Highlights
-
•
Higher sensitivity for self-serving rewards is related to lower peer acceptance.
-
•
Peer acceptance is not related to Nucleus Accumbens activity during vicarious wins.
-
•
Findings highlight the importance of neural reward-processing in peer relationships.
Keywords: Reward processing, Vicarious rewards, Peer relationships, Adolescence, fMRI
Abstract
Peer relationships play an important role in adolescent social development. Adolescence is also a sensitive period for reward-related processing where Nucleus Accumbens (NAcc) shows peak levels of activity. To investigate the role of reward-related neural processes in peer relationships, we scanned 31 adolescents (16 boys, 15 girls) from 12 to 17 years old and had their classmates rate their likability and dislikability. Using these ratings, we calculated levels of peer acceptance (i.e., likability minus dislikability scores). Participants played a social gambling paradigm in the scanner where we examined NAcc responses to winning for self and winning for best friends. We showed that acceptance by peers was related negatively to activation patterns in the NAcc when winning money for self. Peer acceptance was not related to NAcc activity during vicarious reward processing where participants won money for their best friend. These results point in the direction of an underlying neural mechanism indicating that peer interactions of well-liked adolescents are characterized by a lower focus on benefits for self.
1. Introduction
Adolescence is the transitional period from childhood to adulthood and is an important time for social development. Adolescents show increased recognition skills for faces of peers (Picci and Scherf, 2016), and have increased susceptibility to peer influences on decision-making (Knoll et al., 2015; Van Hoorn et al., 2016). In addition, adolescents continue to develop the ability to perceive the environment from another individual’s perspective (Dumontheil et al., 2010) and increasingly incorporate the other’s perspective into their decision-making (Güroğlu et al., 2009; Overgaauw et al., 2012; Van den Bos et al., 2011). Research on social development shows that during adolescence a shift in social orientation occurs from parents to peers. Adolescents spend increasingly more time with peers (Larson et al., 2002) and feelings of belonging and acceptance within the peer group are very important for adolescents (Brown et al., 1986). For many adolescents, positive peer relations provide opportunities in the development of social functioning, academic achievement, and self-esteem (Malti et al., 2012; Valkenburg et al., 2006; Wentzel, 2005). With such relevance to adolescent development, it is important to understand what factors contribute to acceptance among peers.
Previous research shows how behavioral tendencies relate to peer group dynamics in adolescence. Specifically, the crucial role of social skills in general, and other-regarding behaviors in particular, in being accepted by peers have been emphasized (Cillessen et al., 2011). On the one hand, children and adolescents who are highly liked among peers have higher levels of empathy and prosocial motivation and display more cooperative, helping and sharing behaviors (Cillessen and Rose, 2005; De Bruyn and Cillessen, 2006; Meuwese et al., 2016). On the other hand, rejected adolescents tend to show more antisocial behavior than adolescents that are accepted among their peers (Wolters et al., 2013), and they display deficits in social cognition (e.g. lower levels of perspective-taking skills) and executive control (e.g. limited impulse control and emotion regulation) (Dodge et al., 2003; Eisenberg et al., 1997; Fink et al., 2014). Peer relationship literature has so far focused on how behavior relates to peer acceptance. However, the role of neural processes that could be underlying behavioral tendencies related to peer acceptance has not been investigated yet.
The current study aims to investigate the relationship between neural activation patterns in the Nucleus Accumbens (NAcc) during self-serving and vicarious reward-processing, and peer acceptance (i.e., being well-liked by peers). We used a gambling fMRI-paradigm to examine brain responses when receiving monetary rewards for self (i.e., self-serving) or for other individuals (i.e., vicarious). This paradigm has been validated to detect differential activation patterns in the NAcc for winning versus losing and for different reward recipients, in children, adolescents, and adults (Braams et al., 2013, 2014). In the current study we focused on neural responses in the NAcc due to its role in reward processing (for a review see Delgado, 2007; for a meta-analysis see Silverman et al., 2015). Interestingly, the NAcc has been shown to be responsive not only to rewards for self (self-serving rewards), but also shows similar activation when receiving rewards for close others (vicarious rewards) (Braams et al., 2013, 2014). In a recent study, we have further shown that activation patterns in the NAcc are related to a general tendency to approach rewards (Schreuders et al., 2018).
In the current study, we examined the link between NAcc responses to self-serving and vicarious rewards and acceptance by peers. Peer acceptance was assessed by nominations provided by participants’ classmates. Each classmate provided ratings for whom they liked and disliked. Participants’ level of peer acceptance was measured by “like” and “dislike” nominations given by their classmates in school. We hypothesized that stronger responses to self-serving rewards are negatively correlated to levels of peer acceptance, since a stronger orientation towards rewards for self could be perceived as egoistic and non-beneficial for other individuals and the group as a whole. A previous study on vicarious reward processing reports that adolescents who show more reward-related neural activation display less antisocial behavior (Telzer et al., 2013) and well-liked adolescents generally behave less antisocial than their less accepted peers (Wolters et al., 2013). Therefore, we hypothesized that stronger responses to vicarious rewards are positively related to peer acceptance.
