Abstract
Background
Until recently, determining penetrance required large observational cohort studies. Data from the Exome Aggregate Consortium (ExAC) allows a Bayesian approach to calculate penetrance, in that population frequencies of pathogenic germline variants should be inversely proportional to their penetrance for disease. We tested this hypothesis using data from two cohorts for succinate dehydrogenase subunits A, B and C (SDHA–C) genetic variants associated with hereditary pheochromocytoma/paraganglioma (PC/PGL).
Methods
Two cohorts were 575 unrelated Australian subjects and 1240 unrelated UK subjects, respectively, with PC/PGL in whom genetic testing had been performed. Penetrance of pathogenic SDHA–C variants was calculated by comparing allelic frequencies in cases versus controls from ExAC (removing those variants contributed by The Cancer Genome Atlas).
Results
Pathogenic SDHA–C variants were identified in 106 subjects (18.4%) in cohort 1 and 317 subjects (25.6%) in cohort 2. Of 94 different pathogenic variants from both cohorts (seven in SDHA, 75 in SDHB and 12 in SDHC), 13 are reported in ExAC (two in SDHA, nine in SDHB and two in SDHC) accounting for 21% of subjects with SDHA–C variants. Combining data from both cohorts, estimated lifetime disease penetrance was 22.0% (95% CI 15.2% to 30.9%) for SDHB variants, 8.3% (95% CI 3.5% to 18.5%) for SDHC variants and 1.7% (95% CI 0.8% to 3.8%) for SDHA variants.
Conclusion
Pathogenic variants in SDHB are more penetrant than those in SDHC and SDHA. Our findings have important implications for counselling and surveillance of subjects carrying these pathogenic variants.
Keywords: pheochromocytoma, paraganglioma, succinate dehydrogenase, penetrance, pathogenic variant
Introduction
Phaeochromocytomas (PCs, tumours of the adrenal medulla) and paragangliomas (PGLs, tumours of sympathetic or parasympathetic ganglia) are highly heritable, with 14 PC/PGL susceptibility genes identified.1–3 Six of these genes were included in the American College of Medical Genetics recommendations for mandated reporting of incidental findings from clinical exome and genome sequencing4 5: VHL, RET, succinate dehydrogenase subunits B, C, D (SDHB, SDHC, SDHD) and SDHAF2. The high heritability of PC/PGL strongly suggests that germline genetic testing be considered for all affected individuals, enabling predictive genetic testing for at-risk relatives if a pathogenic variant is detected.6 7
Germline mutations in the SDH genes are the most common genetic cause of PC/PGLs (MIM:168000,605373,115310 and 614165), occurring in approximately 15% of cases.1–3 By comparison, the next most common associated genes are VHL (4%–10%), RET (1%–5%) and NFI (1%–5%). VHL and NF1 are associated with Von Hippel Lindau (MIM:193300) and neurofibromatosis type 1 (MIM:162200), respectively, that include PC/PGL as part of a broader syndrome.
Since PC/PGL are rare tumours,8 not only should pathogenic variants be individually extremely rare but their cumulative frequency within disease-associated genes (if fully penetrant) should be <0.0001 in large population cohorts (ie, population prevalence of 1/5000 with allele frequency of 1/10 000 for autosomal dominant disease). Penetrance estimates from cohort studies varies considerably for each gene, ranging from ~3% for NF1 mutations to 90% for SDHD mutations.6 7 As a corollary of the observed imperfect penetrance, the frequency of potentially pathogenic variants within the population should be higher than the empiric figure presented above. It is also appreciated that individual pathogenic variants in the same gene may have differing functional impact and hence penetrance, a good example being the BRCA1 c.5096G>A (p.R1699Q) variant having moderate penetrance as opposed to the highly penetrant c.5095C>T (p.R1699W).9 This variable penetrance complicates genetic counselling.
Penetrance for SDHx variants has been somewhat controversial. Initial estimates suggested penetrance for SDHB variants of between 45% and 77% at ages 40–60 years,10–12 likely inflated, however, by inclusion of index cases. Subsequent analyses excluding index cases have suggested a much lower lifetime penetrance for SDHB variants of 22%–30%,11 13 14 although Rijken et al 15 have reported penetrance for SDHB variants of 42.1% (34.8%–49.5%) at 70 years and Jochmanova et al 16 reported penetrance of 49.80% (95% CI 29 to 74.9) at 85 years. Family-based penetrance studies in SDHB kindreds have suggested penetrance of 26%–35% by age 50 years.17–19 A large number of possible confounders might explain these differences, including referral bias, intensity of carrier screening, genotype–phenotype correlation or other genetic and/or environmental modifiers. A recent study of a relatively small number of SDHA variants reported penetrance of 39% at 40 years, but significantly less (13%) when index cases were removed.20 Penetrance for SDHC variants is as yet unknown.
An elegant approach to estimating penetrance of pathogenic variants was recently proposed by Vassos et al 21 and extended by Minikel et al 22 and Stessman et al,23 using an algorithm that compares variant allelic frequency in disease cases to its frequency in large population control cohorts such as the Exome Aggregate Consortium (ExAC), and accounting for known disease prevalence and proportion of hereditary cases for that disease.
In this study, we tested the hypothesis that allelic frequencies for pathogenic SDHA–C variants present in ExAC would be inversely proportional to their penetrance, using two cohorts of PC/PGL subjects in whom genetic testing had been performed. We excluded SDHD variants from this analysis since this gene is unsuited to Bayesian methodology due to imprinting and suboptimal coverage in whole exome sequencing.
