Abstract
Ecological traps occur due to a mismatch between a habitat's attractiveness and quality, wherein organisms show preference for low-quality habitats over other available high-quality habitats. Our previous research identified leaf litter from common blackberry (Rubus allegheniensis) as a natural ecological trap for an important vector for West Nile virus (Culex pipiens), attracting mosquitoes to oviposit in habitats deleterious to the survival of their larvae. Here we demonstrate that manipulation of leaf litter in stormwater catch basins, an important source of disease vector mosquitoes in urban environments, can increase Cx. pipiens oviposition but reduce survival. In a series of experiments designed to elucidate the mechanisms that explain the attractive and lethal properties of this native plant, behavioural bioassays suggest that oviposition site selection by Cx. pipiens is mediated primarily by chemical cues as leaves decompose. However, we also show that juvenile mosquito survival is mainly related to the suitability of the bacterial community in the aquatic habitat for mosquito nutritional needs, which does not appear to create a cue that influences oviposition choice. This mismatch between oviposition cues and drivers of larval habitat quality may account for the ecological trap phenomenon detected in this study. Our findings provide new insights into potential mechanistic pathways by which ecological traps may occur in nature and proof-of-concept for a new ‘attract-and-kill’ tool for mosquito control.
Keywords: Culex pipiens, habitat selection, integrated pest management, ecological trap
1. Background
Animals' survival and reproductive success in heterogeneous environments depend upon how well they choose where to shelter, feed and reproduce, and consequently, many species have evolved the ability to recognize and select the best available habitats to maximize their fitness [1]. However, it is possible for the attractiveness of a habitat and its suitability for survival and reproduction to be decoupled, leading to judgement errors. Ecological traps occur due to a mismatch between a habitat's attractiveness and quality, wherein organisms show preference for low-quality habitats over other available high-quality habitats [2–4]. Numerous examples of ecological traps created by human activity have been documented in mammals, birds, reptiles, amphibians, fishes and insects, indicating that they are widespread phenomena [5–10]. Few examples of natural ecological traps currently exist, and with limited evidentiary support [11,12], although other such traps probably occur frequently and go undetected.
Ecological traps can arise when changes to an organism's environment lower the suitability of a habitat without affecting its attractiveness, increase the attractiveness of a habitat without affecting its suitability, or simultaneously increase the attractiveness of a habitat while reducing its suitability [4]. Ecological traps are classified based on the degree of decoupling between attractiveness cues and fitness value; an ‘equal-preference trap’ occurs when a low-quality habitat is equally attractive as other high-quality habitats, while a ‘severe trap’ occurs when a low-quality habitat is more attractive than other available high-quality habitats [4]. Severe traps present a major conservation concern because they attract animals away from high-fitness habitats, potentially leading to population decline [13]. Yet there also is evidence that ecological traps may, under certain conditions, offer benefits such as controlling invasive species and inhibiting the spread of infectious diseases in the landscape [14–16]. In these latter cases, the mechanisms generating ecological traps may provide us with tools for creating traps that are more effective in controlling pest species compared to conventional approaches (e.g. exclusive reliance upon insecticides for control of arthropod disease vectors). But despite well-documented evidence for the occurrence and environmental impacts of ecological traps, mechanistic understanding generally is lacking [4]. Understanding the mechanisms responsible for the occurrence of ecological traps is critical to effective exploitation of traps in public health, pest management or conservation settings.
Container-inhabiting mosquitoes provide an ideal model system for study of ecological traps because their aquatic juvenile stages develop in simple, easily manipulated habitats. For example, Culex pipiens, an important vector for West Nile virus (WNV) in the US, exploits engineered structures designed for stormwater management [17–19]. Stormwater catch basins are underground reservoirs that reduce flooding by collecting and conducting surface runoff through the subterranean storm drain system. They create protected and nutrient-rich aquatic habitats that can be ideal for juvenile development of Cx. pipiens. Thus, catch basins are common targets for insecticide-based mosquito abatement efforts [20,21]. Application of insecticides can alter the community structure of aquatic habitats, causing them to become ecological traps for mosquitoes. For example, pyriproxyfen-treated pools act as an ecological trap for Cx. pipiens, associated with high oviposition rates yet low survival to adult emergence [22], although the mechanisms that explain the attractiveness of this trap and how this habitat yielded poor mosquito survival remain unexplored.
Aquatic habitat quality for mosquitoes is influenced strongly by leaf litter subsidies, an important energy source for juveniles. As leaves decay via leaching of soluble compounds [23] and decomposition by detritivorous macroinvertebrates and microorganisms [24], mosquito larvae filter bacteria suspended in the water column and graze on leaf surfaces [25,26]. Mosquito survival to adult emergence varies with species of foliar material in the aquatic habitat [27,28], and multiple mechanisms could explain this pattern. Habitat quality may be altered by plant secondary compounds that leach into the aquatic environment, including toxic phytochemicals that act directly on developing mosquitoes [23] and antimicrobials that reduce the food available to larvae [29]. Alternatively, aquatic habitat quality may be related to the species composition and nutritional quality of the bacteria associated with leaf litter [30]. There also is evidence that leaf litter in the aquatic environment mediates choice of oviposition site by container-inhabiting mosquitoes. Volatiles released during leaf decay may include olfactory attractants to female mosquitoes [31,32] or gustatory stimulants that induce oviposition upon contact with the water surface [33]. Semiochemicals produced by bacterial flora as by-products of metabolism also are linked to habitat selection [33,34]; thus, the latter pathway also may increase aquatic habitat attractiveness due to chemical cues but via an indirect microbial mechanism.
In previous laboratory studies, we discovered that while leaf litter from some plants (e.g. Amur honeysuckle, Lonicera maackii) is attractive to female mosquitoes and yields high juvenile survival, leaf litter from common blackberry (Rubus allegheniensis) acts as an ecological trap for Cx. pipiens [30]. Here, we take advantage of this system to investigate the causal mechanisms that explain the occurrence of this trap. First, we conducted a 2-year field experiment to test whether this trap for Cx. pipiens could be implemented under natural conditions in stormwater catch basins. Subsequently, we used a combination of laboratory bioassays and next-generation sequencing of bacterial flora present in aquatic habitats containing foliar material from different plant species to investigate the mechanisms underlying variation in the attractiveness and lethality of leaf litter to mosquitoes. Our results yield understanding of how mosquito habitat selection and juvenile survival are decoupled, and ultimately suggest the potential to exploit an ecological trap for mosquito control.
