Abstract
The behaviour of a nursing dam influences the development, physiology, and behaviour of her offspring. Maternal behaviours can be modulated both by environmental factors, including diet, and by physical or behavioural characteristics of the offspring. In most studies of the effects of the environment on maternal behaviour, F0 dams nurse their own F1 offspring. Because the F1 are indirectly exposed to the environmental stressor in utero in these studies, it is not possible to differentiate between effects on maternal behaviour from direct exposure of the dam and those mediated by changes in the F1 as a consequence of in utero exposure. In this study, we used a mouse model of high-fat (HF) diet feeding, which has been shown to influence maternal behaviours, combined with cross-fostering to discriminate between these effects. We tested whether the diet of the F0 dam or the exposure experienced by the F1 pups in utero is the most significant predictor of maternal behaviour. Neither factor significantly influenced pup retrieval behaviours. However, strikingly, F1 in utero exposure was a significant predictor of maternal behaviour in the 15 min immediately following pup retrieval while F0 diet had no discernable effect. Our findings suggest that in utero exposure to HF diet programmes physiological changes in the offspring which influence the maternal behaviours of their dam after birth.
Keywords: maternal behaviour, maternal–offspring interactions, high-fat diet, in utero exposure
1. Introduction
Maternal care plays a crucial role in shaping offspring development and physiology across taxonomic groups [1–3]. In mammals, maternal care influences pubertal onset [4], stress response [5,6], and the programming of maternal care behaviours in female offspring [7].
Maternal behaviour can be influenced by environmental and social factors. These diverse factors include exposure to endocrine disruptors [8–11], levels of anxiety in mates [12], and diet, among others. In rodents, maternal high-fat (HF) diet has been reproducibly demonstrated to cause impaired maternal behaviours. Rats fed a HF diet during pregnancy and lactation spend less time licking and grooming their pups [13], a behaviour that programmes altered hypothalamic-pituitary-adrenal responses to stress in the offspring [14]. While the mechanisms underpinning the relationship between diet and maternal behaviour have not been fully elucidated, at least some effects may be mediated through prolactin signalling. Prolactin is a key regulator of maternal behaviours, as demonstrated by the stimulatory effects of ectopic prolactin delivery in rats [15,16] and the behavioural deficiencies exhibited by prolactin receptor knockout mice [17]. Mice with HF diet-induced obesity are resistant to prolactin signalling in the hypothalamus, an important region of the brain for controlling maternal behaviour, and demonstrate an impairment in pup retrieval behaviour [18].
Maternal behaviour can also be influenced by the offspring. Maximizing the resources they can extract from their parents may be advantageous to offspring, but for optimal reproductive success parents may benefit from a more equal distribution of resources among their offspring [19,20]. This conflict has been predicted to lead to the evolution of behaviours or other traits in offspring that influence parental care and investment. Begging behaviour in young birds, for example, is a form of communication that can modulate parental investment and the allocation of food among nestlings [21,22]. Neonatal mice stimulate maternal licking, changing of suckling position, and nest-building through the emission of low-frequency calls [23], and when separated from the nest promote maternal searching and retrieval with ultrasonic vocalizations [24,25].
Although it is clear that both the environment and the behaviour of offspring can modulate maternal behaviour, the interactions between these factors have not been extensively explored.
Previous studies of how the behaviour of a dam is influenced by her consumption of a HF diet have used an experimental design in which dams (the F0 generation) nurse their own offspring (the F1 generation) [13]. Thus, a dam consuming a HF diet nurses pups that were themselves exposed to the dam's HF diet during in utero development. Such an experimental design precludes differentiation between effects on maternal behaviour that result from direct exposure of the F0 dam and those mediated by physiological changes in the F1 offspring as a consequence of in utero exposure.
To further our understanding of the interactions between the environment, F1 in utero exposure, and F0 dam behaviour, we implemented a cross-fostering strategy in which pups born to dams on a HF diet were nursed by dams on a control (CT) diet, and the reciprocal. We also included control groups (figure 1). With this approach, we aimed to determine whether the diet of the F0 dam or the exposure experienced by the F1 pups in utero is the most significant predictor of maternal behaviour. Given that 60% of women are overweight at the time of conception in the USA, the findings of this study will build on our understanding of how nutrition affects maternal care, with potential long-term consequences for offspring physiology.
Figure 1.
