Abstract
In group-living species, social stability is an important trait associated with the evolution of complex behaviours such as cooperation. While the drivers of stability in small groups are relatively well studied, little is known about the potential impacts of unstable states on animal societies. Temporary changes in group composition, such as a social group splitting and recombining (i.e. a disturbance event), can result in individuals having to re-establish their social relationships, thus taking time away from other tasks such as foraging or vigilance. Here, we experimentally split socially stable groups of captive zebra finches (Taeniopygia guttata), and quantified the effects of repeated disturbance events on (1) group foraging efficiency, and (2) co-feeding associations when subgroups were recombined. We found that the efficiency of groups to deplete a rich, but ephemeral, resource patch decreased after just a single short disturbance event. Automated tracking of individuals showed that repeated disturbances reduced efficiency by causing social relationships to become more differentiated and weaker, resulting in fewer individuals simultaneously accessing the patch. Our experiment highlights how short-term disturbances can severely disrupt social structure and group functionality, revealing potential costs associated with group instability that can have consequences for the evolution of animal societies.
Keywords: collective behaviour, foraging efficiency, group dynamics, resilience, social disturbance, zebra finch
1. Introduction
Social stability is an important facet of the social structure of animal populations [1] and plays a key role in the evolution of animal societies (e.g. [2,3]). Characteristics such as cohesion and stable relationships are thought to have underpinned the evolution of group-level traits such as cooperation [4] and collective decision-making [5,6]. Recent evidence from group-living animals has also highlighted the benefits of stable social relationships for reproductive success [7,8], offspring survival [9–11] and longevity [12–15] (reviewed in [16]). Although groups naturally change over time (e.g. due to immigration or mortality), transitory states of instability—when groups experience reversible changes in size and composition—can be caused by extrinsic factors such as human disturbance (e.g. [17]) or unsuccessful predator attacks that cause group members to become temporarily separated. Studies on the effects of permanent changes in group membership support the idea that social instability may yield individual-level costs. For example, free-ranging mares that have been transferred into a new group have higher stress levels [18] and vampire bats that have been transferred to a new group receive fewer food donations than those that stayed in their original groups [19]. However, little is known about how temporary disturbances affect group dynamics in animal societies, and whether these effects translate to changes in group functionality.
Coordination and synchronization can benefit members of a social group [20,21]. Groups that coordinate their foraging by matching activities such as spacing, vigilance bouts or communication signals should be able to more efficiently deplete ephemeral resources, and thus should have higher average food intake rates than otherwise groups of identical characteristics in which individuals are unable to coordinate their actions. Thus, individuals in groups that perform most efficiently—for example, through mechanisms such as cooperative foraging [22]—should have the highest average fitness [23]. Competition between group members when foraging at food patches could limit the number of individuals that can forage simultaneously, which would reduce the rate at which the group depletes resources [24]. If periods of instability disrupt the consistency of social relationships among individuals, then we expect a negative effect on tasks in which group members benefit from social coordination: reduced tolerance among group members foraging on a clumped resource patch would reduce the ability for that group to efficiently extract resources from the environment. Should this be the case, then it would raise questions about how resilient animal societies that feature stable group membership are to increasing disturbances.
To determine whether social instability shapes group-level performance, and therefore can act as an agent of selection on behaviours that promote stable group-living, we experimentally manipulated the stability of two replicated colonies of zebra finches (Taeniopygia guttata). Zebra finches live in relatively compact [25] and stable colonies [26], in which males and females form lifelong pair bonds and maintain these bonds even when they are not breeding. In the wild, zebra finch colonies consisting of many male–female pairs can persist for several years, despite experiencing annual changes in size and composition related to breeding activity [27]. This makes them a suitable biological system for quantifying the costs associated with social instability.
Our colonies were kept in stable social environments for six months prior to the start of the experiment. We then subjected colonies to temporary disturbances by splitting them into three subcolonies for 2 days. When the colonies were recombined, we provided them with an ephemeral high-quality resource to forage from. As outlined above, we predicted that disturbance events would lead colonies to become less efficient at depleting the food patch. Using fine-scale simultaneous tracking of all colony members [28], we then quantified changes in group-level social behaviours during foraging to identify mechanisms that could be responsible for a reduction in foraging efficiency. If social instability disrupts the social relationships among individuals (i.e. outside of the male–female pair bonds), then social instability should lead to a reduction in social tolerance (here, the propensity for individuals to forage with other group members in a competitive setting). Specifically, we predicted that disturbance events can cause a reduction in social tolerance among individuals that forage simultaneously, leading to smaller foraging group sizes. We also predicted that disturbance could increase the skew in access to resources, for example, if patches are more often monopolized by dominant individuals (following [29,30]). Finally, we predicted that disturbance might reduce individuals' propensity to feed with less preferred group members, which should be reflect in the structure of social relationships, or more specifically the exclusivity of social relationships in the social foraging network [31].
