Abstract
Understanding how the brain processes social information and generates adaptive behavioural responses is a major goal in neuroscience. We examined behaviour and neural activity patterns in socially relevant brain nuclei of hermaphroditic mangrove rivulus fish (Kryptolebias marmoratus) provided with different types of social stimuli: stationary model opponent, regular mirror, non-reversing mirror and live opponent. We found that: (i) individuals faced with a regular mirror were less willing to interact with, delivered fewer attacks towards and switched their orientation relative to the opponent more frequently than fish exposed to a non-reversing mirror image or live opponent; (ii) fighting with a regular mirror image caused higher expression of immediate-early genes (IEGs: egr-1 and c-Fos) in the teleost homologues of the basolateral amygdala and hippocampus, but lower IEG expression in the preoptic area, than fighting with a non-reversing mirror image or live opponent; (iii) stationary models elicited the least behavioural and IEG responses among the four stimuli; and (iv) the non-reversing mirror image and live opponent drove similar behavioural and neurobiological responses. These results suggest that the various stimuli provide different types of information related to conspecific recognition in the context of aggressive contests, which ultimately drive different neurobiological responses.
Keywords: social information, mirror-image stimulation, stationary model test, non-reversing mirror, immediate early gene, Kryptolebias marmoratus
1. Background
Animals must process social information to make adaptive decisions that determine their behavioural response towards conspecifics and, ultimately, their fitness. Perceiving and using social information underlies every aspect of social behaviour, including mate choice, affiliation and aggression. Understanding how the brain translates social information into behavioural responses that are appropriate in diverse social contexts is therefore a fundamental goal in neuroscience. To investigate this, several types of standardized tests, such as mirror image stimulation and presentation of stationary models, have been used to understand the neurobiological mechanisms underlying visual perception [1], cognitive abilities [2] and social interaction among conspecifics [3]. Because most vertebrates are not capable of self-recognition [4], mirror image stimulation has become popular for evaluating behavioural displays towards conspecifics, such as shoaling [5], conspecific-directed aggression [3] and mate preference [6].
However, whether individuals perceive these social stimuli in a manner that mimics natural stimuli is not clear [7–9]. Moreover, which types of information an individual perceives when seeing its own image in a mirror, and the neural mechanisms responsible for guiding behavioural output in response to these types of stimuli, remain largely unknown. The appropriateness of mirror image stimulation has been challenged [10–13] because behavioural performance towards, and endocrine and neural responses to mirror images, differ markedly from those exhibited when interacting with live opponents [7–9,14]. Recent studies indicate that the inconsistency of behavioural and physiological responses to mirror images versus live conspecifics may result from brain (and associated behavioural) lateralization [10,14]. Regular mirror images are ideal for species that perform head-head lateral displays during social interaction, while non-reversing mirror stimulation, which provides a true reflection, is better for species that perform head-tail lateral displays [13]. Another standardized test is the model test, which allows animals to perform both head-head and head-tail postures by interacting with a size-matched stationary model, but elicits considerably lower behavioural responses. Given the diverse, sometimes divergent social cues that these stimuli present to a focal animal, it is essential to understand whether animals perceive these stimuli as equivalent.
To determine whether the brain can discriminate live opponents from standardized opponents, we quantified behaviour and brain activation in mangrove rivulus fish, Kryptolebias marmoratus (hereafter rivulus), interacting with a conspecific across transparent glass (control), regular mirror, non-reversing mirror and stationary model opponent. The behaviour of rivulus towards different types of social stimuli and live opponents has been well documented [11,13,15,16]. Also, because head–tail lateral displays are preferred in this species, rivulus exhibit different behavioural response towards regular mirrors and non-reversing mirrors, and behaviour exhibited towards the latter better predicts aggression during real contests [13]. These features make rivulus an ideal species in which to test whether the brain is capable of distinguishing standardized social stimuli from live opponents. We quantified localized differences in immediate early gene (IEG) expression as an index of neural activation [17] in key nodes of the vertebrate social decision-making network, a highly conserved network of fore- and midbrain regions responsible for processing social information and mediating social behaviours [18]. IEGs play a key role in activating signal transduction cascades, which leads to differential gene expression and can mediate neural plasticity and behaviour [17,19]. Expression levels of egr-1 and c-Fos, IEGs commonly used to examine nucleus-specific activation of brain regions in response to social stimuli [20,21], were quantified in the: (i) area dorsolateral telencephalon (Dl), the putative homologue of mammalian hippocampus, which mainly regulates spatial learning and social cognition [22]; (ii) area dorsomedial telencephalon (Dm), the putative homologue of mammalian basolateral amygdala, which is involved with fear responses and emotional learning [23]; and (iii) preoptic area (POA), which mediates aggression, parental behaviour and reproduction across vertebrates [43]. Based on previous research indicating that rivulus behave differently towards standardized stimuli [13], we hypothesized that behaviour and brain IEG expression patterns would be similar for individuals encountering non-reversing mirror images and live opponents, but highly divergent for individuals interacting with regular mirrors or stationary models.