2. Material and methods
2.1. Participants and procedure
A total of 1259 adolescents participated in a behavioral study in school and were afterwards invited to volunteer in an MRI-study in the lab. Initially, 100 of these participants indicated their interest to participate. A follow-up telephone screening for right-handedness and absence of neurological and psychiatric disorders or use of medication known to affect nervous system functioning resulted in a total of 39 adolescents who met our inclusion criteria and who were available on scanning days. The scanning session took place between 4–9 months after testing in school. Five participants were excluded after their scanning session due to excessive movement (>3 mm in any direction) or data artifacts. Two participants from two schools with a grade structure (instead of a classroom structure) were excluded from the analyses. In schools with a grade structure students change classes from period to period and are in contact with students from the entire grade, which contained 52 and 60 students in the two schools involved here. In schools with a classroom structure, students spend time with the same group of classmates for all classes and class sizes were much smaller (class sizes ranged from 10 to 32 (M = 24.91, SD = 4.87). Thus, students from the schools with a grade structure were excluded from analyses because it is likely that peer relationships in schools with a grade versus classroom structure differ and that students from schools with a grade structure know each other less well compared to those from schools with a classroom structure. Data collection took place near the end of the school year to guarantee that the participants in 7th grade (the first grade of secondary school) had spent sufficient time together to know each other. Testing sessions were supervised by trained assistants. All testing was done using an online survey and took between 60 and 90 min. Consent was obtained from schools and parents.
The final sample comprised of 31 adolescents (Mage = 14.43; SDage = 1.32; Rangeage 12–17 years old) from 20 classrooms. For these participants, intelligence was approximated using block design and similarities of the WISC-III for children up to 16 years of age and of the WAIS-IV for 16 years and older. All participants had normal intelligence (MIQ = 107.18, SDIQ = 9.33). Primary caregivers and all participants signed informed consent prior to the MRI session. Participants were prepared for the testing session in a quiet room. They were familiarized with the MRI scanner with a mock scanner and by listening to recordings of the scanner sounds. Next, participants received instructions for the fMRI task and performed six practice trials of the task. At the end of the scanning session, all participants received 30 euros for their participation. The university internal review board approved the study.
2.2. Materials
2.2.1. Peer nominations
All 1259 participants from the original sample, in 48 classrooms from two local secondary schools, were asked to nominate the peers within their school class who they liked the most and the least. The nomination process was aided with an alphabetic list of names of all classmates where participants were asked to provide the number of the classmate they would like to nominate. An unlimited number of nominations could be given; self-nominations were not allowed. The total number of received ‘likes’ (i.e., likability scores) and ‘dislikes’ (i.e., dislikability scores) were calculated for each participant and the resulting scores were standardized within classrooms. We calculated acceptance scores for each participant where dislikability scores were subtracted from likability scores (Coie et al., 1982).
2.2.2. fMRI paradigm
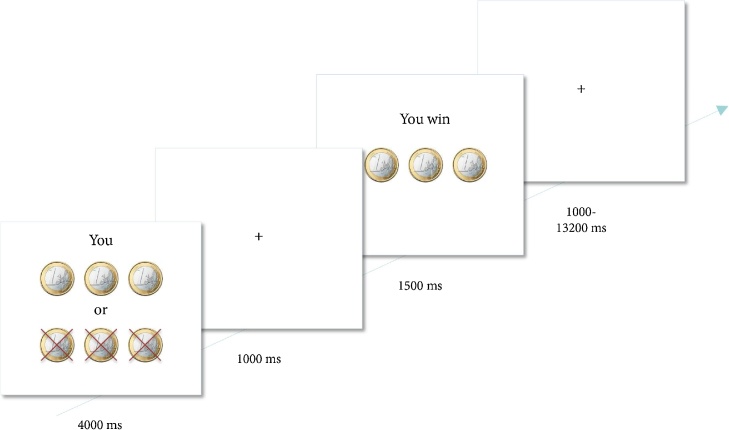
During the scanning session, participants played a heads or tails gambling game in which they could win or lose money (Braams et al., 2013, 2014). On each trial, participants guessed whether the computer would pick heads or tails and they won when the computer selected the chosen side of the coin (see Fig. 1). Each trial started with a trial onset screen (4000 ms) during which the participant made their choice to play for heads or tails. Probabilities for winning were 50%. On the trial onset screen the participants saw how much they could win or lose on that trial.
Fig. 1.
Example of a trial. On trial onset, participants were presented with a screen for 4000 ms indicating how many coins could be won or lost. During this time, participants chose to play heads or tails by pressing the corresponding button. After a 1000 ms delay, trial outcome was presented for 1500 ms. Participants won when the computer randomly selected the same side of the coin as chosen by the participant (Braams et al., 2014).
