The exact pathogenesis of idiopathic inflammatory bowel disease (IBD) in humans as well as dogs remains largely unknown, although it is believed to represent a multifactorial immune-mediated disease, resulting from complex interplay between the mucosal immune system and the intestinal microbiome in genetically susceptible individuals.1 Many studies have attempted to identify inflammatory mediators in the mucosa of affected dogs, with no predominant cytokine pattern emerging.2 3 In human IBD, as well as mouse models of human IBD, activation of the inflammasome pathway with secretion of interleukin (IL)-1β and IL-18 has been shown to play an important role in the pathogenesis of the disease.4 5 In canine IBD, two previous studies have shown that IL-1β may be elevated in the intestinal mucosa of affected dogs.6–8 The purpose of the current study was to investigate duodenal levels of IL-1β protein in a larger population of dogs having varying clinical severity of IBD, and to correlate IL-1β expression profiles with a clinical scoring index (Canine Chronic Enteropathy Clinical Activity Index, CCECAI).9
Duodenal biopsies from 10 dogs each with mild IBD (CCECAI 0–5), moderate IBD (CCECAI 6–8), severe IBD (CCECAI ≥9), but no clinical signs of protein-losing enteropathy (PLE), and with serum albumin concentrations within the reference range, and dogs with PLE with clinical signs such as ascites or peripheral oedema, as well as 10 healthy control beagle dogs were included in the study. The diagnosis of IBD was confirmed in all dogs based on exclusion of any other causes for chronic diarrhoea,10 including a food trial and histopathologic findings of intestinal mucosal biopsies confirming lymphoplasmacytic, eosinophilic or mixed inflammation. In addition, at the time of IBD diagnosis, all dogs had a clinical scoring index (CCECAI) recorded by the attending clinician. The diagnosis of PLE was made if all of the following applied: (1) history of chronic gastrointestinal disease (including weight loss, vomiting, diarrhoea, decreased appetite), clinical signs of ascites and/or peripheral oedema, and diagnosis of IBD as above; (2) panhypoproteinaemia (serum albumin less than 2.8 g/dl and serum globulin less than 2.1 g/dl; reference ranges 2.8–3.9 and 2.1–4.1 g/dl, respectively); (3) exclusion of hepatic dysfunction by serum bile acid stimulation test; and (4) absence of proteinuria by urinalysis. Proteinuria was excluded in all dogs on the basis of a negative urine dipstick or a urine protein:creatinine ratio of less than 0.5 (reference range: less than 0.5). The healthy control dogs had been euthanased for an unrelated study and had duodenal endoscopic biopsies collected before terminal euthanasia. All healthy control dogs had normal duodenal histopathology findings.
Duodenal endoscopic biopsies were acquired at the time of diagnosis, and one additional biopsy was fresh frozen in a plain tube and stored at −80°C until batch analysis. Histopathology was performed in all dogs and was consistent with idiopathic IBD in all cases. In addition, in the PLE group, all cases showed histopathologically significant lacteal dilation. The dogs in the group of severe IBD but with no clinical signs of PLE showed histopathological infiltration with lymphocytes and plasma cells, as well as significant villus blunting; however, lacteal dilation or crypt abscesses were not observed in any of these cases. IL-1β protein expression in duodenal biopsies was measured according to a modified protocol that has been previously published.6 Briefly, biopsy samples were weighed prior to manipulation and equal weighted specimens were subsequently homogenised at 20 Hz for two minutes in 500 µl homogenisation buffer (PBS containing 0.05 per cent Tween-20) with a protease inhibitor cocktail.i Tissue homogenates were centrifuged at 400 g for two minutes and the supernatants frozen at −80°C until quantitative analysis. Quantification of IL-1β protein expression was determined using a commercially available canine-specific IL-1β ELISAii following the manufacturer’s assay instructions. The assay has a lower detection limit of 0.1 pg/ml and intra-assay and interassay coefficients of variation are less than or equal to 2.4 per cent and less than or equal to 11.8 per cent, respectively.ii
All samples were analysed in duplicate and values were expressed as mean protein concentration (pg/ml/mg tissue). Statistical analyses were performed using a Kruskal-Wallis test for comparison of IL-1β expression between disease groups, followed by a Dunn’s post hoc test. The relationships between IL-1β expression and CCECAI score was analysed using a Spearman rank correlation test.
Median IL-1β expression (range) for the total patient group (all IBD groups plus PLE group) was 18.8 (8.0–219.4) pg/ml/mg tissue. Median and range of IL-1β expression for the individual patient groups is summarised in Table 1.