2. Material and methods
(a). Study organism
Kryptolebias marmoratus inhabit mangrove ecosystems ranging from Central America to Florida and the Bahamas [24]. This species is highly aggressive in both the field and laboratory [15,24]. Rivulus is one of only two self-fertilizing hermaphroditic vertebrates, and exclusive selfing results in completely homozygous genotypes whose offspring are genetically identical to the parent and all siblings (i.e. isogenic lineages) [25]. This study used adult hermaphrodites from seven isogenic lineages derived from populations ranging from the Florida Keys to Central Florida. For additional details on animal husbandry, see electronic supplementary material S1.
(b). Behavioural responses to social stimuli
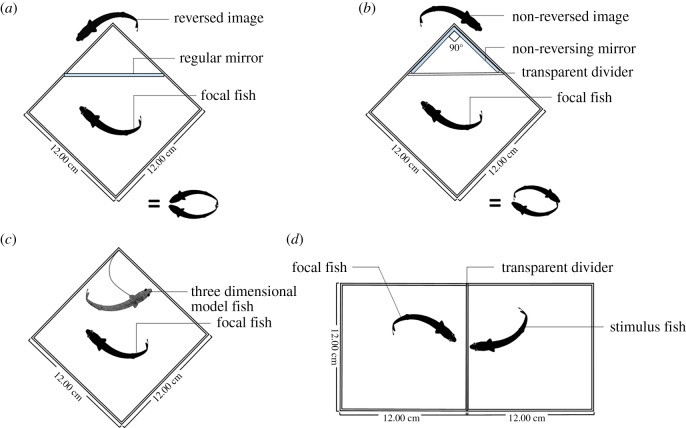
We compared the behaviour of rivulus when pitted against four different types of social stimuli: regular mirror-image stimulation (RMS), non-reversing mirror-image stimulation (NMS), stationary model opponent (SMO) and live opponent (LO) (figure 1). Fifty-six individuals representing seven genetically distinct lineages were used in these social tests (two replicates per lineage per stimulus). All social tests were conducted in a standard aquarium (12 × 12 × 12 cm3) containing water 11 cm deep and 0.5 cm of gravel. For details on the procedures, consult electronic supplementary material S2 and [13]. Descriptions of all behavioural displays are listed in electronic supplementary material, table S3.
Figure 1.
Graphical depiction of the four types of social test: (a) regular mirror-image stimulation; (b) non-reversing mirror-image stimulation; (c) stationary model test; (d) live opponent across transparent divider. (Online version in colour.)
(c). Immediate early gene expression in brain nuclei
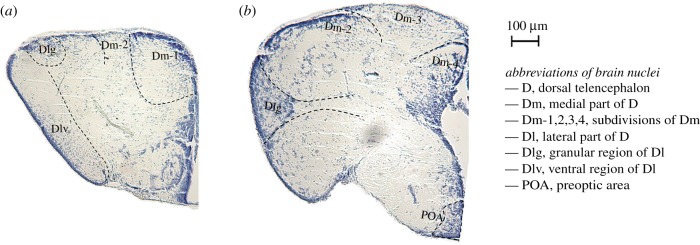
Immediately after social tests, individuals were decapitated and brains were removed by microdissection, fast-frozen in liquid nitrogen, and stored at −80°C. Brains were embedded in optimal cutting temperature medium (OCT Tissue Plus, Fisher HealthCare, PA, USA), sectioned on a cryostat at 200 µm, and targeted brain nuclei (area Dl [including Dlv and Dlg subdivisions], area Dm [including Dm1-4 subdivisions], and POA; see figure 2) were collected using 250 µm and 500 µm (inner diameter) Brain Punch Tissue Set (Leica Biosystems, IL, USA) under a stereomicroscope. Punches of brain nuclei from both hemispheres were pooled in one LoBind microcentrifuge tube (Eppendorf, NY, USA) filled with 400 µl ice-cold TRIzol (Sigma-Aldrich, MO, USA) and stored at −80°C for RNA extraction.
