Abstract
Background
Atrial fibrillation (AF) is frequently associated with enhanced inflammatory response. The “NACHT, LRR and PYD domain containing protein 3” (NLRP3)-inflammasome mediates caspase-1 activation and interleukin-1β release in immune cells, but is not known to play a role in cardiomyocytes (CMs). Here, we assessed the role of CM NLRP3-inflammasome in AF.
Methods
NLRP3-inflammasome activation was assessed by immunoblot in atrial whole-tissue lysates and CMs from patients with paroxysmal (pAF) or long-standing persistent (chronic) AF (cAF). To determine whether CM-specific activation of NLPR3 is sufficient to promote AF, a CM-specific knock-in mouse model expressing constitutively active NLRP3 (CM-KI) was established. In vivo electrophysiology was used to assess atrial arrhythmia vulnerability. To evaluate the mechanism of AF, electrical activation pattern, Ca2+ spark frequency (CaSF), atrial effective refractory period (AERP), and morphology of atria were evaluated in CM-KI mice and WT littermates.
Results
NLRP3-inflammasome activity was increased in atrial CMs of pAF and cAF patients. CM-KI mice developed spontaneous premature atrial contractions and inducible AF, which was attenuated by a specific NLRP3-inflammasome inhibitor, MCC950. CM-KI mice exhibited ectopic activity, abnormal sarcoplasmic-reticulum Ca2+-release, AERP shortening and atrial hypertrophy. Adeno-associated virus subtype-9 mediated CM-specific knockdown of Nlrp3 suppressed AF development in CM-KI mice. Finally, genetic inhibition of Nlrp3 prevented AF development in CREM transgenic mice, a well-characterized mouse model of spontaneous AF.
Conclusions
Our study establishes a novel pathophysiological role for CM NLRP3-inflammasome signaling with a mechanistic link to the pathogenesis of AF, and establishes inhibition of NLRP3 as a potential novel AF-therapy approach.
Keywords: NLRP3 inflammasome, atrial fibrillation, electrical remodeling, AAV9
INTRODUCTION
Atrial fibrillation (AF) is the most common cardiac arrhythmia, with an increasing prevalence worldwide.1 An enhanced inflammatory response is frequently observed in AF patients.2–6 Increased circulating levels of pro-inflammatory cytokines interleukin (IL)-1β, IL-18, and tumor-necrosis-factor α (TNFα) correlate with AF progression in patients.3, 7–9 Moreover, the level of inflammatory cytokines can predict adverse outcomes following AF ablation.10–12 However, the precise role of inflammatory signaling in AF pathogenesis remains elusive.
The “NACHT, LRR and PYD domain containing protein 3” (NLRP3)-inflammasome is a key inflammatory signaling complex that regulates innate immunity.13, 14 Activation of the NLRP3-inflammasome requires two processes: 1) a priming event that induces transcription of NLRP3 and precursor caspase-1 (pro-Casp1) via toll-like receptor (TLR)-NFκB-signaling, and 2) a subsequent triggering mechanism that promotes assembly of NLRP3, adaptor-protein “apoptosis-associated speck-like protein containing a CARD” (ASC) and pro-Casp1, leading to auto-cleavage of pro-Casp1 into active Casp1 heterodimers comprised of p20 and p10 subunits.14, 15 In immune cells, active Casp1 cleaves pro-IL-1β and pro-IL-18, releasing the active products IL-1β and IL-18.16, 17 Although several studies have suggested a role for NLRP3-inflammasome activation in the development of cardiomyopathy,18–20 the function of NLRP3 in cardiomyocytes (CMs) remains largely unknown. In addition, whether the NLRP3-inflammasome affects cardiac electrophysiological function has not been established. Here, we sought to determine whether CM NLRP3-inflammasome activation is involved in the pathogenesis of AF.
METHODS
Expanded methods are available in Supplementary Material. The data, methods, and study materials will be made available to other researchers upon request.
Human atrial samples
Right atrial appendages of patients undergoing open-heart surgery for coronary bypass grafting and/or valve replacement were collected with patients’ written informed consent. All experimental protocols were approved by the institutional review committee of the Medical Faculty Mannheim, University Heidelberg (No. 2011-216N-MA) and Carl Gustav Carus Medical Faculty, Dresden University of Technology (No. EK790799) performed in accordance with the Declaration of Helsinki. After excision, atrial appendages were snap-frozen in liquid-nitrogen for biochemical studies. Patient demographics and characteristics are listed in Supplementary Tables S1 and S2.
Preparation of cardiomyocyte (CM)-enriched cell pellets from human atrial biopsies
Bovine serum albumin (BSA)-gradient filtration was performed to obtain CM-enriched cell pellets as previously described.21, 22 Briefly, after collagenase/protease enzymatic dissociation of right-atrial tissue samples and centrifugation, cells were re-suspended at room temperature in Ca2+-free Tyrode’s solution containing 0.1% BSA. Cell suspensions were layered over a 6% BSA gradient (Ca2+-free Tyrode’s solution), and cells were allowed to sediment for 45-min. Cell debris and undigested tissue fragments were largely located in the upper part of the 6% BSA fraction, whereas non-cardiomyocytes (mostly fibroblasts), were mainly retained at the phase boundary between 0.1% and 6% BSA. Atrial CMs were enriched at the bottom of the gradient as verified by light-microscopy and were used for subsequent biochemistry experiments.
Dog atrial samples and CMs
Mongrel dogs (20–36 kg) were divided into control and atrial-tachycardia-pacing (ATP, 400 bpm for 1-week) groups. Animal care procedures followed National Institutes of Health (NIH) guidelines and were approved by the Animal Research Ethics Committee of the Montreal Heart Institute. The animal model was prepared as previously described in detail.23 Hearts were excised via a left thoracotomy and immersed in oxygenated Tyrode solution containing (in mmol/L): 136 NaCl, 5.4 KCl, 2 CaCl2, 1 MgCl2, 0.33 NaH2PO4, 10 glucose, and 5 HEPES (pH 7.4). Right atrial CMs were isolated as previously described in detail.24 Whole-tissue samples and CM aliquots from the right atria were snap-frozen for biochemical studies.
Animal studies
All studies involving mice were performed according to protocols approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine, and conformed to the Guide for the Care and Use of Laboratory Animals published by NIH. NLRP3 homozygous knockout mice (Nlrp3−/−)25 and CREM-IbΔC-X TG (CREM-TG) mice26–28 were established previously. CREM-TG mice were crossbred to Nlrp3−/− and the offspring maintained expected Mendelian ratios. WT, CREM-TG, CREM-TG:Nlrp3−/−, and Nlrp3−/− from the mixed genetic background were used in the current study. To yield mice with cardiomyocyte-specific expression of constitutively active NLRP3, Myh6Cre mice were crossbred to Nlrp3A350VneoR mice25 (Myh6Cre:Nlrp3A350V/+). The offspring of this cross also maintained expected Mendelian ratios. Since there are no differences between wildtype (WT) and Myh6Cre mice in baseline ECG parameters, AF-inducibility, ventricular morphology, and overall cardiac function at 3 months, WT and Myh6Cre mice were combined and used as a control (Ctl) group for the age-matched Myh6Cre:Nlrp3A350V/+ mice.
Telemetry studies
Telemetry ECG recordings were acquired to evaluate the occurrence of spontaneous arrhythmias in conscious and ambulatory mice. ECG radio telemeters (Data Sciences International, MN, USA) were implanted intraperitoneally, as described.27, 29 Telemetry analyses were performed by an independent person blinded to mouse genotypes. Premature-atrial-contraction (PAC) was defined by the occurrence of a premature P-wave with an abnormal morphology, followed by a conducted QRS complex. The number of PAC-events during 2 hours of recording between 6 and 8 pm was counted.
Programmed intracardiac stimulation
Programmed intracardiac stimulation was performed to assess AF-inducibility as described.27, 30, 31 Briefly, AF was induced by an overdrive pacing protocol, starting with 2-second burst pacing at a cycle length (CL) of 40-ms and decreasing in each successive burst by a 2-ms decrement to a CL of 10-ms. AF was defined as the occurrence of rapid, fragmented atrial electrograms with irregular R-R intervals lasting at least 1-second. To determine whether AF-inducibility was reproducible, mice were subjected to the same atrial burst-pacing protocols for 3 times, and only the mice exhibited 2 or 3 times of AF evoked by burst-pacing was considered as AF-positive. The incidence of inducible AF was calculated as the percentage of the AF-positive mice divided by the total number of mice studied. The experimenter was blinded to genotype or viral gene-transfer status of mice.
Optical mapping
Anesthetized mice were injected with 100 units of heparin intraperitoneally. After cervical dislocation, hearts were removed and washed in oxygenated (95% O2/5% CO2), cold Tyrode’s solution containing 1.3 mmol/L CaCl2. Then, hearts were cannulated via the aorta, retrograde-perfused and superfused with Tyrode’s solution (2–5 mL/min) and the aortic pressure was kept between 80- and 120-mm Hg. Right atrial epicardial pacing was achieved with a PowerLab 26T stimulator (AD Instruments, Australia). Hearts were loaded with blebbistatin (Sigma-Aldrich, 0.5 μmol/L) to eliminate motion artifact and stained with voltage-sensitive dye RH237 (Invitrogen, 0.1 μmol/L). An LED light was used with an excitation wavelength of 530-nm. The emitted fluorescence Vm-signal was long-passed (>700 nm) and acquired via MiCAM CMOS camera (SciMedia, USA). The surface ECG was recorded continuously. Atrial-effective-refractory-period (AERP) was assessed with S1–S2-pacing at a CL of 100-ms.