Methods
Clinical methods
Australian patients with PC/PGL referred to the Cancer Genetics Diagnostic Laboratory, Royal North Shore Hospital, were tested for RET, VHL, SDHA, SDHB, SDHC and SDHD according to previously published methodology.24–26 Genetic testing was triaged initially by an in-house protocol and more recently according to PC/PGL Clinical Practice Guidelines,6 with the additional use of tumour SDHB immunohistochemistry27 to guide testing of SDH subunit genes. Testing of a sample was performed iteratively and stopped when a variant was identified and considered to be pathogenic/likely pathogenic (P/LP) by one of the following criteria: (1) the variant was described as P/LP in a disease-specific database (ARUP, Leiden Open Variation Database (LOVD) or ClinVar); (2) null variant or missense variant predicted to be damaging or deleterious by at least two in silico tools and a functional study to support damaging effect (eg, in the case of SDHx variants, loss of SDHB immunostaining in tumour) or (3) the variant was present in multiple affected family members. UK patients were analysed for SDHB/SDHC/SDHD/VHL mutations by Sanger sequencing (until 2012) and then mostly by a next generation sequencing assay of MAX, RET, SDHA, SDHB, SDHC, SDHD, SDHAF2, TMEM127 or VHL.28 SDHB and SDHC sequence variants were classified as P/LP/benign/variants of uncertain significance by the reporting diagnostic laboratory. The GenBank Accession numbers were as follows: for SDHA NG_012339.1, NM_004168.3; for SDHB NG_012340.1, NM_003000.2 and for SDHC NG_012767.1, NM_003001.3.
Comparison of allele frequencies (table 1) between the Cohort Aus and Cohort UK was performed by G-test of Independence in DescTools R package V.0.99.24.
Table 1.
SDHA-C variants in cases from cohorts 1 (Aus) and 2 (UK) and also present in ExAC
| Gene | Variants† | LOVD ID (26) | Number of probands (allele frequencies, %) | Allele frequencies in ExAC (-TCGA) | |||
| Aus (n=575) | UK (n=1240) | Combined, % | Total, % | European (non-Finnish), % | |||
| SDHA | c.91C>T, p.Arg31* | SDHA_000013 | 3 (0.26) | n/a | 0.014 | 0.026 | |
| c.512G>A, p.Arg171His | Novel | 1 (0.087) | n/a | 0.0009 | 0 | ||
| Non-ExAC variants | 5 (0.43) | ||||||
| Total | 9 (0.78) | n/a | 0.78 | 0.015 | 0.022 | ||
| SDHB | c.79C>T, p.Arg27* | SDHB_000150 | 2 (0.17) | 8 (0.32) | 0.28 | 0.001 | 0 |
| c.88delC, p.Gln30Argfs*47 | SDHB_000017 | 3 (0.26) | 7 (0.28) | 0.28 | 0.0009 | 0.0018 | |
| c.136C>T, p.Arg46* | SDHB_000021 | 4 (0.35) | 11 (0.44) | 0.41 | 0.0019 | 0.0018 | |
| c.268C>T, p.Arg90* | SDHB_000001 | 14 (1.2) | 8 (0.32) | 0.61 | 0.001 | 0.0019 | |
| c.423+1G>A | SDHB_000047 | 2 (0.17) | 3 (0.12) | 0.14 | 0.0009 | 0.0018 | |
| c.343C>T, p.Arg115* | SDHB_000042 | 0 (0) | 6 (0.24) | 0.17 | 0.0019 | 0.0037 | |
| c.649C>G, p.Arg217Gly | novel | 0 (0) | 1 (0.04) | 0.028 | 0.001 | 0.002 | |
| c.688C>T, p.Arg230Cys | SDHB_000058 | 0 (0) | 1 (0.04) | 0.028 | 0.0009 | 0.0018 | |
| c.725G>A, p.Arg242His | SDHB_000004 | 5 (0.43) | 3 (0.12) | 0.22 | 0.0028 | 0.0018 | |
| Non-ExAC variants | 60 (5.2) | 239 (9.6) | 8.20 | ||||
| Total | 90 (7.8) | 287 (11.6) | 10.0 | 0.012 | 0.014 | ||
| SDHC | c.77+2dupT | SDHC_000049 | 0 (0) | 1 (0.04) | 0.028 | 0.0009 | 0.0018 |
| c.397C>T, p.Arg133* | SDHC_000015 | 1 (0.087) | 5 (0.2) | 0.17 | 0.0028 | 0.0018 | |
| Non-ExAC variants | 6 (0.52) | 24 (0.97) | 0.83 | ||||
| Total | 7 (0.61) | 30 (1.2) | 1.0 | 0.0038 | 0.0029 | ||
†RefSeq: for SDHA NG_012339.1, NM_004168.3; for SDHB NG_012340.1, NM_003000.2; for SDHC NG_012767.1, NM_003001.3.
ExAC, Exome Aggregate Consortium; SDHx, succinate dehydrogenase subunits A, B and C; TCGA, The Cancer Genome Atlas.
Bold values are totals.
Northern Sydney Local Health District Human Research Ethics Committee (Executive)noted that this project involves the use of existing data for the purpose of publishing figures on the occurrence of pathogenic variants. All subjects had given written informed consent for clinical genetic testing. The data being used are de-identified. Based on this information and in accordance with the National Health and Medical Research Council National Statement 2007—Section 5.1.22, the NSW Supplement to the National Statement—Section 5.1.6 and NSW Health Guideline GL2007_020: Quality Improvement and Ethics Review: A Practice Guide for NSW, this project was assessed as activity not requiring full HREC review.