2. Methods
(a). Study system and experimental manipulations
This study was conducted at the Max Planck Institute for Ornithology in Radolfzell, Germany. Zebra finch colonies (n1 = 28 individuals and n2 = 30 individuals) were housed in 2 × 2 × 2 m indoor aviaries with a fixed day–night cycle for the course of the experiment. Colonies were composed of an equal sex ratio and had previously reproduced with the same colony composition. We gave birds the opportunity to breed (by providing nesting material) for one month, three months before the start of the experiment, to allow us to identify and later control for male–female pair bonds. Each bird in the study was individually identified with a unique combination of colour leg bands, and a visual-based recognition marker (two-dimensional tag; dimensions 1 × 1 cm; weight: approx. 0.2 g) that can be automatically recognized in digital images [32,33]. Two-dimensional tags were attached to birds using a small backpack (following [28]; electronic supplementary material, figure S1).
The experiment consisted of four phases (figure 1a): (i) the pre-treatment phase, the 14 days immediately prior to the first disturbance event; (ii) the treatment phase, which includes three disturbance events (splitting the colony in three subcolonies for 2 days and recombining them for 3 days, three times over a period of 15 days); (iii) the post-treatment phase, 13 days after the last disturbance event; and (iv) the recovery phase, a 5-day period three weeks after the last disturbance event. In all phases of the experiment, colonies were kept in, or returned to, their original state (the pre-disturbance size and composition of the colony).
Figure 1.
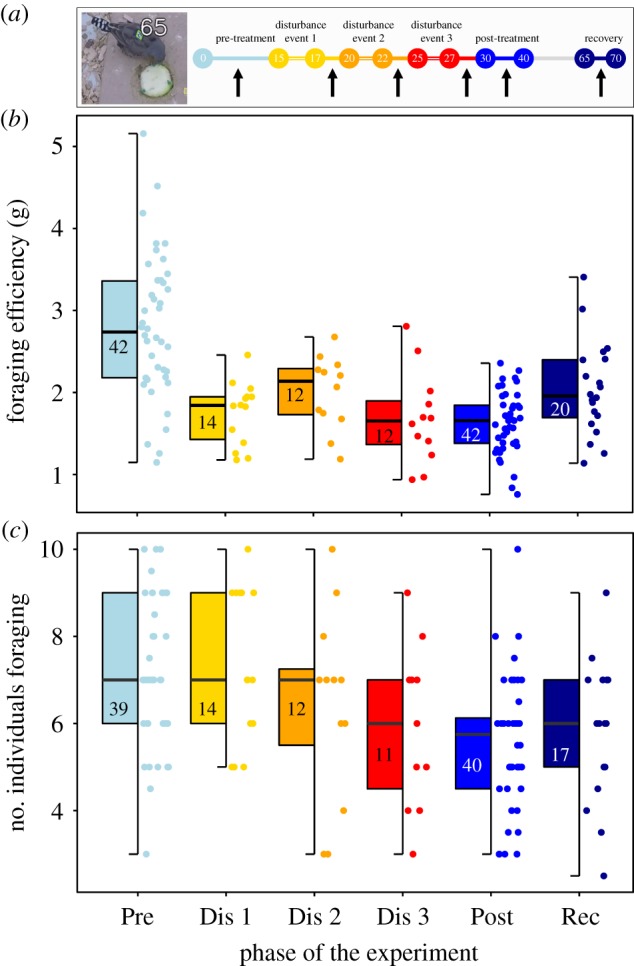
Reduced foraging efficiency and social tolerance during treatment phases of the experiment. (a) Timeline of the experiment with coloured circles corresponding to phases, and numbers representing days since the start of the experiment. Double lines correspond to days in which the colonies were split to simulate a temporary disturbance event, with colonies being in their normal state at all other times (solid lines). The solid arrows show the periods during which the feeding trial data were collected. There were no manipulations from day 30 onwards, but data were collected on days 65–70. The embedded photo shows an individual feeding at the zucchini, the number corresponding to its one-dimensional detected from the two-dimensional tag on its back using automated tracking software. (b) Foraging efficiency (amount of zucchini eaten in 45 min trial) reduced significantly after the first treatment phase (between Pre and Dis 1; reference level is Pre), remaining significantly lower during the following treatment phases and the post-treatment phase (reference level is Pre). Model results are in table 1. (c) The number of individuals simultaneously foraging dropped significantly during the second treatment phase (reference level is Pre). Fewer individuals were simultaneously present at the resource during the last disturbance phase and the post-treatment phase, but numbers increased slightly during the recovery phase. Model results are in table 2. (b,c) The medians are shown by the black line inside the box, with boxes showing the lower and upper quartile, black vertical bars showing the full range of the data and dots showing the raw data. Numbers inside the box indicate the number of trials. Phases of the experiment correspond to: pre-treatment (Pre), disturbance event 1 (Dis 1), disturbance event 2 (Dis 2), disturbance event 3 (Dis 3), post-treatment (Post) and recovery (Rec).