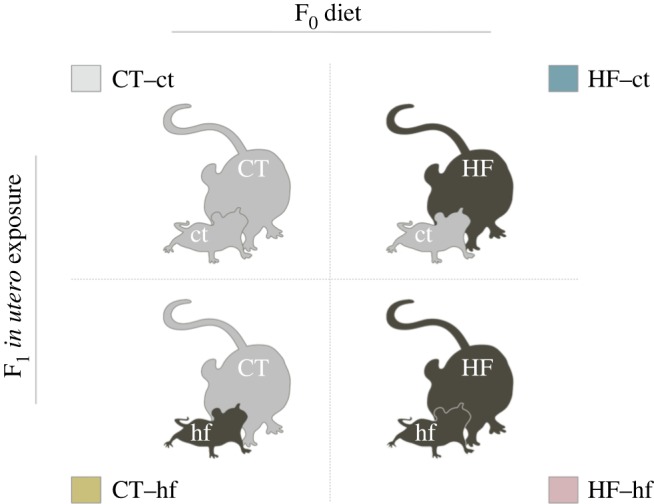
Experimental design. F1 mice were cross-fostered at birth to generate four experimental groups. Group labels indicate the diet of the nursing dam (F0) and the in utero exposure of the pups (F1), in the format F0–F1.
2. Methods
(a). Animals and cross-fostering
C57Bl/6 J mice were obtained from The Jackson Laboratory. This strain was chosen for its strong maternal nurturing behaviours reported by others [26] and for its responsiveness to cross-fostering [27]. All studies were approved by the North Carolina State University Institutional Animal Care and Use Committee. Animals were socially housed and maintained on a 14 h/10 h light/dark cycle at 30–70% humidity, 22°C ± 4°C. From three weeks of age, female mice (F0 generation) were fed a 45% fat diet (‘HF diet’; D12451, Research Diets Inc. (4.7 kcal g−1)) or a micronutrient-matched 10% fat diet (‘CT diet’; D12450H, Research Diets Inc. (3.8 kcal g−1)). Body weight was recorded weekly. After six weeks on the diet, a glucose tolerance test was performed. Mice were fasted overnight for 16 h and basal blood glucose levels measured using an Aimstrip Plus Glucose Meter and strips through tail snips. Glucose (Sigma-Aldrich) was injected at 2 mg g−1 body weight and blood glucose levels were measured at 15, 30, 60, 90, and 120 min.
At nine weeks of age, F0 females were mated to C57Bl/6 J male mice which were maintained on the 10% fat (CT) diet. To allow mating, females were placed in male cages for approximately 8 h during the light cycle for 10 consecutive days. Females were returned to their home cages for the entirety of the dark cycle, thereby ensuring continued exposure to the 45 or 10% fat diets throughout the mating period. Upon observation of pregnancy, females were individualized.
On the day of birth (postnatal day 0, PND0), F1 animals were weighed and litter sizes were normalized to five pups, maintaining an equivalent sex ratio between groups (electronic supplementary material, figure 1; F3,27 = 0.683, p = 0.5702). Extra pups were sacrificed and dissected. The remaining F1 animals were cross-fostered to nurse dams who had given birth on the same day to generate four groups (figure 1). F1 animals in all groups were cross-fostered. Groups are labelled in the format F0–F1, with the dam's diet in uppercase and the offspring's in utero exposure in lower case. CT–ct: dams consuming a CT diet nursing offspring exposed to a maternal CT diet in utero (n = 9 litters). CT–hf: dams consuming a CT diet nursing offspring exposed to a maternal HF diet in utero (n = 8 litters). HF–ct: dams consuming a HF diet nursing offspring exposed to a maternal CT diet in utero (n = 10 litters). HF–hf: dams consuming a HF diet nursing offspring exposed to a maternal HF diet in utero (n = 9 litters).
(b). Behaviour
Behavioural assays were performed during the light cycle on a subset of litters on postnatal day 3 (PND3), i.e. 3 days after cross-fostering (CT–ct, n = 9 litters; CT–hf, n = 8 litters; HF–ct, n = 8 litters; HF–hf, n = 8 litters). Analyses were performed on all animals in all groups concurrently. F1 pups were separated from their dams for 1 h, and placed in nesting material on a heat pad. Dams remained in their home cages during this time, but were briefly removed immediately before pups were replaced. Pups were reintroduced to the home cage at the opposite end to the nest. Each dam was reintroduced to their respective cage, and a modified lid containing a GoPro camera was fitted. The time taken for each pup to be retrieved to the nest was recorded. Pups were manually replaced in the nest if they had not been retrieved within 30 min.