2. Methods
(a). Catch basin manipulation experiment
We selected Paxton, IL, to conduct our field test of an ecological trap due to an absence of mosquito abatement efforts in a residential setting representative of the epidemiological context of WNV transmission. Four experimental treatments (negative control, positive control, ecological trap, attractants only; see below) were applied to 20 catch basins from 16 August to 13 September 2013; the same four treatments plus one additional treatment (toxins only; see below) were applied to 50 catch basins from 18 July to 15 August 2014. Because mosquito abundance in catch basins varies widely in response to seasonal environmental conditions such as ambient temperature, rainfall and water chemistry [19,35], the experiments were confined to four weeks in duration during periods with low rainfall, but repeated across 2 years to confirm the reproducibility of the findings. All catch basins had open grates and were located on street edges in a relatively homogeneous residential neighbourhood. Treatments were spatially aggregated within the study area to minimize carryover effects of one treatment into adjacent catch basins connected via subterranean infrastructure (electronic supplementary material, figure S1). Because water, sediment and bacteria can transport between adjacent catch basins, individual catch basins are not spatially independent. To minimize the confounding of treatment effects, areas of the city where each treatment was applied were selected randomly in 2013, and re-randomized in 2014. Also, during each year, we sampled larvae (see below) prior to allocating treatments to establish that there was no spatial dependence associated with baseline mosquito abundance across treatment areas, and only included catch basins where ≥15 larvae were found.
The five experimental treatments were: (i) negative control: all organic detritus dredged from catch basins weekly throughout the duration of the study; (ii) positive control: no modification of debris in catch basins; (iii) ecological trap: all organic detritus dredged from the catch basins during the first week of the experiment and 100 g fresh blackberry leaves added, submerged underwater in mesh bags; (iv) attractants only: same as previous, except 100 g fresh honeysuckle leaves; and (v) toxins only: FourStar 45-day Bacillus thuringiensis var. israelensis (Bti) briquettes (Central Life Sciences, Phoenix, AZ) added to catch basins, with no additional modification. Bti is a mosquito larvicide widely used in stormwater infrastructure, and does not inhibit mosquito oviposition behaviour [36].
To estimate relative adult mosquito survival, floating emergence traps [37] were established to capture adult mosquitoes flying upward out of the catch basins. Adults were collected from the emergence traps once per week. Abundance of larvae was used as a proxy estimate of relative oviposition rates due to the logistical challenge of collecting egg rafts inside deep catch basins and the effect that removal and handling of egg rafts would have on the abundance of emerging adult mosquitoes. Larvae were collected by passing a 12.7 × 12.7 cm aquarium net over the water surface in two figure eights, and inverting the net into a container and flushing with water. All instars were counted in aggregate and adults and larvae were identified to species using taxonomic keys [38]. Larvae were collected once per week for five weeks, once prior to allocation of treatments and four times after treatment application.
Data analyses were conducted in SAS v. 9.3 (SAS Institute Inc., Cary, NC). To test the hypotheses that inputs of attractive foliar material in catch basins increases mosquito oviposition rates and inputs of deleterious foliar material in catch basins decreases mosquito survival, separate general linear mixed models (GLMMs) with repeated measures were fit to the number of larvae and the number of adults per catch basin, including the random effect of year, the fixed effects of treatment and week, and their interaction. Linear contrasts were used to compare treatments.
(b). Oviposition site selection bioassays
We hypothesized that the high mosquito oviposition associated with certain leaf litter substrates primarily is driven by one of two ecological pathways: a chemical pathway involving the release of volatile attractants and oviposition stimulants during leaf decomposition, or microbial activity in the aquatic habitat, which also may increase attractiveness due to chemical cues but via an indirect microbial mechanism. To discern the relative contributions of these mechanisms, we conducted binary choice bioassays in which oviposition was compared among a ‘whole’ unaltered infusion of blackberry or Amur honeysuckle leaves, a filter-sterilized infusion to remove microbial flora but maintain chemical cues (leachate only), an infusion residue treatment to maintain microbial flora but remove chemical cues (microbes only), and a deionized water negative control. We confirmed that microbial flora had been removed from the filter-sterilized treatment via sequencing (see below).
Infusions of blackberry and Amur honeysuckle leaves were prepared by fermenting 15 g fresh leaves in closed plastic 2 l buckets filled with tap water for 7 days [30]. Large particulates were strained by passing the infusion through cheesecloth. These ‘whole’ infusions were used to prepare three treatments for bioassays: (i) whole infusion; (ii) filter-sterilized infusion to remove microorganisms but retain foliar chemicals (leachate only); and (iii) infusion residue to remove foliar chemicals but retain microorganisms (microbes only). The filter-sterilized infusion treatment was prepared by passing 100 ml whole infusion through 0.22 µm pore Stericup filters (Fisher Scientific, Hampton, NH) to remove microorganisms while retaining foliar chemicals associated with the different leaf detritus mixtures. The infusion residue treatment was prepared by centrifuging 100 ml whole infusion in 25 ml aliquots at 5000 rpm for 15 min, removing the supernatant, and re-suspending the pellet containing the microbes in deionized water.
For binary choice bioassays, gravid female Cx. pipiens were collected using grass infusion-baited gravid traps. Female Cx. pipiens were placed in one of ten 1 ft3 cages (24.88 ± 2.36 mosquitoes per cage). Polypropylene cups containing 100 ml of test and control treatments were placed in opposite diagonal corners of each cage. The five treatment pairs repeated for both Amur honeysuckle and blackberry leaf infusion were: (i) whole infusion versus deionized water; (ii) filter-sterilized infusion (leachate only) versus deionized water; (iii) infusion residue (microbes only) versus deionized water; (iv) filter-sterilized infusion (leachate only) versus whole infusion; and (v) infusion residue (microbes only) versus whole infusion. Treatment cups were fitted with black sleeves to mask the visual cues associated with darker-coloured treatments [19]. The cages were stored in a dark room for 3 days, and egg rafts were counted in each cup every 12 h. Chi-square tests were conducted to compare oviposition for each treatment pair.
(c). Mosquito survival bioassays
We hypothesized that the low survival of juvenile mosquitoes associated with foliar material from blackberry primarily is driven by one of two pathways: low nutritional quality of bacterial food resources, or release of chemicals toxic to larvae during leaf decomposition. Therefore, we designed an experiment to test whether addition of a high-quality food resource could mitigate the deleterious effects of a low-quality resource (i.e. low survival was caused by lack of food rather than by presence of toxins) and to compare differences in microbial resources and mosquito survival across multiple leaf litter substrates. We considered blackberry, Amur honeysuckle, elderberry (Sambucus canadensis) and autumn olive (Elaeagnus umbellata) leaves, which represent a range of survival outcomes for Cx. pipiens larvae [30]. We established a completely randomized design composed of 15 treatments, consisting of leaves from the single plant species and all possible mixtures of two, three and four plant species.
