Abstract
We present a case of disseminated Kaposi’s sarcoma with both cutaneous and extracutaneous involvement in an HIV-infected patient with a relatively high CD4 count of 369 cell/mm3. He developed chronic diarrhea, constitutional symptoms, worsening bilateral pleural effusion with respiratory distress, and progression of skin lesions distributed over his chest and extremities. The temporal relationship between rapid clinical progression and initiation of HAART suggested the possibility of Kaposi’s sarcoma-associated immune reconstitution inflammatory syndrome, which eventually resulted in the death of this patient.
INTRODUCTION
Kaposi’s sarcoma (KS), a low-grade multicentric vascular tumor, is associated with infection of human herpes virus 8 (HHV-8) [1]. Being one of the acquired immunodeficiency syndrome (AIDS) defining illnesses, the clinical course of KS is highly variable, ranging from asymptomatic to rapidly progressing disease [1, 2]. In addition to cutaneous involvement, KS can be present in disseminated form, frequently involving the gastrointestinal (GI) tract, lungs, oral cavity and lymph nodes [1]. We report a case of AIDS-related KS with both cutaneous and extracutaneous involvement.
CASE REPORT
A 34-year-old heterosexual gentleman was found to have HIV infection during his annual employment health screening. He was last tested negative for HIV in 2016. Eruptions of multiple non-pruritic skin lesions over his chest and limbs started three months prior to his medical check-up that led to the diagnosis of HIV. A few months after his diagnosis, he started to develop chronic diarrhea, constitutional symptoms, swelling on his face and lymphedema. However, his Karnofsky performance status at the initial presentation was good. He admitted that he had few unprotected heterosexual exposures in the past but denied having any homosexual contact. There was no history of blood transfusion or intravenous drug abuse. No medication was taken prior to the onset of his skin lesions.
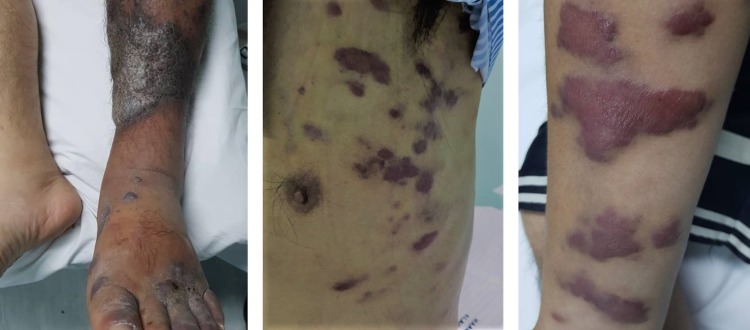
On examination, he looked cachexic and dehydrated. He had bilateral tiny cervical lymphadenopathy with painless parotid gland swelling. There were presence of multiple well-defined, non-scaly, discrete, violaceous to hyper-pigmented skin lesions of variable sizes distributed over his chest and extremities, predominantly over the flexor surface (Fig. 1). In addition, multiple similar but mildly tender and erythematous plaques were noted over his both lower legs region (Fig. 1). Oral cavity was normal. Chest examination was consistent with bilateral pleural effusion. Other physical examinations were unremarkable.
Figure 1:
Cutaneous manifestation of Kaposi’s sarcoma (left, legs; middle, chest; right, arms)
Serial investigations were carried out. Baseline full blood count showed normochromic normocytic anemia (hemoglobin 7.4 g/dL), leukocyte count of 4600/μL and platelet count of 144 000/μL. His renal and liver profile was normal except having low albumin (27 g/L). His LDH was 577 μ/L. Biohazard screening was positive for anti-HIV 1 enzyme immunosorbant assay (EIA) and particle agglutination (PA). It was further confirmed by HIV Western Blot (Immunoblot) test. His CD4 count tested using the ALERE PIMATM Analyzer was 369 cell/mm3. No baseline HIV viral load was taken as our center only performed viral load testing 4 months after commencing on anti-retroviral treatment. His serum was positive for TPPA and VDRL (titer 1:64) in which he received three doses of intramuscular Benzathine Penicillin. Serology screening for Hepatitis B and C were negative. Blood serology for HHV-8 and Esptein Barr Virus (EBV) were not sent as the tests were not readily available and very costly. Ultrasound of face reported bilateral parotiditis with intra-parotid lymphadenopathy. Histologically, the skin biopsy showed proliferation of thin-walled disorganized vascular channel (highlighted by CD31) in between the dermal collagen bundles with surrounding dermal appendages. The endothelial cells showed atypia with infiltration of lympho-plasma cells. These features were in favor of Kaposi’s sarcoma. Skin fungal and mycobacterium tuberculosis (MTB) cultures were negative. Contrasted CT of thorax, abdomen and pelvis demonstrated left upper zone fluffy consolidation, bilateral pleural effusion, axillary and mediastinal lymphadenopathy; rectum and rectosigmoid junction circumferential wall thickening with regional lymphadenopathy. Sputum for acid-fast bacilli (AFB) smear, MTB culture as well as geneXpert MTB/RIF results were negative. Stool for cultures, AFB smear, MTB culture and parasite screening were negative as well. In view of persistent diarrhea, colonoscopy was performed, which showed extensive polypoidal lesion with irregular edematous mucosa scattered throughout the entire rectum and colon. The histopathological examination (HPE) of caecal biopsy showed the presence of chronic active colitis with no evidence of crypt abscesses, malignancy or granulomatous lesions.
