Abstract
Antibodies against programmed cell death protein 1, such as nivolumab and pembrolizumab, are widely used for treating various cancers, including advanced melanoma. Nivolumab significantly prolongs survival in patients with metastatic melanoma, and sequential administration with lipilimumab may improve outcomes when switched at the appropriate time. Biomarkers are therefore needed to evaluate nivolumab efficacy soon after first administration. This study analyzed serum levels of soluble cluster of differentiation 163 (sCD163) in 59 cases of advanced cutaneous melanoma and 16 cases of advanced mucosal melanoma treated using nivolumab. Serum levels of sCD163 were significantly increased after 6 weeks in responders compared to non-responders after initial administration of nivolumab for cutaneous melanoma. In contrast, no significant difference between responders and non-responders was seen among patients with non-cutaneous melanoma. These results suggest that sCD163 may be useful as a biomarker for selecting patients with advanced cutaneous melanoma most likely to benefit from anti-melanoma immunotherapy.
Keywords: sCD163, TAMs, melanoma, nivolumab, prediction of efficacy
Introduction
The programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) pathway plays a critical role in tumor immune response. Anti-PD-1 antibodies such as nivolumab and pembrolizumab are thus in wide use for the treatment of various cancers, including advanced melanoma (1, 2). Nivolumab significantly prolongs survival in patients with metastatic melanoma (1), and co-administration with ipilimumab also leads to improved outcomes (1–3). Ipilimumab is a fully humanized immunoglobulin (Ig)G1 monoclonal antibody that blocks cytotoxic T-lymphocyte antigen (CTLA-4) to activate and increase T cells, and suppress the function of regulatory T cells (Tregs) (4). Previous reports have suggested ipilimumab is useful for treating advanced melanoma, particularly in combination with other anti-melanoma reagents (1–3, 5). Moreover, combination therapy using nivolumab and ipilimumab reportedly increases the response rate for untreated metastasis of melanoma to the brain (6). However, the efficacy of ipilimumab in patients with nivolumab-resistant melanoma is extremely low after objective tumor progression (7). Because both co-administration of nivolumab and ipilimumab and sequential administration of nivolumab and ipilimumab with a planned switch leads to a high frequency of immune-related adverse events (irAEs) in patients with advanced melanoma (1, 3), determining the efficacy of nivolumab monotherapy before the planned switch from nivolumab to ipilimumab is important.
Tumor-associated macrophages (TAMs) are characterized by their heterogeneity and plasticity, and may be functionally reprogrammed to polarized phenotypes by exposure to cancer-related factors, stromal factors, or infection, leading to the production of various chemokines that play significant roles in maintaining the tumor microenvironment (8–13). Because PD-1 expression in TAMs is one of the key factors in M2 macrophage polarization (14), administration of an anti-PD1 antibody might repolarize TAMs, leading to TAM activation in melanoma patients. Because the main population of TAMs in skin cancer is CD163+ M2 macrophages, and soluble cluster of differentiation 163 (sCD163) is a TAM marker that appears in the serum as a result of proteolytic shedding (15), we hypothesized that serum sCD163 may offer a predictive marker for the efficacy of nivolumab in the early stage of disease. This study analyzed serum levels of sCD163 in 59 cases of advanced cutaneous melanoma and 16 cases of advanced mucosal melanoma treated with nivolumab.
Patients and methods
Ethics statement for human experiments
The protocol for this human study was approved by the ethics committee of Tohoku University Graduate School of Medicine, Sendai, Japan (Permit No: 2017-1-064). All methods were performed in accordance with the relevant guidelines and regulations. All patients provided written informed consent.
Patients
Data from patients treated with nivolumab were collected from eight clinical sites in Japan. Patients were eligible if they had unresectable stage III melanoma, the tumor was resectable but the patient had declined resection, or if the patient had stage IV melanoma with accessible cutaneous, subcutaneous, and/or nodal lesions (staging was performed according to the AJCC Staging Manual, 7th edition, 2011). All patients received 2 mg/kg of nivolumab followed by a 3-week rest period or 3 mg/kg of nivolumab followed by 2 weeks of rest, both of which are approved dosing schedules in Japan. Serum from patients was obtained on days 0 and 42.
