Laboratories, bred in-house and used for experimentation approximately at 8 weeks of age. The Tulane University Institutional Animal Care and Use Committee approved all procedures.
Fusion protein generation
Oligos were ordered (IDT, Coralville, IA) containing the 2W1S DNA sequence (peptide: EAWGALANWAVDSA) with BamHI and EcoRI cut sites, GCGCTCTTTGGATCCGAGGCTTGGGGAGCATTGGCTAATTGGGCTGTGGACTCAGCTGAATTCCCCTGTGAG (sense) and CTCACAGGGGAATTCAGCTGAGTCCACAGCCCAATTAGCCAATGCTCCCCAAGCCTCGGATCCAAAGAGCGC (antisense). Oligos were annealed and the resulting dimer was ligated into the pRSET-emeraldGFP cloning/expression vector (Life Technologies, Carlsbad, CA) using New BioLabs Quick Ligation™ Kit. The completed vector was then transformed into DH5α with ampicillin selection. Positive colonies were selected for plasmid isolation. Upon isolation of plasmid, the 2W1S-emGFP plasmid was transformed into Life Technologies BL21 Star™(DE3) T7 expressing E. coli for protein expression. 1L lag-phase cultures were induced with 1 mM IPTG for 16 hours and monitored for emGFP expression. Protein was purified from bacterial lysates using the Novagen His-Bind Purification Kit. Prior to elution, the column was rinsed with 50 column volumes of 0.1% Triton X-114/phosphate-buffered saline at 4°C to clear endotoxin. Eluted protein was then dialyzed into phosphate-buffered saline and concentration was determined via spectrophotometry using extinction coefficient determined by ExPASy ProtParam tool.
dmLT preparation
dmLT was obtained as previously described 50. Briefly, purified dmLT was prepared from E.coli overnight cultures lysed in a micro fluidizer, dialyzed and fractionated by affinity chromatography on immobilized D-galactose columns. After elution, the protein was passed through an endotoxin removing resin resulting in purified adjuvant preparations with endotoxin levels less than 1 EU/mg.
Immunization and tissue processing
Mice were immunized in the dermis of the ear pinna in 7 μl of antigen containing 15 μg of 2W1S-emGFP fusion protein plus either 10 μg Invivogen Vaccigrade™ CpG ODN 2395 (San Diego, CA) or 2 μg of dmLT. Local T cells responses were assayed by harvesting both superficial CLN and distal responses were assayed by harvesting the MLN and spleen. To isolate leukocytes, lymphoid tissue was mechanically dissociated and passed through a 100-micron filter. Filtered cells were subsequently stained for flow cytometry and/or subjected to cell enrichment for antigen-specific T cells. I-Ab:2W1S expressing S2 cells were grown in shaking 2L flask with Schneider’s drospholia medium supplemented with 10% serum and blasticidin to a density of 107 cell/mL. CuSO4 and biotinylated monomers were purified using a Novagen His-purification kit. Following elution, monomers were conjugated to APC in a 1:4 (SA-APC:monomer) ratio. Antigen-specific cells were enriched as previously described 14. Briefly, cells were incubated with 10 nM of I-Ab:2W1S tetramer for 1 hour at RT in the presence of FcBlock. Samples were washed and incubated with Miltenyi (Bergisch Gladbach, Germany) anti-APC microbeads for 20 minutes on ice. Resulting samples were washed, passed over Miltenyi LS columns and eluted cells were stained for further analysis.