Whole-cell patch-clamp
Kv1.5-current was assessed with a Port-a-Patch (Nanion Technologies, USA) connected to an EPC10-USB amplifier (HEKA Instruments, Germany) as previously described.32 Borosilicate glass microelectrodes had tip resistances of 2- to 3.5-MΩ. Once gigaseal resistances were achieved, series resistance and cell-capacitance were compensated. Currents were elicited with 200-ms pulses from a holding potential of −80 mV to 40 mV, applied at intervals of 30-s. Myocytes were superfused with buffer containing (mmol/L): 160 NaCl, 4.5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES, pH 7.4, 318 mOsm/L. A Ca2+-free pipette solution was used containing (mmol/L): 145 KF, 10 HEPES, 10 EGTA, 2 MgCl2, pH 7.2, 308 mOsm/L. Kv1.5-current (IKur) was isolated as the current inhibited by the selective Kv1.5-channel inhibitor DPO-1,33 applied via a rapid solution-exchange system. IKur was defined as DPO-1-sensitive decrease in total steady-state peak current expressed as density (pA/pF).
Adeno-associated virus (AAV) production and delivery
The pAAV-stop-GFP vector34 was a gift from Dr. Matthias Klugmann (University of New South Wales, Australia). The mCherry-tagged shNlrp3 targeting the sequence of ggttctactctatcaaggaca at site 654 of mus musculus Nlrp3 (NM_145827.3) or scrambled sequence were obtained from GeneCopoeia (MD, USA), and were cloned into the Mlu I site of the pAAV-stop-GFP vector to replace the GFP sequence. The adenoviral helper plasmid pAdDeltaF6 (PL-F-PVADF6) and the AAV9 packaging vector pAAV2/9 (PL-T-P0008-R2) were obtained from Puresyn Inc. AAV9 were packaged in HEK293T cells by the triple transfection method and purified by CsCl density gradient centrifugation as described.35, 36 Two-month old CM-KI mice were injected with one dose of AAV9-scramble or AAV9-shNlrp3 virus (5x1011 GC/mouse) via retro-orbital route.37
Statistical Analysis
For numerical data which were presented as mean ± SEM, two-tailed Student’s t-test was used for data comparison of two groups with normal distribution; one-way ANOVA and Holm-Sidak’s test were used for data comparison of multiple groups with normal distribution; Mann-Whitney and Kruskal-Wallis test followed by Dunn’s test were used to compare groups when the data were not normally distributed. Fisher’s exact or Chi-squared tests were used to compare categorical data. P-value of less than 0.05 was considered statistically significant.
RESULTS
Enhanced activity of NLRP3-inflammasomes in AF patients
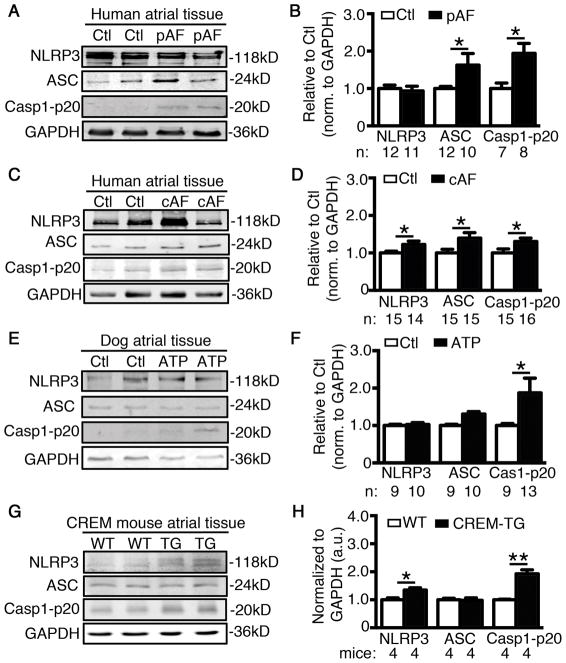
To determine whether the activation of NLRP3-inflammasomes is altered in AF patients, we assessed the protein levels of NLRP3, ASC, and active Casp1-p20 in atrial whole-tissue lysates from patients with a history of paroxysmal (pAF) or long-standing persistent (chronic) AF (cAF). While protein levels of NLRP3 remained unchanged, levels of active Casp1-p20 were significantly increased, indicating increased NLRP3-inflammasome activity, in atria of pAF-patients compared to sinus-rhythm controls with no history of AF (Ctl) (P<0.05, Figure 1A–B, patient characteristics in Supplementary Table S1). Total and phosphorylated NFκB (pNFκB) were unchanged in pAF patients, suggesting that assembly (“triggering”) is the major mechanism for NLRP3-inflammasome activation (Supplementary Figure S1A–B). In contrast, protein levels of NLRP3, ASC, and Casp1-p20 were all increased in patients with cAF compared to Ctl (Figure 1C–D), indicating that besides “triggering”, “priming” of NLRP3-inflammasomes accompanies AF-progression to persistent forms, as evidenced by increased expression and phosphorylation of NFκB in cAF patients (Supplementary Figure S1C–D). In addition, dogs subjected to AF-mimicking ATP38 also showed increased expression of active Casp1-p20, without changes in NLRP3 or ASC (Figure 1E–F). Similarly, CREM-TG mice that develop spontaneous AF and recapitulate the progressive nature of AF seen in humans,26, 27 also showed increased atrial levels of NLRP3, ASC, and Casp1-p20 at the age of 2-months, along with atrial ectopic events, prior to the onset of spontaneous AF (Figure 1G–H). Upregulation of the NLRP3-inflammasome components in CREM-TG mice was associated with an enhanced activation of TLR4-NFκB pathways (Supplementary Figure S1E–F) and an increased nuclear localization of pNFκB (Supplementary Figure S1G–H).
Figure 1. Enhanced activation of NLRP3-inflammatory signaling pathway in AF patients, dogs subjected to atrial tachycardia pacing (ATP), and CREM-TG mice with spontaneous AF.
Representative Western blots (WBs) and quantification of NLRP3, ASC, and Casp1-p20 in atrial tissue of patients with paroxysmal AF (pAF, A–B) and chronic AF (cAF, C–D). Representative WBs (E) and quantification (F) of NLRP3, ASC, and p20 in atrial tissue of ATP and control (Ctl) dogs. Representative WBs (G) and quantification (H) of NLRP3, ASC, and Casp1-p20 in atrial tissues of CREM-TG mice and WT littermates. *P<0.05, **P<0.01.
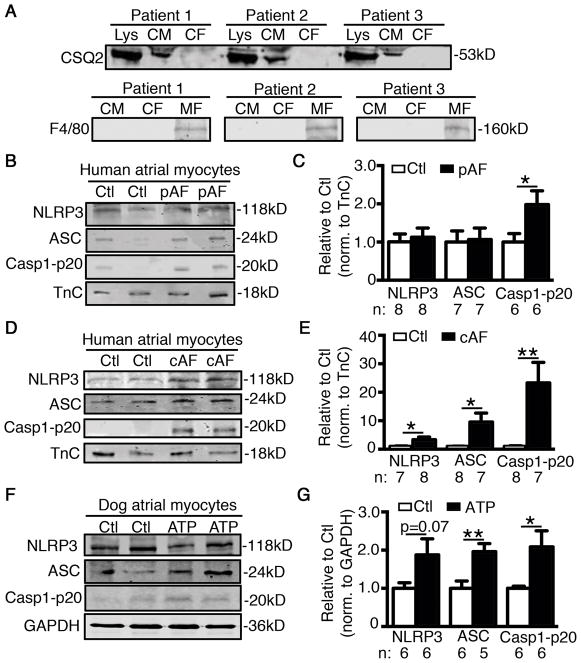
Since NLRP3-inflammasomes play an important role in innate immune cells, we assessed whether enhanced macrophage infiltration might contribute to AF development in patients. The protein levels of CD68 - a macrophage marker - were unchanged in pAF patients, but increased in cAF (Supplementary Figure S2A). Similarly, CD68 levels were unchanged in ATP dogs (Supplementary Figure S2B). These data suggest that the initial activation of NLRP3-inflammasomes in the atria of pAF patients is not due to macrophage activation. In a previously-established murine model of macrophage-specific deletion of autophagy related 7 (LysMCre+;Atg7f/f),39 increased macrophage NLRP3-inflammasome activity39 did not increase AF-inducibility (Supplementary Figure S2C). These results suggest that macrophage NLRP3 activation is insufficient to cause AF and suggest that macrophage NLRP3 is unlikely to account for clinical AF, pointing to a role for NLRP3 in other cell types. To further determine whether the activity of the NLRP3-inflammasome increases in CMs of AF patients, we separated atrial CMs from other atrial cell-types, mostly cardiac fibroblasts (CFs) (Figure 2A, top). Moreover, these CMs and CFs preparations were negative for the highly-specific macrophage/eosinophilic granulocyte marker F4/8040, 41 (Figure 2A, bottom), suggesting that the levels of macrophages/eosinophilic granulocytes in the human atrium (as a source of NLRP3-inflammasome) are rather low and lie below the detection limit of Western blotting. Consistent with the findings in atrial whole-tissue lysates, the activity of NLRP3-inflammasome was increased (reflected by the increased level of active Casp1-p20) in the atrial CMs of pAF and cAF patients compared to Ctl (Figure 2B–E), as well as in the atrial CMs of ATP compared to Ctl dogs42 (Figure 2F–G). Overall, these results indicate that increases in NLRP3-inflammasome in CMs might contribute to the evolution of atrial remodeling that promotes AF induction and maintenance.