LOVD search method
The LOVD (http://www.lovd.nl)29 was manually searched for variants in SDHA–C subunit genes and retrieved 59 unique variants in SDHA, 260 variants in SDHB and 66 variants in SDHC. Variants common both to LOVD and ExAC (http://exac.broadinstitute.org) and absent from The Cancer Genome Consortium (ftp://ftp.broadinstitute.org/pub/ExAC_release/release1/subsets/ExAC_nonTCGA.r1.sites.vep.vcf.gz) were tabulated according to allelic frequency in ExAC.
Penetrance and CI calculation
Bayesian calculation of the conditional probability of disease (penetrance) given the genotype was performed using the following formula23:
where = disease, = genotype, and = absence of disease.
The denominator, equivalent to P(G), is the sum of joint probabilities of with respect to both and which are mutually exclusive and collectively exhaustive of all possible events.
is the penetrance (the probability of disease given a genotype); is the genotype frequency in cases; is the allele frequency in ExAC and is the general population prevalence for PC/PGL, assumed ~1/3000.8
CI was obtained on the binomial probability as described by Rosenfeld et al.30 Upper bound CI for penetrance using upper bound on and the lower bound on . Lower bound CI for penetrance using lower bound on and the upper bound on . Data from cases and from ExAC were used to estimate these frequencies.
Figures were plotted by using a modification of Minikel et al,22 and source code is provided online at https://github.com/ericminikel/prnp_penetrance.
Results
Cohort 1 consisted of 575 Australian subjects presenting with PC/PGL for whom genetic testing was performed between 1998 and 2016. Overall, 172 subjects (29.9%) with PC/PGL were diagnosed with a P/LP variant in one of nine genes. P/LP SDHA–C variants were identified in 106 subjects (nine SDHA, 90 SDHB and seven SDHC). By comparison, P/LP variants in other genes were: 36 SDHD, nine RET, 15 VHL, four TMEM127, one FH and one MAX.
Cohort 2 consisted of 1240 UK subjects presenting with PC/PGL for whom genetic testing was performed between 2001 and 2017.14 Overall, 446 subjects (36%) with PC/PGL were diagnosed with a P/LP variant in one of nine genes. P/LP SDHA–C variants were identified in 317 subjects (287 SDHB and 30 SDHC) and P/LP variants in other genes were 96 SDHD, 25 VHL, two RET, two FH, one TMEM127, two MAX and one SDHAF2.
We inferred pathogenicity for each variant from published evidence29 and/or based on segregation or loss of heterozygosity or the absence of protein on immunohistochemistry (table 1, online Supplementary table 1). Criteria for P/LP variants were consistent with standards for the interpretation of sequence variants issued by the American College of Medical Genetics and Genomics (ACMG).31
jmedgenet-2018-105427supp001.docx (118.6KB, docx)
We compared SDHA–C variants considered P/LP in either cohort against the high confidence variant calls in the ExAC database from which The Cancer Genome Atlas (TCGA) cases had been removed (obtained from ftp://ftp.broadinstitute.org/pub/ExAC_release/release1/subsets/, 22 June 2017) in order to diminish the risk of confounding by disease inclusion in cases. The allelic frequency of 13 variants that are present in ExAC are shown in table 1 and those that are not reported in ExAC are shown in the online Supplementary table 1. For completeness, we have listed in online Supplementary table 2 all previously reported SDHA–C variants from ClinVar that are also present in ExAC.
jmedgenet-2018-105427supp002.docx (108.9KB, docx)
For variants in SDHB and SDHC, allelic frequencies were not significantly different between Aus and UK cohorts: using G-test of independence, G=1.2858, 10 df, p=0.9995 and post hoc pairwise G-test found a coefficient value of 0.8897441 between the two cohorts. We therefore combined the two cohorts for subsequent analyses. We note that when all variants (including those not present in ExAC) were considered, SDHB variants were collectively more frequent in cohort 2 (23.2%) than in cohort 1 (15.6%) (table 1). This difference was not confined to a particular type of mutation (online Supplementary table 1) and therefore unlikely to be due to any systematic difference in variant detection method. The collective frequency of SDHC variants was similar in both cohorts (table 1).
Of these 13 P/LP SDHx variants in ExAC, all are individually rare with the exception of SDHA variant c.91C>T, p.Arg31* (frequency 1/3036). Although individually rare, when the population frequencies for these variants were combined together (excluding SDHA p.Arg31*), the estimated population prevalence of these hereditary PGL syndromes assuming complete penetrance would be 1/6000.
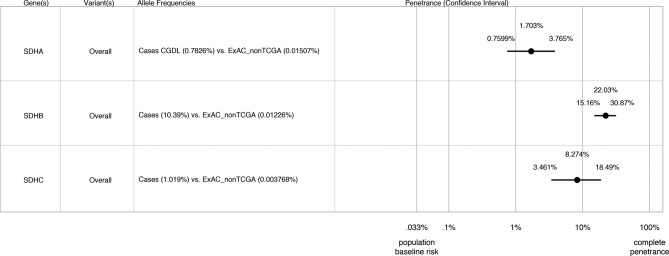
We next applied the principles described in Minikel et al 22, using the algorithm described by Stessman et al 23 and with CIs calculated as described by Rosenfeld et al 30 to estimate the lifetime penetrance of PC/PGL for SDHA–C variants taking into account allelic frequencies in our cases versus ExAC controls and estimated population prevalence of these disorders. Our penetrance estimates are shown in figure 1: predicted lifetime penetrance for SDHB variants is 22.0% (95% (CI 15.2% to 30.9%), for SDHC variants 8.3% (95% CI 3.5% to 18.5%) and for SDHA variants 1.7% (95% CI 0.8% to 3.8%). Penetrance estimates did not vary significantly by individual allele either in separate cohorts or in the combined analysis (online Supplementary figure 1). Although population stratification is not relevant when considering pathogenic variants causing monogenic disease (in the absence of a founder effect), nevertheless, to account for possible confounding by ethnicity, we also compared allelic frequencies in our cases against the European non-Finnish exome data from ExAC. As shown in the online Supplementary figure 2, exclusion of non-European/Finnish alleles from the control data set did not significantly alter our penetrance estimates although did result in wider CIs due to inclusion of fewer variants (for SDHA 1.2%, 95% CI 0.5% to 2.7%; for SDHB 20.1%, 95% CI 12.8% to 30.0% and for SDHC 10.4%, 95% CI 3.4% to 27.6%.