Flow cytometry
Antibodies used for flow cytometry were as follows: Lineage negative cells were stained in vFluor450 including anti-CD11c (clone N418), anti-CD11b (clone M1/70), anti-F4/80 (clone BM8.1), and anti-CD19 (clone 6D5) purchased from Tonbo Biosciences (San Diego, CA). Anti-α4β7 PE (clone DAK32), anti-CD326 PE-Cy7 (clone G8.8), anti-CD4+4 PE-Cy7 (clone IM7), anti-CD3ε FITC (clone 145-2C11), anti-CD19 PE-Dazzle (clone 6D5), anti-CD80 BV605 (clone 16-10A1), anti-CD86 BV605 (clone GL-1), anti-CD103 PerCP-Cy5.5 (clone 2E7) and streptavidin BV421 were purchased from BioLegend (San Diego, CA). Anti-I-A/I-E APC (clone M5/114), DEC-205 PE (clone 205yekta) and anti-CD11b AF700 (clone M1/70) were purchased from ebioscience (San Diego, CA). For intracellular cytokine staining, anti-IFN-γ PerCP-Cy5.5 (clone XMG1.2), anti-IL-4 BV605 (clone 11B11), and anti-IL-17A PE-Cy7 (clone TC11-18H10.1) were purchased from BioLegend (San Diego, CA). Anti-IL-5 PE (clone TRK5) was purchased from ebioscience. GATA3 PE (clone L50-823) was purchased from BD Biosciences. For all surface staining protocols, cells were blocked with Fc Block mixed with 2% mouse and rat serum for 10 minutes on ice, then surface stained at 1:100 dilutions (DAK32 was used at 1:25 dilution; 2E7 at 1:50). Samples were collected using a BD Biosciences LSRII Fortessa cytometer. All FACS data was analyzed in TreeStar, Inc FlowJo software v9.9.3.
Intracellular Staining
For restimulation, whole lymph node cell suspensions were stimulated with 25 μg 2W1S peptide in 1 mL volume for two hours in 37°C, 5% CO2. Without rinsing, 10 μg/mL of Brefeldin A was added to the media and allowed to continue incubating for four more hours (six total). Cells were washed and tetramer and surface stained as above. For intracellular staining, cells were permeabilized and fixed using BD Biosciences Perm/Fix intracellular staining kit and were stained for cytokines overnight. For transcription factor staining, cells were permeabilized using ebioscience FoxP3 staining buffer kit and stained overnight.
Anti-I-Ab:2W1S Antibody purification and biotinylation
The monoclonal anti-I-Ab:2W1S antibody (called W6) was generated in-house as previously described 31. Hybridoma cells were grown for 14 days and media was harvested and filtered. Cleared lystate was buffer exchanged to 50 mM sodium acetate (pH 5.0). Antibody was isolated using a Thermofisher (Carlsbad, CA) Pierce™ Protein G chromatography column according to manufacturing specifications, with a flow rate of 1-3 mL/minute. Bound antibody was stripped from the column using 0.1 M glycine, pH 2. Purified antibody was then exchanged into 0.1 M sodium bicarbonate buffer. Two mg/mL Biotin-X-NHS mixture prepared in DMSO was added to the antibody solution and incubated for 1 hour in the dark at RT. After incubation, the solution was then exchanged into PBS (pH 7.2) and filtered.
In vivo antigen presentation assay
To assay the binding of the W6 antibody, C57BL/6 mice were immunized intradermally in the ear pinna with either Ovalbumin or 2W1S-GFP plus dmLT. CLN were dissociated and the cell suspension was then digested with 300 MandlU/mL Collagenase D (Roche Applied Sciences) for 30 minutes at 37°C. After digestion the cell suspension was filtered and stained for 30 minutes on ice with the W6 antibody, washed and stained with SA-PE for 15 minutes on ice.
Statistics
All data sets collected or exported from FlowJo were analyzed in GraphPad Prism version 6. Statistical significance was determined using multiple t-tests with the Holm-Sidak correction for multiple tests or by individual t-tests. Significance was assigned as: *, p<0.05; **, p<0.01; ***p<0.005. Outliers were removed using the Grubb’s outliers test. All graphs were generated in Prism 6.
Supplementary Material
Figure S1. Time Course of CD4+ T cell expansion in response to intradermal immunization with dmLT or CpG. C57BL/6 mice were immunized with 2W1S-GFP protein plus either CpG or dmLT in the pinna of each ear. At each indicated time point, the mice were assayed the number indicated cells in CLN and spleen: A) Total 2W1S-specific CD4+ cells. B) Total α4β7+ 2W1S-specific T cells. Each time point represents three mice and is representative of two independent experiments.