TABLE 1:
Summary of median (range) IL-1β protein expression (pg/ml/mg tissue)
| Severe IBD |
Moderate IBD |
Mild IBD |
PLE | Healthy controls |
| 121.6 (38.3–219.4) |
19.7 (10.9–24.8) |
13.3 (8.0–29.8) |
13.0 (8.0–27.9) |
0.9 (0.3–1.4) |
IBD, inflammatory bowel disease; IL, interleukin; PLE, protein-losing enteropathy.
There was a statistically significant difference in IL-1β expression between all IBD dogs and the healthy controls (P<0.0001), with a 20-fold increase in IL-1β concentrations between the combined severity IBD group (mild, moderate or severe IBD) versus healthy control dogs (Fig 1). In addition, there was a statistically significant difference between the PLE and the severe IBD group (P<0.01), between the severe and moderate IBD group (P<0.001), and between the severe and mild IBD group (P<0.0001). There was no statistically significant difference between the moderate and mild IBD groups and the PLE and the healthy control groups (P=0.7). There was a moderate positive correlation between IL-1β protein expression and severity of disease as measured by CCECAI scores (r2=0.53, P<0.0001) (Fig 2).
FIG 1:
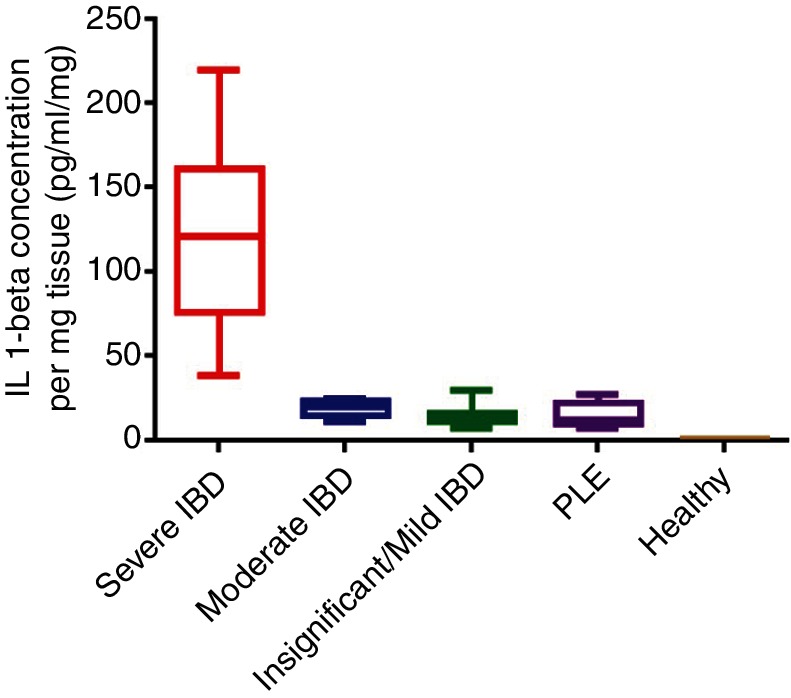
Box and whisker plots of interleukin (IL)-1β expression in the different groups of inflammatory bowel disease (IBD) and in healthy dogs. The plots show median (line within box), 25th and 75th percentiles (box), and minimum and maximum values (whiskers). There was a statistically significant difference in IL-1β expression between all IBD dogs and the healthy controls (P<0.0001). Mucosal IL-1β was increased 20-fold in the combined severity IBD group (mild, moderate or severe IBD) versus control dogs. PLE, protein-losing enteropathy.