Figure 1.
Estimated lifetime penetrance of pheochromocytoma/paraganglioma (PC/PGL) in subjects heterozygous for genetic variants in succinate dehydrogenase subunits A, B and C (SDHA, SDHB and SDHC) from cohorts 1 and 2 combined. The algorithm used to calculate these estimates is based on Minikel et al 22 and takes into account allelic frequencies in cases versus Exome Aggregate Consortium (ExAC) controls and estimated population prevalence of these disorders.
jmedgenet-2018-105427supp003.jpg (2.7MB, jpg)
jmedgenet-2018-105427supp004.jpg (264.1KB, jpg)
Discussion
We have systematically addressed the possibility of low penetrance alleles in hereditary PC/PGL syndromes, using a recently described approach for correlating penetrance with allelic frequency.22 Correct assignment of pathogenecity for genetic variants has become an urgent problem facing the clinical genetics community, particularly with increasing use of whole exome/genome sequencing technology.31 32 Our study has several notable results: first, P/LP SDHB, SDHC and SDHA variants are more common in ExAC than expected; second, our Bayesian estimate of lifetime penetrance for SDHB variants is close to empiric data from cohort studies and third, SDHC and SDHA variants have low penetrance.
Although each are individually rare, the collective frequency of known P/LP SDHB, SDHC and SDHA variants in ExAC was highly surprising and may have several explanations: (1) these hereditary endocrine disorders are more common than previously thought due to the presence either of subpenetrant alleles or incomplete case ascertainment; (2) development of these disorders requires additional genetic modifiers, the absence of which diminishes disease risk in carriers of P/LP alleles; (3) the ExAC database is inadvertently enriched for PC/PGL subjects (unlikely) or (4) that the ExAC database contains sequencing errors (unlikely). On one hand, it is attractive to dismiss these findings as variant calling artefacts present in the ExAC database; however, population frequency estimates for pathogenic BRCA mutations inferred in a similar fashion are extremely close to sequencing estimates from a randomly selected Australian patient pool in the Lifehouse study.33 Again, these estimates and population screening findings are at least twofold higher than previously perceived population estimates. That these variants are more common than expected is non-trivial, if whole exome/genome sequencing is performed at a population level, when apparently healthy subjects carrying so-called pathogenic alleles will outnumber subjects identified on the basis of disease expression34: if indeed present in 0.017% of the population, then ~4000 subjects in Australia and ~11 000 subjects in the UK are carrying these P/LP SDHx variants.
We deliberately excluded SDHD variants from our analysis, since this gene is unsuited to Bayesian methodology due to imprinting and suboptimal coverage in whole exome sequencing. (Only one of 37 different P/LP SDHD variants from our cohorts was present in ExAC, data not shown.) Paternally inherited SDHD variants are associated with high penetrance of disease35 and would therefore be expected to be rare in the general population.
The finding that SDHA c.91C>T, p.Arg31* occurs in ExAC at a population frequency >10−4 is at first glance surprising: several reports have shown an association between this variant with either PGLs or gastrointestinal stromal tumours36 37; it is more frequently reported in PGLs than expected by chance, and bona fide loss of function was inferred from tumoural loss of heterozygosity at this locus and by the absence of SDHA assessed by immunohistochemistry.37 However, familial disease appears to be rare in association with this variant20 consistent with low penetrance.
For SDHB variants, calculated lifetime penetrance estimates appear close to recent empiric data,11–14 and the lower penetrance estimates for SDHA and SDHC conform to our anecdotal experience. It is interesting to note that penetrance and risk of multifocal disease seem to be related, that is, ~30% subjects with SDHB variants will have more than one PGL or PC, whereas very few subjects with SDHA variants develop multifocal disease.20 This deserves further study with larger cohorts of specific genotypes.
Shah et al 38 recently used whole genome sequence data from 10 495 unrelated individuals (with replication in public data from more than 138 000 exomes/genomes in gnomAD) to study population frequency of pathogenic variants in ACMG-recommended 59 gene-condition sets, including SDHB and SDHD. They found that SDHB and SDHD P/LP variants were more than 10-fold inflated in the population compared with expected population prevalence of hereditary PC/PGL, and with one possible explanation being that some variants may have been misclassified. The alternate explanation that the inflation is due to incomplete penetrance is supported by our data with some frequent variants being significantly inflated in two clinically ascertained datasets in a consistent manner. Those 13 SDHx variants in ExAC that we have observed in our PC/PGL cases all have very strong evidence of pathogenicity in LOVD and/or ClinVar. Indeed, 12 of these variants have a ClinVar star rating of 2 (multiple submitters with assertion criteria). Moreover, nine of these variants are loss-of-function (premature termination or splice site) variants. That these variants are more frequent in the population than expected for the corresponding disease prevalence can only mean either that they are subpenetrant and/or that the disease itself is more common than realised. The fact that our Bayesian estimates for SDHB are so close to empiric findings from recent cohort studies11–14 and to family-based studies17–19 gives us confidence that our estimates for SDHC and SDHA are also reliable.