During his ward admission, he developed respiratory distress with worsening pleural effusion, in which bilateral chest tubes were inserted. Pleural fluid cytology was negative for malignant cells. Pleural fluid culture and sensitivity, AFB smear, MTB culture and fungal culture were all negative. Fundoscopy examination by ophthalmologist was consistent with features of HIV retinopathy, with presence of target lesions and intra-retinal bleed but no features of retinitis.
Our working diagnosis was HIV with disseminated KS which involved the lung, skin and GI tract. A multidisciplinary team discussion was conducted. A decision was made to start on highly active anti-retroviral therapy (HAART) first to control his HIV viral load and improve his immune function before commencing on chemotherapy after his general condition was optimized. Patient commenced on HAART—tenofovir–emtricitabine and lopinavir/ritonavir combination regimen. However, his condition progressively deteriorated and he succumbed to his illness a month after commencing on HAART despite good supportive care.
DISCUSSION/CONCLUSION
KS has been reported among all groups at risk for HIV infection [3]. It generally occurs in patients with a low CD4 count, particularly below 100 cell/mm3 and high viral load count of >10 000 copies/mL. It can be present at any time during the course of HIV infection [4]. Our patient had a relatively high CD4 count (369 cell/mm3), however, his disease progressed rapidly with widespread dissemination. He gave a history that he had annual medical check-ups which included HIV testing but was last tested negative in 2016. However, we could not assess his HIV test report in 2016. If indeed he was tested negative in 2016, he was likely at an early stage of HIV infection whereby his CD4 count was still relatively high (more than 300 cell/mm3).
Based on the staging system developed by the AIDS Clinical Trial Group (ACTG) of the National Institute of Health [5], our patient falls under ‘poor risk’ prognostic category. This can be explained by the presence of tumor-associated lymphedema, GI involvement as well as ‘B’ symptoms.
Our patient had a disseminated form of KS which involved the skin, pulmonary and GI tract. The incidence of GI tract involvement was more than 50% in AIDS patients with cutaneous KS [6]. The clinical manifestation varies from asymptomatic to a combination of symptoms, including weight loss, nausea, vomiting, abdominal pain, malabsorption and/or diarrhea [7]. These findings are consistent with our patient’s clinical presentation, representing part of the clinical spectrum of disseminated KS. Pulmonary involvement was found in ~45% of patients with AIDS-related KS with cutaneous or GI involvement [8]. It can be difficult to differentiate from other common pulmonary opportunistic infection such as MTB and lymphoproliferative disorder with pulmonary involvement. Investigations performed in this patient did not reveal any other evidence to suggest other viral, bacterial, mycobacterium or parasitic infections. There was also no evidence to suggest lymphoproliferative disorder.
Pulmonary involvement of KS, when it occurs, is often fatal and has a median survival rate which was quoted to be between 3 and 10 months in pre-HAART era as compared to 1.6 years in era of HAART [9]. Due to the extensiveness of disease in this case, protease inhibitor (PI)-based HAART was chosen for our patient aimed at reducing HIV viral load and improving the immune function prior to chemotherapy. Besides, PI is known to have potent anti-angiogenic and anti-tumor effect against KS [10, 11].
Studies have shown that HAART alone can improve immune response towards KS by decreasing plasma HIV viral load, resulting in stabilization and complete or partial regression of KS lesions. This often alleviates the need for chemo-radiotherapy [8]. In addition, the initial response to KS could be induced by either HAART or chemotherapy, but only HAART was associated with complete resolution of disease [12].
Despite the fact that HAART improves KS lesions in patients with AIDS-related KS, there is a risk of KS-associated immune reconstitution inflammatory syndrome (IRIS) [13]. IRIS-KS occurred more commonly in HAART-naïve individuals and patients with higher CD4 counts [13, 14], just like in our case. IRIS-KS was also reported to be more common in patients with KS-associated edema and those who received anti-retroviral combination therapy of either protease or non-nucleoside inhibitor [13, 14]. The supporting evidence of IRIS-KS in this case was the temporal relationship between rapid clinical progression of KS and initiation of HAART. It was likely due to dysregulated or intensified HHV-8 specific immune response or ineffective control of HHV-8 antigens in blood and tissue cells, as a result of the shift in cytokine profile from Th2 to Th1 [14]. This patient progressively deteriorated within a month upon commencing HAART and was not started on any chemotherapy.