Serum levels of sCD163
On days 0 and 42 after nivolumab administration, we stored the serum and analyzed serum levels of soluble sCD163 by enzyme-linked immunoassay (ELISA) according to the protocol provided by the manufacturer (catalog number DY1607; R&D Systems, Minneapolis, MN). Data from each donor were obtained as the mean of duplicate assays. Serum levels on day 42 were compared to baseline (day 0) and statistically analyzed.
Statistical methods
Receiver operating characteristic (ROC) curves were applied to calculate cut-off values for serum levels of sCD163 and areas under the curve (AUCs). Cut-offs were determined using Youden's index (16) (sensitivity + specificity −1) to determine the point of maximum index value. ROC curves were established to evaluate serum levels of sCD163 in patients administered nivolumab. For a single comparison between two groups, the Mann-Whitney U-test was used. Levels of significance were set at p < 0.01. All statistical analyses were performed using JMP version 14.1 software (SAS Institute, Tokyo, Japan).
Results
Patients
We collected data from 75 melanoma patients treated with nivolumab, including 59 patients with cutaneous melanoma (Table 1) and 16 patients with non-cutaneous (e.g., digestive tract, vagina, nasal cavity) melanoma (Table 2). Mean patient age was 68.0 years for cutaneous melanoma (range, 31–93 years) and 66.0 years for non-cutaneous melanoma (range, 54–82 years). The percentages of male patients with cutaneous and non-cutaneous melanoma were 57.6 and 56.3%, respectively. The most common primary tumor site was the extremities (55.9%), followed by the head and neck (15.3%), trunk (11.9%), mucosal origin (8.5%), and unknown origin (8.5%).
Table 1.
Characteristics and serum levels of sCD163 in patients with cutaneous melanoma.
| Age (y) | Sex | Location | Body weight (kg) | Prior systemic therapy | Efficacy at 3 months | Increase of sCD163 | Change ratio of sCD163 (%) | |
|---|---|---|---|---|---|---|---|---|
| No. 1 | 61–70 | M | Extremities | 63.2 | DAV+IFN-β | PR | 11.556 | 149 |
| No. 2 | 61–70 | M | Extremities | 48 | – | PR | 50.8525 | 227.8 |
| No. 3 | 81–90 | F | Extremities | 48.7 | DPCP | PR | 12.676 | 152.7 |
| No. 4 | 61–70 | M | Extremities | 70.4 | DAV+IFN-β | PR | 13.27122 | 146.2 |
| No. 5 | 61–70 | M | Extremities | 58.5 | DAV+IFN-β | PR | 7.17709 | 127 |
| No. 6 | 61–70 | M | Lip | 69.8 | DAV+IFN-β | PR | 5.834 | 123.1 |
| No. 7 | 71–80 | M | Trunk | 59.4 | – | PR | 21.49956 | 133.4 |
| No. 8 | 71–80 | F | Extremities | 68 | – | PR | −12.9704 | 81.6 |
| No. 9 | 31–40 | F | Extremities | 55.4 | DTIC+IFN-β | PR | 24.5042321 | 189.5 |
| No. 10 | 71–80 | M | Extremities | 61.2 | – | PR | 3.36944 | 109.5 |
| No. 11 | 51–60 | F | Extremities | 41.7 | – | CR | 2.109187 | 170.7 |
| No. 12 | 81–90 | M | Unknown | 68.3 | – | PR | 3.07118 | 111.3 |
| No. 13 | 71–80 | F | Extremities | 50.1 | IFN-α | PR | 35.7011169 | 158.5 |
| No. 1 | 51–60 | M | Trunk | 68.7 | – | SD | 1.5625 | 107.5 |
| No. 2 | 31–40 | F | Extremities | 60.8 | CBDCA+PTX | PD | −9.6755 | 82.4 |
| No. 3 | 61–70 | M | Trunk | 62 | DTIC+IFN-β | PD | 2.884 | 109.6 |
| No. 4 | 81–90 | F | Extremities | 44.9 | IFN-α | PD | −0.333 | 98.7 |
| No. 5 | 71–80 | M | Head and neck | 59.2 | IFN-β | SD | 0.998 | 103.6 |