During the treatment phase, we split each colony into three subcolonies as follows: we first removed all birds from the main aviary, assigning half the birds to be placed back into it. We then divided the other half into two subcolonies of six or eight birds each, which we kept in separated aviaries (of smaller size compared to the main aviary) with no visual contact and minimum acoustic contact. We randomly picked different individuals to allocate into each subcolony in each disturbance event, meaning that subcolony membership, and which aviary each bird experienced, varied from one disturbance event to another. However, we always kept mated pairs together to avoid effects of our experimental treatment on pair re-formation that would have been caused by splitting established male–female pairs that had previously bred with each other (as shown in [34,35]). That is, our focus was on studying the effects of instability at the colony level, while maintaining stable male–female pairs.
(b). Foraging efficiency
To first determine whether disturbance events affect group performance, we measured and compared the foraging efficiency of the colony before any disturbance (pre-treatment phase) and after each subsequent phase of the experiment by presenting colonies with an ephemeral high-quality food patch. We conducted feeding trials only when colonies were returned to their original composition (figure 1a). This process involved fasting colonies for one hour prior to the trial to ensure they were motivated to feed, and then providing each colony with one slice of zucchini (courgette), equating to one feeding trial. Feeding trials were run once in the morning approximately 9.00 and once in the afternoon approximately 14.30, and ran for 45 min. We measured foraging efficiency as total weight of the zucchini slice eaten by each colony during each trial by weighing the zucchini slice before and after each trial (to 1/100th of a gram). We gave colonies a piece of zucchini (similar to the ones used in the experimental phase) every day for several weeks prior to the experiment to avoid any novelty effects. We also controlled for water loss by measuring the change in weight of an extra identical piece of zucchini placed into the aviary room during each trial, and subtracted the average amount of weight lost due to water evaporation from the total amount of zucchini eaten each day.
(c). Co-feeding dynamics
To investigate the social factors that could explain a reduction in foraging efficiency, we video recorded and tracked individuals at and around the zucchini during each feeding trial using a GoPro Hero 4 camera fitted 50 cm above the feeding area (electronic supplementary material, figure S2). We used software [33] to extract the position (coordinates) and orientation of each bird by automatically detecting its two-dimensional tag, in each frame of the video. We also marked the centre of the zucchini with a two-dimensional tag, allowing us to identify which individuals were feeding at the zucchini in each frame, defined as individuals detected within one body length (8 cm) of the centre of the zucchini and oriented to face the zucchini. Although we recorded data from every trial, nine video recordings were lost due to camera failure or file corruption, resulting in a total of 133 (out of 142) trials with recordings.
(i). Foraging group size
To determine whether a reduction in foraging efficiency was caused by a decrease in the number of individuals foraging simultaneously, we extracted the median number of individuals co-feeding in each video frame (when at least one individual was feeding), which we defined as foraging group size. We used only frames in the video that had at least one individual present at the zucchini because our hypothesis is based on the dynamics between individuals, to which frames with no individuals would be uninformative. Finally, we summarized the foraging group size data for a given trial as the median number of individuals simultaneously present at the food in 3 min bins (15 per 45 min trial).
(ii). Skewed access to food
To determine whether reduced foraging efficiency could be explained by an increase in the skew in who accesses the resource, we calculated the amount of time (number of video frames) each individual spent (was detected) at the food patch for each trial. Using these data, we calculated the binomial skew index (B index) [36], defined as:
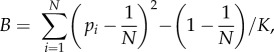 |
2.1 |
where N is the total number of individuals that were colony members, and pi is the proportion of the total group benefit (K; measured as time spent at the zucchini) gained by the ith individual. We also kept track of which subcolony each individual had been allocated to during each disturbance event in order to test for any residence effects, where individuals might have benefited from having remained in the main aviary.
(iii). Social dynamics
To test whether the disturbance events altered the social structure of colonies, we calculated the propensity for each dyadic pair of individuals to synchronize their feeding bouts. That is, we generated a social network for each colony for each phase of the experiment. We defined the edge weight (association strength) connecting each dyadic pair of individuals as the probability of observing individuals i and j feeding together given that either i or j was detected feeding (the simple ratio index, SRI [31]). An SRIij value of 1 represents two individuals always observed feeding together, whereas an SRIij of 0 represents birds always feeding separately. We then quantified changes in the social structure by calculating the mean association strength (mean SRIij values) and the coefficient of variation of each individuals’ SRIij, which we defined as the exclusivity of their social bonds. To test for broader network-level changes in social structure, we measured the correlation between the network from the pre-treatment phase to the networks from all the subsequent phases of the experiment.