Three different distributions of coins were included: trials on which 2 coins could be won and 5 lost, trials on which 3 coins could be won or 3 lost and finally trials on which 5 coins could be won or 2 could be lost. These different distributions of coins were included to keep participants engaged in the task, but were not analyzed separately (see Braams et al., 2013, 2014). Participants were informed about the different distributions of coins and were familiarized with them during the practice session prior to scanning. The trial onset screen was followed by a fixation screen (1000 ms) and a feedback screen, which showed whether participants won or lost on that trial (1500 ms). Trials ended with a variable jitter (1000–13200 ms). Trial sequence and timing was optimized using OptSeq (Dale, 1999); see also (http://surfer.nmr.mgh.harvard.edu/optseq/). Participants played 30 trials in the gambling game for themselves, 30 trials for their best friend and 30 trials for another unfamiliar person. The trials for different players were intermixed within the same block but outcomes for the self and other were independent of each other on each trial. The likability of the unknown person was manipulated by an unfair offer in an ultimatum game so that this was a disliked person. Results for this condition have been reported previously (Braams et al., 2013, 2014). The trials for this player were modeled here in the analyses, but the effects were of no interest for the current study and are thus not included here; a similar analysis strategy has been employed in previous work using this task (see Braams and Crone, 2016a,b; Schreuders et al., 2018).
Half of the trials were win trials and half were lose trials and participants were instructed that the coins won during the experiment would translate to real money at the end of the experiment; however, it was not specified how coins won would translate to real money. Unbeknownst to the participants, the total earnings on the task did not relate to the amount won during the task. All participants received 4, 5 or 6 euro’s bonus earnings at the end of the task for themselves or for their friend; the amount that a participant received was determined randomly. If their best friend won, participants were given money for the best friend and were asked to give the money to their best friend.
2.3. FMRI data
2.3.1. MRI data acquisition
Scanning was performed on a 3 T Philips scanner, with a standard whole- head coil. The functional scans were acquired using T2*-weighted echo-planar imaging (EPI) (TR = 2.2 s, TE = 30 ms, sequential acquisition, 38 slices of 2.75 mm, field of view 220 mm, 80 × 80 matrix, in-plane resolution 2.75 mm). The first two volumes were discarded to allow for equilibration of T1 saturation effects. After the two functional runs, a high-resolution 3D T1- weighted anatomical image was collected (TR = 9.751 ms, TE = 4.59 ms, flip angle = 8°, 140 slices, 0.875 mm × 0.875 mm×1.20 mm, and FOV = 224.0 × 168.0 × 177.3). Visual stimuli were displayed on a screen in the magnet bore. A mirror attached to the head coil allowed participants to view the screen. Foam inserts inside the coil were used to limit head movement. All anatomical scans were reviewed and cleared for gross abnormalities by a radiologist.
2.3.2. FMRI preprocessing and statistical analyses
All data were analyzed with SPM8 (Wellcome Trust Centre for Neuroimaging, London). Images were corrected for slice timing acquisition and differences in rigid body motion. Structural and functional volumes were spatially normalized to T1 templates. Translational movement parameters of the included sample never exceeded 1 voxel (<3 mm) in any direction for any participant or scan. The normalization algorithm used a 12-parameter affine transform together with a nonlinear transformation involving cosine basis functions and resampled the volumes to 3 mm cubic voxels. Templates were based on the MNI305 stereotaxic space. Functional volumes were spatially smoothed with a 6 mm FWHM isotropic Gaussian kernel. Statistical analyses were performed on individual subjects data using the general linear model in SPM8. The fMRI time series were modeled as a series of zero duration events convolved with the hemodynamic response function (HRF). The task was an event related design. On trial onset events were modeled separately for playing for self, friend and the other person. On feedback onset winning and losing for self, friend and other were modeled. This resulted in three conditions at trial onset (self, friend, other) and six conditions at feedback onset (self win, self lose, friend win, friend lose, other win, other lose). Trials on which the participants failed to respond were modeled separately as a covariate of no interest and were excluded from further analyses. The modeled events were used as regressors in a general linear model, along with a basic set of cosine functions that high-pass filtered the data (cutoff 120 s), and a covariate for session effects. The least-squares parameter estimates of height of the best-fitting canonical HRF for each condition were used in pairwise contrasts. The resulting contrast images, computed on a subject-by-subject basis, were submitted to random-effects group analyses.