| No. 6 | 81–90 | F | Trunk | 41.9 | IFN-β | PD | 6.76453 | 116.9 |
| No. 7 | 91–100 | M | Extremities | 55 | IFN-β | PD | 4.02405 | 113 |
| No. 8 | 71–80 | M | Extremities | 85.9 | IFN-β | SD | −5.10307 | 89.9 |
| No. 9 | 31–40 | M | Extremities | 73 | TMZ | PD | −16.806 | 67.8 |
| No. 10 | 61–70 | M | Extremities | 55 | anti-CCR4 Abs | PD | 2.706 | 115.4 |
| No. 11 | 61–70 | F | Extremities | 66.3 | DAV+IFNβ | PD | 0.653 | 103.4 |
| No. 12 | 61–70 | M | Head and neck | 55 | DTIC | PD | −0.094 | 99.6 |
| No. 13 | 61–70 | M | Extremities | 115.4 | DAV+IFN-β | SD | 0.177 | 100.5 |
| No. 14 | 61–70 | M | Extremities | 77.6 | IFN-β | PD | −61.59461 | 35.5 |
| No. 15 | 81–90 | F | Unknown | 47.8 | – | SD | −6.54815 | 86.5 |
| No. 16 | 71–80 | M | Extremities | 54.4 | IFN-β | SD | −4.74553 | 80.8 |
| No. 17 | 61–70 | F | Trunk | 46.5 | – | PD | 17.3346 | 140.4 |
| No. 18 | 71–80 | M | Extremities | 53.7 | – | PD | −1.595225 | 93 |
| No. 19 | 51–60 | F | Head and neck | 49.2 | DTIC+IFN-β | PD | 5.457395 | 115.9 |
| No. 20 | 31–40 | M | Trunk | 78.8 | IFN-α | SD | −12.615365 | 53.7 |
| No. 21 | 61–70 | F | Trunk | 48.8 | IFN-β | PD | −1.7178157 | 96.2 |
| No. 22 | 31–40 | F | Head and neck | 52.3 | IFN-β | PD | 3.88229 | 120.1 |
| No. 23 | 71–80 | F | External genitalia | 59.1 | – | SD | −3.34502 | 92.1 |
| No. 24 | 71–80 | F | External genitalia | 53.4 | IFN-β | SD | −7.38773 | 70.5 |
| No. 25 | 61–70 | F | Extremities | 47.0 | – | PD | −4.11263 | 64 |
| No. 26 | 61–70 | M | Unknown | 53.6 | – | PD | −3.78005 | 75.6 |
| No. 27 | 61–70 | F | Extremities | 60.9 | IFN-β | PD | −0.54818 | 95.9 |
| No. 28 | 61–70 | F | Head and neck | 41.6 | IFN-α | PD | −1.456879 | 80.8 |
| No. 29 | 71–80 | M | Trunk | 56 | – | PD | −0.71335 | 77.2 |
| No. 30 | 41–50 | F | External genitalia | 69.2 | – | PD | −0.170996 | 94.4 |
| No. 31 | 71–80 | F | Extremities | 53.3 | – | PD | −0.682222 | 85.8 |
| No. 32 | 61–70 | M | Extremities | 61 | – | SD | 2.202349 | 183.2 |
| No. 33 | 71–80 | M | Unknown | 55 | – | PD | 0.328041 | 107.4 |
| No. 34 | 61–70 | F | Extremities | 68 | – | SD | 0.228017 | 106.3 |
| No. 35 | 71–80 | M | Extremities | 59.3 | IFN-β | SD | −1.7178157 | 96.2 |
| No. 36 | 61–70 | M | Unknown | 49.1 | – | PD | 0.428485 | 117.4 |
| No. 37 | 61–70 | M | Head and neck | 61 | – | SD | 0.164125 | 110.7 |
| No. 38 | 41–50 | F | External genitalia | 56 | – | SD | 1.268748 | 247.2 |
| No. 39 | 71–80 | F | Extremities | 47 | – | PD | −0.721186 | 86.1 |
| No. 40 | 41–50 | M | Head and neck | 103 | DTIC | PD | −0.111639 | 98 |
| No. 41 | 61–70 | M | Extremities | 73 | IFN-β | SD | 0.421323 | 113.8 |
| No. 42 | 31–40 | M | Extremities | 63.5 | – | PD | 0.9897167 | 102.1 |
| No. 43 | 71–80 | F | Extremities | 66.2 | – | SD | 7.3643392 | 116.5 |
| No. 44 | 71–80 | M | Extremities | 63.7 | IFN-β | PD | −7.0989841 | 91.9 |
| No. 45 | 71–80 | M | Head and neck | 57.4 | – | PD | −0.5957191 | 98.4 |
| No. 46 | 41–50 | M | Extremities | 62.7 | IFN-β | PD | 2.4247196 | 105.7 |
CBDCA, carboplatin; PTX, paclitaxel; DTIC; dacarbazine; TMZ, temozolomide; DAV, dacarbazine + nimustine hydrochloride + vincristine. Serum level changes of sCD163 from each patient (n = 59) between day 42 and day 0 were examined by ELISA. Data for each donor represent the means of duplicate assays.