We performed a laboratory study to directly measure impacts of individual and mixed terrestrial leaf species on development and survival of juvenile mosquitoes. Culex pipiens larvae were obtained by collecting egg rafts using oviposition traps. Green leaves were collected from each of four plant species (Amur honeysuckle, autumn olive, blackberry and elderberry). Four treatments consisted of leaves from a single plant species as a baseline for comparison of mixture treatments. Six treatments consisted of two-species mixtures, four treatments consisted of three-species mixtures and one treatment was a mixture of all four species. Infusions were prepared by fermenting a total of 10 g green leaves in tap water for 7 days. Leaves and large particulates were strained out of the buckets by passing the infusion through cheesecloth.
Using these ‘whole’ infusions, two mosquito development bioassays were prepared. In the first bioassay, 20 first instar Cx. pipiens were reared in 320 ml whole infusion from each treatment. The containers were monitored daily until all mosquitoes had either died or emerged. The experiment was conducted under ambient conditions of 25°C, 70% relative humidity, and a 16 : 8 (L : D) photoperiod. In the second assay, identical experimental conditions and design were maintained. However, instead of supplying the larvae with whole infusion, 320 ml whole infusion were passed through 0.22 µm pore filters to remove microorganisms while retaining plant secondary components associated with the different leaf detritus mixtures. Every 2 days, 0.1 mg ground TetraMin fish food flakes were added to each container as a uniform diet across all treatments.
To test the hypothesis that addition of a high-quality food resource could mitigate the deleterious effects of a low-quality resource (i.e. low survival was caused by lack of food and not presence of toxins), a GLMM with the fixed effects of number of leaf species and mixture nested within number of species was fit to the proportion of mosquitoes emerged. Tukey's mean separation test was used to detect significant pairwise differences. To clarify the magnitude and direction of non-additive effects of resource diversity, linear contrasts were performed. We compared mean survival for a leaf mixture to the combined mean response for its constituent individual leaves. A non-significant contrast indicated an additive effect of mixing leaf species on survival, whereas a significant contrast indicated a synergistic or antagonistic effect [39]. To test the hypothesis that variation in survival is related to the microbial community, we repeated the same analyses for the assay in which microbes were replaced with a uniform diet.
(d). Bacterial community sequencing and analyses
To investigate differences in microbial communities among the leaf mixtures, before adding larvae, 15 ml aliquots of 7-day-old whole infusion were taken from each container. Genomic DNA was extracted using the UltraClean Soil DNA Isolation Kit (Mo Bio Laboratories Inc., Carlsbad CA, cat. no. 12800-50) with modifications described in [40]. The DNA extracts were sequenced using Illumina MiSeq Bulk v3 analysis at the W. M. Keck Center for Comparative and Functional Genomics and bacterial operational taxonomic units (OTUs) were profiled across the leaf litter mixtures. We targeted three hypervariable regions of the 16S rRNA gene using the primer sets: V1–V3 forward 5′-GAGTTTGATCNTGGCTCAG-3′, reverse 5′-GTNTTACNGCGGCKGCTG-3′; V3–V5 forward 5′-CCTACGGGAGGCAGCAG-3′, reverse 5′-CCGTCAATTCMTTTRAGT-3′; V4 forward 5′-GTGCCAGCMGCCGCGGTAA-3′, reverse 5′-GGACTACHVGGGTWTCTAAT-3′. For Illumina library preparation by Fluidigm protocols, DNA was amplified using the three primer sets added with Fluidigm Illumina linkers and unique barcodes [41]. The final Fluidigm libraries were pooled for sequencing at the Keck Center's DNA Services laboratory. The V4 region yielded 8.5 million reads, compared with 3.9 million reads for the V3–V5 region and 2.6 million reads for the V1–V3 region; therefore the V4 region was used for subsequent analyses.
The raw reads were processed using IM-TORNADO v2.0.3.2 pipeline [42] following previously described procedures [43]. IM-TORNADO trims low-quality bases, forward and reverse primers, and adaptor sequences using Trimmomatic [44] before de novo OTU picking. For this analysis, we used R1_TRIM = 150 as the cut-off length for the R1 read and R2_TRIM = 150 as the cut-off length for the R2 read. Because paired reads from the V4 region overlap, PEAR 0.9.2 was used to merge the trimmed reads using default parameters [45]. The RDP10 database [46] was used for the taxonomy assignment with a bootstrap cut-off of 50% to assign the sequences to different taxonomy levels. Sampling effort was standardized per sample using rarefaction to normalize read depth to 37 832 reads per sample. Reads were filtered using the criterion that an OTU must account for at least 0.5% of the total sequences for retention, resulting in 936 OTUs retained for bacterial amplicons (electronic supplementary material, figure S2).
To test the hypothesis that abundance and composition of bacterial OTUs vary predictably among aquatic habitats containing different leaf detritus species, nonmetric multidimensional scaling (NMDS) was conducted with bacterial OTU abundances as the raw explanatory variables and a Bray–Curtis dissimilarity index. Multi-response permutation procedure (MRPP) tests were used to compare among leaf mixture treatments using PCORD 6 [47]. Finally, similarity percentage (SIMPER) analysis was used to identify OTUs that were primarily responsible for observed differences between the single leaf species treatments using PAST [48].
3. Results
(a). Ecological traps inhibit mosquito production from stormwater catch basins
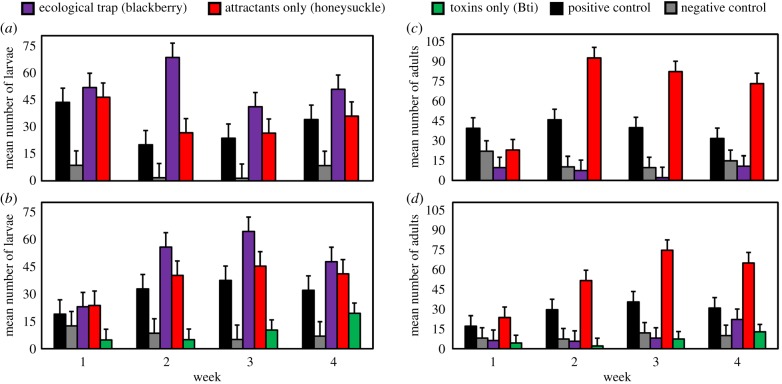
A total of 7105 Cx. pipiens larvae and 6848 Cx. pipiens adults were collected throughout the study, with no other mosquito species observed. Treatment significantly affected abundance of Cx. pipiens larvae (F = 8.42; d.f. = 8, 61; p < 0.01; figure 1a,b); in particular, the abundance of larvae in the ecological trap (blackberry leaves) treatment was significantly higher than in the positive control (figure 1a,b; table 1). Treatment also significantly affected abundance of adult Cx. pipiens emerging from catch basins (F = 22.20; d.f. = 8, 61; p < 0.01; figure 1c,d); the abundance of adults emerging from the trap and the toxins only treatments were lower than the positive control and not significantly different from the negative control. Moreover, the abundance of emerging Cx. pipiens adults was higher in the attractants only treatment compared to the positive control (table 2). We found significant interactions between treatment and time (weeks) for abundance of larvae (F = 2.52; d.f. = 24, 161; p < 0.01) and adults (F = 4.04; d.f. = 24, 177; p < 0.01), both reflecting changes in magnitude (i.e. increase) but not direction of the treatment effect sizes one week after the initial application of treatments.