Figure 2. Enhanced activation of NLRP3-inflammatory signaling pathway in cardiomyocytes (CMs) of AF patients.
(A) Validation of atrial CM-enriched cell pellets isolated from human atrial biopsies by detecting a CM marker - calsequestrin (CSQ) - using Western blotting (top). Tissue lysate (Lys) was used as positive control and cardiac fibroblasts (CFs) as negative control. Validation of absence of immune cells including macrophages (MFs) in human CMs and CFs preparations by detecting the specific MF/eosinophilic granulocyte marker F4/80 (bottom). Differentiated bone marrow-derived mouse MFs served as a positive control. (B–E) Representative WBs and quantification of NLRP3, ASC, and Casp1-p20 in atrial CMs of patients with paroxysmal AF (pAF, B–C) or chronic AF (cAF, D–E). Representative WBs and quantification of NLRP3, ASC, and Casp1-p20 in atrial CMs of ATP dogs (F–G). *P<0.05, **P<0.01.
CM-specific activation of NLRP3 enhances AF-susceptibility
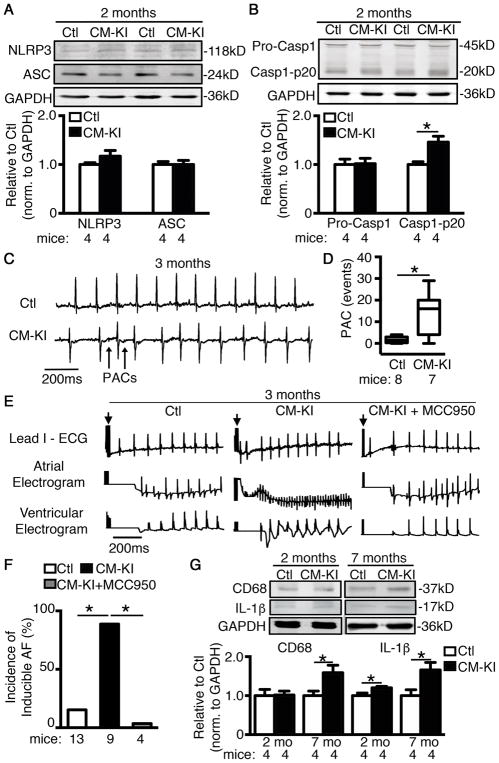
To test whether CM-restricted activation of NLPR3 is sufficient to promote AF, we developed CM-specific knockin mice expressing a gain-of-function NLRP3 (Myh6Cre;Nlrp3A350V/+, CM-KI) by crossbreeding Nlrp3neoR-A350V with CM-specific Cre mice (Myh6Cre) (Supplementary Figure S3).25, 43 qPCR and immunocytochemistry confirmed expression of NLRP3 in atrial CMs of CM-KI mice (Supplementary Figure S3). The A350V mutation in NLRP3 facilitates interdomain interactions, leading to constitutive activation of NLRP3-inflammasomes.25 Similar to pAF patients, 2-month old CM-KI mouse atria showed unchanged protein expression of NLRP3 and ASC compared to controls (Ctls, including WT and Myh6Cre mice; Figure 3A), whereas Casp1-p20 was significantly upregulated (P<0.05, Figure 3B), confirming constitutive activation of NLRP3-inflammasomes in CM-KI mouse CMs.
Figure 3. Constitutive activation of NLRP3 in cardiomyocytes (CMs) predisposes mice to atrial arrhythmias.
(A–B) Representative WBs and quantification of NLRP3, ASC, and Caspase-1 in atrial tissue of CM-KI and Ctl mice. (C) Representative telemetry ECG recording demonstrated sinus rhythm in Ctl mice and premature atrial contractions (PACs, indicated by arrows) in CM-KI mice. (D) Box plot summarizing the average events of PACs with whiskers indicating the minimum and maximum values in Ctl and CM-KI mice. (E) Representative simultaneous recordings of surface ECG (lead 1) and intracardiac electrograms in CM-KI and Ctl mice following programmed intracardiac stimulation (indicated by arrows). (F) Incidence of pacing-induced AF in Ctl mice (combining WT and Myh6Cre) and CM-KI mice. (G) Representative WBs and quantification of CD68 (macrophage marker) and IL-1β in atrial tissue of CM-KI and Ctl mice. *P<0.05.
To determine whether CM-restricted activation of NLRP3-inflammasomes promotes atrial arrhythmias, we performed ECG telemetry recordings. At the age of 3 months, baseline ECG parameters (RR, HR, PR, QRS, QTc), sinus-node-recovery-time (SNRT), atrioventricular-node-effective-refractory-period (AVNERP), and ventricular-effective-refractory-period (VERP) were unaltered in CM-KI mice (Supplementary Table S3 and Figure S4), suggesting that conduction system and ventricular electrophysiology were not affected in CM-KI mice. Further analysis of 2-hour recordings between 6pm and 8pm showed that PAC-events were significantly more frequent in CM-KI (13.3±3.9 events, n=8) versus Ctl mice (1.7±0.5 events, n=7, P<0.05, Figure 3C–D). These results indicate that CM-KI mice exhibit atrial ectopic activity that might contribute to AF-initiation. To verify whether an AF-maintaining substrate also developed, we performed programmed electrical stimulation as previously described.27, 30, 31 The incidence of reproducible pacing-induced AF was significantly higher in CM-KI (88.9%, n=9, P<0.05) than in Ctl mice (15.4%, n=13, Figure 3E–F). These findings suggest that CM-KI mice likely develop a substrate for AF. Echocardiography revealed that left ventricular ejection fraction was mildly reduced in 3-month old CM-KI mice (Supplementary Table S4 & Figure S5) suggesting that the enhanced susceptibility to AF in CM-KI mice is not caused by clinically relevant ventricular dysfunction. Importantly, a newly-developed selective inflammasome-inhibitor MCC950 (10mg/kg i.p. daily for 10-days) that interrupts assembly of the NLRP3-inflammasome complex44 prevented AF-inducibility in CM-KI mice (0%, n=4, P<0.05 vs CM-KI, Figure 3F), validating the notion that enhanced NLRP3-inflammatory signaling is sufficient and necessary for AF-susceptibility in CM-KI mice. Similar to AF-patient results, protein levels of CD68 were unchanged in 2-month old CM-KI mice (Figure 3G), arguing against significant contributions of macrophage activation to AF promotion in 2-month old CM-KI mice. Despite unchanged CD68 abundance, levels of IL-1β protein were increased in CM-KI mice as early as 2 months of age, and further increased at 7 months (Figure 3G).
CM-specific activation of NLRP3 promotes abnormal sarcoplasmic reticulum (SR) Ca2+ release and electrical remodeling
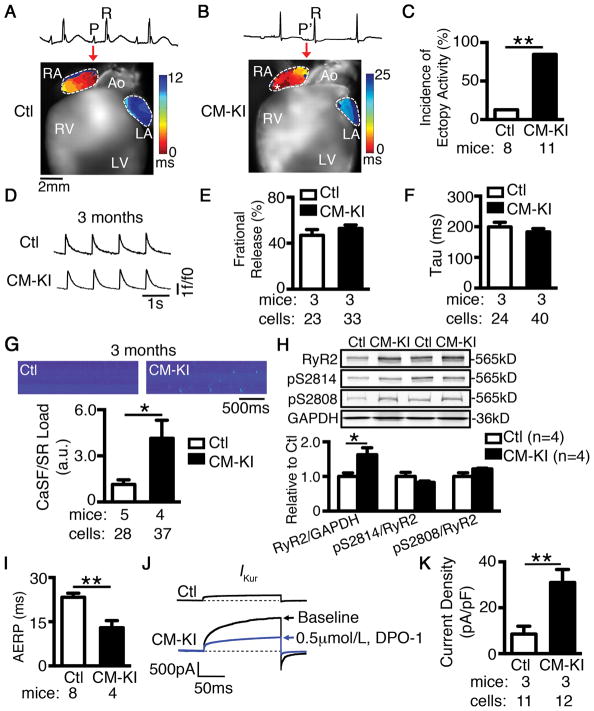
To study the consequences of CM NLRP3-inflammasome activation for atrial electrophysiology, we assessed the electrical activation pattern in CM-KI mice atria by optical mapping. We found that CM-KI mice manifested ectopic activity that can initiate PACs more frequently than Ctl mice (85% vs 12%, P<0.05; Figure 4A–C). Emerging evidence shows that abnormal SR Ca2+ release frequently underlies ectopic activity.30, 45–47 We therefore assessed intracellular Ca2+ homeostasis in atrial CMs of CM-KI mice using confocal imaging. While fractional Ca2+ release and decay of Ca2+-transients (Figure 4D–F) were similar in both groups, suggesting that systolic Ca2+ release was not altered in CM-KI mice, the frequency of spontaneous SR Ca2+-release events (Ca2+ spark frequency, CaSF) was significantly increased in atrial CMs from CM-KI mice (Figure 4G). These data indicate that the function of ryanodine receptor type-2 (RyR2) clusters is augmented in CM-KI mice, potentially because of increased RyR2 phosphorylation or increased total RyR2 protein expression29, 48. Western blotting showed that the relative levels of phosphorylated RyR2-pS2808 (PKA-phosphorylation site) and RyR2-pS2814 (CaMKII-phosphorylation site) to total RyR2 were unchanged in CM-KI mice (Figure 4H), suggesting that PKA and CaMKII mediated post translational modification was not altered in CM-KI mice. In contrast, total RyR2 protein levels were upregulated by ~60% in CM-KI atria compared to Ctl (P<0.05, Figure 4H), similar to results in pAF patients.30 Increased RyR2 expression was atrial-specific in CM-KI mice, since both the protein and phosphorylation levels of RyR2 were similar in ventricles of Ctl and CM-KI mice (Supplementary Figure S6). Moreover, increased RyR2 protein in atria of CM-KI mice was associated with enhanced Ryr2 mRNA expression in CM-KI mice (P<0.01 vs Ctl) (Supplementary Figure S7A), which could not be attributed to miR-106b-25 cluster mediated post-transcriptional regulation of RyR2 as described previously29 (Supplementary Figure S7B).