Figure 2.
Transverse sections of the adult Kryptolebias marmoratus forebrain. (a) and (b) indicate two representative coronal sections of the forebrain. Adult brains (independent of those used in this experiment) were fixed in Bouin's fixative at 23°C for 72 h, dehydrated, embedded in paraffin, sectioned on a microtome at 16 µm, and stained with cresyl violet. Brain atlases from swordtail fish (Xiphophorus helleri [26]) and cichlids (Astatotilapia burtoni [27,28]) were used to identify putative brain regions. (Online version in colour.)
Brain punches were homogenized in TRIzol, and total RNA was extracted using phenol-chloroform, converted to cDNA, and stored at −80°C for quantification of gene expression (see electronic supplementary material S4–S6 for additional details on brain processing, RNA extraction and qPCR). We designed nested sets of primers for the immediate early genes (egr-1 and c-Fos); primer sequences spanned two neighbouring exons to prevent amplifying genomic DNA. Forward and reverse primers used in this experiment are listed in electronic supplementary material, table S7. We quantified gene expression using quantitative PCR (qPCR) performed on a Mastercycler ep realplex System with SYBR green (Kapa Biosystems, MA, USA) following the manufacturer's instructions. All data were expressed relative to the housekeeping gene RPL8 with ΔCt values calculated as Cttarget gene-CtRPL8 [29]. qPCR requires the use of this sort of ‘housekeeping’ gene to normalize differences in expression that might be owing to differences among individuals in the amount of starting template. In this study, RPL8 expression did not vary across the four social tests within any of the brain regions (electronic supplementary material, figure S8). ΔCt is negatively correlated with gene expression; we therefore used −ΔCt values to conduct all statistical analyses such that higher values indicate higher relative IEG expression levels.
(d). Data analysis
We used JMP (v. 12; SAS Institute, Cary, NC, USA) for all statistical analyses. General linear mixed models examined whether individuals performed differently in the four types of standardized social test, and whether patterns of immediate early gene expression were different among individuals that encountered different social stimuli. Latency to first move, latency to first approach, latency to first opercular display, latency to first attack, the number of switches, the number of attacks, total interacting time, and immediate early gene expression (−ΔCt) in different brain nuclei (Dl, Dm, and POA) were the response variables (run in separate models). Type of social test (RMS, NMS, SMO, LO) was a fixed predictor variable. Standard length of focal individuals was included in the models as a covariate. Lineage was included as a random effect to account for variation among genotypes. Data are presented as boxplots including all data points. The lower and upper edges of each box correspond to 25% and 75% quantiles, respectively. The median (50% quantile) is shown as a horizontal line in each box and the whiskers depict the minimum and maximum values. The mean for each treatment group is shown as a cross in each box. Tukey's honest significant difference (HSD) post hoc test, which adjusts for compounding Type I error with multiple comparisons, was used to determine significant differences among levels of the main effects. The results of Tukey's HSD are reported with p-values only. Details on data transformation are provided in the electronic supplementary material S9.