Figure 1.
Mean (±s.e.) number of Culex pipiens larvae (a,b), and adults (c,d) collected across treatments in catch basins in Paxton, Illinois over four-week study periods. The five treatments included a natural ecological trap (blackberry leaves), toxins only (Bti larvicidal briquets), attractants only (honeysuckle leaves), and positive and negative controls. (Online version in colour.)
Table 1.
Comparison of abundance of Culex pipiens larvae across catch basin treatments and controls. p-values were calculated using linear contrasts to test the hypothesis that inputs of attractive leaf detritus species increase mosquito oviposition rates in these habitats.
| catch basin treatment | estimate | s.e. | T | p |
|---|---|---|---|---|
| blackberry versus positive control | 19.96 | 5.93 | 3.37 | <0.01 |
| honeysuckle versus positive control | 5.37 | 5.94 | 0.90 | 0.37 |
| negative control versus positive control | −23.50 | 5.97 | −3.94 | <0.01 |
Table 2.
Comparison of abundance of Culex pipiens adults across catch basin treatments and controls. p-values were calculated using linear contrasts to test the hypothesis that inputs of toxins (i.e. larvicides and deleterious leaf detritus species) reduce mosquito emergence rates in these habitats while inputs of high-quality leaf detritus resources increase emergence rates.
| catch basin treatment | estimate | s.e. | T | p |
|---|---|---|---|---|
| blackberry versus positive control | −24.56 | 4.58 | −5.36 | <0.01 |
| Bti versus positive control | −10.69 | 2.65 | −4.03 | <0.01 |
| blackberry versus negative control | −2.76 | 4.58 | −0.60 | 0.55 |
| Bti versus negative control | −1.32 | 2.65 | −0.50 | 0.62 |
| honeysuckle versus positive control | 26.85 | 4.55 | 5.90 | <0.01 |
| negative control versus positive control | −21.80 | 4.55 | −4.79 | <0.01 |
(b). Chemical cues mediate oviposition site selection by mosquitoes
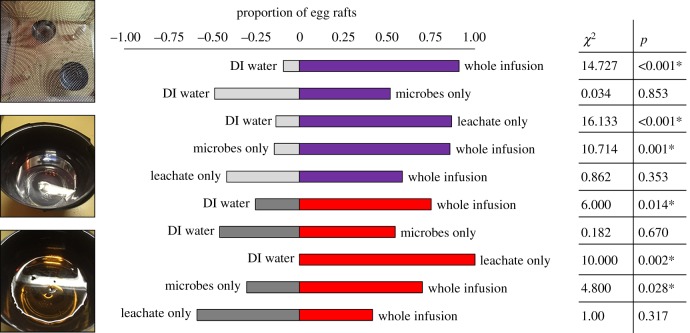
In binary choice oviposition bioassays, for both blackberry and Amur honeysuckle leaf litter treatments, we found that a larger proportion of Cx. pipiens egg rafts were laid in whole infusion (positive control) and filter-sterilized infusion treatments (leachate only) compared with deionized water (negative control) (figure 2). A significantly greater number of egg rafts also were laid in the whole infusion compared to the infusion residue treatment (microbes only). There were no significant differences in the number of egg rafts laid in the whole infusion versus the filter-sterilized infusion treatment (leachate only) or infusion residue treatment (microbes only) versus deionized water treatment (figure 2).
Figure 2.
Proportion of Culex pipiens egg rafts laid in binary choice behavioural cage bioassays for blackberry leaf infusion (top; purple) and honeysuckle leaf infusion (bottom; red). Pictured left is the configuration of treatment pairs in insect rearing cages (top), and absence (centre) and presence (bottom) of egg rafts in experimental treatments. Asterisks indicate significant pairwise differences at α = 0.05. (Online version in colour.)
(c). Bacterial community composition determines mosquito survival
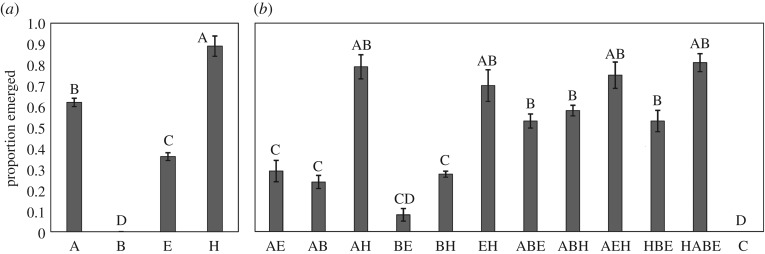
In a laboratory study that measured impacts of foliar material from mixed terrestrial plant species on survival of juvenile mosquitoes, Cx. pipiens survival varied considerably among treatments (F = 42.16; d.f. = 11, 58; p < 0.01; figure 3a,b). Blackberry leaves consistently resulted in low juvenile survival, and even in the two-species leaf mixtures containing blackberry leaves, addition of a higher-quality resource was not sufficient to mitigate the deleterious effects of the blackberry leaves (table 3). However, we found a synergistic survival response to leaf mixtures containing three or four species, all of which yielded higher mosquito survival than any of the single-species or two-species treatments regardless of the species identities of the leaves in the mixture (F = 34.08; d.f. = 3, 58; p < 0.01; table 3). This outcome correlates strongly with changes in the microbial community under different leaf species combinations (see below). When filter-sterilized infusions were prepared for all treatments and the infusion residue was replaced with a uniform diet, we found no variation in mosquito survival among treatments (electronic supplementary material, figure S3A,B).
Figure 3.
Adult emergence of Culex pipiens from whole leaf infusion across (a) single and (b) mixed leaf species treatments. Grouping letters represent significant pairwise differences at α = 0.05. Leaves include autumn olive (‘A’), blackberry (‘B’), elderberry (‘E’), Amur honeysuckle (‘H’) and a grass infusion positive control (‘C’).
Table 3.