Figure 4. Constitutive activation of NLRP3 in cardiomyocytes (CMs) promotes electrical remodeling associated with AF development.
(A–B) Representative activation maps revealed an early ectopic activation (indicated by asterisk) corresponding to premature atrial contraction (P′-wave on ECG, B) in the right atrium of a CM-KI mouse, at a location different from sinus rhythm origin corresponding to the normal P-wave in a Ctl mouse (A). (C) Incidence of ectopic activity. (D) Representative recordings of Ca2+ transients. (E–F) Quantification of fractional release and decay (time constant, Tau) in atrial CMs of Ctl and CM-KI mice. (G) Representative line-scan confocal images and the quantification of Ca2+ sparks (CaSF) normalized to SR Ca2+ load in CMs of Ctl and CM-KI mice. (H) Representative WBs and quantification of total whole-tissue RyR2 and the phosphorylated RyR2-pS2808 or RyR2-pS2814 relative to RyR2. (I) Quantification of AERP in Ctl and CM-KI mice. (J) Representative recordings of IKur in atrial CMs of Ctl and CM-KI mice (in the presence and absence of the selective IKur inhibitor DPO-1, 0.5 μmol/L), respectively. (K) Quantification of IKur density. *P<0.05, **P<0.01.
In addition to ectopic activity, CM-KI mice also had abbreviated AERP (Figure 4I), which provides a validated substrate for AF-promoting reentry.49, 50 To examine how abnormal NLRP3-inflammasome activation in CMs leads to electrical remodeling, we evaluated the expression of genes encoding IKur, acetylcholine-activated K+ current (IK,ACh), and L-type Ca2+ current (ICa,L), which are known to contribute to electrical remodeling in AF.51–53 qPCR analysis showed that CM-KI mice exhibited a pronounced increase in the mRNA level of Kcn5a (encoding the Kv1.5 subunit that carries IKur) (P<0.05 vs Ctl, Supplementary Figure S7A), and a moderate increase in the expression of Girk1 and Girk4 (encoding GIRK1 and GIRK4, which underlie the G-protein-activated K+ current, IK,ACh; P<0.01, P<0.05 vs Ctl respectively, Supplementary Figure S7A). The expression of Cacna1c mRNA (encoding the L-type Ca2+ channel α-subunit) was unaltered in CM-KI mice. Because Kcna5 mRNA exhibited the largest upregulation relative to other genes studied, we studied the corresponding current in atrial cardiomyocytes of CM-KI mice. Whole-cell voltage-clamp recordings showed a strong increase in DPO-1-sensitive IKur in CM-KI mice (31.01±5.72 pA/pF, n=12/3 myocytes/mice) versus Ctl (8.54±3.48 pA/pF, n=11/3, P<0.01, Figure 4J–K). These results provide a potential molecular mechanism underlying the abbreviation of AERP (electrical remodeling) caused by constitutive activation of NLRP3 in this model.
CM-specific activation of NLRP3 promotes structural remodeling
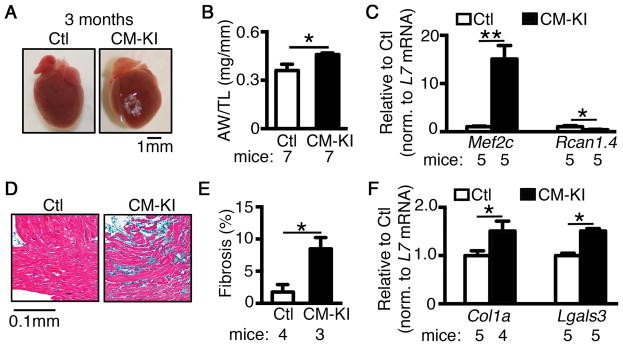
To elucidate whether CM NLRP3-inflammasome activation also promotes structural remodeling, we assessed atrial morphology. CM-KI mice exhibited larger atria, along with an increased ratio of atrial weight to tibia length (AW/TL, P<0.05 vs Ctl, Figure 5A–B), while the ratio of ventricular weight to tibia length (VW/TL) was unchanged (P=0.153, Supplementary Figure S8), suggesting that atrial hypertrophy may be a primary consequence of increased activity of atrial NLRP3-inflammasome. Atrial hypertrophy in CM-KI mice was associated with enhanced CaMKII-mediated hypertrophic signaling, since Mef2c (activated by CaMKII signaling,54 Figure 5C) was upregulated in CM-KI mice. In contrast, Rcan1, a prohypertrophic regulator of calcineurin-NFAT signaling,55 was downregulated (Figure 5C). Masson trichrome staining demonstrated atrial fibrosis in CM-KI mice (Figure 5D–E). The mRNA levels of the fibrosis markers Col1a (encoding collagen 1a) and Lgals (encoding galectin 3) were increased in CM-KI mice (Figure 5F), suggesting CF activation.56–58
Figure 5. Constitutive activation of NLRP3 in cardiomyocytes (CMs) promotes structural remodeling associated with AF development.
(A) Whole mount images of hearts and (B) quantification of atrial weight to tibia length (AW/TL) ratio in Ctl and CM-KI mice. (C) qPCR revealed increased levels of hypertrophic marker Mef2c, but not Rcan1 in CM-KI mice compared to Ctl mice. (D) Masson’s trichrome staining and (E) quantification of atrial fibrosis (blue) in CM-KI and Ctl mice. (F) qPCR revealed elevated levels of Col1a (encoding collagen-1a) and Lgals3 (encoding galectin-3) mRNA in the atria of CM-KI compared to Ctl mice. *P<0.05, **P<0.01.
CM-specific inhibition of NLRP3 reduces AF-inducibility
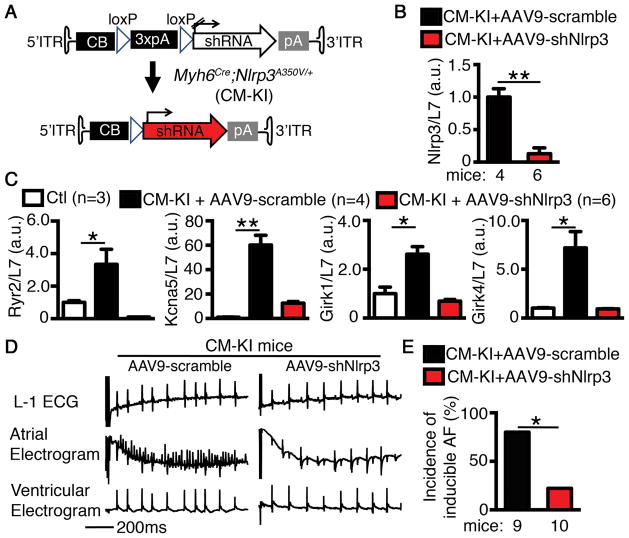
To further evaluate the role of NLRP3 in AF-development, we developed an adeno-associated virus 9 (AAV9) to knock down Nlrp3 specifically in CM-KI-mouse CMs (Figure 6A). We injected 2-month old CM-KI mice with either AAV9-scramble or AAV9-shNlrp3 (5x1011 GC/mouse) virus. Four weeks later, qPCR confirmed that Nlrp3 mRNA was reduced by 87% in atria of CM-KI mice treated with AAV9-shNlrp3 (P<0.01 vs AAV9-scramble treated CM-KI, Figure 6B). CM-specific knockdown of Nlrp3 restored the levels of Ryr2, Kcna5, Girk1, and Girk4 mRNAs (upregulated in CM-KI mice) towards Ctl values (Figure 6C). AAV9-shNlrp3 reduced the incidence of inducible AF in CM-KI mice (20.0% vs 77.8% in AAV9-scramble treated CM-KI mice, p<0.05. Figure 6D–E).