Table 2.
Characteristics and serum levels of sCD163 in patients with non-cutaneous melanoma.
| Age (y) | Sex | Location | Body weight (kg) | Prior systemic therapy | Efficacy at 3 months | Increase of sCD163 | Change ratio of sCD163 (%) | |
|---|---|---|---|---|---|---|---|---|
| No. 1 | 61–70 | M | Digestive duct | 62 | – | PR | −0.444523 | 81.5 |
| No. 2 | 61–70 | F | Palate | 52.8 | – | PR | −0.799493 | 77.6 |
| No. 3 | 61–70 | M | Paranasal | 71 | – | PR | −1.803397 | 62.8 |
| No. 4 | 61–70 | F | Vagina | 52.5 | – | PR | 0.469173 | 114.6 |
| No. 1 | 61–70 | M | Vagina | 53.4 | – | SD | −17.52087 | 78.7 |
| No. 2 | 61–70 | M | Vagina | 80.4 | – | PD | 49.95895 | 134.6 |
| No. 3 | 51–60 | F | Conjunctiva | 84 | – | SD | 8.27076 | 115.4 |
| No. 4 | 81–90 | M | Digestive duct | 49.5 | – | PD | 19.6379 | 158.1 |
| No. 5 | 61–70 | F | Digestive duct | 49.3 | – | SD | 20.26377 | 155.2 |
| No. 6 | 71–80 | F | Vagina | 50.1 | – | PD | 28.525981 | 192.1 |
| No. 7 | 61–70 | M | Nasal cavity | 47.2 | DTIC | PD | 17.0558 | 132.6 |
| No. 8 | 61–70 | F | Vagina | 53.1 | DAV + IFN-β | PD | −17.787 | 64.2 |
| No. 9 | 61–70 | M | Paranasal | 56 | DTIC | PD | 0.16109 | 132.6 |
| No. 10 | 51–60 | M | Palate | 52.9 | – | PD | 1.086 | 107.3 |
| No. 11 | 71–80 | M | Nasal cavity | 53.1 | DAV + IFN-β | SD | 2.52838 | 107.6 |
| No. 12 | 71–80 | F | Nasal cavity | 53 | – | SD | −0.345647 | 89.4 |
DAV, dacarbazine + nimustine hydrochloride + vincristine; DTIC, dacarbazine Serum level changes of sCD163 from each patient (n = 16) between day 42 and day 0 were examined by ELISA. Data for each donor represent the means of duplicate assays.
Efficacy of nivolumab at 3 months after first administration
Among patients with cutaneous melanoma, complete response (CR) was seen in 1 patient (1.7%; 95% confidence interval [CI], 0–3.4%), partial response (PR) in 12 patients (20.3%; 95%CI, 0–40.6%), stable disease (SD) in 16 patients (27.1%; 95%CI, 0–54.2%), and progressive disease (PD) in 29 patients (49.2%; 95%CI, 0–98.4%). The objective response rate (ORR) at 3 months after first administration was thus 22.0% (95%CI, 0–44.0%). Tumor responses of individual patients are listed in Table 1. Among patients with mucosal melanoma, PR was seen in 4 patients (25.0%; 95%CI, 0–50.0%), SD in 5 patients (31.3%; 95%CI, 0–62.6%), and PD in 7 patients (43.8%; 95%CI, 0–87.6%). The ORR at 3 months after first administration was thus 25.0% (95%CI, 0–50.0%). Tumor responses of individual patients are listed in Table 2.