Our study has some important limitations. We deliberately chose a validation cohort from a population with close genetic similarity to the discovery cohort,39 and naturally our findings may not apply to populations with different ethnic backgrounds; indeed, it will be interesting to compare allelic frequencies of these variants in populations worldwide. These algorithms may underestimate penetrance for variants not present in ExAC; some studies12 14 15 have suggested that certain SDHB variants are more penetrant. With respect to using ExAC data as controls, we attempted to minimise confounding by using the data set from which TCGA cases had been removed; it is remotely possible that PC/PGL cases were inadvertently enriched in other cohorts contributing to ExAC (eg, within cardiovascular cohorts). Finally, it is possible that an iterative testing process may have missed combinations of pathogenic variants in two or more genes; although our subsequent experience using massively parallel sequencing approaches suggests that the presence of two germline pathogenic variants is rare (data not shown).
While our manuscript was under review, Maniam et al 40 reported a similar Bayesian approach to calculate penetrance for SDHA variants at 0.1%–4.9%, although their study was based on published series of SDHA cases rather than as we have done using PPGL case cohorts. Despite these differences in case ascertainment, the similarity of penetrance estimates between the two studies is striking and consistent with our conclusion that pathogenic SDHA variants are likely to have low penetrance for disease expression.
We conclude that this approach of using population frequency of suspected P/LP variants in ExAC is extremely useful to validate empiric calculations from cohort studies. Our data suggests that at least for P/LP variants present in ExAC, penetrance is approximately 22% for SDHB variants, 8.3% for SDHC variants and 1.7% for SDHA variants. Our findings will have critical value for genetic counselling and screening of subjects carrying these P/LP variants. By more robust stratification of risk, rational allocation of biochemical and imaging surveillance could reduce both the cost and anxiety associated with carrying a germline mutation.
Electronic database information.
ExAC Browser, http://exac.broadinstitute.org.
Mutation Taster, http://www.mutationtaster.org/.
OMIM, http://www.omim.org/.
PolyPhen-2, http://genetics.bwh.harvard.edu/pph2/.
PROVEAN, http://provean.jcvi.org.
Acknowledgments
We are grateful to Dr Warren Kaplan and Dr Marcel Dinger for helpful discussions.
Footnotes
Contributors: MF and RJC-B conceived the study and wrote the manuscript. DEB, TD, CL, ALR, BR and RJC-B were responsible for curating the genetic test results for Cohort 1, and KAA and ERM were responsible for Cohort 2. Additional oversight of the clinical cohorts was provided by JB, AC, AJG, RJH, HM, RS, LT, AT, and KT. DEB, MW and EK confirmed pathogenicity for each variant. DEB, YZ, MF and RJC-B performed the initial analyses, with input from ELD, RWT and AS. All authors had full access to the data, and contributed to review of the manuscript.
Funding: This work was supported by NHMRC Project 1108032 to DEB, RT, ED, TD, KT, AJG, BGR, RH, AT and RJC-B and Hillcrest Foundation (Perpetual Trustees) to DB and TD.
Competing interests: None declared.
Patient consent: Not requried.
Ethics approval: Northern Sydney Local Health District Human Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Gimenez-Roqueplo AP, Dahia PL, Robledo M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm Metab Res 2012;44:328–33. 10.1055/s-0031-1301302 [DOI] [PubMed] [Google Scholar]
- 2. Dahia PL. Pheochromocytoma and paraganglioma pathogenesis: learning from genetic heterogeneity. Nat Rev Cancer 2014;14:108–19. 10.1038/nrc3648 [DOI] [PubMed] [Google Scholar]
- 3. Castro-Vega LJ, Lepoutre-Lussey C, Gimenez-Roqueplo AP, Favier J. Rethinking pheochromocytomas and paragangliomas from a genomic perspective. Oncogene 2016;35:1080–9. 10.1038/onc.2015.172 [DOI] [PubMed] [Google Scholar]
- 4. Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, McGuire AL, Nussbaum RL, O’Daniel JM, Ormond KE, Rehm HL, Watson MS, Williams MS, Biesecker LG. American College of Medical Genetics and Genomics. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med 2013;15:565–74. 10.1038/gim.2013.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kalia SS, Adelman K, Bale SJ, Chung WK, Eng C, Evans JP, Herman GE, Hufnagel SB, Klein TE, Korf BR, McKelvey KD, Ormond KE, Richards CS, Vlangos CN, Watson M, Martin CL, Miller DT. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med 2017;19:249–55. 10.1038/gim.2016.190 [DOI] [PubMed] [Google Scholar]