3. Results
(a). Behavioural performance in different standardized social tests
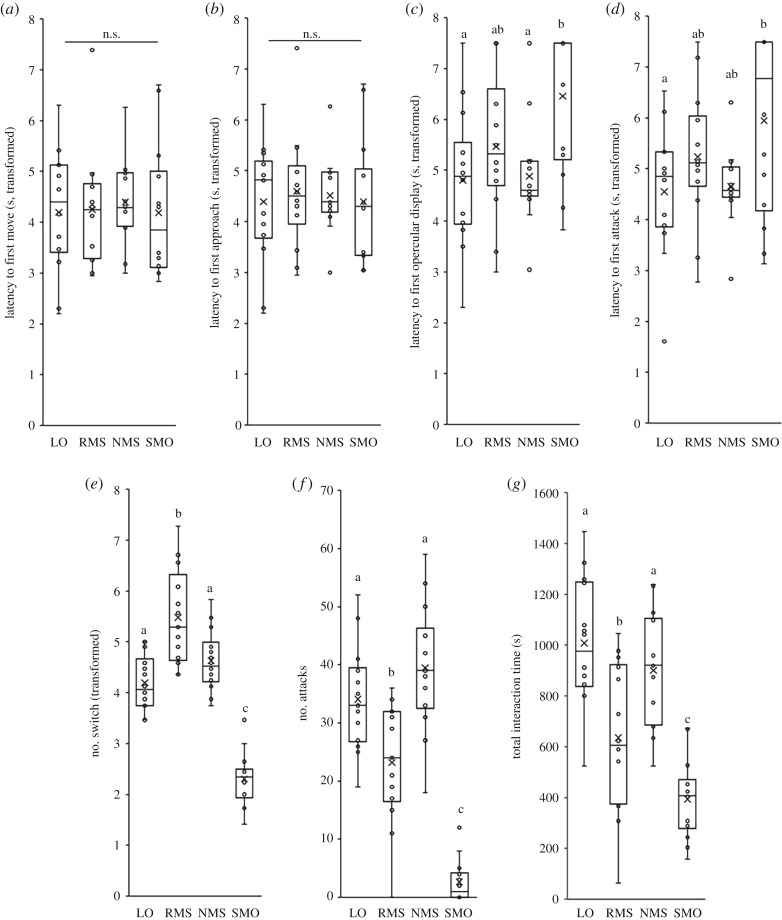
Latency to first move and latency to first approach did not vary as a function of opponent type (figure 3a,b), but there were considerable differences among tests in latencies to first opercular display and attack, frequencies of attack and switching between different lateral displays, and total interaction time (electronic supplementary material, table S10). Individuals encountering model opponents showed delayed latencies to opercular display (typical threat behaviour used during contests) and took longer to launch a first attack relative to those encountering non-reversing mirrors (opercular display: p = 0.012; latency to first attack: p = 0.055) and live opponents (opercular display: p = 0.008; latency to first attack: p = 0.033; figure 3c,d).
Figure 3.
Behavioural differences between individuals exposed to the three standardized social stimuli or live opponents: (a) latency to first move; (b) latency to first approach; (c) latency to first opercular display; (d) latency to first attack; (e) frequencies of switch and (f) attack; (g) time interacting with the various opponent types. Different lower case letters indicate significant differences between treatments within each behavioural category (Tukey's honest significant difference pairwise comparisons, p < 0.05). (LO, live opponent; RMS, regular mirror-image stimulation; NMS, non-reversing mirror-image stimulation; SMO, stationary model opponent).
Against model opponents, individuals also switched between different types of lateral display less frequently than those encountering the other three social stimuli (SMO versus RMS: p < 0.001; SMO versus NMS: p < 0.001; SMO versus LO: p < 0.001; figure 3e). Additionally, individuals encountering regular mirror images tended to switch more frequently than those encountering non-reversing mirrors (p = 0.064) and live opponents (p = 0.007; figure 3e). Model opponents elicited significantly fewer attacks than the other three social stimuli (SMO versus RMS: p < 0.001; SMO versus NMS: p < 0.001; SMO versus LO: p < 0.001; figure 3f). Individuals encountering regular mirror images also launched fewer attacks than those encountering non-reversing mirror images (p = 0.001) or live opponents (p = 0.011) but, there was no significant difference in attack frequency between non-reversing mirrors and live opponents (p = 0.373; figure 3f).
Individuals also spent significantly less time interacting with model opponents than the other social stimuli (SMO versus RMS: p = 0.043; SMO versus NMS: p < 0.001; SMO versus LO: p < 0.001), and less time interacting with regular mirrors than non-reversing mirrors (p = 0.022) and live opponents (p = 0.001; figure 3g). However, there was no significant difference in time spent interacting with non-reversing mirrors and live opponents (p = 0.626; figure 3g).
(b). Immediate early gene expression in response to different social stimuli
The different types of social stimuli drove divergent patterns of IEG expression across the three brain nuclei (electronic supplementary material, table S11).