Serum levels of sCD163
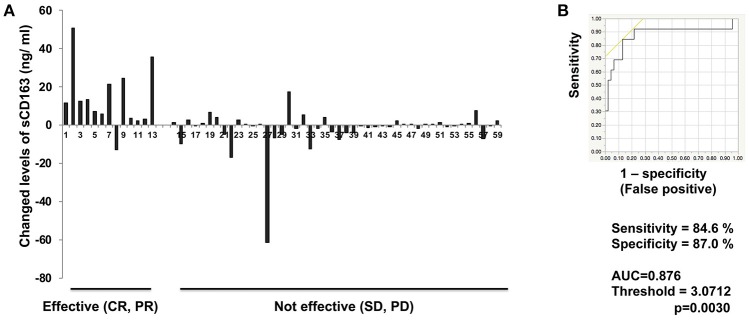
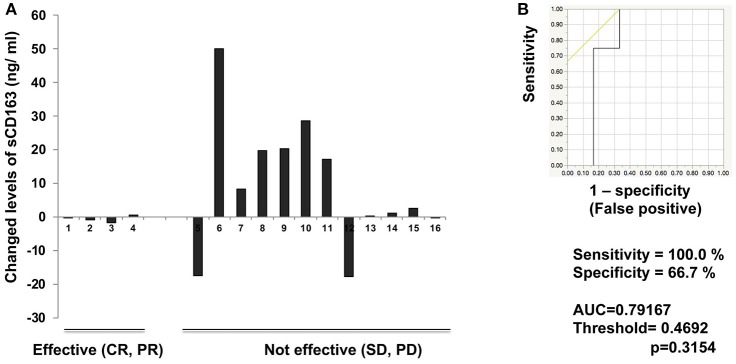
To determine whether serum levels of sCD163 may predict early response in melanoma patients treated with nivolumab, we evaluated levels in 75 patients with advanced melanoma treated using nivolumab (Supplemental Figure 1). Compared to baseline (day 0), serum levels of sCD163 at day 42 were significantly increased in the group showing objective response (p < 0.0001; Figure 1A) among patients with advanced cutaneous melanoma, whereas no significant difference in serum sCD163 levels was seen among patients with advanced mucosal melanoma (Figure 2A). Increases in serum sCD163 and efficacy at 3 months after the first administration of nivolumab in each patient are described in Tables 1, 2. The thresholds for sCD163 change between day 42 and day 0 to distinguish responders from non-responders were respectively 3.07 ± 0.07 ng/mL and 0.47 ± 0.05 ng/mL in cutaneous and non-cutaneous melanoma. In the cutaneous melanoma cohort, the mean change ratio in sCD163 serum level was 144.6% for those patients showing objective response, compared to 101.0% for non-responders (Table 1). In the mucosal melanoma cohort, the mean change ratio in sCD163 serum level was 84.1% for those patients showing objective response, and 122.3% for non-responders (Table 2). The threshold for increased serum sCD163 in cutaneous melanoma was 3.07 ± 0.07 ng/mL, whereas that in non-cutaneous melanoma was 0.47 ± 0.05 ng/mL. The sensitivity and specificity of serum sCD163 in cutaneous melanoma were 84.6 and 87.0%, respectively (p = 0.0030; Figure 1B), whereas the sensitivity and specificity of serum sCD163 in non-cutaneous melanoma were 100 and 66.7%, respectively (p = 0.3154; Figure 2B).
Figure 1.
Serum levels of sCD163 and ROC curve in cutaneous melanoma. Serum level changes of sCD163 in each patient (n = 59) (A) on day 42. The ROC curve was applied to calculate cut-offs for sCD163 serum levels and AUC (B). Cut-offs were determined to distinguish responders from nonresponders using Youden's index.
Figure 2.
Serum levels of sCD163 and ROC curve in non-cutaneous melanoma. Serum level changes of sCD163 in each patient (n = 16) (A) on day 42. The ROC curve was applied to calculate cut-offs for sCD163 serum levels and AUC (B). Cut-offs were determined to distinguish responders from nonresponders using Youden's index.