Tests for additive effects of mixing four leaf detritus species on Culex pipiens adult emergence rates. p-values were calculated using linear contrasts to compare mean emergence rates for leaf mixture treatments to the combined mean response for their constituent individual leaf species. Leaves include autumn olive (‘A’), blackberry (‘B’), elderberry (‘E’) and Amur honeysuckle (‘H’).
| leaf mixture | estimate | s.e. | T | p | effect |
|---|---|---|---|---|---|
| HA | 0.035 | 0.053 | 0.67 | 0.508 | additive |
| HB | −0.170 | 0.057 | −2.99 | 0.004 | antagonistic |
| HE | 0.075 | 0.053 | 1.43 | 0.159 | additive |
| AB | −0.073 | 0.057 | −1.28 | 0.207 | additive |
| AE | −0.200 | 0.053 | −3.80 | <0.001 | antagonistic |
| BE | −0.100 | 0.053 | −1.90 | 0.062 | antagonistic |
| HAB | 0.077 | 0.050 | 1.55 | 0.127 | additive |
| HAE | 0.127 | 0.050 | 2.56 | 0.013 | synergistic |
| HBE | 0.113 | 0.050 | 2.29 | 0.026 | synergistic |
| ABE | 0.203 | 0.050 | 4.10 | <0.001 | synergistic |
| HABE | 0.343 | 0.048 | 7.14 | <0.001 | synergistic |
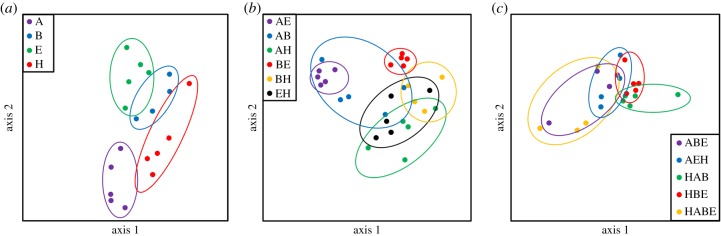
Composition of bacterial communities varied among leaf litter treatments (T = 19.92; d.f. = 1; p < 0.01; figure 4). Significant pairwise differences among the treatments generally reflected those observed in comparisons of mosquito survival (electronic supplementary material, table S1). SIMPER revealed 19 OTUs that largely explained observed differences between blackberry, Amur honeysuckle, autumn olive and elderberry leaf litter treatments, contributing an average dissimilarity equal to or greater than 1% between treatments (electronic supplementary material, table S2). While we found significant variation in bacterial composition among the single-species and two-species mixtures, there were no significant pairwise differences among bacterial communities between leaf mixtures containing three or four species.
Figure 4.
Comparisons of bacterial community composition across treatments of single leaf species (a), two leaf species (b), and three and four leaf species (c). Analyses were conducted using non-metric multidimensional scaling (NMDS) for 70 replicate containers on 936 bacterial taxa with a Bray–Curtis dissimilarity index. Leaves include autumn olive (‘A’), blackberry (‘B’), elderberry (‘E’) and Amur honeysuckle (‘H’). (Online version in colour.)
4. Discussion
Our findings extend the ecological trap concept to an important mosquito disease vector and provide mechanistic insights into why traps may occur in nature. The results of a field test that intentionally created an ecological trap for mosquitoes in stormwater catch basins demonstrate that leaf litter subsidies can attract and kill Cx. pipiens, increasing oviposition while reducing survival, despite the high environmental variability of stormwater habitats. In experiments designed to identify the mechanisms that explain the attractive yet lethal properties of certain plant species to mosquito disease vectors, behavioural bioassays suggested that oviposition site selection by Cx. pipiens is mediated primarily by chemical cues as leaves decompose. However, juvenile mosquito survival mainly appeared related to the suitability of the bacterial community for nutritional needs, which does not appear to create a cue that influences oviposition site selection. This mismatch between oviposition cues and drivers of habitat quality for survival to adult emergence may explain mechanistically the occurrence of this ecological trap phenomenon.
Behavioural bioassays suggest that Cx. pipiens oviposition behaviour is not driven by bacterial communities per se but rather by the chemical cues emanating from decaying leaf litter. These results are comparable to those of a similar laboratory experiment which found bamboo and white oak leaf infusions collect large numbers of Aedes aegypti eggs due to bacteria-associated carboxylic acids and methyl esters that stimulate oviposition [33]. Plant secondary compounds extracted from leaf tissue using acetone, ethyl acetate and methanol alter oviposition site selection of Anopheles stephensi and An. subpictus, indicating that leaf material itself as well as microbial flora can provide the source of chemicals that drive oviposition choice [49,50]. A variety of phenolic compounds are abundant in blackberry [51] and Amur honeysuckle leaves [52] that previously have been shown to attract mosquito oviposition [53,54]. Further research is needed to establish the metabolic source of the chemical cues that drove oviposition site selection in the current study. These studies should investigate whether these chemical cues are derived from detritus-based microbes [33] or from plant secondary compounds [32]. Our study also contributed to a well-established literature suggesting that leaf litter species vary in quality for mosquito development and adult emergence [28,55,56]. Our observation that survival is not driven primarily by plant secondary compounds and may be related to the bacterial community was consistent with the findings of other studies that suggest the importance of microbial flora to the fitness of filter-feeding invertebrates [24,57].
Previous research has addressed effects of leaf litter from individual plant species on mosquito performance. However, container habitats typically contain a mix of foliar material from different plants, and comparatively few experiments have examined leaf litter mixtures. Further, these studies often focus on mixtures of only two leaf species, potentially yielding biased conclusions regarding the impact of leaf resource diversity if one of the leaf species selected was highly nutritious [58,59]. Here, we selected leaf species representing a range of qualities for Cx. pipiens development and a larger number of leaf combination treatments. We found that increasing diversity of foliar material yields higher mosquito survival, consistent with the result of Walker & Merritt [57]. Mixtures of leaf litter from three or more plant species yielded equal emergence rates and consistently produced a synergistic effect compared to the mean emergence rate for the constituent single leaf species, regardless of leaf identity. However, while the majority of two leaf mixtures produced an additive effect, the mixtures containing blackberry leaves often yielded an antagonistic effect on emergence, indicating that addition of a higher-quality resource did not offset the deleterious effect of the blackberry leaves.
Patterns in survival of juvenile Cx. pipiens in different leaf litter combinations reflected our analyses of bacterial community composition associated with those leaf litter combinations. We found that the bacterial community varies predictably among individual leaf litter species and likely is the mechanism which determines juvenile mosquito survival from the different combinations of leaf infusions in the field and laboratory portions of this study. With increasing leaf species diversity, the microbial community becomes homogenized, apparently to the benefit of mosquito survival. This observation was consistent with the findings of other studies that demonstrate the importance of microbial flora to the fitness of filter-feeding invertebrates [24].