Figure 6. Cardiomyocyte (CM)-specific knockdown of Nlrp3 reduces AF-inducibility.
(A) Design of AAV9 vector to achieve the CM-specific shRNA-mediated Nlrp3 knockdown after injecting CM-KI mice. (B) qPCR confirmed the Nlrp3 knockdown efficacy by AAV9-shNlrp3 in CM-KI atria. (C) qPCR demonstrated that knockdown of Nlrp3 by AAV9-shNlrp3 reduced the levels of Ryr2, Kcna5, Girk1, and Girk4 mRNA in CM-KI mice. (D) Representative simultaneous recordings of surface ECG (lead 1) and intracardiac electrograms in CM-KI treated with either AAV9-scramble or AAV9-shNlrp3. (E) Incidence of pacing-induced AF in CM-KI mice treated with either AAV9-scramble or AAV9-shNlrp3. *P<0.05, **P<0.01.
Genetic inhibition of NLRP3 prevents spontaneous AF in CREM-transgenic mice
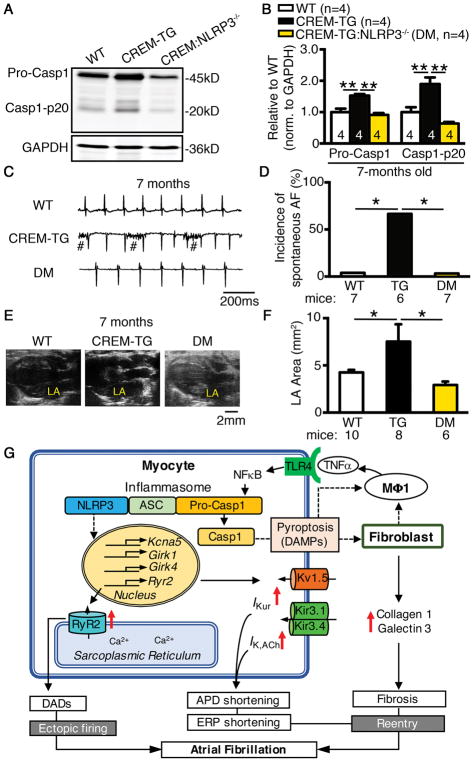
To establish whether NLRP3 is involved in the development of spontaneous AF in an established mouse model, we sought to determine whether inhibition of NLRP3 could prevent spontaneous AF in CREM-TG mice, which exhibited minimal ventricular dysfunction without beta-adrenoceptor stimulation.27, 59 Of note, the NLRP3-inflammasome activity was increased in CREM-TG mice at the age of 2 months, before the onset of spontaneous AF (Figure 1G–H). To inhibit NLRP3, we crossbred CREM-TG mice with NLRP3−/− mice. The offspring of CREM-TG:NLRP3−/− double-mutant (DM) mice exhibited Mendelian inheritance and were maintained on a mixed genetic background. The levels of CREM transcript in CREM-TG and DM mice were similar (Supplementary Figure S9A), excluding a potential differential impact of uneven expression of CREM protein on atrial electrophysiology. The level of active Casp1-p20 was reduced in DM mice (Figure 7A–B), suggesting reduced NLRP3-inflammasome activation. 24-hr ECG recordings revealed that 67% of CREM-TG mice (n=6) developed spontaneous AF, whereas spontaneous AF was absent in WT littermates (0%, n=7, P<0.05, Figure 7C–D). None of the DM mice (n=7, P<0.05 vs CREM-TG mice) exhibited spontaneous AF (Figure 7C–D) at the age of 7 months. Echocardiography demonstrated that left atrial chamber area was significantly reduced in DM compared to CREM-TG mice (Figure 7E–F) at 7 months, suggesting that NLRP3 inhibition prevents the structural substrate for AF25 in this model.
Figure 7. Genetic ablation of Nlrp3 prevents the development of spontaneous AF.
Representative WBs (A) and quantification (B) of caspase-1 in atrial tissues of WT, CREM-TG and CREM-TG:NLRP3−/− (DM) mice at the age of 7 months. Representative telemetry ECG recordings (C) and incidence of spontaneous AF (D) in WT, CREM-TG and DM mice (# indicated breathing artifact). Representative B-mode echocardiography recordings (E) and quantification (F) of left-atrial (LA) area in WT, CREM-TG and DM mice. *P<0.05, **P<0.01. (G) Working model of AF-promoting mechanisms resulting from CM-restricted NLRP3-inflammasome activation. Solid lines indicate proven mechanisms, and dash lines indicate putative mechanisms that need further investigation. DADs, delayed afterdepolarizations; DAMPs, danger-associated molecular patterns; ERP, effective refractory period; MΦ1, M1 macrophage; TNFα, tumor necrosis factor alpha.
DISCUSSION
In this study, we discovered that the activity of NLRP3-inflammasomes is enhanced in atrial cardiomyocytes of AF patients and dogs subjected to atrial tachycardia pacing (ATP dogs). CM-restricted activation of NLRP3 in mice promotes both the ectopic firing and AF-maintaining substrate that ultimately lead to AF. These results provide new pathophysiological insights of potential therapeutic relevance into a common and challenging cardiac condition. Furthermore, they put forward a novel paradigm of disease-inducing inflammasome-mediated signaling in non-immune cells.
Our proposed working model of the NLRP3 activation-mediated AF mechanisms is multifactorial (Figure 7G). On one hand, overactive CM NLRP3-signaling enhances the expression of Ryr2, leading to increased protein expression and RyR2-mediated arrhythmic SR Ca2+ release events,30 which ultimately cause ectopic firing via the generation of delayed afterdepolarizations (DADs). Meanwhile, overactive NLRP3-signaling in CMs enhances the transcription of Kcna5, which could lead to augmented IKur that abbreviate AERP, creating a reentry substrate. On the other hand, the enhanced activation of NLRP3-inflammasomes in atrial CMs could increase caspase-1 cleavage and caspase-1-mediated pyroptosis,60 which can activate CFs via danger-associated molecular factors (e.g. ATP, DNA, etc.). Thereafter, activated CMs and CFs can secret inflammatory cytokines (and collagen), recruit macrophages and other immune cells, and induce fibrosis, promoting the development of an AF-maintaining substrate.
Despite the increasing evidence demonstrating a clear association between the level of inflammatory cytokines and AF progression in patients,3, 7–12 it is unclear which and whether inflammatory signaling pathways play a direct role in AF promotion. The NLRP3-inflammasome is a well-established signaling pathway responsible for IL-1β and IL-18 releases from innate immune cells. NLRP3-inflammasome signaling has never been linked to arrhythmogenesis prior to this study. Our results point to enhanced activation of NLRP3-inflammasomes in both pAF and cAF patients. The activity of NLRP3-inflammasomes is altered not only in AF patients, but also in a dog model of atrial-tachycardia-remodeling and in a murine model of spontaneous AF (CREM-TG mice), establishing a major role for NLRP3-inflammasome in AF pathophysiology in the context of different pathologies. To the best of our knowledge, our study is the first to demonstrate a direct relationship between the augmented activity of NLRP3-inflammasomes in atrial cardiomyocytes and AF in patients.
The canonical function of NLRP3 in immune cells like macrophages, T-lymphocytes, eosinophils/neutrophils, etc. is to facilitate the oligomerization of the inflammasome, thereby activating caspase-1 and promoting the release of IL-1β and IL-18.15, 25, 61 Our results demonstrate a novel non-canonical role of NLRP3-inflammasomes in excitable cells like atrial CMs. Employing a unique CM-specific knockin model producing NLRP3 gain-of-function, we demonstrated detrimental cardiac electrophysiological consequences resulting from the CM-specific activation of NLRP3. The constitutive activation of CM NLRP3-inflammasomes led to an array of well-known arrhythmic events including frequent ectopic activity and AF-susceptibility. Ectopic activity due to increased SR Ca2+ release events via RyR2 clusters can initiate AF, while electrical remodeling like AERP shortening and atrial fibrosis can provide a substrate for AF maintenance.62 Our results have thus revealed a multi-functional role of inflammatory signaling in promoting AF pathophysiology.
The proarrhythmic consequences of increased RyR2 protein levels as a molecular correlate of triggered (ectopic) activity are supported by a number of previous studies in human atrial CMs or mouse models, which have shown that the abnormal SR Ca2+ release via RyR2 promotes DADs by enhancing the activity of Na+/Ca2+ exchanger (NCX).27, 45, 63 Many binding partners of RyR2 (such as FKBP12.6 and junctophilin-2) can stabilize RyR2 channels in the closed state.64, 65 When RyR2 protein levels are increased, the stoichiometry between RyR2 and its binding partners could be disproportionally altered; thus, despite the unchanged phosphorylation level of RyR2 channels (a known mechanism causing hyperactive RyR2), the “extra” un-bound RyR2 channels could be hyperactive due to the lack of the stabilizing binding partners.29 Furthermore, increased numbers of RyR2 channels may cause the formation of larger RyR2-clusters, releasing more Ca2+ during activation. In addition, a reduced distance between RyR2-clusters may also improve synchronization between RyR2-clusters. Further extensive work is needed to directly address these possibilities.