3. Statistical analyses
(a). Foraging efficiency
We constructed a linear mixed model with amount of zucchini eaten as the response variable, phase of the experiment (i.e. pre-treatment, disturbance event 1–3, post-treatment, recovery period) and time of the day (i.e. morning versus afternoon trial) as fixed effects, and colony number (ID) as random effects. Because for a few days we had a larger (wider diameter) zucchini from which we cut our slices, we also included a random effect of zucchini type (large or small). We used a Gaussian model (identity link error structure) from the lme4 package v. 1.1.12 [37] in R v. 3.3.1 [38].
(b). Co-feeding dynamics
(i). Foraging group size
To evaluate the effects of social instability on foraging group size we constructed a linear mixed model with median number of individuals as the response variable, phase of the experiment, time of the day and zucchini type as fixed effects, and colony number (ID) as random effects. We fitted a Gaussian model in the lme4 package v. 1.1.12 [37] in R v. 3.3.1 [38].
(ii). Skewed access to food
To determine if disturbance events resulted in an increase in the skew, we calculated the B index for each trial, and then calculated the difference in the mean B indices between the pre-treatment phase and each subsequent phase. Because skew data represent non-independent social data, we used a simple randomisation test (see [39]) to confirm whether there was a significant effect of experimental phase on the skew in the access to the food resource. To do this, we permutated the B-index values within each flock across trials and phases, and recalculated the difference in mean B indices for the current pair of phases. We calculated statistical significance (Prand) by comparing the observed mean to 9999 randomly generated differences in means.
We tested whether the birds that remained in the main aviary (i.e. residents) outcompeted those that were moved into external aviaries (i.e. non-residents) during the disturbance event (residence effect). For each treatment phase, we used the difference between the mean number of frames in which residents were detected and the mean number of frames that non-residents were detected as the test statistics. We then implemented another simple randomization test to recalculate this difference by randomly re-assigning the resident and non-resident category in the data from that phase. We calculated statistical significance (Prand) by repeating the randomisation process 9999 times.
(iii). Social dynamics
To determine if disturbance events affected co-feeding associations, we calculated the mean strength of the co-feeding associations and the coefficient of variation of the co-feeding association strengths for each individual in each of the phases of the experiment. We fitted these phase-level network metrics to test for a quadratic effect using a linear mixed model, with phase number (1–6) and its square as fixed effects, and colony number as a random effect. Because each network and subsequent metric was measured identically, we interpreted the model output directly. We fitted a Gaussian model in the lme4 package v. 1.1.12 [37] in R v. 3.3.1 [38]. As the mean strength data largely replicates the mean foraging group size data, we report those results in the electronic supplementary material.
To test whether the structure of the co-feeding networks were stable over time, we used a Mantel test (vegan package [40] in R [38]) to compare the network from the pre-disturbance phase to the networks in each of the subsequent phases (pre-treatment versus disturbance event 1, versus disturbance event 2, versus disturbance event 3, versus post-treatment and versus recovery). Statistical significance of the Mantel test (Prand) was calculated using 9999 node permutations.
4. Results
(a). Colonies foraged less efficiently after disturbance events
We found that birds in both colonies foraged significantly less efficiently on days following disturbance events (i.e. when subgroups were recombined; n = 142 trials; table 1). The reduction in foraging efficiency continued through the post-treatment and the recovery phases, indicating a slow recovery period for group functionality (figure 1b).
Table 1.
Reduced foraging efficiency after disturbance events. The foraging efficiency model describes the effect of treatment phase on the amount of zucchini consumed by colonies (in grams) per 45 min trial. The model estimates are relative to trial data from the pre-treatment phase, and the effect of time is relative to morning trials.
| estimate | s.e. | t-value | p-value | |
|---|---|---|---|---|
| intercept | 2.420 | 0.254 | 9.541 | <0.0001 |
| disturbance event 1 | −0.951 | 0.180 | −5.291 | <0.0001 |
| disturbance event 2 | −0.761 | 0.186 | −4.086 | 0.0002 |
| disturbance event 3 | −1.206 | 0.188 | −6.399 | <0.0001 |
| post-treatment | −1.161 | 0.124 | −9.375 | <0.0001 |
| recovery phase | −0.664 | 0.160 | −4.155 | 0.0001 |
| time (afternoon trial) | −0.390 | 0.095 | −4.096 | 0.0001 |
| initial zucchini weight | 0.061 | 0.024 | 2.489 | 0.0109 |
| random effects | variance | s.d. | ||
| colony | 0 | 0 | ||
| zucchini slice size (normal/large) | 0 | 0 |
(b). Foraging group size decreased after disturbance events
Foraging group size decreased as the number of disturbance events increased (n = 133 trials; table 2 and figure 1c). This effect lasted until the recovery phase, although the number of individuals feeding simultaneously did not fully return to pre-treatment levels, suggesting a slow recovery of group coordination.