Figure S2. Quantification of total numbers of antigen-specific CD4+ T cells in different tissues of dmLT and CpG immunized mice. Mice immunized with CpG or dmLT plus 2W1S-GFP were assayed nine days later for 2W1S-specific CD4+ T cell responses which were then quantified. A) Total number of 2W1S-specific CD4 T cells in the CLN, MLN, and spleen, B) total number of a4b7+, 2W1S-specific CD4 T cells in the CLN, MLN, and spleen, C) Total number of 2W1S-specific CD4 T cells in the small and large intestine. Five mice per group representative of two independent experiments. Significance was determined by student’s two-tailed t-test. Statistical significance is shown on each graph. Error bars represent SEM.
Figure S3. Antigen-specific CD4+ T cell lung migration was comparable between dmLT and CpG immunized mice. Mice immunized with CpG or dmLT plus 2W1S-GFP were assayed nine days later for lung 2W1S-specific CD4+ T cell responses. Tetramer+ cells were magnetically enriched before analysis. A) representative flow cytometry plots and B) quantification of T cell numbers are shown. Representative of two independent experiments with two to four mice per group. Significance was determined by student’s two-tailed t-test. Statistical significance is defined as follows: *, P<0.05; **, P<0.01; ***, P<0.005. Error bars represent SEM.
Figure S4. dmLT induces greater expression of α4β7 and MLN migration compared to Pam3CSK(4). C57BL/6 mice were intradermally immunized with 2W1S-GFP plus either Pam3CSK(4) or dmLT. Nine days post immunization, CLN, MLN, and spleen were harvested and dissociated to yield lymphocytes. Single-cell suspensions were then magnetically enriched for 2W1S-specific CD4+ T cells. A) Representative plots on the left demonstrate the enriched 2W1S-specific fraction of CD4+ T cells. B) To the right are the representative plots showing α4β7 expression on 2W1S-specific CD4+ T cells. The bottom figure is the graphical representation of %α4β7+ 2W1S-specific cells. From one independent experiment of two experiments with three mice per group. Significance was determined by Student’s two-tailed test with Holm-Sidak correction for multiple comparisons where *=p<0.05; **=p<0.01; ***=p<0.001.
Figure S5. Modifying Route of dmLT Administration Does Not Impact α4β7 Imprinting on 2W1S-specific T cells. C57BL/6 mice were immunized with 2W1S-GFP plus dmLT intradermally in each ear pinna, the flank near the hind leg, or intramuscularly in the hind leg. At nine days post immunization, the draining CLN and MLN were harvested and dissociated to yield lymphocytes. Single-cell suspensions were then magnetically enriched for 2W1S-specific CD4+ T cells. A) Representative plots showing comparison of intradermal ear pinna versus flank injections and B) representative plots showing the comparison between intradermal and intramuscular immunizations. From two independent experiments with two to three mice per group.
Figure S6. dmLT induces IL-17 and IFN-γ following in vivo restimulation with 2W1S peptide in 2W1S-specific CD4+ T cells. C57BL/6 mice were immunized with CpG or dmLT plus 2W1S-GFP and then immunized mice were intravenously pulsed with 100 μg of purified 2W1S peptide nine days after prime. Two hours after peptide stimulation, mice were sacrificed and spleens harvested. Tissues were directly homogenized in media containing Brefeldin A. 2W1S-specific cells were labeled with 2W1S:MHCII and magnetically enriched. Enriched cells were then fixed, permeabilized and stained for cytokines. A) Representative FACS plots of splenic 2W1S-specific CD4+ T cell IL-17A and IFN-γ cytokine staining, B) Graphs of cytokine production for two independent experiments pooled are shown with three mice per group. Significance was determined by Student’s two-tailed t test where *=p<0.05; **=p<0.01; ***=p<0.001.
Figure S7. Batf3−/− mice lack CD103 dDCs. Batf3−/− mice and WT mice were intradermally immunized with dmLT. One day after immunization, CLN were harvested and stained for the presence of CD11c+ CD103+ DCs and the absence of CD103+ was confirmed by FACS analysis. Gated events represent CD19- MHCII+ bulk DCs. Representative of three mice.