Recent work by Bruchard et al. has shown that NLRP3 could act as a coactivator of transcription factor interferon regulatory factor 4 in T-lymphocytes to promote TH2 differentiation, in addition to its canonical function.66 Our data demonstrate that NLRP3 activation enhances the mRNA expression of key ion channel subunits (RyR2, Kv1.5, GIRK1 and GIRK4) involved in AF arrhythmogenesis, while CM-specific knockdown of Nlrp3 via AAV9-mediated gene transfer reverses their overexpression. These results support an alternative function of NLRP3 in modulating gene transcription or RNA stability. Further investigation is required to elucidate the precise mechanisms by which the inflammasome complex alters the expression of ion channel subunits.
Emerging evidence supports a synergistic action of CMs and CFs in promoting a substrate for AF development,67 and fibrosis inevitably increases the heterogeneity of conduction, producing a reentrant substrate for AF maintenance.57 The elevated levels of collagen-1a and galectin-3, both markers of fibrosis, in CM-KI mice suggest that the CF activation is induced by CM-restricted NLRP3-inflammasome upregulation. This could be explained by the fact that CFs also express NLRP3-inflammasomes that can be activated by DAMPs like ATP and DNA fragments released from CMs (Figure 7G).68, 69 Previous work has shown that activation of NLRP3-inflammasomes can activate gasdermin-D, which promotes the formation of permeable pores in immune cells.70 Similarly, we speculate that NLRP3 activation could promote the release of DAMPs from CMs into the extracellular space to activate CFs, further promoting cytokine release and collagen production (Figure 7G). Alternatively, the active caspase-1 could also promote pyroptosis – a type of programmed cell death.60, 71 Since CMs undergo pyroptosis in response to mature caspase-1, this would also cause the release of DAMPs. Our results point to a novel form of interplay between CMs and CFs caused by the activation of NLRP3-inflammasomes. Future studies are required to establish the potential role of the NLRP3-inflammasome in CFs for AF pathophysiology.
Since CM NLRP3-inflammasome activation can promote ectopic activity and reentry, both critical components of AF pathophysiology, NLRP3 inhibition might be a potentially effective intervention in AF. In the light of the recent CANTOS trial72 showing that anti-IL-1β neutralizing antibody can be beneficial in reducing cardiac events, one might speculate that therapeutic targeting of the NLRP3-inflammasome could be a viable therapeutic option for certain forms of AF. Here, we found that pharmacological inhibition by MCC950, AAV9-mediated shRNA gene transfer to knockdown NLRP3, or genetic inhibition by NLRP3 knockout prevented the development of AF. These results point to potential novel anti-AF approaches. Several pharmacological inhibitors of NLRP3-inflammasomes have been developed, like caspase-1 inhibitors (Ac-YVAD-cho, Ac-YVAD-CMK, etc), the IL-1β inhibitor canakinumab, and the IL-1β-receptor antagonist anakinra.15, 73, 74 MCC950 appears to be a highly-selective NLRP3 inhibitor.44, 73 Given the substantial limitations of current AF therapies, the establishment of new targetable pathophysiological mechanisms like those related to NLRP3-inflammasome activation is of great clinical importance. Further extensive experimentation would be required to evaluate whether these pharmacological options are beneficial in preventing the onset of AF or reducing AF burden in both animal models of AF and AF patients with activated NLRP3-inflammasome.
Our study has several limitations. First, although our data revealed an enhanced activity of NLRP3-inflammasome in CMs of AF patients, the detailed upstream factors causing the activation of NLRP3-inflammasome were not investigated in the current study. Due to the limited amount of human atrial tissue available to us, we were unable to prepare nuclear fractions from human atrial biopsies to assess the nuclear levels of pNFκB in patients with AF. Thus, it is unclear whether an enhanced nuclear translocation of pNFκB contributes to the activation of NLRP3-inflammasome in AF patients. In addition, because Myh6Cre is expressed in both atrial and ventricular CMs, the constitutively active NLRP3 should affect both atrial and ventricular functions in the CM-KI model. However, ventricular function was only mildly altered at the age we investigated. A similar atrial-specific remodeling response was seen in an intense exercise-induced AF-model,75 in which TNFα (a proinflammatory regulator interacting importantly with NLRP3 activation)76 was implicated for atrial remodeling and AF-promotion.75 Nevertheless, an atrial specific knockin or knockout of NLRP3 would be the ultimate model to demonstrate that the enhanced atrial NLRP3 activity is the primary cause underlying AF development. Finally, although MCC950 effectively reduced AF-inducibility in CM-KI mice, we did not determine the precise pharmacokinetics of MCC950 and its potential adverse effects on ventricular function. Future work is needed to address these important aspects.
In summary, our work has demonstrated that NLRP3-inflammasome activation in atrial cardiomyocytes is associated with the pathogenesis of AF. Cardiomyocyte-restricted activation of NLRP3 is sufficient and required to promote AF and the underlying atrial remodeling events. To our knowledge, this study is the first to demonstrate a mechanistic link between NLRP3-inflammasome signaling and the pathophysiology of arrhythmias like AF. Our results suggest that inhibition of the NLRP3-inflammasome prevents AF promotion and may constitute a novel anti-AF approach targeting both electrical and structural remodeling.
Supplementary Material
Clinical Perspective.
What Is New?
This is the first study to demonstrate a causal link between the NLRP3 inflammasome (an innate inflammation signaling-complex) and the pathophysiology of atrial fibrillation (AF).
The activity of the NLRP3 inflammasome in atrial cardiomyocytes is enhanced in patients with paroxysmal or long-lasting persistent AF.
Constitutive activation of the cardiomyocyte NLRP3 inflammasome in an animal model produces ectopic activity and a reentry substrate for AF development.
What Are the Clinical Implications?
We discovered a novel paradigm of disease-inducing inflammasome-mediated signaling in non-immune cells.
Targeting the NLRP3 inflammasome is a novel therapeutic possibility for AF.
Antagonizing downstream effectors activated by the NLRP3 inflammasome, like IL-1β or Caspase-1, for which clinical options are already available, may also be of value against AF.
Acknowledgments
The authors thank Barbara Langer, Ramona Nagel, Simone Olesch, Kirsten Baden, Monika Hagedorn, and Annette Kötting-Dorsch for excellent technical assistance and the cardiac surgeon teams in Heidelberg and Dresden for provision of human atrial biopsies.
Funding Sources
This work was supported by the NIH (R56-HL131649 to N.L., R01-HL136389 to N.L. and D.D., R01-HL089598, R01-HL091947, R01-HL117641, and R41-HL129570 to X.H.T.W., R01-HL131517 to D.D., and U54-HG006348 to MPC at Baylor College of Medicine), the American Heart Association (14SDG20080008 to N.L. and 13EIA14560061 to X.H.T.W.), the German Research Foundation DFG (Mu1376/11-3 to F.U.M. and Do 769/4-1 to D.D.), the German Center for Cardiovascular Research (DZHK to D.D.), and the Canadian Institutes of Health Research and Heart and Stroke Foundation of Canada (to S.N.).
Footnotes
Disclosures
None.