Table 2.
Reduced foraging group sizes after disturbance events. The foraging group size model describes the effect of treatment phase on the number of individuals foraging simultaneously (median per 3 min bin per trial). The estimates are relative to data collected in the pre-treatment phase. The effects of time and zucchini type are relative to morning trials and normally sized zucchinis, respectively.
| estimate | s.e. | t-value | p-value | |
|---|---|---|---|---|
| disturbance event 1 | 0.131 | 0.471 | 0.278 | 0.781 |
| disturbance event 2 | −1.099 | 0.523 | −2.102 | 0.038 |
| disturbance event 3 | −2.486 | 0.701 | −3.548 | 0.001 |
| post-treatment | −1.848 | 0.360 | −5.130 | <0.0001 |
| recovery phase | −1.2413 | 0.439 | −2.824 | 0.006 |
| time (afternoon trial) | −0.272 | 0.262 | −1.037 | 0.302 |
| zucchini size (large) | 1.197 | 0.474 | 2.523 | 0.013 |
| random effects | variance | s.d. | ||
| colony | 1.188 | 1.090 |
(c). Foraging did not become more skewed after disturbance events
The reduction in foraging group size was not caused by individuals becoming more competitive about accessing the resource. Our data did not show any evidence for significant changes in skewed access to the food (electronic supplementary material, table S1). We also found no residence effect favouring birds that remained in the main aviary during disturbances (phase 1: Prand = 0.592; phase 2: Prand = 0.832; phase 3: Prand = 0.923).
(d). Social dynamics were affected by disturbance events
The mean association strength cumulatively decreased (electronic supplementary material, figure S3). Our general linear model suggested significant linear and quadratic terms, suggesting a significant decrease during experimental phases was followed by a slight recovery (electronic supplementary material, table S2). Relationships also became more exclusive (figure 2), with significant linear and quadratic terms in the general linear model suggesting that the initial increase in the coefficient of variation was followed by a recovery (table 3). Thus, relationships among group members became weaker, corroborating the data on reduction in the size of foraging groups. Changes in co-feeding relationships among individuals also had a global impact on social dynamics. The correlation to the pre-treatment network decreased throughout the subsequent treatment phases (electronic supplementary material, table S3), indicating that the social structure of foraging groups is not resilient to repeated disturbances.
Figure 2.
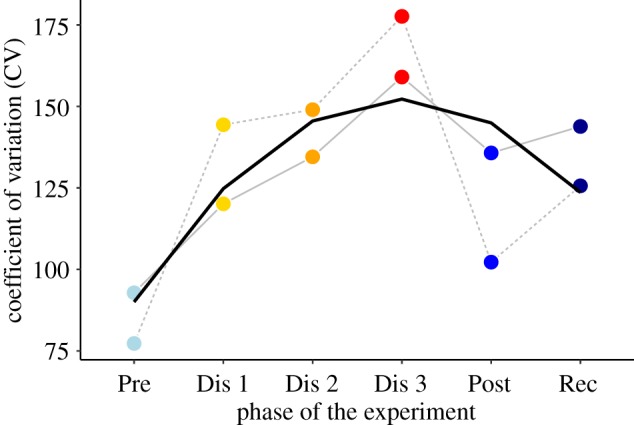
Increased social exclusivity during treatment phases of the experiment. The co-feeding relationships among individuals in the colony became more exclusive—that is, they increased coefficient of variation together with reduced mean association strengths (electronic supplementary material, figure S3) suggest that weaker relationships became weaker—during the treatment phases, and showed a tendency to stabilize during the post-treatment and recovery phases. Model results are in table 3. Colours represent the phases of the experiment. Abbreviations correspond to: pre-treatment (Pre), disturbance event 1 (Dis 1), disturbance event 2 (Dis 2), disturbance event 3 (Dis 3), post-treatment (Post) and recovery (Rec). Solid and dashed lines represent colony 1 and 2, respectively. Thicker line shows the fit of the linear mixed models.
Table 3.