- 6. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF. Endocrine Society. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014;99:1915–42. 10.1210/jc.2014-1498 [DOI] [PubMed] [Google Scholar]
- 7. Toledo RA, Burnichon N, Cascon A, Benn DE, Bayley JP, Welander J, Tops CM, Firth H, Dwight T, Ercolino T, Mannelli M, Opocher G, Clifton-Bligh R, Gimm O, Maher ER, Robledo M, Gimenez-Roqueplo AP, Dahia PL. NGS in PPGL (NGSnPPGL) Study Group. Consensus Statement on next-generation-sequencing-based diagnostic testing of hereditary phaeochromocytomas and paragangliomas. Nat Rev Endocrinol 2017;13:233–47. 10.1038/nrendo.2016.185 [DOI] [PubMed] [Google Scholar]
- 8. Pacak K, Lenders JWM, Eisenhofer G. Pheochromocytoma: diagnosis, localization and treatment. Blackwell; Malden, MA, 2007:41–71. [Google Scholar]
- 9. Spurdle AB, Whiley PJ, Thompson B, Feng B, Healey S, Brown MA, Pettigrew C, Van Asperen CJ, Ausems MG, Kattentidt-Mouravieva AA, van den Ouweland AM, Lindblom A, Pigg MH, Schmutzler RK, Engel C, Meindl A, Caputo S, Sinilnikova OM, Lidereau R, Couch FJ, Guidugli L, Hansen T, Thomassen M, Eccles DM, Tucker K, Benitez J, Domchek SM, Toland AE, Van Rensburg EJ, Wappenschmidt B, Borg Å, Vreeswijk MP, Goldgar DE. kConFab Dutch Belgium UV Consortium German Consortium of Hereditary Breast and Ovarian Cancer French COVAR group collaborators ENIGMA Consortium. BRCA1 R1699Q variant displaying ambiguous functional abrogation confers intermediate breast and ovarian cancer risk. J Med Genet 2012;49:525–32. 10.1136/jmedgenet-2012-101037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Benn DE, Gimenez-Roqueplo AP, Reilly JR, Bertherat J, Burgess J, Byth K, Croxson M, Dahia PL, Elston M, Gimm O, Henley D, Herman P, Murday V, Niccoli-Sire P, Pasieka JL, Rohmer V, Tucker K, Jeunemaitre X, Marsh DJ, Plouin PF, Robinson BG. Clinical presentation and penetrance of pheochromocytoma/paraganglioma syndromes. J Clin Endocrinol Metab 2006;91:827–36. 10.1210/jc.2005-1862 [DOI] [PubMed] [Google Scholar]
- 11. Srirangalingam U, Walker L, Khoo B, MacDonald F, Gardner D, Wilkin TJ, Skelly RH, George E, Spooner D, Monson JP, Grossman AB, Akker SA, Pollard PJ, Plowman N, Avril N, Berney DM, Burrin JM, Reznek RH, Kumar VK, Maher ER, Chew SL. Clinical manifestations of familial paraganglioma and phaeochromocytomas in succinate dehydrogenase B (SDH-B) gene mutation carriers. Clin Endocrinol 2008;69:587–96. 10.1111/j.1365-2265.2008.03274.x [DOI] [PubMed] [Google Scholar]
- 12. Ricketts CJ, Forman JR, Rattenberry E, Bradshaw N, Lalloo F, Izatt L, Cole TR, Armstrong R, Kumar VK, Morrison PJ, Atkinson AB, Douglas F, Ball SG, Cook J, Srirangalingam U, Killick P, Kirby G, Aylwin S, Woodward ER, Evans DG, Hodgson SV, Murday V, Chew SL, Connell JM, Blundell TL, Macdonald F, Maher ER. Tumor risks and genotype-phenotype-proteotype analysis in 358 patients with germline mutations in SDHB and SDHD . Hum Mutat 2010;31:41–51. 10.1002/humu.21136 [DOI] [PubMed] [Google Scholar]
- 13. Schiavi F, Milne RL, Anda E, Blay P, Castellano M, Opocher G, Robledo M, Cascón A. Are we overestimating the penetrance of mutations in SDHB? Hum Mutat 2010;31:761–2. 10.1002/humu.21269 [DOI] [PubMed] [Google Scholar]
- 14. Andrews KA, Ascher DB, Pires DEV, Barnes DR, Vialard L, Casey RT, Bradshaw N, Adlard J, Aylwin S, Brennan P, Brewer C, Cole T, Cook JA, Davidson R, Donaldson A, Fryer A, Greenhalgh L, Hodgson SV, Irving R, Lalloo F, McConachie M, McConnell VPM, Morrison PJ, Murday V, Park SM, Simpson HL, Snape K, Stewart S, Tomkins SE, Wallis Y, Izatt L, Goudie D, Lindsay RS, Perry CG, Woodward ER, Antoniou AC, Maher ER. Tumour risks and genotype-phenotype correlations associated with germline variants in succinate dehydrogenase subunit genes SDHB, SDHC and SDHD . J Med Genet 2018;55:384–94. 10.1136/jmedgenet-2017-105127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rijken JA, Niemeijer ND, Jonker MA, Eijkelenkamp K, Jansen JC, van Berkel A, Timmers H, Kunst HPM, Bisschop P, Kerstens MN, Dreijerink KMA, van Dooren MF, van der Horst-Schrivers ANA, Hes FJ, Leemans CR, Corssmit EPM, Hensen EF. The penetrance of paraganglioma and pheochromocytoma in SDHB germline mutation carriers. Clin Genet 2018;93 10.1111/cge.13055 [DOI] [PubMed] [Google Scholar]