After all five pups had been retrieved, the behaviour of the dam was scored every minute for the subsequent 15 min. ‘Interactive behaviours were classified as ‘in the nest with pups, not moving’, ‘in the nest with pups, moving’, and ‘nest-building’. ‘Non-interactive’ behaviours were classified as ‘self-grooming’, ‘resting’, ‘exploring’, and ‘wall-rearing’. No dam in any group was observed performing self-grooming or resting behaviours.
(c). Statistical analyses
Means comparison by Student's t-test (unpaired, two-tailed) was used to compare CT diet versus HF diet groups for body mass of dams, body mass of F1 pups at birth, litter size, and the area under the curve for the glucose tolerance test performed on F0 dams.
For pup retrieval analysis, Fisher's Exact test was performed on all groups, testing the null hypothesis that there is no significant difference in retrieval success between groups. For maternal behaviour analyses, data were expressed as counts for each exhibited behaviour, including a count variable summing across observed interactive behaviours. For each behaviour, the mean counts of each group were compared to the CT–ct group using one-way analysis of variance (ANOVA) with Dunnett's test for multiple comparisons. The same approach was used to compare the sex ratios of groups at PND3. To determine whether F0 diet or F1 in utero exposure were significant predictors of maternal behaviour, their individual effects were analysed using a Poisson generalized linear model using CT or ct as the reference group, respectively. We also determined whether the interaction of F0 diet and F1 in utero exposure (F0*F1) were significant predictors. Hypothesis tests for the resulting p-values test that the diet coefficient does (Estimate = 0) or does not (Estimate ≠ 0) have an effect on maternal behaviour. Analyses were performed using R version 3.4.3 [28].
3. Results
(a). Mouse model
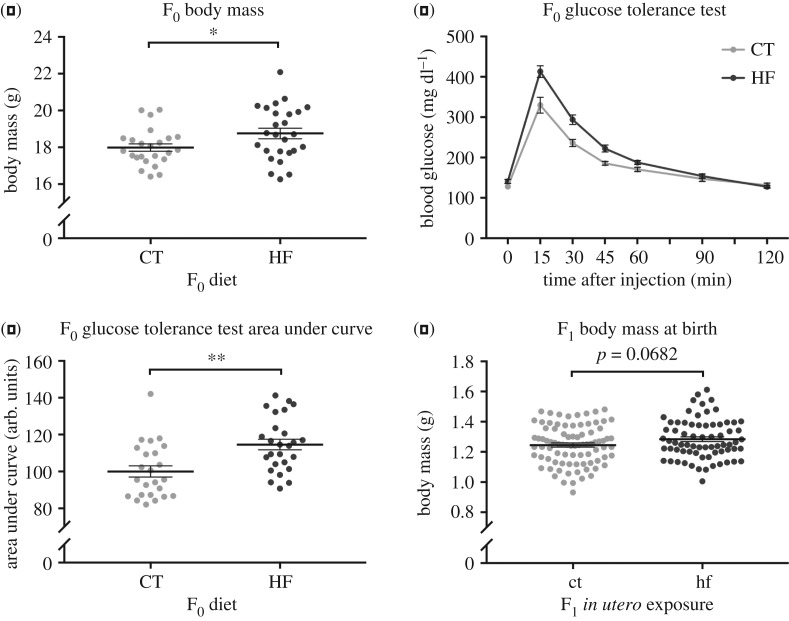
Female mice consuming the HF diet for six weeks had significantly elevated body weights (t48 = 2.141, p = 0.04; figure 2a) and impaired glucose tolerance (t48 = 3.488, p = 0.001 for area under curve; figure 2b,c) compared to mice consuming the CT diet. Females in both groups were mated to males consuming a CT diet. At birth, F1 pups born to dams on the HF diet showed slightly increased body weights compared with those born to dams on the CT diet, although this was not statistically significant (t153 = 1.837, p = 0.07; figure 2d). No effects on litter size associated with diet were observed (t34 = 0, p > 0.99). F1 mice were cross-fostered on the day of birth and maternal behaviours assessed on PND3, as described in the Methods (figure 1).
Figure 2.
Effects of diet on F0 and F1 body mass, and F0 glucose homeostasis. (a) Body mass of F0 dams prior to mating, after consuming CT or HF diets for six weeks. (b) Glucose tolerance test of F0 dams at the same time point as described for (a). (c) Quantification of the area under the curve in (b). (d) Body mass of F1 pups on the day of birth after in utero exposure to a maternal control (ct) or high-fat (hf) diet. Individual data points are shown for (a), (c), and (d), with horizontal black lines representing means and error bars representing standard error. Data in (b) are presented as mean ± s.e. *p < 0.05, **p < 0.005; Student's t-test, two-tailed.