2.3.3. Region of interest selection
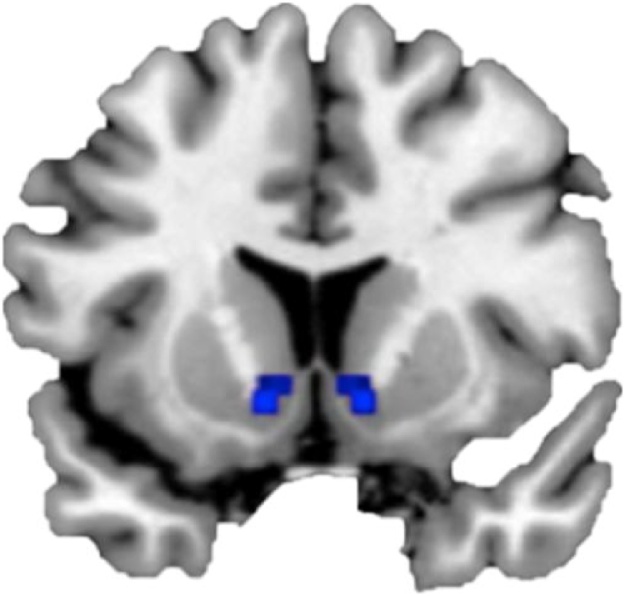
Whole-brain analyses for the t-contrasts win > lose for self and friend resulted in activation clusters in the bilateral ventral striatum, including the NAcc, see Table 1. In this study we specifically focused on the NAcc, since previous studies have highlighted this part of the ventral striatum as a key region in reward-based processing (Braams et al., 2015; Delgado, 2007). We used an anatomical mask of the left and right NAcc extracted from the Harvard-Oxford subcortical atlas, thresholded at 40% (see also Braams et al., 2015 for a similar approach; see Fig. 2). The MarsBaR toolbox (Brett et al., 2002) (http://marsbar.sourceforge.net/) for SPM8 was used to extract patterns of activation in the left and right NAcc. Average beta values, also known as parameter estimates, for the contrasts win > fixation and lose > fixation for both self and friend were extracted and used for subsequent ROI analyses.
Table 1.
Table for neural activation for the whole brain t-contrast win > lose when playing for friend and win > lose when playing for self. Reported clusters are p < .001, uncorrected. Only clusters comprised of 10 voxels or more are reported. All reported coordinates are in Montreal Neurological Institute (MNI) space. Right and left side of the brain are indicated by R and L respectively. For clusters with multiple peaks, each peak is reported separately.
| MNI |
||||||
|---|---|---|---|---|---|---|
| Region | R/L | x | y | z | t(198) | Voxels |
| Friend Win > Friend Lose | ||||||
| Caudate | L | −3 | 2 | 7 | 3.95 | 15 |
| Superior Medial Gyrus | L | −3 | 56 | 1 | 4.73 | 62 |
| Mid Orbital Gyrus | R | 9 | 47 | −5 | 3.71 | 62 |
| Precuneus | R | 9 | −52 | 22 | 4.49 | 237 |
| Precuneus | L | −6 | −55 | 19 | 4.09 | 237 |
| Cuneus | L | −9 | −61 | 25 | 3.99 | 237 |
| Middle Temporal Gyrus | L | −63 | −10 | −14 | 4.09 | 19 |
| Middle Temporal Gyrus | L | −60 | −7 | −23 | 3.77 | 19 |
| Superior Temporal Gyrus | R | 45 | −40 | 7 | 4.03 | 11 |
| Superior Frontal Gyrus | L | −18 | 32 | 49 | 3.61 | 10 |
| Hippocampus | L | −21 | −13 | −23 | 3.51 | 10 |
| Self Win > Self Lose | ||||||
| Caudate Nucleus | L | −12 | 14 | −5 | 5.56 | 72 |
| Putamen | R | 18 | 14 | −5 | 5.59 | 120 |
| Caudate Nucleus | R | 12 | 17 | 13 | 3.66 | 120 |
| Putamen | R | 30 | −10 | 10 | 4.06 | 25 |
| Caudate Nucleus | L | −21 | 2 | 25 | 3.63 | 20 |
| Putamen | L | −24 | 5 | 10 | 3.52 | 20 |
| Pallidum | L | −18 | −4 | 10 | 3.39 | 20 |
| Angular Gyrus | R | 48 | −70 | 43 | 4.37 | 38 |
| Middle Cingulate Gyrus | R | 3 | 2 | 31 | 4.35 | 61 |
| Middle Cingulate Gyrus | L | −3 | −10 | 31 | 4.26 | 61 |
| Putamen | R | 30 | −10 | 10 | 4.06 | 25 |
| Precentral Gyrus | R | 48 | −1 | 31 | 3.99 | 6 |
| Superior Frontal Gyrus | L | −15 | 62 | 28 | 3.77 | 10 |
| Middle Frontal Gyrus | L | −42 | 50 | 28 | 3.76 | 27 |
| Middle Frontal Gyrus | L | −39 | 56 | 19 | 3.64 | 27 |
| Middle Frontal Gyrus | L | −30 | 62 | 19 | 3.37 | 27 |
| Middle Frontal Gyrus | R | 36 | 59 | 25 | 3.67 | 18 |
Fig. 2.