Acknowledgements
We thank Dr. Lisa Morici and members of the McLachlan Lab for critical reading of the manuscript. This work is supported by NIH grants R01 AI103343 and U01 AI124289 (to J.B.M.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386, 743–800 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grassly NC et al. New strategies for the elimination of polio from India. Science 314, 1150–1153 (2006). [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization Collaborative Study Group on Oral Poliovirus Vaccine Factors affecting the immunogenicity of oral poliovirus vaccine: a prospective evaluation in Brazil and the Gambia. World Health Organization Collaborative Study Group on Oral Poliovirus Vaccine. J. Infect. Dis 171, 1097–1106 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Posey DL, Linkins RW, Oliveria MJ, Monteiro D & Patriarca PA The effect of diarrhea on oral poliovirus vaccine failure in Brazil. J. Infect. Dis 175 Suppl 1, S258–63 (1997). [DOI] [PubMed] [Google Scholar]
- 5.Heine SJ et al. Intradermal delivery of Shigella IpaB and IpaD type III secretion proteins: kinetics of cell recruitment and antigen uptake, mucosal and systemic immunity, and protection across serotypes. J Immunol 192, 1630–1640 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norton EB et al. The novel adjuvant dmLT promotes dose sparing, mucosal immunity and longevity of antibody responses to the inactivated polio vaccine in a murine model. Vaccine 33, 1909–1915 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Gloudemans AK et al. The mucosal adjuvant cholera toxin B instructs non-mucosal dendritic cells to promote IgA production via retinoic acid and TGF-β. PLoS ONE 8, e59822 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J et al. Transcutaneous immunization using colonization factor and heat-labile enterotoxin induces correlates of protective immunity for enterotoxigenic Escherichia coli. Infect Immun 70, 1056–1068 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavelle EC et al. Effects of cholera toxin on innate and adaptive immunity and its application as an immunomodulatory agent. J Leukoc Biol 75, 756–763 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Welty NE et al. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. Journal of Experimental Medicine 210, 2011–2024 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruane D et al. Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract. Journal of Experimental Medicine 210, 1871–1888 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stock A, Napolitani G & Cerundolo V Intestinal DC in migrational imprinting of immune cells. Immunol Cell Biol 91, 240–249 (2013). [DOI] [PubMed] [Google Scholar]
- 13.McLachlan JB, Catron DM, Moon JJ & Jenkins MK Dendritic cell antigen presentation drives simultaneous cytokine production by effector and regulatory T cells in inflamed skin. Immunity 30, 277–288 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon JJ et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27, 203–213 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huseby ES et al. How the T cell repertoire becomes peptide and MHC specific. Cell 122, 247–260 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Rees W et al. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc Natl Acad Sci USA 96, 9781–9786 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta SK et al. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. Proc. Natl. Acad. Sci. U.S.A 107, 10638–10643 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clements JD, Hartzog NM & Lyon FL Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine 6, 269–277 (1988). [DOI] [PubMed] [Google Scholar]
- 19.Moon JJ et al. Tracking epitope-specific T cells. Nat Protoc 4, 565–581 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berlin C et al. α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993). [DOI] [PubMed] [Google Scholar]
- 21.Petrovic A et al. LPAM (alpha 4 beta 7 integrin) is an important homing integrin on alloreactive T cells in the development of intestinal graft-versus-host disease. Blood 103, 1542–1547 (2004). [DOI] [PubMed] [Google Scholar]
- 22.Iwata M et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 21, 527–538 (2004). [DOI] [PubMed] [Google Scholar]
- 23.Berlin C et al. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell 74, 185–195 (1993). [DOI] [PubMed] [Google Scholar]
- 24.Wu HY, Nikolova EB, Beagley KW, Eldridge JH & Russell MW Development of antibody-secreting cells and antigen-specific T cells in cervical lymph nodes after intranasal immunization. Infect Immun 65, 227–235 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNeal MM et al. CD4 T cells are the only lymphocytes needed to protect mice against rotavirus shedding after intranasal immunization with a chimeric VP6 protein and the adjuvant LT(R192G). J Virol 76, 560–568 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattsson J et al. Cholera toxin adjuvant promotes a balanced Th1/Th2/Th17 response independently of IL-12 and IL-17 by acting on Gsa in CD11b+ DCs. Mucosal Immunol 8, 815–827 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Leach S, Clements JD, Kaim J & Lundgren A The adjuvant double mutant Escherichia coli heat labile toxin enhances IL-17A production in human T cells specific for bacterial vaccine antigens. PLoS ONE 7, e51718 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meza-Sánchez D, Pérez-Montesinos G, Sánchez-García J, Moreno J & Bonifaz LC Intradermal immunization in the ear with cholera toxin and its non-toxic β subunit promotes efficient Th1 and Th17 differentiation dependent on migrating DCs. Eur J Immunol 41, 2894–2904 (2011). [DOI] [PubMed] [Google Scholar]