(b). Pup retrieval
Following separation of the dam and pups, no significant differences were observed between the four groups in the latency to retrieve the first pup (F3,25 = 0.21, p = 0.89), or in the latency to retrieve all pups (F3,23 = 0.17, p = 0.92). Sixty-seven per cent of dams in the HF–hf group successfully retrieved all pups, compared to 88–100% of dams in other groups (figure 3), but this did not represent a statistically significant difference (p = 0.40).
Figure 3.
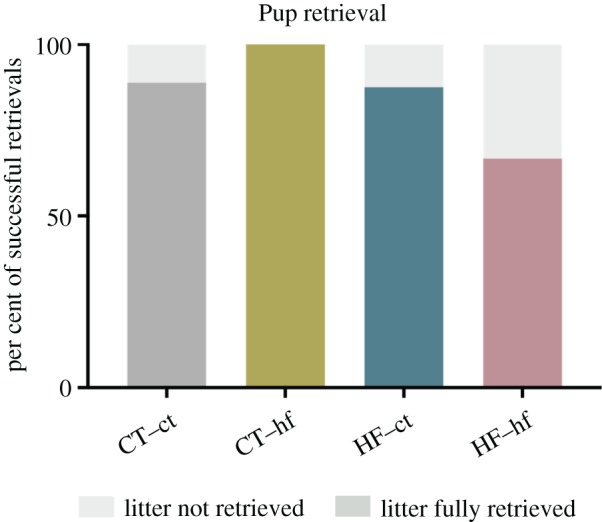
Pup retrieval. Per cent of dams within each experimental group that showed successful retrieval of all pups to the nest within 30 min of reunion.
(c). Post-retrieval maternal behaviour
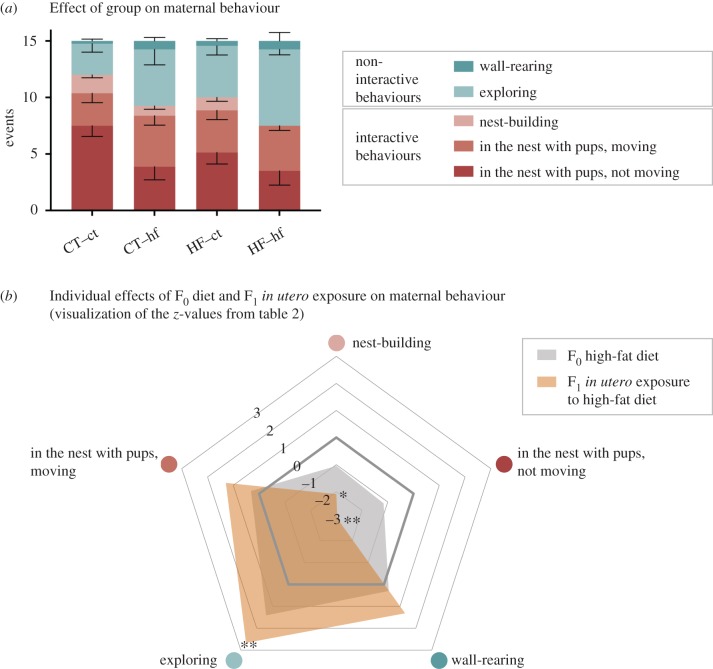
Following retrieval of the last pup, dams in the CT–ct group spent an average of 12 out of 15 of the measured time points (75%) engaged in interactive behaviours (figure 4a and table 1), more than dams in any other group. In particular, dams in the HF–hf group spent significantly fewer time points (an average of 7.5 out of 15 time points, 50%) engaged in interactive behaviours in comparison to dams in the CT–ct group. These differences were predominantly explained by a reduction in the number of time points that dams in the HF–hf group were ‘in the nest with pups, not moving’ and ‘nest-building’ with a concomitant increase in the number of time points that these dams were observed ‘exploring’.
Figure 4.
Post-retrieval maternal behaviour. (a) Effect of group on maternal behaviour. Number of time points (events) within the 15 min following reunion that dams were observed engaging in non-interactive and interactive behaviours. Error bars represent standard error. (b) Radar chart illustrating the predictability of F0 HF diet and F1 in utero exposure to a HF diet on specific maternal behaviours. The plot provides a graphical illustration of the z-scores from table 2, including the directionality of the effect. Non-interactive behaviours are grouped at the bottom of the plot (behaviours with blue shades) and interactive behaviours are grouped at the top (red shades).