Anatomical mask of the left and right NAcc extracted from the Harvard-Oxford subcortical atlas, thresholded at 40%.
2.4. Statistical analysis
We tested whether peer acceptance was related to neural activation in NAcc during the gambling task. To this end we performed a multiple linear regression analysis on standardized acceptance ratings. We included Self Win > Fixation, Self Lose > Fixation, Friend Win > Fixation and Friend Lose > Fixation contrast parameter estimates; to control for effect of age and sex, these were also included in the regression. For the contrast values we calculated an average of left and right NAcc since we did not expect laterality for any of the effects. Effects of laterality were checked and we confirmed no differences between left and right NAcc. In the regression analysis, NAcc activation for both winning and losing were both included in order to investigate whether the obtained effects are specific for the win or loss domain. All data were checked for outliers before running the multiple regressions. When outliers were detected we used robust analyses. Robust statistics minimize the influence of outliers and as such they produce a more stable estimation of parameters.
3. Results
3.1. Descriptives
Table 2 presents all means, standard deviations and intercorrelations between all variables. These correlations were not corrected for age and sex.
Table 2.
Descriptives and correlations of study variables. For the Nucleus Accumbens (NAcc) activations, Self Win refers to the condition in which the participant won for themselves, Self Lose refers to the condition in which the participant lost for themselves, Friend Win refers to the condition in which the participant won for their friend and Friend Lose refers to the condition in which the participant lost for their friend. All neural activation is against baseline. Stars indicate significance at p < .05 (*), p < .01 (**) and p < .001 (***).
| 1 | 2 | 3a | 3b | 3c | 3d | ||
|---|---|---|---|---|---|---|---|
| Means and standard deviations | |||||||
| Means | 14.43 | 0 | −0.99 | −4.11 | −0.5 | −2.23 | |
| Standard deviations | 1.32 | 0.91 | 4.13 | 3.88 | 4.13 | 5 | |
| Uncorrected correlations | |||||||
| 1 | Age | −0.08 | −0.07 | −0.22 | −0.36* | −0.14 | |
| 2 | Acceptance | −0.42** | 0.08 | 0.07 | −0.06 | ||
| 3 | NAcc | ||||||
| 3a | Self Win | 0.44* | 0.21 | 0.46** | |||
| 3b | Self Lose | 0.24 | 0.23 | ||||
| 3c | Friend Win | 0.29 | |||||
| 3d | Friend Lose | ||||||
3.2. Nucleus Accumbens activation and peer acceptance
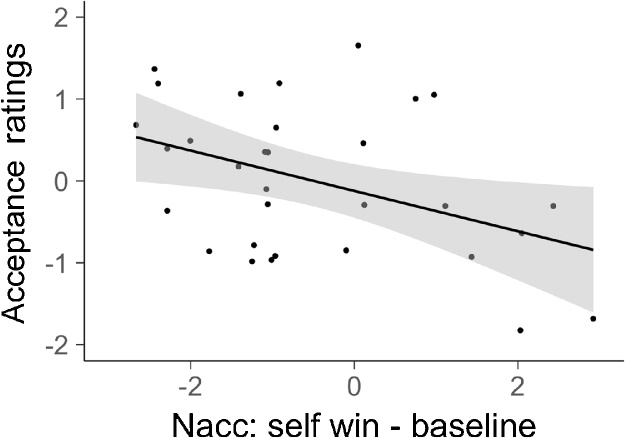
To test whether acceptance scores were related to NAcc activation while winning and losing for self and friend we performed a multiple linear regression. Due to outliers in the data robust regressions were run. In the model we included the NAcc activation when winning for self, when losing for self, when winning for a friend and when losing for friend. We also added age and gender in the analysis to control for these variables. The model showed that acceptance was negatively related to NAcc activation when winning for self. This shows that participants who showed higher NAcc activation when winning for self were less preferred by their peers, see Fig. 3. The other predictors were not significant in this model, see Table 3.
Fig. 3.
Figure displays the relationship between Nucleus Accumbens (NAcc) activation when winning for the self and acceptance ratings. Confidence intervals are indicated by the shaded area. Those participants who were less accepted showed higher activation in the Nucleus Accumbens when winning for self.
Table 3.