Nonlinear dynamics of network properties over the course of the experiment. The models describe the effect of phase on the coefficient of variation of the association strength of individuals in the colonies. The estimates indicate the direction of the change in each of the network metrics analysed.
| coefficient of variation |
||||
|---|---|---|---|---|
| estimate | s.e. | t-value | p-value | |
| intercept | 43.068 | 26.880 | 1.602 | 0.143 |
| phase | 54.513 | 17.586 | 3.100 | 0.013 |
| phase2 | −7.009 | 2.459 | −2.806 | 0.020 |
| random effects | variance | s.d. | ||
| colony | 0 | 0 | ||
5. Discussion
Our results support our hypothesis that temporary social instability can affect the benefits gained through coordinated actions, and thus reduced group performance. Previous studies have shown that social instability can negatively affect cooperative activities such as allo-parental care [41,42], group hunting [43] and reciprocal food sharing [44]; however, most of these studies focused on permanent changes in group membership. Our study shows that temporary changes in group size and composition, resulting from repeated short-term disturbance events, can reduce the ability of groups to forage efficiently. Further, by tracking in detail how groups foraged when re-united, we have identified potential social mechanisms underlying the effects of disturbances on foraging efficiency. Disturbances can disrupt the social relationships among members of the group, subsequently reducing the coordination and synchronisation of foraging groups.
Benefits of social tolerance while co-feeding at monopolizable resources facilitate cooperation (e.g. bonobos Pan paniscus [45]). In our system, social tolerance in a collective task was negatively affected by repeated disturbance events, indicated by a decrease in foraging group size. This effect could have arisen because of differences in competitive abilities when accessing the food resource, resulting in dominant individuals monopolising the resource. However, our data provide little evidence for competitive dynamics among colony members (no skewed access to the resource nor any resident effects), which is consistent with zebra finches having relatively shallow dominance hierarchies [46].
Coordinated actions are important for group living species. To remain cohesive, groups must be able to collectively decide when to move or where to forage [21]. Our results suggest that social instability can affect coordination and synchronisation in otherwise stable groups by reshaping social relationships among individuals. These findings support previous evidence that social instability can drive reconfiguration of social systems. For example, changes in social relationships resulting from removal of key individual(s) have been previously reported in bottlenose dolphins (Tursiops spp.) [47] and pigtailed macaques (Macaca nemestrina) [48]. However, our results extend the findings of these studies by demonstrating that even temporary disturbance events can have relatively permanent consequences on group-level functions without any permanent changes in group membership.
We have shown, for the first time, that social instability poses a measurable cost to group-living individuals. Temporary changes in group membership generated significant costs in terms of performance at a task in which individuals benefit from coordinated action. By reducing the ability for individuals living in social groups to coordinate and synchronise their actions, disturbance events could negatively impact their survival and reproduction. Our study, therefore, suggests that instability can act as an agent of selection, acting on social traits that facilitate stable social relationships. This largely unexplored impact could have widespread significance on the evolution and maintenance of social complexity in animal societies faced with increasingly disturbed environments.
Supplementary Material
Acknowledgements
We thank Alex Bruttel, Daniel Zuñiga and the animal caretaker team at the Max Planck Institute for Ornithology in Radolfzell for assistance with data collection and the outstanding monitoring animal health each day. We thank J. Graving for providing the processing software to decode the 2D barcodes and assistance with setting up the tracking procedure. We also thank Dr N. Boogert, Dr G. Carter and members of the Farine laboratory for useful criticisms of the manuscript.
Ethics
This study was conducted under permit no. 35-9185.81/G16/73 approved by Ethical Committee of Baden-Wurttemberg.
Data accessibility
We provide the complete code and data to replicate the results described in this study. These are available for download from: https://edmond.mpdl.mpg.de/imeji/collection/bcVWl9YKhHcPvwh9
Authors' contributions
D.R.F. and A.A.M.-C. designed the experiment; G.A.-N. and J.A.K.-I. collected the data; A.A.M.-C. and D.R.F. analysed the data and wrote the manuscript. All authors contributed to the developing the experiment and its methodology, and to the revision and editing of the final paper.
Competing interests
We declare we have no competing interests.
Funding
This work was funded by the Max Planck Society and the German Research Foundation (DFG grant no. FA 1420/4-1 awarded to D.R.F.).