- 29.Chu RS, Targoni OS, Krieg AM, Lehmann PV & Harding CV CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med 186, 1623–1631 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Itano AA et al. Distinct dendritic cell populations sequentially present antigen to CD4 T cells and stimulate different aspects of cell-mediated immunity. Immunity 19, 47–57 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Spanier JA et al. Efficient generation of monoclonal antibodies against peptide in the context of MHCII using magnetic enrichment. Nature Communications 7, 11804 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD & Shlomchik MJ Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity 23, 611–620 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Kaplan DH In vivo function of Langerhans cells and dermal dendritic cells. Trends Immunol 31, 446–451 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seré K et al. Two distinct types of Langerhans cells populate the skin during steady state and inflammation. Immunity 37, 905–916 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Edelson BT et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8alpha+ conventional dendritic cells. Journal of Experimental Medicine 207, 823–836 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis DJM et al. Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS ONE 4, e6999 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davitt CJH & Lavelle EC Delivery strategies to enhance oral vaccination against enteric infections. Adv. Drug Deliv. Rev (2015). doi:10.1016/j.addr.2015.03.007 [DOI] [PubMed] [Google Scholar]
- 38.Apte SH et al. Subcutaneous cholera toxin exposure induces potent CD103(+) dermal dendritic cell activation and migration. Eur J Immunol 43, 2707–2717 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Olvera-Gomez I et al. Cholera toxin activates nonconventional adjuvant pathways that induce protective CD8 T-cell responses after epicutaneous vaccination. Proc. Natl. Acad. Sci. U.S.A 109, 2072–2077 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kashem SW et al. Candida albicans morphology and dendritic cell subsets determine T helper cell differentiation. Immunity 42, 356–366 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuklin NA et al. alpha(4)beta(7) independent pathway for CD8(+) T cell-mediated intestinal immunity to rotavirus. J Clin Invest 106, 1541–1552 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker AM et al. GPR18 Controls Reconstitution of Mouse Small Intestine Intraepithelial Lymphocytes following Bone Marrow Transplantation. PLoS ONE 10, e0133854 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stenstad H et al. Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine. Blood 107, 3447–3454 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Kaplan DH et al. Autocrine/paracrine TGFβ1 is required for the development of epidermal Langerhans cells. Journal of Experimental Medicine 204, 2545–2552 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang SG, Park J, Cho JY, Ulrich B & Kim CH Complementary roles of retinoic acid and TGF-β1 in coordinated expression of mucosal integrins by T cells. Mucosal Immunol 4, 66–82 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim SV et al. GPR15-mediated homing controls immune homeostasis in the large intestine mucosa. Science 340, 1456–1459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen LP et al. Role and species-specific expression of colon T cell homing receptor GPR15 in colitis. Nat Immunol 16, 207–213 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammerschmidt SI et al. Retinoic acid induces homing of protective T and B cells to the gut after subcutaneous immunization in mice. J Clin Invest 121, 3051–3061 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johanns TM, Ertelt JM, Rowe JH & Way SS Regulatory T cell suppressive potency dictates the balance between bacterial proliferation and clearance during persistent Salmonella infection. PLoS Pathog 6, e1001043 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norton EB, Lawson LB, Freytag LC & Clements JD Characterization of a mutant Escherichia coli heat-labile toxin, LT(R192G/L211A), as a safe and effective oral adjuvant. Clin Vaccine Immunol 18, 546–551 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Time Course of CD4+ T cell expansion in response to intradermal immunization with dmLT or CpG. C57BL/6 mice were immunized with 2W1S-GFP protein plus either CpG or dmLT in the pinna of each ear. At each indicated time point, the mice were assayed the number indicated cells in CLN and spleen: A) Total 2W1S-specific CD4+ cells. B) Total α4β7+ 2W1S-specific T cells. Each time point represents three mice and is representative of two independent experiments.