Table 1.
Effect of group on maternal behaviour. Values are mean ± s.e. for the number of time points dams in each group were observed engaging in each activity. p-values are calculated using a one-way ANOVA with Dunnett's test for multiple comparisons, comparing each group to the CT–ct group.
| CT–ct | CT–hf | HF–ct | HF–hf | |
|---|---|---|---|---|
| interactive behaviours | ||||
| all interactive behaviours | 12.0 ± 0.7 | 9.3 ± 1.3 p = 0.120 |
10.0 ± 0.8 p = 0.346 |
7.5 ± 1.2 p = 0.028* |
| in the nest with pups, not moving | 7.5 ± 0.9 | 3.9 ± 1.2 p = 0.048* |
5.1 ± 1.0 p = 0.284 |
3.5 ± 1.3 p = 0.081 |
| in the nest with pups, moving | 2.9 ± 0.8 | 4.5 ± 0.8 p = 0.321 |
3.7 ± 0.8 p = 0.792 |
4.0 ± 0.4 p = 0.729 |
| nest-building | 1.6 ± 0.3 | 0.9 ± 0.3 p = 0.153 |
1.1 ± 0.3 p = 0.492 |
0 ± 0.0 p = 0.006** |
| non-interactive behaviours | ||||
| all non-interactive behaviours | 3.0 ± 0.7 |
5.8 ± 1.3 p = 0.120 |
5.0 ± 0.8 p = 0.346 |
7.5 ± 1.2 p = 0.028* |
| exploring | 2.8 ± 0.8 | 5.0 ± 1.4 p = 0.248 |
4.6 ± 0.8 p = 0.431 |
6.8 ± 0.5 p = 0.059 |
| wall-rearing | 0.3 ± 0.2 | 0.8 ± 0.3 p = 0.487 |
0.4 ± 0.2 p = 0.952 |
0.8 ± 0.8 p = 0.635 |
*p < 0.05, **p < 0.01.
Our data show that dams consuming a HF diet who nurse pups exposed to HF diet in utero (i.e. the HF–hf group) have impairments in maternal behaviour, consistent with observations by others. Interestingly, dams in the CT–hf and HF–ct groups also showed differences when compared to CT–ct dams in the number of time points spent on certain behaviours. For example, CT–hf dams were observed ‘in the nest with pups, not moving’ fewer times than CT–ct dams (table 1), suggesting that the in utero exposure experienced by the F1 pups might contribute to influencing maternal behaviour.
To gain further insight into this possibility, we fitted the data to generalized linear models and tested the individual effects of F0 diet and F1 in utero exposure, as well as the interaction between these two terms (F0*F1), on maternal behaviour. F0 diet was not a predictor of the number of time points spent on interactive or non-interactive behaviours overall (z = −0.995, p = 0.32), or indeed on any specific behaviour (table 2). Strikingly, F1 in utero exposure was predictive of maternal interactive and non-interactive behaviours overall (z = −2.071, p = 0.04). F1 exposure to a HF diet during in utero development was associated with the nursing dam being more engaged in non-interactive behaviours and less engaged in interactive behaviours than dams nursing pups exposed to the CT diet in utero (table 2). This effect was predominantly explained by a reduction in the time spent ‘inside the nest, not moving’ (z = −2.9651, p < 0.01) and ‘nest-building’ (z = −2.0894, p = 0.04), with a concomitant increase in time spent ‘exploring’ (z = 2.6657, p < 0.01) (table 2 and figure 4b). F0*F1 interaction was not a significant predictor of any maternal behaviour (electronic supplementary material, table S1).
Table 2.
Effect of F0 diet and F1 in utero exposure on maternal behaviour. Results from the Poisson generalized linear model for predicting the given maternal behaviour using single predictors for either F0 diet or F1 in utero exposure, with CT or ct as the reference group, respectively.