Discussion
Because nivolumab shows higher efficacy than other anti-melanoma drugs (e.g., ipilimumab and dacarbazine) (1, 17), and induces a longer duration of anti-tumor response than BRAF/MEK inhibitors (e.g., vemurafenib, dabrafenib, trametinib) (18, 19), oncologists have been particularly interested in combining nivolumab with agents that enhance anti-tumor immune responses in patients with metastatic melanoma (1–3, 20). The efficacy of nivolumab appears significantly increased when combined with ipilimumab (57.7%), but the rate of severe treatment-related AEs (Grade 3 or 4) is unfortunately also significantly increased with this particular combination (59.0%) (1, 2). To avoid severe AEs caused by ipilimumab, predictive biomarkers are needed to evaluate the efficacy of nivolumab monotherapy at 2–3 months after first administration, to prepare for any planned switch from nivolumab to ipilimumab.
CD163 is a member of the scavenger receptor cysteine-rich family, and is exclusively expressed on cells of the monocyte/macrophage lineage (15). As previously reported, in metastatic melanoma, a substantial number of CD163+ TAMs are present in metastatic melanoma and are activated by stromal factors, leading to an increase in serum levels of sCD163 as a result of proteolytic shedding (21–23). In addition, Jensen et al. (21) previously reported sCD163 and CD163+ TAMs as a prognostic marker for early-stage cutaneous melanoma. Notably, as Gordon et al. (14) reported, PD-1 expression is a key factor in maintaining TAMs as M2-polarized, and blockade of PD-1/PD-L1 leads to conversion of TAMs into M1-polarized activated macrophages. Because TAMs in melanoma comprise a heterogeneous, mainly immunosuppressive population (9, 23), and because repolarization of TAMs into the activated subtype by immunomodulatory drugs has been reported to significantly suppress melanoma growth in a spontaneous mouse melanoma model (8), we hypothesized that one of the targets for nivolumab in melanoma is activated CD163+ TAMs.
To prove our hypothesis, we analyzed serum levels of sCD163 in 59 cases of advanced cutaneous melanoma and 16 cases of advanced mucosal melanoma treated with nivolumab. Serum levels of sCD163 were significantly increased 6 weeks after initial administration of nivolumab in the response group compared to the non-response group in cutaneous melanoma. In contrast, no significant difference between nivolumab responders and non-responders was seen for non-cutaneous melanoma. Interestingly, in non-cutaneous melanoma, serum levels of sCD163 in non-responders tended to be even higher than those in responders. This discrepancy might be explained by differences in cancer stroma that could stimulate TAMs in the tumor microenvironment of each organ. Such studies should be performed in the future.
According to the present results, sCD163 may represent a predictive biomarker for evaluating the efficacy of nivolumab at 3 months after first administration for advanced cutaneous melanoma. Because sequential administration of ipilimumab followed by nivolumab is only effective for advanced melanoma before the melanoma develops tolerance for nivolumab (7), and administration of ipilimumab leads to the development of irAEs more frequently than nivolumab (2, 3), predicting the efficacy of nivolumab before first tumor estimation is crucial to determining whether the patient will successfully adapt to the planned switch from nivolumab to ipilimumab therapy. The present study suggested that sCD163 may be a useful biomarker for the selection of those cutaneous melanoma patients most likely to benefit from anti-melanoma immunotherapy using nivolumab and ipilimumab. Because this was a pilot study, future independent studies with larger patient cohorts are needed to confirm our findings.
Author contributions
TFuj designed the research study. TFuj, YS, KT, and YK performed and analyzed the ELISA data. TFuj, YK, AO, YF, KY, SM, TFun, HH, YY, HU, YN, RT, MA, KI, HO, NW, HI, TH, and AH treated the patients and acquired the clinical data and samples. TFuj wrote the manuscript. TFuj and SA supervised the study.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported in part by the Japan Agency for Medical Research and Development (l8cm01643h0001) and grants-in-aid for scientific research from the Japan Society for the Promotion of Science (16K10143).
Footnotes
Funding. This study was partially funded by Ono Pharmaceutical Co., Ltd. and Bristol-Myers Squibb.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2018.00530/full#supplementary-material
Serum levels of sCD163 at day 0 in patients with cutaneous and non-cutaneous melanoma. Mean serum levels of sCD163 in responders (n = 13) and non-responders (n = 46) at day 0 (A). Mean serum levels of sCD163 in responders (n = 4) and non-responders (n = 12) at day 0 (B) n.s, not significant.