Mosquito-borne diseases exact a huge toll on human health globally, and abundance of adult mosquitoes generally is considered the most important predictor of human risk of exposure to mosquito-borne pathogens, necessitating effective vector control [60]. Within recent decades, mosquito management strategies that rely exclusively upon insecticide use in aquatic larval habitats have fallen short, due to evolution of insecticide resistance in mosquitoes, impacts of insecticides on non-target species, and perceived and actual risks regarding the environmental and public health safety of insecticides [61–63]. Thus, to enhance the long-term sustainability of juvenile mosquito abatement efforts and thereby protect human health, there is an urgent need to develop ecologically based complements to insecticides for mosquito management [64]. Our findings demonstrate that certain leaf litter types offer potential for the development of novel mosquito control strategies. The approach used here of attract-and-kill has been used for decades to control agricultural and forest pests but remains underexplored for arthropod vector species [22,65], and has the potential to yield an integrated vector management tool that may enhance the efficacy of and reduce the need for insecticide use. Future research should assess the duration of efficacy of leaf litter-derived attractants and toxins, including whether further ageing of the ecological trap treatment continues to attract mosquitoes [30] and whether its deleterious effect on mosquitoes may diminish over time, as well as potential dose-dependence of the attractant properties of leaf detritus [31]. Collectively, our results suggest that mosquito abatement may be enhanced by use of oviposition attractants to lure females to lay eggs in habitats deleterious to the survival of larvae, potentially increasing the efficacy of control efforts by simultaneously wasting female production and effectively reducing juvenile survival.
Supplementary Material
Acknowledgements
We thank Millon Blackshear, Jackie Duple, Matt Edinger, Brit'nee Haskins, Reanna Kayser, Chang-Hyun Kim, Brandon Lieberthal, Noor Malik, Leah Overmier, Allison Parker and Chase Robinson for their technical assistance. We thank the DNA Services laboratory at the W. M. Keck Center for Comparative and Functional Genomics for sequencing services.
Data accessibility
Supporting data from the catch basin experiment, mosquito oviposition cage bioassay, and leaf mixture mosquito emergence bioassay, are available in Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.g3t3cj8 [66]. All bacterial sequences have been deposited in NCBI Bioproject ID PRJNA488445.
Authors' contributions
A.M.G., E.J.M. and B.F.A. defined and directed the research questions. A.M.G. conceptualized and carried out the statistical analysis and was lead author in the writing of the manuscript. A.M.G. collected all data. All authors read and approved the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
This study was supported by a US Environmental Protection Agency STAR Graduate Fellowship to A.M.G., a University of Illinois Institute for Sustainability, Energy, and Environment grant to B.F.A., and the Used Tire Fund and Emergency Public Health Act from the State of Illinois to E.J.M. This project was supported further by the USDA National Institute of Food and Agriculture, Hatch Project no. ME021826 through the Maine Agricultural and Forest Experiment Station (Publication no. ME0-21826). Any opinions, findings, conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. The mention of firm names or trade products does not imply that they are endorsed or recommended by the U.S. Department of Agriculture over other firms or similar products not mentioned; the USDA is an equal opportunity employer.
References
- 1.Fretwell SD, Lucas HJ. 1970. On territorial behavior and other factors influencing habitat distribution in birds. Acta Biotheor. 19, 16–36. ( 10.1007/BF01601953) [DOI] [Google Scholar]
- 2.Gripenberg S, Mayhew PJ, Parnell M, Roslin T. 2010. A meta-analysis of preference-performance relationships in phytophagous insects. Ecol. Lett. 13, 383–393. ( 10.1111/j.1461-0248.2009.01433.x) [DOI] [PubMed] [Google Scholar]
- 3.Schlaepfer MA, Runge MC, Sherman PW. 2002. Ecological and evolutionary traps. Trends Ecol. Evol. 17, 474–480. ( 10.1016/S0169-5347(02)02580-6) [DOI] [Google Scholar]
- 4.Robertson BA, Hutto RL. 2006. A framework for understanding ecological traps and an evaluation of existing evidence. Ecology 87, 1075–1085. ( 10.1890/0012-9658(2006)87%5B1075:AFFUET%5D2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 5.Kriska G, Horvath G, Andrikovics S. 1998. Why do mayflies lay their eggs en masse on dry asphalt roads? Water-imitating polarized light reflected from asphalt attracts Ephemeroptera. J. Exp. Biol. 201, 2273–2286. [DOI] [PubMed] [Google Scholar]
- 6.Weldon AJ, Haddad NM. 2005. The effects of patch shape on indigo buntings: evidence for an ecological trap. Ecology 86, 1422–1431. ( 10.1890/04-0913) [DOI] [Google Scholar]
- 7.D'Amore A, Kirby E, Hemingway V. 2009. Reproductive interference by an invasive species: an evolutionary trap? Herpetol. Conserv. Biol. 4, 325–330. [Google Scholar]
- 8.Hagman M, Phillips BL, Shine R. 2009. Fatal attraction: adaptations to prey on native frogs imperil snakes after invasion of toxic toads. Proc. R. Soc. B 276, 2813–2818. ( 10.1098/rspb.2009.0192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balme GA, Slotow R, Hunter LTB. 2010. Edge effects and the impact of non-protected areas in carnivore conservation: leopards in the Phinda-Mkhuze Complex, South Africa. Anim. Conserv. 13, 315–323. ( 10.1111/j.1469-1795.2009.00342.x) [DOI] [Google Scholar]
- 10.Jaquemet S, Potier M, Menard F. 2011. Do drifting and anchored Fish Aggregating Devices (FADs) similarly influence tuna feeding habits? A case study from the western Indian Ocean. Fish. Res. 107, 283–290. ( 10.1016/j.fishres.2010.11.011) [DOI] [Google Scholar]
- 11.Horváth G, Kriska G, Malik P, Robertson B. 2009. Polarized light pollution: a new kind of ecological photopollution. Front. Ecol. Environ. 7, 317–325. ( 10.1890/080129) [DOI] [Google Scholar]
- 12.Pečnerová P, et al. 2017. Genome-based sexing provides clues about behavior and social structure in the woolly mammoth. Curr. Biol. 27, 3505–3510. ( 10.1016/j.cub.2017.09.064) [DOI] [PubMed] [Google Scholar]