References
- 1.Hinde RA. 1976. Interactions, relationships and social-structure. Man 11, 1–17. ( 10.2307/2800384) [DOI] [Google Scholar]
- 2.Shultz S, Opie C, Atkinson QD. 2011. Stepwise evolution of stable sociality in primates. Nature 479, 219–222. ( 10.1038/nature10601) [DOI] [PubMed] [Google Scholar]
- 3.Kappeler PM. In press. A framework for studying social complexity. Behav. Ecol. Sociobiol. [Google Scholar]
- 4.Nowak MA, Tarnita CE, Antal T. 2010. Evolutionary dynamics in structured populations. Phil. Trans. R Soc. B 365, 19–30. ( 10.1098/rstb.2009.0215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strandburg-Peshkin A, Farine DR, Couzin ID, Crofoot MC. 2015. Shared decision-making drives collective movement in wild baboons. Science 348, 1358–1361. ( 10.1126/science.aaa5099) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sueur C, et al. 2011. Collective decision-making and fission–fusion dynamics: a conceptual framework. Oikos 120, 1608–1617. [Google Scholar]
- 7.McDonald DB. 2007. Predicting fate from early connectivity in a social network. Proc. Natl Acad. Sci. USA 104, 10 910–10 914. ( 10.1073/pnas.0701159104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulke O, Bhagavatula J, Vigilant L, Ostner J. 2010. Social bonds enhance reproductive success in male macaques. Curr. Biol. 20, 2207–2210. ( 10.1016/j.cub.2010.10.058) [DOI] [PubMed] [Google Scholar]
- 9.Cameron EZ, Setsaas TH, Linklater WL. 2009. Social bonds between unrelated females increase reproductive success in feral horses. Proc. Natl Acad. Sci. USA 106, 13 850–13 853. ( 10.1073/pnas.0900639106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silk JB, Alberts SC, Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1231–1234. ( 10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 11.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2009. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc. R. Soc. B 276, 3099–3104. ( 10.1098/rspb.2009.0681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Archie EA, Tung J, Clark M, Altmann J, Alberts SC. 2014. Social affiliation matters: both same-sex and opposite-sex relationships predict survival in wild female baboons. Proc R. Soc. B 281, 20141261 ( 10.1098/rspb.2014.1261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brent LJN, Ruiz-Lambides A, Platt ML. 2017. Family network size and survival across the lifespan of female macaques. Proc. R. Soc. B 284, 20170515 ( 10.1098/rspb.2017.0515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lehmann J, Majolo B, McFarland R. 2016. The effects of social network position on the survival of wild Barbary macaques, Macaca sylvanus. Behav. Ecol. 27, 20–28. ( 10.1093/beheco/arv169) [DOI] [Google Scholar]
- 15.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2010. Strong and consistent social bonds enhance the longevity of female baboons. Curr. Biol. 20, 1359–1361. ( 10.1016/j.cub.2010.05.067) [DOI] [PubMed] [Google Scholar]
- 16.Silk J. 2014. Evolutionary perspectives on the links between close social bonds, health, and fitness. In Sociality, hierarchy, health: comparative biodemography: a collection of papers (eds Weinstein M, Lane MA), pp. 121–144. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 17.Shannon G, Slotow R, Durant SM, Sayialel KN, Poole J, Moss C, McComb K. 2013. Effects of social disruption in elephants persist decades after culling. Front. Zool. 10, 62 ( 10.1186/1742-9994-10-62) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nunez CM, Adelman JS, Smith J, Gesquiere LR, Rubenstein DI. 2014. Linking social environment and stress physiology in feral mares (Equus caballus): group transfers elevate fecal cortisol levels. Gen. Comp. Endocrinol. 196, 26–33. ( 10.1016/j.ygcen.2013.11.012) [DOI] [PubMed] [Google Scholar]
- 19.Carter G, Wilkinson G. 2013. Does food sharing in vampire bats demonstrate reciprocity? Commun. Integr. Biol. 6, e25783 ( 10.4161/cib.25783) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biro D, Sasaki T, Portugal SJ. 2016. Bringing a time-depth perspective to collective animal behaviour. Trends Ecol. Evol. 31, 550–562. ( 10.1016/j.tree.2016.03.018) [DOI] [PubMed] [Google Scholar]
- 21.King AJ, Sueur C. 2011. Where Next? Group coordination and collective decision making by primates. Int. J. Primatol. 32, 1245–1267. ( 10.1007/s10764-011-9526-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suchak M, Eppley TM, Campbell MW, Feldman RA, Quarles LF, de Waal FB. 2016. How chimpanzees cooperate in a competitive world. Proc. Natl Acad. Sci. USA 113, 10 215–10 220. ( 10.1073/pnas.1611826113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farine DR, Montiglio PO, Spiegel O. 2015. From individuals to groups and back: the evolutionary implications of group phenotypic composition. Trends Ecol. Evol. 30, 609–621. ( 10.1016/j.tree.2015.07.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beauchamp G. 2014. Social predation: How group living benefits predators and prey. San Diego, CA: Academic Press. [Google Scholar]