Parameter estimates for all parameters in the multiple regression model for the acceptance scores. Stars indicate significance at p < .05 (*), p < .01 (**) and p < .001 (***).
| B | Std. Error | t | p | |
|---|---|---|---|---|
| Intercept | 2.36 | 1.97 | 1.20 | |
| NAcc self win | −0.33 | 0.13 | −2.63 | .013 |
| NAcc self lose | 0.10 | 0.09 | 1.08 | .283 |
| NAcc friend win | 0.00 | 0.08 | 0.06 | .955 |
| NAcc friend lose | 0.05 | 0.07 | 0.70 | .484 |
| Age | −0.14 | 0.13 | −1.04 | .328 |
| Sex | −0.64 | 0.33 | −1.93 | .068 |
4. Discussion
This study aimed to investigate the relation between neural processing of rewards in the NAcc and acceptance by peers. We related NAcc activity during an fMRI reward-processing paradigm to peer acceptance, which was measured by the difference between the number of received like and dislike peer nominations in the classroom. We distinguished between NAcc responses when receiving self-serving rewards and when receiving vicarious rewards. Our results showed that neural responses in the NAcc during processing of self-serving rewards were inversely related to peer acceptance. In other words, increased neural responsiveness to stimuli that involve benefits for the self was related to lower acceptance among peers. Contrary to expectations, we did not find associations between NAcc activation during vicarious wins and peer acceptance. This study shows that primary neural processing of rewards is related to real-life adolescent peer relationships.
4.1. Self-serving reward processing and peer acceptance
In the current study we found a negative correlation between NAcc responses to rewards for self and peer acceptance. The Nacc is a primary reward area (Schultz, 2000; Delgado, 2007) and NAcc activation is positively related to a drive to obtain rewards (Schreuders et al., 2018). Potentially, the higher NAcc responses during receipt of self-serving rewards and the subsequent higher drive to obtain rewards for oneself might not be appreciated within the peer group. This is in line with prior findings that relate peer rejection to selfish behaviors (Carlson et al., 1984) and with results from research stressing the importance of other-regarding acts in successful peer relationships (Cillessen and Rose, 2005; Meuwese et al., 2016; Peters et al., 2010; Poorthuis et al., 2012).
4.2. Vicarious reward processing and peer acceptance
In the current study we did not find evidence for a relationship between NAcc responses during vicarious reward processing and peer acceptance. This was surprising since prior research showed that NAcc activity when winning money for close others was related to prosocial tendencies (Telzer et al., 2013) and that prosocial behavior is related to increased peer acceptance (Layous et al., 2012). We therefore expected that NAcc sensitivity for vicarious rewards could serve as a driving mechanism behind the prosocial tendencies that increase the chances of being accepted by peers. In the current study we tested a relatively small sample of participants and this enabled us to only detect larger effects. It could be that the relationship between vicarious reward processing and peer acceptance is more complex than the relationship between self-serving reward processing and peer acceptance. Friendships are multi-faceted and are characterized not only by positive feelings, but also by feelings of competition (Hartup and Stevens, 1997). Vicarious reward processing might therefore be more complex than processing of self-serving rewards and therefore more power would be needed to detect a relationship between vicarious reward processing and peer acceptance.
Furthermore, we used NAcc activation in vicarious reward processing for best friend (on a dyadic level) and related this to being accepted with peers (group level). There is evidence for a positive relation between peer relationships at the dyadic and the group level. For example, adolescents with higher quality friendships are also more popular and well-liked in the peer group (Meuwese et al., 2016). However, there is also evidence that many children who are less liked and less accepted in the peer group have best friends and are highly satisfied with these relationships (Parker and Asher, 1993). Such variance across the links between friendships and peer status (e.g., being accepted by peers) is likely to weaken the possible link between NAcc activation during vicarious wins for best friends and being liked by peers.
4.3. Limitations and future directions
The current study employed an interdisciplinary approach through the connection of neuroimaging research and sociometric research in a developmental perspective. Measures based on peer nominations are able to capture essential adolescent real-life social dynamics and therefore contribute considerably to the ecological validity of research on social development in adolescence. Combining this with fMRI provides unique insight in the neural mechanism related to peer acceptance. However, there are also several limitations that should be noted.
The current study unveils a hint of “what lies beneath” peer acceptance, yet does not provide information about “what lies in-between”. In other words, we have not investigated here what variable(s) could explain the link between neural sensitivity in the NAcc and peer acceptance. Brain activation patterns in response to rewards and appreciation by peers are connected to one another possibly through observable behaviors; future studies should incorporate measures of social behavior in order to investigate this link further. For example, a direct measure of prosocial behavior can shed light on the mechanism behind our finding. It is important to note that, although prosocial behavior would be a candidate mediator in this link, prosociality contains a wide array of behaviors and therefore a future direction would also be to investigate what type of prosocial behaviors are most relevant in the adolescent social context.
In the current study we focused on NAcc activation during reward processing. Although, the NAcc is a primary reward area, it is not the only neural region associated with reward processing. It is likely that other neural regions also contribute to relevant behavior. The orbitofrontal cortex has been shown to be an integrative region for processing value and future work could investigate how value computations in the orbitofrontal cortex are related to peer status. Furthermore, a host of work has shown activation in a network of brain regions related to social cognition, such as the temporoparietal junction, precuneus and temporal poles. Neural activation in these regions has been shown to relate to perspective taking. As such, these regions might be related to social cognitive processes important for navigating the peer group.