Figure S2. Quantification of total numbers of antigen-specific CD4+ T cells in different tissues of dmLT and CpG immunized mice. Mice immunized with CpG or dmLT plus 2W1S-GFP were assayed nine days later for 2W1S-specific CD4+ T cell responses which were then quantified. A) Total number of 2W1S-specific CD4 T cells in the CLN, MLN, and spleen, B) total number of a4b7+, 2W1S-specific CD4 T cells in the CLN, MLN, and spleen, C) Total number of 2W1S-specific CD4 T cells in the small and large intestine. Five mice per group representative of two independent experiments. Significance was determined by student’s two-tailed t-test. Statistical significance is shown on each graph. Error bars represent SEM.
Figure S3. Antigen-specific CD4+ T cell lung migration was comparable between dmLT and CpG immunized mice. Mice immunized with CpG or dmLT plus 2W1S-GFP were assayed nine days later for lung 2W1S-specific CD4+ T cell responses. Tetramer+ cells were magnetically enriched before analysis. A) representative flow cytometry plots and B) quantification of T cell numbers are shown. Representative of two independent experiments with two to four mice per group. Significance was determined by student’s two-tailed t-test. Statistical significance is defined as follows: *, P<0.05; **, P<0.01; ***, P<0.005. Error bars represent SEM.
Figure S4. dmLT induces greater expression of α4β7 and MLN migration compared to Pam3CSK(4). C57BL/6 mice were intradermally immunized with 2W1S-GFP plus either Pam3CSK(4) or dmLT. Nine days post immunization, CLN, MLN, and spleen were harvested and dissociated to yield lymphocytes. Single-cell suspensions were then magnetically enriched for 2W1S-specific CD4+ T cells. A) Representative plots on the left demonstrate the enriched 2W1S-specific fraction of CD4+ T cells. B) To the right are the representative plots showing α4β7 expression on 2W1S-specific CD4+ T cells. The bottom figure is the graphical representation of %α4β7+ 2W1S-specific cells. From one independent experiment of two experiments with three mice per group. Significance was determined by Student’s two-tailed test with Holm-Sidak correction for multiple comparisons where *=p<0.05; **=p<0.01; ***=p<0.001.
Figure S5. Modifying Route of dmLT Administration Does Not Impact α4β7 Imprinting on 2W1S-specific T cells. C57BL/6 mice were immunized with 2W1S-GFP plus dmLT intradermally in each ear pinna, the flank near the hind leg, or intramuscularly in the hind leg. At nine days post immunization, the draining CLN and MLN were harvested and dissociated to yield lymphocytes. Single-cell suspensions were then magnetically enriched for 2W1S-specific CD4+ T cells. A) Representative plots showing comparison of intradermal ear pinna versus flank injections and B) representative plots showing the comparison between intradermal and intramuscular immunizations. From two independent experiments with two to three mice per group.
Figure S6. dmLT induces IL-17 and IFN-γ following in vivo restimulation with 2W1S peptide in 2W1S-specific CD4+ T cells. C57BL/6 mice were immunized with CpG or dmLT plus 2W1S-GFP and then immunized mice were intravenously pulsed with 100 μg of purified 2W1S peptide nine days after prime. Two hours after peptide stimulation, mice were sacrificed and spleens harvested. Tissues were directly homogenized in media containing Brefeldin A. 2W1S-specific cells were labeled with 2W1S:MHCII and magnetically enriched. Enriched cells were then fixed, permeabilized and stained for cytokines. A) Representative FACS plots of splenic 2W1S-specific CD4+ T cell IL-17A and IFN-γ cytokine staining, B) Graphs of cytokine production for two independent experiments pooled are shown with three mice per group. Significance was determined by Student’s two-tailed t test where *=p<0.05; **=p<0.01; ***=p<0.001.
Figure S7. Batf3−/− mice lack CD103 dDCs. Batf3−/− mice and WT mice were intradermally immunized with dmLT. One day after immunization, CLN were harvested and stained for the presence of CD11c+ CD103+ DCs and the absence of CD103+ was confirmed by FACS analysis. Gated events represent CD19- MHCII+ bulk DCs. Representative of three mice.