FIG 2:
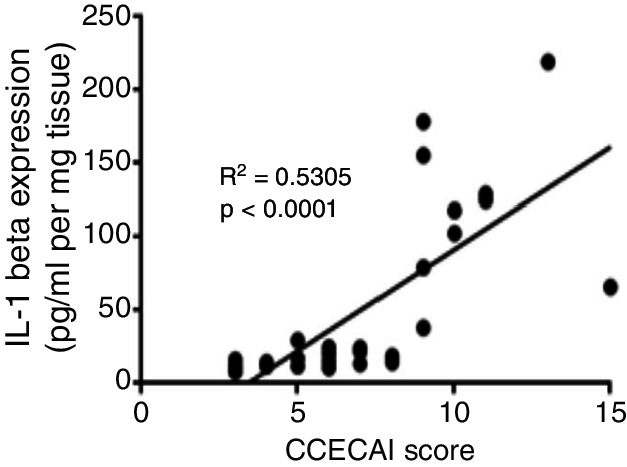
Spearman rank correlation between interleukin (IL)-1β expression in all groups of dogs with inflammatory bowel disease (IBD) and clinical disease activity score as measured by Canine Chronic Enteropathy Clinical Activity Index (CCECAI).9
Similar to what has been shown in human IBD, the data in this current study confirm previous reports of IL-1β protein expression being increased in the intestinal mucosa of dogs diagnosed with IBD.6–8 Interestingly, the highest expression levels were found in intestinal mucosa of dogs with severe IBD with serum albumin concentrations within the reference range, and not in dogs with PLE. This may point to a different pathogenesis between the clinical syndromes of severe IBD and PLE. However, it is also possible that there was an effect of the diet, treatments or supplements that was not taken into account. The number of cells producing IL-1β was not standardised because of the method used to detect IL-1β, which will have an effect on the amount of IL-1β measured per mg tissue. A further potential limitation of this study is that the group of dogs with severe IBD but without clinical signs of PLE and the PLE group may have overlapped, as faecal alpha1-protease inhibitor was not measured and would represent a more sensitive method for detection of PLE than hypoalbuminaemia.11 In addition, correlation of IL-1β concentrations with the severity of histological score was not performed and would have added additional information. Whole crushed biopsies were used for the measurement of IL-1β, which may falsely increase the amount of IL-1β in the supernatant. Furthermore, the cellular source of the IL-1β production cannot be determined with this method.
Footnotes
Aprotinin-based protease inhibitor cocktail, P3840, Sigma Aldrich.
Rabbit anti-canine IL-1β ELISA development kit, Kingfisher Biotech, St Paul, MN.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Ethics approval: The collection of additional duodenal samples for this study has been approved by the Royal Veterinary College Ethics Committee and the home office (licence 70/7393).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sartor RB. Clinical applications of advances in the genetics of IBD. Rev Gastroenterol Disord 2003;3(Suppl 1):S9–17. [PubMed] [Google Scholar]
- 2.Heilmann RM, Suchodolski JS. Is inflammatory bowel disease in dogs and cats associated with a Th1 or Th2 polarization? Vet Immunol Immunopathol 2015;168:131–4. 10.1016/j.vetimm.2015.10.008 [DOI] [PubMed] [Google Scholar]
- 3.Jergens AE, Sonea IM, O’Connor AM, et al. Intestinal cytokine mRNA expression in canine inflammatory bowel disease: a meta-analysis with critical appraisal. Comp Med 2009;59:153–62. [PMC free article] [PubMed] [Google Scholar]
- 4.Hirota SA, Ng J, Lueng A, et al. NLRP3 inflammasome plays a key role in the regulation of intestinal homeostasis. Inflamm Bowel Dis 2011;17:1359–72. 10.1002/ibd.21478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaki MH, Lamkanfi M, Kanneganti TD. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol 2011;32:171–9. 10.1016/j.it.2011.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maeda S, Ohno K, Nakamura K, et al. Mucosal imbalance of interleukin-1β and interleukin-1 receptor antagonist in canine inflammatory bowel disease. Vet J 2012;194:66–70. 10.1016/j.tvjl.2012.02.026 [DOI] [PubMed] [Google Scholar]
- 7.Schmitz S, Henrich M, Neiger R, et al. Stimulation of duodenal biopsies and whole blood from dogs with food-responsive chronic enteropathy and healthy dogs with Toll-like receptor ligands and probiotic Enterococcus faecium. Scand J Immunol 2014;80:85–94. 10.1111/sji.12186 [DOI] [PubMed] [Google Scholar]
- 8.Schmitz S, Werling D, Allenspach K. Effects of ex-vivo and in-vivo treatment with probiotics on the inflammasome in dogs with chronic enteropathy. PLoS One 2015;10:e0120779 10.1371/journal.pone.0120779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allenspach K, Wieland B, Gröne A, et al. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med 2007;21:700–8. 10.1111/j.1939-1676.2007.tb03011.x [DOI] [PubMed] [Google Scholar]
- 10.Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010;24:10–26. [DOI] [PubMed] [Google Scholar]
- 11.Murphy KF, German AJ, Ruaux CG, et al. Fecal alpha1-proteinase inhibitor concentration in dogs receiving long-term nonsteroidal anti-inflammatory drug therapy. Vet Clin Pathol 2003;32:136–9. 10.1111/j.1939-165X.2003.tb00326.x [DOI] [PubMed] [Google Scholar]