Lastly, due to the correlational nature of our design no solid conclusions about the directionality of the effects should be drawn. It is, for example, possible that resources in the peer group are not shared with individuals low in acceptance and that cumulative experiences of not having access to resources in the peer context can shape NAcc-sensitivity to self-serving benefits. Future research using longitudinal designs should test whether adolescents with higher neural sensitivity act in ways that lead them to become less accepted over time or whether lower acceptance enhances neural sensitivity in the NAcc to self-serving rewards.
4.4. Conclusions
The current results show that adolescents who are accepted by their peers show less self-serving reward sensitivity compared to those adolescents who are less accepted by their peers. Given the link between neural activation to rewards and the drive to obtain rewards, our findings provide support for the idea that accepted peers are less likely to act in ways that signal selfish motivations to peers. This study highlights the importance of lower order neural reward-processing in social interactions with peers and provide a first insight into the role of adolescent neural reward sensitivity in peer relationships.
Conflict of Interest
None.
References
- Braams B.R., Crone E.A. Longitudinal changes in social brain development: processing outcomes for friend and self. Child Dev. 2016;88(6):1952–1965. doi: 10.1111/cdev.12665. [DOI] [PubMed] [Google Scholar]
- Braams B.R., Crone E.A. Peers and parents: a comparison between neural activation when winning for friends and mothers in adolescence. Soc. Cogn. Affect. Neurosci. 2016;12(3):417–426. doi: 10.1093/scan/nsw136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams B.R., Güroğlu B., de Water E., Meuwese R., Koolschijn P.C., Peper J.S., Crone E.A. Reward-related neural responses are dependent on the beneficiary. Soc. Cogn. Affect. Neurosci. 2013;9(7):1030–1037. doi: 10.1093/scan/nst077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams B.R., Peters S., Peper J.S., Güroğlu B., Crone E.A. Gambling for self, friends, and antagonists: differential contributions of affective and social brain regions on adolescent reward processing. Neuroimage. 2014;100:281–289. doi: 10.1016/j.neuroimage.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Braams B.R., van Duijvenvoorde A.C.K., Peper J.S., Crone E.A. Longitudinal changes in adolescent risk-taking: a comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. J. Neurosci. 2015;35(18):7226–7238. doi: 10.1523/JNEUROSCI.4764-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J.-L., Valabregue R., Poline J.-B. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage. 2002;16(2):S497. [Google Scholar]
- Brown B.B., Eicher Sa, Petrie S. The importance of peer group (“crowd”) affiliation in adolescence. J. Adolesc. 1986;9(1):73–96. doi: 10.1016/s0140-1971(86)80029-x. [DOI] [PubMed] [Google Scholar]
- Carlson C.L., Lahey B.B., Neeper R. Peer assessment of the social behavior of accepted rejected and neglected children. J. Abnorm. Child Psychol. 1984;12(2):187–198. doi: 10.1007/BF00910662. [DOI] [PubMed] [Google Scholar]
- Cillessen A.H., Rose A.J. Understanding popularity in the peer system. Curr. Dir. Psychol. Sci. 2005;14(2):102–105. [Google Scholar]
- Cillessen A.H., Schwartz D., Mayeux L. Guilford Press; 2011. Popularity in the Peer System. [Google Scholar]
- Coie J.D., Dodge K.A., Coppotelli H. Dimensions and types of social status: a cross-age perspective. Dev. Psychol. 1982;18(4):557. [Google Scholar]
- Dale A.M. Optimal experimental design for event-related fMRI. Hum. Brain Mapp. 1999;8(2–3):109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bruyn E.H., Cillessen A.H. Popularity in early adolescence: prosocial and antisocial subtypes. J. Adolesc. Res. 2006;21(6):607–627. [Google Scholar]
- Delgado M.R. Reward-related responses in the human striatum. Ann. N. Y. Acad. Sci. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Dodge K.A., Lansford J.E., Burks V.S., Bates J.E., Pettit G.S., Fontaine R., Price J.M. Peer rejection and social information-processing factors in the development of aggressive behavior problems in children. Child Dev. 2003;74:374–393. doi: 10.1111/1467-8624.7402004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumontheil I., Apperly I.A., Blakemore S.J. Online usage of theory of mind continues to develop in late adolescence. Dev. Sci. 2010;13(2):331–338. doi: 10.1111/j.1467-7687.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- Eisenberg N., Guthrie I.K., Fabes R.A., Reiser M., Murphy B., Holgren R., Losoya S. The relations of regualation and emotionality to resiliency and competent social functioning in elementary school children. Child Dev. 1997;68:295–311. [PubMed] [Google Scholar]