| estimate | s.e. | z-value | Pr(>|z|) | |
|---|---|---|---|---|
| interactive behaviours | ||||
| all interactive behaviours | ||||
| F0 diet | −0.1207 | 0.1214 | −0.995 | 0.32 |
| F1 exposure | −0.25531 | 0.12330 | −2.071 | 0.0384* |
| in the nest with pups, not moving | ||||
| F0 diet | −0.1978 | 0.1698 | −1.1648 | 0.2441 |
| F1 exposure | −0.5306 | 0.179 | −2.9651 | 0.003** |
| in the nest with pups, moving | ||||
| F0 diet | 0.0603 | 0.1955 | 0.3084 | 0.7578 |
| F1 exposure | 0.2499 | 0.1943 | 1.2864 | 0.1983 |
| nest-building | ||||
| F0 diet | −0.4055 | 0.3873 | −1.0469 | 0.2951 |
| F1 exposure | −0.9019 | 0.4317 | −2.0894 | 0.0367* |
| non-interactive behaviours | ||||
| exploring | ||||
| F0 diet | 0.2549 | 0.1811 | 1.4075 | 0.1593 |
| F1 exposure | 0.4850 | 0.1820 | 2.6657 | 0.0077** |
| wall-rearing | ||||
| F0 diet | 0.1542 | 0.5175 | 0.2979 | 0.7658 |
| F1 exposure | 0.6931 | 0.5270 | 1.3152 | 0.1884 |
*p < 0.05, **p < 0.01.
4. Discussion
Previous rodent studies have demonstrated that HF diet modulates maternal behaviour. In these studies, dams raised their own biological offspring which were exposed to maternal HF diet during in utero development, which precluded discrimination between effects of F0 diet and F1 in utero exposure on maternal behaviour. By using a cross-fostering study design, in which mice born to dams on a CT diet were fostered to dams on a HF diet, and the reciprocal, we were able to assess these effects independently.
Pup retrieval behaviour, including success at retrieving the entire litter within the experimental period and latency to retrieve pups, was not significantly affected by group, F0 diet, or F1 exposure, although dams in the HF–hf group showed a non-significant impairment in retrieval compared to other groups. Other studies have suggested a link between diet and pup retrieval behaviours, but this is context- and age-dependent [18,29]. For example, in one study, dams on a HF diet demonstrated impaired pup retrieval on postnatal day 7 but not on postnatal day 4 [18], with the latter time point being close to our own study.
During the 15 min immediately following pup retrieval, F1 in utero exposure, but not F0 diet, was found to be a significant predictor of maternal behaviour. Specifically, F1 in utero exposure to HF diet was negatively associated with the number of time points during which the dam was observed ‘in the nest with pups, not moving’ and ‘nest-building’, and positively associated with ‘exploring’. These findings suggest that alterations to maternal behaviours previously associated with the consumption of a HF diet may in part be mediated through the offspring. F1 in utero exposure to polychlorinated byphenyls has been similarly reported to impact F0 maternal behaviour [10], suggesting that our general observations may apply to a range of environmental stressors.
A parent has finite resources to invest in reproduction. Assuming identical fitness among all offspring, the best reproductive strategy would be to invest equally in each. However, offspring are likely to differ in their fitness and thus the optimal strategy is to invest more heavily in the offspring of greater quality [30]. This has been demonstrated empirically for a range of species, implying that parents can respond to cues from offspring that reflect their fitness [21,31,32].
In the context of maternal HF diet, in utero exposure causes reduced fitness in later life, including metabolic and behavioural dysfunction. Early physiological alterations in exposed pups may provide cues that influence the time spent on interactive behaviours by dams, representing a strategy to limit investment and enable greater resource allocation to future, potentially fitter, broods. However, the molecular nature of these cues—and how they are perceived by the dam—is unclear.
Genetic studies in mice have identified loci in offspring that can influence maternal behaviour. By fostering genetically variable mouse pups to genetically uniform dams, Ashbrook et al. [33] were able to map maternal behaviours as a function of genetic variation in offspring, identifying loci on chromosomes 5 and 7 that modify maternal behaviours. Together, these loci contain greater than 400 genes, and a small number of these were identified as strong candidates for modulating maternal behaviour because of their involvement in steroid hormone biosynthesis. Other loci that influence maternal behaviour have been identified through targeted approaches. Deletion of the imprinted gene Peg3 in offspring is associated with impaired maternal behaviours, including delayed pup retrieval and increased anxiety, in wild-type nurse dams [34]. Offspring deficient for Peg3 demonstrated a reduction in ultrasonic vocalizations upon separation from the dam, suggesting a possible mechanism through which they may influence maternal behaviour.
The findings of our study suggest that the previously described effects of diet on maternal behaviour may be partly attributed to physiological influences from offspring, motivating further study of the molecular mechanisms involved.
Supplementary Material
Acknowledgements
The authors thank Emilie Rissman for comments on the manuscript, and the staff at the Biological Resources Facility at NC State University for their support.
Ethics
All studies were approved by the North Carolina State University Institutional Animal Care and Use Committee (protocol #15-013-B).
Data accessibility
Data are provided as tables in the manuscript or in the electronic supplementary material.