In summary, we present a case of HIV-infected patient with relatively high CD4 count of 369 cell/mm3 with disseminated KS. Pulmonary involvement in disseminated KS was associated with poor outcome. KS-IRIS can occur in patients with higher CD4 count and result in death. Chemotherapy initiated in tandem with HAART might be a better treatment option for this case.
ACKNOWLEDGEMENTS
No other persons have made substantial contributions to the article other than the authors.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interest.
FUNDING
The authors declare no financial disclosure.
ETHICAL APPROVAL
No ethical approval is required. Each of the authors warrants that the enclosed article is original and does not infringe any copyright or violate any other right of any third parties, and the article has not been published elsewhere, has not been submitted for publication, and is not being considered for publication elsewhere in any form, except as explained to the Editor.
CONSENT FOR PUBLICATION
Written permission for publication has been obtained from the patient’s wife.
GUARANTOR
Siaw Tze Yeo was nominated as a guarantor of this report.
REFERENCES
- 1. Dezube BJ. Clinical presentation and natural history of AIDS-related Kaposi’s sarcoma. Hematol Oncol Clin North Am 1996;10:1023. [DOI] [PubMed] [Google Scholar]
- 2. Brodt HR, Kamps BS, Gute P, Knupp B, Staszewski S, Helm EB. Changing incidence of AIDS-defining illness in the era of antiretroviral combination therapy. AIDS 1997;11:1731–8. [DOI] [PubMed] [Google Scholar]
- 3. Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi’s sarcoma among persons with AIDS: a sexually transmitted infection? Lancet 1990;335:123. [DOI] [PubMed] [Google Scholar]
- 4. Nnoruka EN, Chukwuka JC, Anisuiba B. Correlation of mucocutaneous manifestations of HIV/AIDS infection with CD4 counts and disease progression. Int J Dermatol 2007;46:14–8. [DOI] [PubMed] [Google Scholar]
- 5. Krown SE, Metroka C, Wernz JC. Kaposi’s sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 1989;7:1201. [DOI] [PubMed] [Google Scholar]
- 6. Huang WY, Pantanowitz L, Dezube BJ. Unusual sites of malignancies: Case 3. AIDS-related Kaposi’s sarcoma of gastrointestinal tract. J Clin Oncol 2005;23:2098–9. [DOI] [PubMed] [Google Scholar]
- 7. Bower M, Nelson M, Young AM, Thirwell C, Newson-Davis T, Mandalia A, et al. s Immune reconstitution inflammatory syndrome associated with Kaposi’s sarcoma. J Clin Oncol 2005;23:5224–8. [DOI] [PubMed] [Google Scholar]
- 8. Gasparetto TD, Marchiori E, Lourenco S, Zanetti G, Vianna AD, Santos AA, et al. Pulmonary involvement in Kaposi sarcoma: correlation between imaging and pathology. Orphanet J Rare Dis 2009;4:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Palmieri C, Dhillon T, Thirlwell C, Newsom-Davis T, Young AM, Nelson M, et al. Pulmonary Kaposi sarcoma in the era of highly active antiretroviral therapy. HIV Med 2006;7:291–3. [DOI] [PubMed] [Google Scholar]
- 10. La Ferla L, Pinzone MR, Nunnari G, Martellotta F, Lleshi A, Tirelli U, et al. Kaposi’s sarcoma in HIV-positive patients: the state of art in the HAART-era. Eur Rev Med PharmacolSci 2013;17:2354–65. [PubMed] [Google Scholar]
- 11. Sgadari C, Barillari G, Toschi E, Carlei D, Bacigalupo L, Baccarini S, et al. HIV protease inhibitors are potent anti-angiogenic molecules and promote regression of Kaposi sarcoma. Nat Med 2002;8:225–32. [DOI] [PubMed] [Google Scholar]
- 12. Huong QN, Amalia SM, Mari MK, Stephen EVR, Anna W, Corey C. Persistent Kaposi sarcoma in the era of HAART: characterizing the predictors of clinical response. AIDS 2008;22:937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Connick E, Kane MA, White IE, Ryder J, Campbell TB. Immune reconstitution inflammatory syndrome associated with Kaposi sarcoma during potent antiretroviral therapy. Clin Infect Dis 2004;39:1852–5. [DOI] [PubMed] [Google Scholar]
- 14. Feller L, Lemmer J. Insights into pathogenic events of HIV-associated Kaposi sarcoma and immune reconstitution syndrome related Kaposi sarcoma. Infect Agents Cancer 2008;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]