- 16. Jochmanova I, Wolf KI, King KS, Nambuba J, Wesley R, Martucci V, Raygada M, Adams KT, Prodanov T, Fojo AT, Lazurova I, Pacak K. SDHB-related pheochromocytoma and paraganglioma penetrance and genotype-phenotype correlations. J Cancer Res Clin Oncol 2017;143:1421–35. 10.1007/s00432-017-2397-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solis DC, Burnichon N, Timmers HJ, Raygada MJ, Kozupa A, Merino MJ, Makey D, Adams KT, Venisse A, Gimenez-Roqueplo AP, Pacak K. Penetrance and clinical consequences of a gross SDHB deletion in a large family. Clin Genet 2009;75:354–63. 10.1111/j.1399-0004.2009.01157.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hes FJ, Weiss MM, Woortman SA, de Miranda NF, van Bunderen PA, Bonsing BA, Stokkel MP, Morreau H, Romijn JA, Jansen JC, Vriends AH, Bayley JP, Corssmit EP. Low penetrance of a SDHB mutation in a large Dutch paraganglioma family. BMC Med Genet 2010;11:92 10.1186/1471-2350-11-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rijken JA, Niemeijer ND, Corssmit EP, Jonker MA, Leemans CR, Menko FH, Hensen EF. Low penetrance of paraganglioma and pheochromocytoma in an extended kindred with a germline SDHB exon 3 deletion. Clin Genet 2016;89:128–32. 10.1111/cge.12591 [DOI] [PubMed] [Google Scholar]
- 20. Bausch B, Schiavi F, Ni Y, Welander J, Patocs A, Ngeow J, Wellner U, Malinoc A, Taschin E, Barbon G, Lanza V, Söderkvist P, Stenman A, Larsson C, Svahn F, Chen JL, Marquard J, Fraenkel M, Walter MA, Peczkowska M, Prejbisz A, Jarzab B, Hasse-Lazar K, Petersenn S, Moeller LC, Meyer A, Reisch N, Trupka A, Brase C, Galiano M, Preuss SF, Kwok P, Lendvai N, Berisha G, Makay Ö, Boedeker CC, Weryha G, Racz K, Januszewicz A, Walz MK, Gimm O, Opocher G, Eng C, Neumann HPH. European-American-Asian Pheochromocytoma-Paraganglioma Registry Study Group. Clinical characterization of the pheochromocytoma and paraganglioma susceptibility genes SDHA, TMEM127, MAX, and SDHAF2 for gene-informed prevention. JAMA Oncol 2017;3:1204 10.1001/jamaoncol.2017.0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vassos E, Collier DA, Holden S, Patch C, Rujescu D, St Clair D, Lewis CM. Penetrance for copy number variants associated with schizophrenia. Hum Mol Genet 2010;19:3477–81. 10.1093/hmg/ddq259 [DOI] [PubMed] [Google Scholar]
- 22. Minikel EV, Vallabh SM, Lek M, Estrada K, Samocha KE, Sathirapongsasuti JF, McLean CY, Tung JY, Yu LP, Gambetti P, Blevins J, Zhang S, Cohen Y, Chen W, Yamada M, Hamaguchi T, Sanjo N, Mizusawa H, Nakamura Y, Kitamoto T, Collins SJ, Boyd A, Will RG, Knight R, Ponto C, Zerr I, Kraus TF, Eigenbrod S, Giese A, Calero M, de Pedro-Cuesta J, Haïk S, Laplanche JL, Bouaziz-Amar E, Brandel JP, Capellari S, Parchi P, Poleggi A, Ladogana A, O’Donnell-Luria AH, Karczewski KJ, Marshall JL, Boehnke M, Laakso M, Mohlke KL, Kähler A, Chambert K, McCarroll S, Sullivan PF, Hultman CM, Purcell SM, Sklar P, van der Lee SJ, Rozemuller A, Jansen C, Hofman A, Kraaij R, van Rooij JG, Ikram MA, Uitterlinden AG, van Duijn CM, Daly MJ, MacArthur DG. Exome Aggregation Consortium (ExAC). Quantifying prion disease penetrance using large population control cohorts. Sci Transl Med 2016;8:322ra9 10.1126/scitranslmed.aad5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stessman HAF, Willemsen MH, Fenckova M, Penn O, Hoischen A, Xiong B, Wang T, Hoekzema K, Vives L, Vogel I, Brunner HG, van der Burgt I, Ockeloen CW, Schuurs-Hoeijmakers JH, Klein Wassink-Ruiter JS, Stumpel C, Stevens SJC, Vles HS, Marcelis CM, van Bokhoven H, Cantagrel V, Colleaux L, Nicouleau M, Lyonnet S, Bernier RA, Gerdts J, Coe BP, Romano C, Alberti A, Grillo L, Scuderi C, Nordenskjöld M, Kvarnung M, Guo H, Xia K, Piton A, Gerard B, Genevieve D, Delobel B, Lehalle D, Perrin L, Prieur F, Thevenon J, Gecz J, Shaw M, Pfundt R, Keren B, Jacquette A, Schenck A, Eichler EE, Kleefstra T. Disruption of POGZ is associated with intellectual disability and autism spectrum disorders. Am J Hum Genet 2016;98:541–52. 10.1016/j.ajhg.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marsh DJ, Theodosopoulos G, Howell V, Richardson AL, Benn DE, Proos AL, Eng C, Robinson BG. Rapid mutation scanning of genes associated with familial cancer syndromes using denaturing high-performance liquid chromatography. Neoplasia 2001;3:236–44. 10.1038/sj.neo.7900154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meyer-Rochow GY, Smith JM, Richardson AL, Marsh DJ, Sidhu SB, Robinson BG, Benn DE. Denaturing high performance liquid chromatography detection of SDHB, SDHD, and VHL germline mutations in pheochromocytoma. J Surg Res 2009;157:55–62. 10.1016/j.jss.2008.07.043 [DOI] [PubMed] [Google Scholar]
- 26. Dwight T, Benn DE, Clarkson A, Vilain R, Lipton L, Robinson BG, Clifton-Bligh RJ, Gill AJ. Loss of SDHA expression identifies SDHA mutations in succinate dehydrogenase-deficient gastrointestinal stromal tumors. Am J Surg Pathol 2013;37:226–33. 10.1097/PAS.0b013e3182671155 [DOI] [PubMed] [Google Scholar]