Authors' contributions
M.B. and M.C. conceived the project, designed the experiments, performed the experiments, analysed data, interpreted findings, and wrote the manuscript. H.L., C.B., and J.T. performed experiments and contributed to data analysis. K.T. and D.R. analysed data and contributed to data visualization. All authors gave final approval for publication.
Competing interests
The authors declare no competing interests.
Funding
This work was supported by a Ralph E. Powe Junior Faculty Enhancement Award (to M.C.), the National Institutes of Health through P30ES025128 and North Carolina State University.
References
- 1.Dulac C, O'Connell LA, Wu Z. 2014. Neural control of maternal and paternal behaviors. Science 345, 765–770. ( 10.1126/science.1253291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mas F, Kölliker M. 2008. Maternal care and offspring begging in social insects: chemical signalling, hormonal regulation and evolution. Anim. Behav. 76, 1121–1131. ( 10.1016/j.anbehav.2008.06.011) [DOI] [Google Scholar]
- 3.Eriksen MS, Faerevik G, Kittilsen S, McCormick MI, Damsgård B, Braithwaite VA, Braastad BO, Bakken M. 2011. Stressed mothers - troubled offspring: a study of behavioural maternal effects in farmed Salmo salar. J Fish Biol. 79, 575–586. ( 10.1111/j.1095-8649.2011.03036.x) [DOI] [PubMed] [Google Scholar]
- 4.Cameron N, Del Corpo A, Diorio J, McAllister K, Sharma S, Meaney MJ. 2008. Maternal programming of sexual behavior and hypothalamic-pituitary-gonadal function in the female rat. PLoS ONE 3, e2210 ( 10.1371/journal.pone.0002210) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu D, et al. 1997. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277, 1659–1662. ( 10.1126/science.277.5332.1659) [DOI] [PubMed] [Google Scholar]
- 6.Weaver ICG, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. 2004. Epigenetic programming by maternal behavior. Nat. Neurosci. 7, 847–854. ( 10.1038/nn1276) [DOI] [PubMed] [Google Scholar]
- 7.Francis D, Diorio J, Liu D, Meaney MJ. 1999. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science 286, 1155–1158. ( 10.1126/science.286.5442.1155) [DOI] [PubMed] [Google Scholar]
- 8.Palanza PL, Howdeshell KL, Parmigiani S, vom Saal FS. 2002. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ. Health Perspect. 110(Suppl. 3), 415–422. ( 10.1289/ehp.02110s3415) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palanza P, Morellini F, Parmigiani S, vom Saal FS. 2002. Ethological methods to study the effects of maternal exposure to estrogenic endocrine disrupters: a study with methoxychlor. Neurotoxicol. Teratol. 24, 55–69. ( 10.1016/S0892-0362(01)00191-X) [DOI] [PubMed] [Google Scholar]
- 10.Cummings JA, Nunez AA, Clemens LG. 2005. A cross-fostering analysis of the effects of PCB 77 on the maternal behavior of rats. Physiol Behav. 85, 83–91. ( 10.1016/j.physbeh.2005.04.001) [DOI] [PubMed] [Google Scholar]
- 11.Catanese MC, Vandenberg LN. 2016. Bisphenol S (BPS) alters maternal behavior and brain in mice exposed during pregnancy/lactation and their daughters. Endocrinology 158, 516–530. ( 10.1210/en.2016-1723) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mashoodh R, Franks B, Curley JP, Champagne FA. 2012. Paternal social enrichment effects on maternal behavior and offspring growth. Proc. Natl Acad. Sci. USA 109(Suppl. 2), 17 232–17 238. ( 10.1073/pnas.1121083109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connor KL, Vickers MH, Beltrand J, Meaney MJ, Sloboda DM. 2012. Nature, nurture or nutrition? Impact of maternal nutrition on maternal care, offspring development and reproductive function. J. Physiol. 590, 2167–2180. ( 10.1113/jphysiol.2011.223305) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meaney MJ, Szyf M. 2005. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin. Neurosci. 7, 103–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. 1990. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc. Natl Acad. Sci. USA 87, 8003–8007. ( 10.1073/pnas.87.20.8003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridges RS, DiBiase R, Loundes DD, Doherty PC. 1985. Prolactin stimulation of maternal behavior in female rats. Science 227, 782–784. ( 10.1126/science.3969568) [DOI] [PubMed] [Google Scholar]