References
- 1.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. (2017) 377:1345–56. 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. (2015) 373:23–34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber JS, Gibney G, Sullivan RJ, Sosman JA, Slingluff CL Jr, Lawrence DP, et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): an open-label, randomised, phase 2 trial. Lancet Oncol. (2016) 17:943–55. 10.1016/S1470-2045(16)30126-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. (2010) 37 :473–84. 10.1053/j.seminoncol.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. (2011) 364:2517–26. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- 6.Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. (2018); 379:722–30. 10.1056/NEJMoa1805453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujisawa Y, Yoshino K, Otsuka A, Funakoshi T, Fujimura T, Yamamoto Y, et al. Retrospective study of advanced melanoma patients treated with ipilimumab after nivolumab: analysis of 60 Japanese patients. J Dermatol Sci. (2018) 89:60–6. 10.1016/j.jdermsci.2017.10.009 [DOI] [PubMed] [Google Scholar]
- 8.Perry CJ, Muñoz-Rojas AR, Meeth KM, Kellman LN, Amezquita RA, Thakral D, et al. Myeloid-targeted immunotherapies act in synergy to induce inflammation and antitumor immunity. J Exp Med. (2018) 215:877–93. 10.1084/jem.20171435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity (2014) 41:49–61. 10.1016/j.immuni.2014.06.010.Review [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimura T, Kambayashi Y, Furudate S, Asano M, Kakizaki A, Aiba S. Receptor activator of nuclear factor kappa-B ligand (RANKL) promotes the production of CCL17 from RANK+ M2 macrophages. J Invest Dermatol. (2015) 135:2884–7. 10.1038/jid.2015.209 [DOI] [PubMed] [Google Scholar]
- 11.Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev. (2016) 99(Pt B):180–5. 10.1016/j.addr.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 12.Caronni N, Savino B, Bonecchi R. Myeloid cells in cancer-related inflammation. Immunobiology (2015) 220:249–53. 10.1016/j.imbio.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 13.Furudate S, Fujimura T, Kakizaki A, Kambayashi Y, Asano M, Watabe A, et al. The possible interaction between periostin expressed by cancer stroma and tumor-associated macrophages in developing mycosis fungoides. Exp Dermatol. (2016) 25:107–12. 10.1111/exd.12873 [DOI] [PubMed] [Google Scholar]
- 14.Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature (2017) 545:495–9. 10.1038/nature22396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol. (2010) 47:1650–60. 10.1016/j.molimm.2010.02.008 [DOI] [PubMed] [Google Scholar]
- 16.Youden WJ: Index for rating diagnostic tests. Cancer (1950) 3:32–35. [DOI] [PubMed] [Google Scholar]
- 17.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. (2015) 372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 18.McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. (2014) 15:323–32. 10.1016/S1470-2045(14)70012-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med. (2014) 371:1877–88. 10.1056/NEJMoa1406037 [DOI] [PubMed] [Google Scholar]
- 20.Fujimura T, Hidaka T, Kambayashi Y, Furudate S, Kakizaki A, Tono H, et al. Phase I study of nivolumab combined with IFN-β for patients with advanced melanoma. Oncotarget (2017) 8:71181–7. 10.18632/oncotarget.17090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen TO, Schmidt H, Møller HJ, Høyer M, Maniecki MB, Sjoegren P, et al. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. (2009) 27:3330–7. 10.1200/JCO.2008.19.9919 [DOI] [PubMed] [Google Scholar]
- 22.Fujimura T, Sato Y, Tanita K, Kambayashi Y, Otsuka A, Fujisawa Y, et al. Serum soluble CD163 and CXCL5 could be predictive markers for immune related adverse event in patients with advanced melanoma treated with nivolumab. Oncotarget (2018) 9:15542–51. 10.18632/oncotarget.24509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujimura T, Kambayashi Y, Fujisawa Y, Hidaka T, Aiba S. Tumor-associated macrophages: therapeutic targets for skin cancer. Front Oncol. (2018) 8:3. 10.3389/fonc.2018.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Serum levels of sCD163 at day 0 in patients with cutaneous and non-cutaneous melanoma. Mean serum levels of sCD163 in responders (n = 13) and non-responders (n = 46) at day 0 (A). Mean serum levels of sCD163 in responders (n = 4) and non-responders (n = 12) at day 0 (B) n.s, not significant.