- 27. Gill AJ, Benn DE, Chou A, Clarkson A, Muljono A, Meyer-Rochow GY, Richardson AL, Sidhu SB, Robinson BG, Clifton-Bligh RJ. Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma-pheochromocytoma syndromes. Hum Pathol 2010;41:805–14. 10.1016/j.humpath.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 28. Rattenberry E, Vialard L, Yeung A, Bair H, McKay K, Jafri M, Canham N, Cole TR, Denes J, Hodgson SV, Irving R, Izatt L, Korbonits M, Kumar AV, Lalloo F, Morrison PJ, Woodward ER, Macdonald F, Wallis Y, Maher ER. A comprehensive next generation sequencing-based genetic testing strategy to improve diagnosis of inherited pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 2013;98:E1248–56. 10.1210/jc.2013-1319 [DOI] [PubMed] [Google Scholar]
- 29. Bayley JP, Devilee P, Taschner PE. The SDH mutation database: an online resource for succinate dehydrogenase sequence variants involved in pheochromocytoma, paraganglioma and mitochondrial complex II deficiency. BMC Med Genet 2005;6:39 10.1186/1471-2350-6-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosenfeld JA, Coe BP, Eichler EE, Cuckle H, Shaffer LG. Estimates of penetrance for recurrent pathogenic copy-number variations. Genet Med 2013;15:478–81. 10.1038/gim.2012.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–23. 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Toledo RA, Dahia PL. Next-generation sequencing for the diagnosis of hereditary pheochromocytoma and paraganglioma syndromes. Curr Opin Endocrinol Diabetes Obes 2015;22:169–79. 10.1097/MED.0000000000000150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maxwell KN, Domchek SM, Nathanson KL, Robson ME. Population frequency of germline BRCA1/2 mutations. J Clin Oncol 2016;34:4183–5. 10.1200/JCO.2016.67.0554 [DOI] [PubMed] [Google Scholar]
- 34. Ding LE, Burnett L, Chesher D. The impact of reporting incidental findings from exome and whole-genome sequencing: predicted frequencies based on modeling. Genet Med 2015;17:197–204. 10.1038/gim.2014.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neumann HP, Pawlu C, Peczkowska M, Bausch B, McWhinney SR, Muresan M, Buchta M, Franke G, Klisch J, Bley TA, Hoegerle S, Boedeker CC, Opocher G, Schipper J, Januszewicz A, Eng C. European-American Paraganglioma Study Group. Distinct clinical features of paraganglioma syndromes associated with SDHB and SDHD gene mutations. JAMA 2004;292:943–51. 10.1001/jama.292.8.943 [DOI] [PubMed] [Google Scholar]
- 36. Korpershoek E, Favier J, Gaal J, Burnichon N, van Gessel B, Oudijk L, Badoual C, Gadessaud N, Venisse A, Bayley JP, van Dooren MF, de Herder WW, Tissier F, Plouin PF, van Nederveen FH, Dinjens WN, Gimenez-Roqueplo AP, de Krijger RR. SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab 2011;96:E1472–6. 10.1210/jc.2011-1043 [DOI] [PubMed] [Google Scholar]
- 37. Miettinen M, Killian JK, Wang ZF, Lasota J, Lau C, Jones L, Walker R, Pineda M, Zhu YJ, Kim SY, Helman L, Meltzer P. Immunohistochemical loss of succinate dehydrogenase subunit A (SDHA) in gastrointestinal stromal tumors (GISTs) signals SDHA germline mutation. Am J Surg Pathol 2013;37:234–40. 10.1097/PAS.0b013e3182671178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shah N, Hou YC, Yu HC, Sainger R, Caskey CT, Venter JC, Telenti A. Identification of misclassified ClinVar variants via disease population prevalence. Am J Hum Genet 2018;102:609–19. 10.1016/j.ajhg.2018.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Reveille JD, Sims AM, Danoy P, Evans DM, Leo P, Pointon JJ, Jin R, Zhou X, Bradbury LA, Appleton LH, Davis JC, Diekman L, Doan T, Dowling A, Duan R, Duncan EL, Farrar C, Hadler J, Harvey D, Karaderi T, Mogg R, Pomeroy E, Pryce K, Taylor J, Savage L, Deloukas P, Kumanduri V, Peltonen L, Ring SM, Whittaker P, Glazov E, Thomas GP, Maksymowych WP, Inman RD, Ward MM, Stone MA, Weisman MH, Wordsworth BP, Brown MA. Australo-Anglo-American Spondyloarthritis Consortium (TASC). Genome-wide association study of ankylosing spondylitis identifies non-MHC susceptibility loci. Nat Genet 2010;42:123–7. 10.1038/ng.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maniam P, Zhou K, Lonergan M, Berg JN, Goudie DR, Newey PJ. Pathogenicity and penetrance of germline SDHA variants in pheochromocytoma and paraganglioma (PPGL). J Endocr Soc 2018;2:806–16. 10.1210/js.2018-00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jmedgenet-2018-105427supp001.docx (118.6KB, docx)
jmedgenet-2018-105427supp002.docx (108.9KB, docx)
jmedgenet-2018-105427supp003.jpg (2.7MB, jpg)
jmedgenet-2018-105427supp004.jpg (264.1KB, jpg)