References
- 1.Freeman JV, Wang Y, Akar J, Desai N, Krumholz H. National Trends in Atrial Fibrillation Hospitalization, Readmission, and Mortality for Medicare Beneficiaries, 1999–2013. Circulation. 2017;135:1227–1239. doi: 10.1161/CIRCULATIONAHA.116.022388. [DOI] [PubMed] [Google Scholar]
- 2.Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 3.Cheng T, Wang XF, Hou YT, Zhang L. Correlation between atrial fibrillation, serum amyloid protein A and other inflammatory cytokines. Mol Med Rep. 2012;6:581–584. doi: 10.3892/mmr.2012.934. [DOI] [PubMed] [Google Scholar]
- 4.Giannopoulos G, Cleman MW, Deftereos S. Inflammation fueling atrial fibrillation substrate: seeking ways to “cool” the heart. Med Chem. 2014;10:663–671. doi: 10.2174/1573406410666140318110100. [DOI] [PubMed] [Google Scholar]
- 5.Harada M, Van Wagoner DR, Nattel S. Role of inflammation in atrial fibrillation pathophysiology and management. Circ J. 2015;79:495–502. doi: 10.1253/circj.CJ-15-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu YF, Chen YJ, Lin YJ, Chen SA. Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol. 2015;12:230–243. doi: 10.1038/nrcardio.2015.2. [DOI] [PubMed] [Google Scholar]
- 7.Gungor B, Ekmekci A, Arman A, Ozcan KS, Ucer E, Alper AT, Calik N, Yilmaz H, Tezel T, Coker A, Bolca O. Assessment of interleukin-1 gene cluster polymorphisms in lone atrial fibrillation: new insight into the role of inflammation in atrial fibrillation. Pacing Clin Electrophysiol. 2013;36:1220–1227. doi: 10.1111/pace.12182. [DOI] [PubMed] [Google Scholar]
- 8.Luan Y, Guo Y, Li S, Yu B, Zhu S, Li S, Li N, Tian Z, Peng C, Cheng J, Li Q, Cui J, Tian Y. Interleukin-18 among atrial fibrillation patients in the absence of structural heart disease. Europace. 2010;12:1713–1718. doi: 10.1093/europace/euq321. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Yan HM, Tang MX, Wang ZH, Zhong M, Zhang Y, Deng JT, Zhang W. Increased serum levels of microvesicles in nonvalvular atrial fibrillation determinated by ELISA using a specific monoclonal antibody AD-1. Clin Chim Acta. 2010;411:1700–1704. doi: 10.1016/j.cca.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Kimura T, Takatsuki S, Inagawa K, Katsumata Y, Nishiyama T, Nishiyama N, Fukumoto K, Aizawa Y, Tanimoto Y, Tanimoto K, Fukuda K. Serum inflammation markers predicting successful initial catheter ablation for atrial fibrillation. Heart Lung Circ. 2014;23:636–643. doi: 10.1016/j.hlc.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Okumura Y, Watanabe I, Nakai T, Ohkubo K, Kofune T, Kofune M, Nagashima K, Mano H, Sonoda K, Kasamaki Y, Hirayama A. Impact of biomarkers of inflammation and extracellular matrix turnover on the outcome of atrial fibrillation ablation: importance of matrix metalloproteinase-2 as a predictor of atrial fibrillation recurrence. J Cardiovasc Electrophysiol. 2011;22:987–993. doi: 10.1111/j.1540-8167.2011.02059.x. [DOI] [PubMed] [Google Scholar]
- 12.Wu G, Cheng M, Huang H, Yang B, Jiang H, Huang C. A variant of IL6R is associated with the recurrence of atrial fibrillation after catheter ablation in a Chinese Han population. PLoS One. 2014;9:e99623. doi: 10.1371/journal.pone.0099623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haneklaus M, O’Neill LA, Coll RC. Modulatory mechanisms controlling the NLRP3 inflammasome in inflammation: recent developments. Curr Opin Immunol. 2013;25:40–45. doi: 10.1016/j.coi.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- 15.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 17.Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schroder GF, Fitzgerald KA, Wu H, Egelman EH. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bracey NA, Beck PL, Muruve DA, Hirota SA, Guo J, Jabagi H, Wright JR, Jr, Macdonald JA, Lees-Miller JP, Roach D, Semeniuk LM, Duff HJ. The Nlrp3 inflammasome promotes myocardial dysfunction in structural cardiomyopathy through interleukin-1beta. Exp Physiol. 2013;98:462–472. doi: 10.1113/expphysiol.2012.068338. [DOI] [PubMed] [Google Scholar]
- 19.Marchetti C, Chojnacki J, Toldo S, Mezzaroma E, Tranchida N, Rose SW, Federici M, Van Tassell BW, Zhang S, Abbate A. A novel pharmacologic inhibitor of the NLRP3 inflammasome limits myocardial injury after ischemia-reperfusion in the mouse. J Cardiovasc Pharmacol. 2014;63:316–322. doi: 10.1097/FJC.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toldo S, Marchetti C, Mauro AG, Chojnacki J, Mezzaroma E, Carbone S, Zhang S, Van Tassell B, Salloum FN, Abbate A. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. Int J Cardiol. 2016;209:215–220. doi: 10.1016/j.ijcard.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 21.Graf EM, Bock M, Heubach JF, Zahanich I, Boxberger S, Richter W, Schultz JH, Ravens U. Tissue distribution of a human Ca v 1.2 alpha1 subunit splice variant with a 75 bp insertion. Cell Calcium. 2005;38:11–21. doi: 10.1016/j.ceca.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Heijman J, Kirchner D, Kunze F, Chretien EM, Michel-Reher MB, Voigt N, Knaut M, Michel MC, Ravens U, Dobrev D. Muscarinic type-1 receptors contribute to IK,ACh in human atrial cardiomyocytes and are upregulated in patients with chronic atrial fibrillation. Int J Cardiol. 2018;255:61–68. doi: 10.1016/j.ijcard.2017.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shinagawa K, Mitamura H, Ogawa S, Nattel S. Effects of inhibiting Na(+)/H(+)-exchange or angiotensin converting enzyme on atrial tachycardia-induced remodeling. Cardiovasc Res. 2002;54:438–446. doi: 10.1016/s0008-6363(01)00515-6. [DOI] [PubMed] [Google Scholar]
- 24.Ehrlich JR, Cha TJ, Zhang L, Chartier D, Villeneuve L, Hebert TE, Nattel S. Characterization of a hyperpolarization-activated time-dependent potassium current in canine cardiomyocytes from pulmonary vein myocardial sleeves and left atrium. J Physiol. 2004;557:583–597. doi: 10.1113/jphysiol.2004.061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brydges SD, Mueller JL, McGeough MD, Pena CA, Misaghi A, Gandhi C, Putnam CD, Boyle DL, Firestein GS, Horner AA, Soroosh P, Watford WT, O’Shea JJ, Kastner DL, Hoffman HM. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity. 2009;30:875–887. doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirchhof P, Marijon E, Fabritz L, Li N, Wang W, Wang T, Schulte K, Hanstein J, Schulte JS, Vogel M, Mougenot N, Laakmann S, Fortmueller L, Eckstein J, Verheule S, Kaese S, Staab A, Grote-Wessels S, Schotten U, Moubarak G, Wehrens XH, Schmitz W, Hatem S, Muller FU. Overexpression of cAMP-response element modulator causes abnormal growth and development of the atrial myocardium resulting in a substrate for sustained atrial fibrillation in mice. Int J Cardiol. 2013;166:366–374. doi: 10.1016/j.ijcard.2011.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, Horrigan FT, Schmitz W, Muller FU, Valderrabano M, Nattel S, Dobrev D, Wehrens XH. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation. 2014;129:1276–1285. doi: 10.1161/CIRCULATIONAHA.113.006611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muller FU, Lewin G, Baba HA, Boknik P, Fabritz L, Kirchhefer U, Kirchhof P, Loser K, Matus M, Neumann J, Riemann B, Schmitz W. Heart-directed expression of a human cardiac isoform of cAMP-response element modulator in transgenic mice. J Biol Chem. 2005;280:6906–6914. doi: 10.1074/jbc.M407864200. [DOI] [PubMed] [Google Scholar]
- 29.Chiang DY, Kongchan N, Beavers DL, Alsina KM, Voigt N, Neilson JR, Jakob H, Martin JF, Dobrev D, Wehrens XH, Li N. Loss of microRNA-106b-25 cluster promotes atrial fibrillation by enhancing ryanodine receptor type-2 expression and calcium release. Circ Arrhythm Electrophysiol. 2014;7:1214–1222. doi: 10.1161/CIRCEP.114.001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li N, Wang T, Wang W, Cutler MJ, Wang Q, Voigt N, Rosenbaum DS, Dobrev D, Wehrens XH. Inhibition of CaMKII phosphorylation of RyR2 prevents induction of atrial fibrillation in FKBP12.6 knockout mice. Circ Res. 2012;110:465–470. doi: 10.1161/CIRCRESAHA.111.253229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li N, Wehrens XH. Programmed electrical stimulation in mice. J Vis Exp. 2010 doi: 10.3791/1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beeton C, Pennington MW, Wulff H, Singh S, Nugent D, Crossley G, Khaytin I, Calabresi PA, Chen CY, Gutman GA, Chandy KG. Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol Pharmacol. 2005;67:1369–1381. doi: 10.1124/mol.104.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagrutta A, Wang J, Fermini B, Salata JJ. Novel, potent inhibitors of human Kv1.5 K+ channels and ultrarapidly activating delayed rectifier potassium current. J Pharmacol Exp Ther. 2006;317:1054–1063. doi: 10.1124/jpet.106.101162. [DOI] [PubMed] [Google Scholar]
- 34.Guggenhuber S, Monory K, Lutz B, Klugmann M. AAV vector-mediated overexpression of CB1 cannabinoid receptor in pyramidal neurons of the hippocampus protects against seizure-induced excitoxicity. PLoS One. 2010;5:e15707. doi: 10.1371/journal.pone.0015707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jarrett KE, Lee CM, Yeh YH, Hsu RH, Gupta R, Zhang M, Rodriguez PJ, Lee CS, Gillard BK, Bissig KD, Pownall HJ, Martin JF, Bao G, Lagor WR. Somatic genome editing with CRISPR/Cas9 generates and corrects a metabolic disease. Sci Rep. 2017;7:44624. doi: 10.1038/srep44624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lagor WR, Johnston JC, Lock M, Vandenberghe LH, Rader DJ. Adeno-associated viruses as liver-directed gene delivery vehicles: focus on lipoprotein metabolism. Methods Mol Biol. 2013;1027:273–307. doi: 10.1007/978-1-60327-369-5_13. [DOI] [PubMed] [Google Scholar]