- 25.Dunn AM, Zann RA. 1996. Undirected song encourages the breeding female zebra finch to remain in the nest. Ethology 102, 540–548. [Google Scholar]
- 26.Zann R. 1990. Social and call learning in wild zebra finches in south-east Australia. Anim. Behav. 40, 811–828. [Google Scholar]
- 27.Zann R. 1996. The zebra finch: a synthesis of field and laboratory studies. Oxford, UK: Oxford University Press. [Google Scholar]
- 28.Alarcon-Nieto G, Graving J, Klarevas-Irby JA, Maldonado-Chaparro A, Mueller I, Farine DR. 2018. An automated barcode tracking system for behavioural studies in birds. Methods Ecol. Evol. 9, 1536–1547. ( 10.1101/201590) [DOI] [Google Scholar]
- 29.Maynard SJ, Parker GA. 1976. The logic of assymetric contests. Anim. Behav. 24, 159–175. [Google Scholar]
- 30.Davies NB. 1978. Territorial defence in the speckled wood butterfly (Pararge aegeria): the resident always wins. Anim. Behav. 26, 138–147. [Google Scholar]
- 31.Farine DR, Whitehead H. 2015. Constructing, conducting and interpreting animal social network analysis. J. Anim. Ecol. 84, 1144–1163. ( 10.1111/1365-2656.12418) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crall JD, Gravish N, Mountcastle AM, Combes SA. 2015. BEEtag: a low-cost, image-based tracking system for the study of animal behavior and locomotion. PLoS ONE 10, e0136487 ( 10.1371/journal.pone.0136487) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Graving JM. 2017. pinpoint: behavioral tracking using 2D barcode tags v0.0.1-alpha See 10.5281/zenodo.1008969, Zenodo. [DOI]
- 34.Wiley EM, Ridley AR. 2018. The benefits of pair bond tenure in the cooperatively breeding pied babbler (Turdoides bicolor). Ecol. Evol. 8, 7178–7185. ( 10.1002/ece3.4243) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunn AM, Zann RA. 1996. Undirected song in wild zebra finch flocks: contexts and effects of mate removal. Ethology 102, 529–539. [Google Scholar]
- 36.Nonacs P. 2000. Measuring and using skew in the study of social behavior and evolution. Am. Nat. 156, 577–589. ( 10.1086/316995) [DOI] [PubMed] [Google Scholar]
- 37.Bates D, Machler M, Bolker BM, Walker SC. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 38.R Core Team. 2016. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 39.Farine DR. 2017. A guide to null models for animal social network analysis. Methods Ecol. Evol. 8, 1309–1320. ( 10.1111/2041-210x.12772) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oksanen J, et al. 2017. vegan: community ecology package. See https://cran.r-project.org/web/packages/vegan/index.html .
- 41.Ebensperger LA, Aracena S, Avendano N, Toro A, Leon C, Ramirez-Estrada J, Abades S. 2017. Social instability decreases alloparental care and quality of weaned offspring in a communally rearing rodent. Anim. Behav. 133, 195–205. ( 10.1016/j.anbehav.2017.09.021) [DOI] [Google Scholar]
- 42.Ebensperger LA, Correa LA, Leon C, Ramirez-Estrada J, Abades S, Villegas A, Hayes LD. 2016. The modulating role of group stability on fitness effects of group size is different in females and males of a communally rearing rodent. J. Anim. Ecol. 85, 1502–1515. ( 10.1111/1365-2656.12566) [DOI] [PubMed] [Google Scholar]
- 43.Gazda SK, Connor RC, Edgar RK, Cox F. 2005. A division of labour with role specialization in group-hunting bottlenose dolphins (Tursiops truncatus) off Cedar Key, Florida. Proc. Biol. Sci. 272, 135–140. ( 10.1098/rspb.2004.2937) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carter GG, Wilkinson GS. 2015. Social benefits of non-kin food sharing by female vampire bats. Proc. R. Soc. B 282, 20152524 ( 10.1098/rspb.2015.2524) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hare B, Melis AP, Woods V, Hastings S, Wrangham R. 2007. Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Curr. Biol. 17, 619–623. ( 10.1016/j.cub.2007.02.040) [DOI] [PubMed] [Google Scholar]
- 46.Bonoan R, Clodius F, Dawson A, Caetano S, Yeung E, Paz-y-Miño-C G. 2013. Dominance hierarchy formation in a model organism, the zebra finch (Taeniopygia guttata), and its potential application to laboratory research. Bios 84, 201–209. ( 10.1893/0005-3155-84.4.201) [DOI] [Google Scholar]
- 47.Lusseau D, Newman ME. 2004. Identifying the role that animals play in their social networks. Proc. R. Soc. Lond. B 271(Suppl. 6), S477–S481. ( 10.1098/rsbl.2004.0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flack JC, Girvan M, de Waal FB, Krakauer DC. 2006. Policing stabilizes construction of social niches in primates. Nature 439, 426–429. ( 10.1038/nature04326) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We provide the complete code and data to replicate the results described in this study. These are available for download from: https://edmond.mpdl.mpg.de/imeji/collection/bcVWl9YKhHcPvwh9