- Fink E., Begeer S., Hunt C., Rosnay M. False-Belief understanding and social preference over the first 2 years of school: a longitudinal study. Child Dev. 2014;85:2389–2403. doi: 10.1111/cdev.12302. [DOI] [PubMed] [Google Scholar]
- Güroğlu B., van den Bos W., Crone E.A. Neural correlates of social decision making and relationships. Ann. N. Y. Acad. Sci. 2009;1167(1):197–206. doi: 10.1111/j.1749-6632.2009.04502.x. [DOI] [PubMed] [Google Scholar]
- Hartup W.W., Stevens N. Friendships and adaptation in the life course. Psychol. Bull. 1997;121(3):355–370. [Google Scholar]
- Knoll L.J., Magis-Weinberg L., Speekenbrink M., Blakemore S.J. Social influence on risk perception during adolescence. Psychol. Sci. 2015;26(5):583–592. doi: 10.1177/0956797615569578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson R.W., Brown B.B., Mortimer J.T. Blackwell; London, UK: 2002. Adolescents’ Preparation for the Future: Perils and Promise–a Report of the Study Group on Adolescence in the Twenty-first Century. [Google Scholar]
- Layous K., Nelson S.K., Oberle E., Schonert-Reichl K.A., Lyubomirsky S. Kindness counts: prompting prosocial behavior in preadolescents boosts peer acceptance and well-being. PLoS One. 2012;7(12):e51380. doi: 10.1371/journal.pone.0051380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malti T., Gummerum M., Keller M., Chaparro M.P., Buchmann M. Early sympathy and social acceptance predict the development of sharing in children. PLoS One. 2012;7(12):e52017. doi: 10.1371/journal.pone.0052017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuwese R., Cillessen A.H., Güroğlu B. Friends in high places: a dyadic perspective on peer status as predictor of friendship quality and the mediating role of empathy and prosocial behavior. Soc. Dev. 2016;26(3):503–519. [Google Scholar]
- Overgaauw S., Güroğlu B., Crone E.A. Fairness considerations when I know more than you do: developmental comparisons. Front. Psychol. 2012;3 doi: 10.3389/fpsyg.2012.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J.G., Asher S.R. Friendship and friendship quality in middle childhood: links with peer group acceptance and feelings of loneliness and social dissatisfaction. Dev. Psychol. 1993;29(4):611. [Google Scholar]
- Peters E., Cillessen A.H., Riksen-Walraven J.M., Haselager G.J. Best friends’ preference and popularity: associations with aggression and prosocial behavior. Int. J. Behav. Dev. 2010;34(5):398–405. [Google Scholar]
- Picci G., Scherf K.S. From caregivers to peers: puberty shapes human face perception. Psychol. Sci. 2016;27(11):1461–1473. doi: 10.1177/0956797616663142. [DOI] [PubMed] [Google Scholar]
- Poorthuis A.M., Thomaes S., Denissen J.J., van Aken M.A., de Castro B.O. Prosocial tendencies predict friendship quality, but not for popular children. J. Exp. Child Psychol. 2012;112(4):378–388. doi: 10.1016/j.jecp.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Schreuders E., Braams B.R., Blankenstein N.E., Peper J.S., Güroğlu B., Crone E.A. Contributions of reward sensitivity to ventral striatum activity across adolescence and early adulthood. Child Dev. 2018 doi: 10.1111/cdev.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nat. Rev. Neurosci. 2000;1(3):199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Silverman M.H., Jedd K., Luciana M. Neural networks involved in adolescent reward processing: an activation likelihood estimation meta-analysis of functional neuroimaging studies. Neuroimage. 2015;122:427–439. doi: 10.1016/j.neuroimage.2015.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Galván A. Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Dev. Cogn. Neurosci. 2013;3:45–52. doi: 10.1016/j.dcn.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valkenburg P.M., Peter J., Schouten A.P. Friend networking sites and their relationship to adolescents’ well-being and social self-esteem. Cyberpsychol. Behav. 2006;9(5):584–590. doi: 10.1089/cpb.2006.9.584. [DOI] [PubMed] [Google Scholar]
- Van den Bos W., van Dijk E., Westenberg M., Rombouts S.A., Crone E.A. Changing brains, changing perspectives: the neurocognitive development of reciprocity. Psychol. Sci. 2011;22(1):60–70. doi: 10.1177/0956797610391102. [DOI] [PubMed] [Google Scholar]
- Van Hoorn J., Van Dijk E., Güroğlu B., Crone E.A. Neural correlates of prosocial peer influence on public goods game donations during adolescence. Soc. Cogn. Affect. Neurosci. 2016;11(6):923–933. doi: 10.1093/scan/nsw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentzel K.R. Peer relationships, motivation, and academic performance at school. In: Elliot A., Dweck C., editors. Handbook of Competence and Motivation. Guilford; New York: 2005. pp. 279–296. [Google Scholar]
- Wolters N., Knoors H., Cillessen A.H.N., Verhoeven L. Behavioral, personality, and communicative predictors of acceptance and popularity in early adolescence. J. Early Adolesc. 2013;34(5):585–605. [Google Scholar]