- 37.Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. Retro-orbital injections in mice. Lab Anim (NY) 2011;40:155–160. doi: 10.1038/laban0511-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yue L, Melnyk P, Gaspo R, Wang Z, Nattel S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ Res. 1999;84:776–784. doi: 10.1161/01.res.84.7.776. [DOI] [PubMed] [Google Scholar]
- 39.Shin JN, Fattah EA, Bhattacharya A, Ko S, Eissa NT. Inflammasome activation by altered proteostasis. J Biol Chem. 2013;288:35886–35895. doi: 10.1074/jbc.M113.514919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamann J, Koning N, Pouwels W, Ulfman LH, van Eijk M, Stacey M, Lin HH, Gordon S, Kwakkenbos MJ. EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur J Immunol. 2007;37:2797–2802. doi: 10.1002/eji.200737553. [DOI] [PubMed] [Google Scholar]
- 41.Hansen MJ, Achini Bandara N, Low PS. Folate receptor expression on murine and human adipose tissue macrophages. Inflamm Res. 2015;64:697–706. doi: 10.1007/s00011-015-0849-2. [DOI] [PubMed] [Google Scholar]
- 42.Makary S, Voigt N, Maguy A, Wakili R, Nishida K, Harada M, Dobrev D, Nattel S. Differential protein kinase C isoform regulation and increased constitutive activity of acetylcholine-regulated potassium channels in atrial remodeling. Circ Res. 2011;109:1031–1043. doi: 10.1161/CIRCRESAHA.111.253120. [DOI] [PubMed] [Google Scholar]
- 43.Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res. 2006;98:837–845. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- 44.Coll RC, Robertson AA, Chae JJ, Higgins SC, Munoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Nunez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–2070. doi: 10.1161/CIRCULATIONAHA.111.067306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014;114:1483–1499. doi: 10.1161/CIRCRESAHA.114.302226. [DOI] [PubMed] [Google Scholar]
- 48.Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–156. doi: 10.1161/CIRCULATIONAHA.113.006641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anyukhovsky EP, Sosunov EA, Chandra P, Rosen TS, Boyden PA, Danilo P, Jr, Rosen MR. Age-associated changes in electrophysiologic remodeling: a potential contributor to initiation of atrial fibrillation. Cardiovasc Res. 2005;66:353–363. doi: 10.1016/j.cardiores.2004.10.033. [DOI] [PubMed] [Google Scholar]
- 50.Hsueh CH, Chang PC, Hsieh YC, Reher T, Chen PS, Lin SF. Proarrhythmic effect of blocking the small conductance calcium activated potassium channel in isolated canine left atrium. Heart Rhythm. 2013;10:891–898. doi: 10.1016/j.hrthm.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wettwer E, Hala O, Christ T, Heubach JF, Dobrev D, Knaut M, Varro A, Ravens U. Role of IKur in controlling action potential shape and contractility in the human atrium: influence of chronic atrial fibrillation. Circulation. 2004;110:2299–2306. doi: 10.1161/01.CIR.0000145155.60288.71. [DOI] [PubMed] [Google Scholar]
- 52.Voigt N, Friedrich A, Bock M, Wettwer E, Christ T, Knaut M, Strasser RH, Ravens U, Dobrev D. Differential phosphorylation-dependent regulation of constitutively active and muscarinic receptor-activated IK, ACh channels in patients with chronic atrial fibrillation. Cardiovasc Res. 2007;74:426–437. doi: 10.1016/j.cardiores.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 53.Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kuhlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–131. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 54.Lu J, McKinsey TA, Nicol RL, Olson EN. Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases. Proc Natl Acad Sci U S A. 2000;97:4070–4075. doi: 10.1073/pnas.080064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Q, Busby JC, Molkentin JD. Interaction between TAK1-TAB1-TAB2 and RCAN1-calcineurin defines a signalling nodal control point. Nat Cell Biol. 2009;11:154–161. doi: 10.1038/ncb1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen D, Procter N, Goh V, Liu S, Chua SJ, Assadi-Khansari B, Stewart S, Horowitz JD, Sverdlov AL, Ngo DT. New onset atrial fibrillation is associated with elevated galectin-3 levels. Int J Cardiol. 2016;223:48–49. doi: 10.1016/j.ijcard.2016.08.172. [DOI] [PubMed] [Google Scholar]
- 57.Jalife J. Mechanisms of persistent atrial fibrillation. Curr Opin Cardiol. 2014;29:20–27. doi: 10.1097/HCO.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 58.Takemoto Y, Ramirez RJ, Yokokawa M, Kaur K, Ponce-Balbuena D, Sinno MC, Willis BC, Ghanbari H, Ennis SR, Guerrero-Serna G, Henzi BC, Latchamsetty R, Ramos-Mondragon R, Musa H, Martins RP, Pandit SV, Noujaim SF, Crawford T, Jongnarangsin K, Pelosi F, Bogun F, Chugh A, Berenfeld O, Morady F, Oral H, Jalife J. Galectin-3 Regulates Atrial Fibrillation Remodeling and Predicts Catheter Ablation Outcomes. JACC Basic Transl Sci. 2016;1:143–154. doi: 10.1016/j.jacbts.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schulte JS, Fehrmann E, Tekook MA, Kranick D, Fels B, Li N, Wehrens XH, Heinick A, Seidl MD, Schmitz W, Muller FU. Cardiac expression of the CREM repressor isoform CREM-IbDeltaC-X in mice leads to arrhythmogenic alterations in ventricular cardiomyocytes. Basic Res Cardiol. 2016;111:15. doi: 10.1007/s00395-016-0532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, Zhuang Y, Cai T, Wang F, Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 61.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 62.Nattel S, Dobrev D. Electrophysiological and molecular mechanisms of paroxysmal atrial fibrillation. Nat Rev Cardiol. 2016;13:575–590. doi: 10.1038/nrcardio.2016.118. [DOI] [PubMed] [Google Scholar]
- 63.Yeh YH, Wakili R, Qi XY, Chartier D, Boknik P, Kaab S, Ravens U, Coutu P, Dobrev D, Nattel S. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythm Electrophysiol. 2008;1:93–102. doi: 10.1161/CIRCEP.107.754788. [DOI] [PubMed] [Google Scholar]
- 64.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 65.Beavers DL, Wang W, Ather S, Voigt N, Garbino A, Dixit SS, Landstrom AP, Li N, Wang Q, Olivotto I, Dobrev D, Ackerman MJ, Wehrens XH. Mutation E169K in junctophilin-2 causes atrial fibrillation due to impaired RyR2 stabilization. J Am Coll Cardiol. 2013;62:2010–2019. doi: 10.1016/j.jacc.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruchard M, Rebe C, Derangere V, Togbe D, Ryffel B, Boidot R, Humblin E, Hamman A, Chalmin F, Berger H, Chevriaux A, Limagne E, Apetoh L, Vegran F, Ghiringhelli F. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol. 2015;16:859–870. doi: 10.1038/ni.3202. [DOI] [PubMed] [Google Scholar]
- 67.Yue L, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 2011;89:744–753. doi: 10.1093/cvr/cvq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sandanger O, Ranheim T, Vinge LE, Bliksoen M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G, Christensen G, Fitzgerald KA, Lien E, Valen G, Espevik T, Aukrust P, Yndestad A. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia-reperfusion injury. Cardiovasc Res. 2013;99:164–174. doi: 10.1093/cvr/cvt091. [DOI] [PubMed] [Google Scholar]
- 69.Feldman N, Rotter-Maskowitz A, Okun E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res Rev. 2015;24:29–39. doi: 10.1016/j.arr.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 70.Russo HM, Rathkey J, Boyd-Tressler A, Katsnelson MA, Abbott DW, Dubyak GR. Active Caspase-1 Induces Plasma Membrane Pores That Precede Pyroptotic Lysis and Are Blocked by Lanthanides. J Immunol. 2016;197:1353–1367. doi: 10.4049/jimmunol.1600699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, Yang ZH, Zhong CQ, Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 73.Kim RY, Pinkerton JW, Essilfie AT, Robertson AA, Baines KJ, Brown AC, Mayall JR, Ali MK, Starkey MR, Hansbro NG, Hirota JA, Wood LG, Simpson JL, Knight DA, Wark PA, Gibson PG, O’Neill LA, Cooper MA, Horvat JC, Hansbro PM. Role for NLRP3 Inflammasome-mediated, IL-1beta-dependent Responses in Severe, Steroid-resistant Asthma. Am J Respir Crit Care Med. 2017;196:283–297. doi: 10.1164/rccm.201609-1830OC. [DOI] [PubMed] [Google Scholar]
- 74.Iannitti RG, Napolioni V, Oikonomou V, De Luca A, Galosi C, Pariano M, Massi-Benedetti C, Borghi M, Puccetti M, Lucidi V, Colombo C, Fiscarelli E, Lass-Florl C, Majo F, Cariani L, Russo M, Porcaro L, Ricciotti G, Ellemunter H, Ratclif L, De Benedictis FM, Talesa VN, Dinarello CA, van de Veerdonk FL, Romani L. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat Commun. 2016;7:10791. doi: 10.1038/ncomms10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aschar-Sobbi R, Izaddoustdar F, Korogyi AS, Wang Q, Farman GP, Yang F, Yang W, Dorian D, Simpson JA, Tuomi JM, Jones DL, Nanthakumar K, Cox B, Wehrens XH, Dorian P, Backx PH. Increased atrial arrhythmia susceptibility induced by intense endurance exercise in mice requires TNFalpha. Nat Commun. 2015;6:6018. doi: 10.1038/ncomms7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ridker PM, Luscher TF. Anti-inflammatory therapies for cardiovascular disease. Eur Heart J. 2014;35:1782–1791. doi: 10.1093/eurheartj/ehu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.