- 13.Fletcher RJ, Orrock JL, Robertson BA. 2012. How the type of anthropogenic change alters the consequences of ecological traps. Proc. R. Soc. B 279, 2546–2552. ( 10.1098/rspb.2012.0139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson BA, Ostfeld RS, Keesing F. 2017. Trojan females and Judas goats: evolutionary traps as tools in wildlife management. Bioscience 67, 983–994. ( 10.1093/biosci/bix116) [DOI] [Google Scholar]
- 15.Keesing F, Brunner J, Duerr S, Killilea M, LoGiudice K, Schmidt K, Vuong H, Ostfeld RS. 2009. Hosts as ecological traps for the vector of Lyme disease. Proc. R. Soc. B 276, 3911–3919. ( 10.1098/rspb.2009.1159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allan BF, et al. 2017. Can integrating wildlife and livestock enhance ecosystem services in central Kenya? Front. Ecol. Environ. 15, 328–335. ( 10.1002/fee.1501) [DOI] [Google Scholar]
- 17.Geery P, Holub R. 1989. Seasonal abundance and control of Culex spp. in catch basins in Illinois. J. Am. Mosq. Control Assoc. 5, 537–540. [PubMed] [Google Scholar]
- 18.Su T, Webb JP, Meyer RP, Mulla MS. 2003. Spatial and temporal distribution of mosquitoes in underground storm drain systems in Orange County, California. J. Vector Ecol. 28, 79–89. [PubMed] [Google Scholar]
- 19.Gardner AM, Hamer GL, Hines AM, Newman CM, Walker ED, Ruiz MO. 2012. Weather variability affects abundance of larval Culex (Diptera: Culicidae) in storm water catch basins in suburban Chicago. J. Med. Entomol. 49, 270–276. ( 10.1603/ME11073) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson JF, Ferrandino FJ, Dingman DW, Main AJ, Andreadis TG, Becnel JJ. 2011. Control of mosquitoes in catch basins in Connecticut with Bacillus thuringiensis israelensis, Bacillus sphaericus, and spinosad. J. Am. Mosq. Control Assoc. 27, 45–55. ( 10.2987/10-6079.1) [DOI] [PubMed] [Google Scholar]
- 21.Harbison JE, Henry M, Xamplas C, Berry R, Bhattacharya D, Dugas LR. 2014. A comparison of FourStar briquets and Natular XRT tablets in a North Shore suburb of Chicago, IL. J. Am. Mosq. Control Assoc. 30, 68–70. ( 10.2987/13-6355.1) [DOI] [PubMed] [Google Scholar]
- 22.Duchet C, et al. 2018. Pesticide-mediated trophic cascade and an ecological trap for mosquitoes. Ecosphere 9, e4 ( 10.1002/ecs2.2179) [DOI] [Google Scholar]
- 23.Kuiters A, Sarink H. 1986. Leaching of phenolic compounds from leaf and needle litter of several deciduous and coniferous trees. Soil Biol. Biochem. 18, 475–480. ( 10.1016/0038-0717(86)90003-9) [DOI] [Google Scholar]
- 24.Fish D, Carpenter SR. 1982. Leaf litter and larval mosquito dynamics in tree-hole ecosystems. Ecology 63, 283–288. ( 10.2307/1938943) [DOI] [Google Scholar]
- 25.Wallace JB, Merritt RW. 1980. Filter-feeding ecology of aquatic insects. Annu. Rev. Entomol. 25, 103–132. ( 10.1146/annurev.en.25.010180.000535) [DOI] [Google Scholar]
- 26.Merritt R, Dadd R, Walker E. 1992. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu. Rev. Entomol. 37, 349–374. ( 10.1146/annurev.en.37.010192.002025) [DOI] [PubMed] [Google Scholar]
- 27.Walker ED, Merritt RW, Kaufman MG, Ayres MP, Riedel MH. 1997. Effects of variation in quality of leaf detritus on growth of the eastern tree-hole mosquito, Aedes triseriatus (Diptera: Culicidae). Can. J. Zool. 75, 706–718. ( 10.1139/z97-091) [DOI] [Google Scholar]
- 28.Yanoviak SP. 1999. Effects of leaf litter species on macroinvertebrate community properties and mosquito yield in Neotropical tree hole microcosms. Oecologia 120, 147–155. ( 10.1007/s004420050843) [DOI] [PubMed] [Google Scholar]
- 29.Rios J, Recio M, Villar A. 1988. Screening methods for natural products with antimicrobial activity: a review of the literature. J. Ethnopharmacol. 23, 127–149. ( 10.1016/0378-8741(88)90001-3) [DOI] [PubMed] [Google Scholar]
- 30.Gardner AM, Allan BF, Frisbie LA, Muturi EJ. 2015. Asymmetric effects of native and exotic invasive shrubs on ecology of the West Nile virus vector Culex pipiens (Diptera: Culicidae). Parasit. Vectors 8, 329 ( 10.1186/s13071-015-0941-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kramer WL, Mulla MS. 1979. Oviposition attractants and repellents of mosquitoes: oviposition responses of Culex mosquitoes to organic infusions. Environ. Entomol. 8, 1111–1117. ( 10.1093/ee/8.6.1111) [DOI] [Google Scholar]
- 32.Bentley MD, Day JF. 1989. Chemical ecology and behavioral aspects of mosquito oviposition. Annu. Rev. Entomol. 34, 401–421. ( 10.1146/annurev.en.34.010189.002153) [DOI] [PubMed] [Google Scholar]
- 33.Ponnusamy L, Xu N, Nojima S, Wesson DM, Schal C, Apperson CS. 2008. Identification of bacteria and bacteria-associated chemical cues that mediate oviposition site preferences by Aedes aegypti. Proc. Natl Acad. Sci. USA 105, 9262–9267. ( 10.1073/pnas.0802505105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ponnusamy L, Wesson DM, Arellano C, Schal C, Apperson CS. 2010. Species composition of bacterial communities influences attraction of mosquitoes to experimental plant infusions. Microb. Ecol. 59, 158–173. ( 10.1007/s00248-009-9565-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardner AM, Anderson TK, Hamer GL, Johnson DE, Varela KE, Walker ED, Ruiz MO. 2013. Terrestrial vegetation and aquatic chemistry influence larval mosquito abundance in catch basins, Chicago, USA. Parasit. Vectors 6, e9 ( 10.1186/1756-3305-6-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stoops CA. 2005. Influence of Bacillus thuringiensis var. israelensis on oviposition of Aedes albopictus (Skuse). J. Vector Ecol. 30, 41–44. [PubMed] [Google Scholar]
- 37.Hamer GL, Kelly PH, Focks DA, Goldberg TL, Walker ED. 2011. Evaluation of a novel emergence trap to study Culex mosquitoes in urban catch basins. J. Am. Mosq. Control Assoc. 27, 142–147. ( 10.2987/10-6090.1) [DOI] [PubMed] [Google Scholar]
- 38.Andreadis TG, Thomas MC, Shepard JJ. 2005. Identification guide to the mosquitoes of Connecticut. New Haven, CT: Connecticut Agricultural Experiment Station. [Google Scholar]