- 17.Lucas BK, Ormandy CJ, Binart N, Bridges RS, Kelly PA. 1998. Null mutation of the prolactin receptor gene produces a defect in maternal behavior. Endocrinology 139, 4102–4107. ( 10.1210/endo.139.10.6243) [DOI] [PubMed] [Google Scholar]
- 18.Buonfiglio DC, Ramos-Lobo AM, Freitas VM, Zampieri TT, Nagaishi VS, Magalhães M, Cipolla-Neto J, Cella N, Donato J Jr. 2016. Obesity impairs lactation performance in mice by inducing prolactin resistance. Sci. Rep. 6, 22421 ( 10.1038/srep22421) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trivers RL. 1974. Parent-offspring conflict. Am. Zool. 14, 249–264. ( 10.1093/icb/14.1.249) [DOI] [Google Scholar]
- 20.Godfray HCJ. 1995. Evolutionary theory of parent–offspring conflict. Nature 376, 133–138. ( 10.1038/376133a0) [DOI] [PubMed] [Google Scholar]
- 21.Kilner R. 1995. When do canary parents respond to nestling signals of need? Proc. R. Soc. Lond. B 260, 343–348. ( 10.1098/rspb.1995.0102) [DOI] [Google Scholar]
- 22.Smith HG, Montgomerie R. 1991. Nestling American robins compete with siblings by begging. Behav. Ecol. Sociobiol. 29, 307–312. ( 10.1007/BF00163989) [DOI] [Google Scholar]
- 23.Ehret G, Bernecker C. 1986. Low-frequency sound communication by mouse pups (Mus musculus): wriggling calls release maternal behaviour. Anim. Behav. 34, 821–830. ( 10.1016/S0003-3472(86)80067-7) [DOI] [Google Scholar]
- 24.Ehret G. 2005. Infant rodent ultrasounds - a gate to the understanding of sound communication. Behav. Genet. 35, 19–29. ( 10.1007/s10519-004-0853-8) [DOI] [PubMed] [Google Scholar]
- 25.Yin X, et al. 2016. Maternal deprivation influences pup ultrasonic vocalizations of C57BL/6 J mice. PLoS ONE 11, e0160409 ( 10.1371/journal.pone.0160409) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Priebe K, Romeo RD, Francis DD, Sisti HM, Mueller A, McEwen BS, Francis DD. 2005. Maternal influences on adult stress and anxiety-like behavior in C57BL/6 J and BALB/cJ mice: a cross-fostering study. Dev. Psychobiol. 47, 398–407. ( 10.1002/dev.20098) [DOI] [PubMed] [Google Scholar]
- 27.Francis DD, Szegda K, Campbell G, Martin WD, Insel TR. 2003. Epigenetic sources of behavioral differences in mice. Nat. Neurosci. 6, 445–446. ( 10.1038/nn1038) [DOI] [PubMed] [Google Scholar]
- 28.R Core Team. 2017. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (https://www.R-project.org) [Google Scholar]
- 29.Rocha JBT, Soares FAA, De Mello CF. 2002. Influence of the test situation on pup retrieval behavior of normal and undernourished lactating rats. Braz. J. Med. Biol. Res. 35, 91–97. ( 10.1590/S0100-879X2002000100013) [DOI] [PubMed] [Google Scholar]
- 30.Haig D. 1990. Brood reduction and optimal parental investment when offspring differ in quality. Am. Nat. 136, 550–566. ( 10.1086/285113) [DOI] [Google Scholar]
- 31.Thünken T, Meuthen D, Bakker TCM, Kullmann H. 2010. Parental investment in relation to offspring quality in the biparental cichlid fish Pelvicachromis taeniatus. Anim. Behav. 80, 69–74. ( 10.1016/j.anbehav.2010.04.001) [DOI] [Google Scholar]
- 32.Rytkönen S. 2002. Nest defence in great tits Parus major: support for parental investment theory. Behav. Ecol. Sociobiol. 52, 379–384. ( 10.1007/s00265-002-0530-y) [DOI] [Google Scholar]
- 33.Ashbrook DG, Gini B, Hager R. 2015. Genetic variation in offspring indirectly influences the quality of maternal behaviour in mice. Elife 4, e11814 ( 10.7554/eLife.11814) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McNamara GI, Creeth HDJ, Harrison DJ, Tansey KE, Andrews RM, Isles AR, John RM. 2018. Loss of offspring Peg3 reduces neonatal ultrasonic vocalizations and increases maternal anxiety in wild-type mothers. Hum. Mol. Genet. 27, 440–450. ( 10.1093/hmg/ddx412) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are provided as tables in the manuscript or in the electronic supplementary material.