- 39.Reiskind MH, Greene KL, Lounibos LP. 2009. Leaf species identity and combination affect performance and oviposition choice of two container mosquito species. Ecol. Entomol. 34, 447–456. ( 10.1111/j.1365-2311.2008.01067.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muturi EJ, Orindi BO, Kim CH. 2013. Effect of leaf type and pesticide exposure on abundance of bacterial taxa in mosquito larval habitats. PLoS ONE 8, e71812 ( 10.1371/journal.pone.0071812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muturi EJ, Donthu RK, Fields CJ, Moise IK, Kim CH. 2017. Effect of pesticides on microbial communities in container aquatic habitats. Sci. Rep. 7, 44565 ( 10.1038/srep44565) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jeraldo P, Kalari K, Chen X, Bhavsar J, Mangalam A, White B, Nelson H, Kocher JP, Chia N. 2014. IM-TORNADO: a tool for comparison of 16S reads from paired-end libraries. PLoS ONE 9, e114804 ( 10.1371/journal.pone.0114804) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muturi EJ, Ramirez JL, Rooney AP, Kim CH. 2017. Comparative analysis of gut microbiota of mosquito communities in central Illinois. PLoS Negl. Trop. Dis. 11, e0005377 ( 10.1371/journal.pntd.0005377) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. ( 10.1093/bioinformatics/btu170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Kobert K, Flouri T, Stamatakis A. 2013. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics 30, 614–620. ( 10.1093/bioinformatics/btt593) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cole JR, et al. 2008. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 37, D141–D145. ( 10.1093/nar/gkn879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCune B, Mefford MJ. 2011. PC-ORD: multivariate analysis of ecological data. Version 6 Gleneden Beach, OR: MjM Software. [Google Scholar]
- 48.Hammer Ř, Harper D, Ryan P. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electronica 4, 4–9. [Google Scholar]
- 49.Rajkumar S, Jebanesan A. 2009. Larvicidal and oviposition activity of Cassia obtusifolia Linn (Family: Leguminosae) leaf extract against malarial vector, Anopheles stephensi Liston (Diptera: Culicidae). Parasitol. Res. 104, 337–340. ( 10.1007/s00436-008-1197-8) [DOI] [PubMed] [Google Scholar]
- 50.Elango G, Bagavan A, Kamaraj C, Zahir AA, Rahuman AA. 2009. Oviposition-deterrent, ovicidal, and repellent activities of indigenous plant extracts against Anopheles subpictus Grassi (Diptera: Culicidae). Parasitol. Res. 105, 1567–1576. ( 10.1007/s00436-009-1593-8) [DOI] [PubMed] [Google Scholar]
- 51.Wang SY, Lin HS. 2000. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 48, 140–146. ( 10.1021/jf9908345) [DOI] [PubMed] [Google Scholar]
- 52.Cipollini D, Stevenson R, Enright S, Eyles A, Bonello P. 2008. Phenolic metabolites in leaves of the invasive shrub, Lonicera maackii, and their potential phytotoxic and anti-herbivore effects. J. Chem. Ecol. 34, 144–152. ( 10.1007/s10886-008-9426-2) [DOI] [PubMed] [Google Scholar]
- 53.Beehler J, Millar J, Mulla M. 1994. Field evaluation of synthetic compounds mediating oviposition in Culex mosquitoes (Diptera: Culicidae). J. Chem. Ecol. 20, 281–291. ( 10.1007/BF02064436) [DOI] [PubMed] [Google Scholar]
- 54.Du Y, Millar JG. 1999. Electroantennogram and oviposition bioassay responses of Culex quinquefasciatus and Culex tarsalis (Diptera: Culicidae) to chemicals in odors from Bermuda grass infusions. J. Med. Entomol. 36, 158–166. ( 10.1093/jmedent/36.2.158) [DOI] [PubMed] [Google Scholar]
- 55.Reiskind MH, Zarrabi AA, Lounibos LP. 2010. Invasive leaf resources alleviate density dependence in the invasive mosquito, Aedes albopictus. Biol. Invasions 12, 2319–2328. ( 10.1007/s10530-009-9646-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muturi EJ, Spencer JL, Allan BF. 2014. Influence of biofuel crops on mosquito production and oviposition site selection. GCB Bioenergy 6, 61–66. ( 10.1111/gcbb.12038) [DOI] [Google Scholar]
- 57.Walker ED, Merritt RW. 1988. The significance of leaf detritus to mosquito (Diptera: Culicidae) productivity from treeholes. Environ. Entomol. 17, 199–206. ( 10.1093/ee/17.2.199) [DOI] [Google Scholar]
- 58.Reiskind M, Zarrabi A, Lounibos L. 2012. Effects of combination of leaf resources on competition in container mosquito larvae. Bull. Entomol. Res. 102, 424–434. ( 10.1017/S0007485311000861) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muturi EJ, Gardner AM, Bara JJ. 2015. Impact of an alien invasive shrub on ecology of native and alien invasive mosquito species (Diptera: Culicidae). Environ. Entomol. 44, 1308–1315. ( 10.1093/ee/nvv121) [DOI] [PubMed] [Google Scholar]
- 60.Bowman LR, Runge-Ranzinger S, McCall P. 2014. Assessing the relationship between vector indices and dengue transmission: a systematic review of the evidence. PLoS Negl. Trop. Dis. 8, e2848 ( 10.1371/journal.pntd.0002848) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Milam C, Farris J, Wilhide J. 2000. Evaluating mosquito control pesticides for effect on target and nontarget organisms. Arch. Environ. Contam. Toxicol. 39, 324–328. ( 10.1007/s002440010111) [DOI] [PubMed] [Google Scholar]
- 62.Hemingway J, Ranson H. 2000. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 45, 371–391. ( 10.1146/annurev.ento.45.1.371) [DOI] [PubMed] [Google Scholar]
- 63.Tedesco C, Ruiz M, McLafferty S. 2010. Mosquito politics: local vector control policies and the spread of West Nile Virus in the Chicago region. Health Place 16, 1188–1195. ( 10.1016/j.healthplace.2010.08.003) [DOI] [PubMed] [Google Scholar]
- 64.Bellini R, Zeller H, Van Bortel W. 2014. A review of the vector management methods to prevent and control outbreaks of West Nile virus infection and the challenge for Europe. Parasit. Vectors 7, 323 ( 10.1186/1756-3305-7-323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Müller G, Junnila A, Qualls W, Revay E, Kline D, Allan S, Schlein Y, Xue R. 2010. Control of Culex quinquefasciatus in a storm drain system in Florida using attractive toxic sugar baits. Med. Vet. Entomol. 24, 346–351. ( 10.1111/j.1365-2915.2010.00876.x) [DOI] [PubMed] [Google Scholar]
- 66.Gardner AM, Muturi EJ, Allan BF. 2018. Data from: Discovery and exploitation of a natural ecological trap for a mosquito disease vector Dryad Digital Repository. ( 10.5061/dryad.g3t3cj8) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Gardner AM, Muturi EJ, Allan BF. 2018. Data from: Discovery and exploitation of a natural ecological trap for a mosquito disease vector Dryad Digital Repository. ( 10.5061/dryad.g3t3cj8) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Supporting data from the catch basin experiment, mosquito oviposition cage bioassay, and leaf mixture mosquito emergence bioassay, are available in Dryad Digital Repository at http://dx.doi.org/10.5061/dryad.g3t3cj8 [66]. All bacterial sequences have been deposited in NCBI Bioproject ID PRJNA488445.