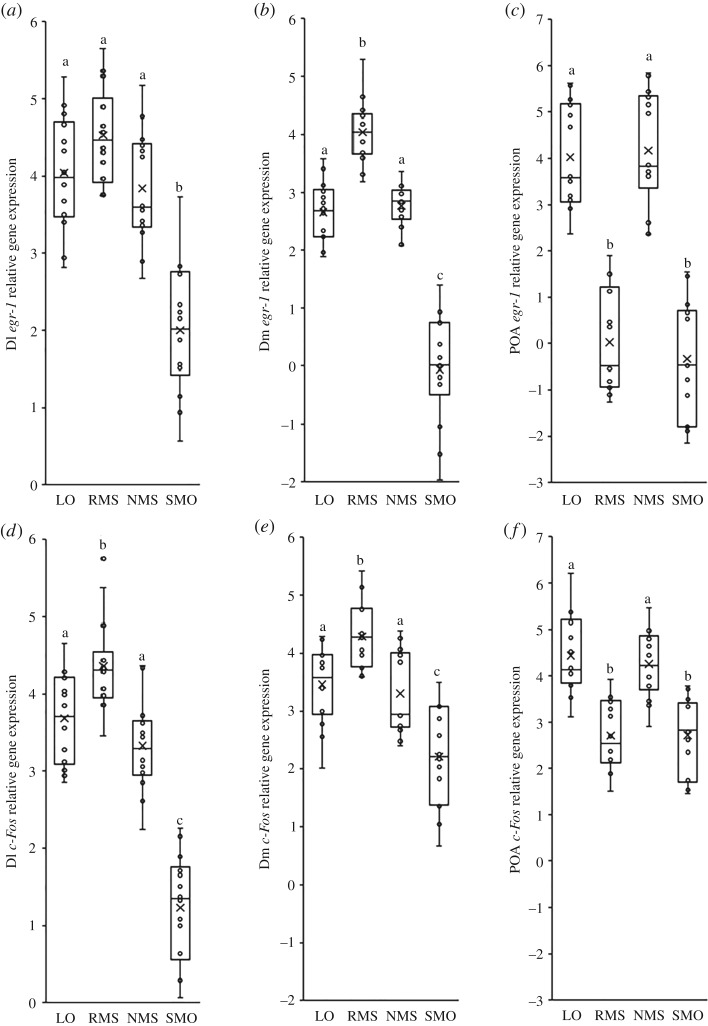
In Dl, model opponents elicited significantly lower levels of IEG expression than regular mirrors (egr-1: p < 0.001, c-Fos: p < 0.001), non-reversing mirrors (egr-1: p < 0.001, c-Fos: p < 0.001) and live opponents (egr-1: p < 0.001, c-Fos: p < 0.001; figure 4a,d). Also, interactions with regular mirrors were associated with significantly higher levels of c-Fos expression in Dl than interactions with non-reversing mirrors (p = 0.001) and live opponents (p = 0.038; figure 4d).
Figure 4.
Differences in immediate early gene, egr-1 and c-Fos, expression for (a,d) area Dl (putative homologue of mammalian hippocampus), (b,e) area Dm (putative homologue of mammalian basolateral amygdala), and (c,f) POA, between individuals exposed to the four types of social stimuli. Different lower case letters indicate significant differences between treatments within each behavioural category (Tukey's honest significant difference pairwise comparisons, p < 0.05). (LO, live opponent; RMS, regular mirror-image stimulation; NMS, non-reversing mirror-image stimulation; SMO, stationary model opponent).
In Dm, model opponents elicited significantly lower levels of IEG expression than regular mirrors (egr-1: p < 0.001, c-Fos: p < 0.001), non-reversing mirrors (egr-1: p < 0.001, c-Fos: p < 0.001) and live opponents (egr-1: p < 0.001, c-Fos: p < 0.001; figure 4b,e). In addition, interactions with regular mirrors led to significantly higher levels of IEG expression in Dm than interactions with non-reversing mirrors (egr-1: p < 0.001, c-Fos: p = 0.003) and live opponents (egr-1: p < 0.001, c-Fos: p = 0.018; figure 4b,e).
In the POA, model opponents and regular mirrors elicited significantly lower levels of IEG expression than non-reversing mirrors (SMO versus NMS—egr-1: p < 0.001, c-Fos: p < 0.001; RMS versus NMS—egr-1: p < 0.001, c-Fos: p < 0.001) and live opponents (SMO versus LO—egr-1: p < 0.001, c-Fos: p < 0.001; RMS versus LO—egr-1: p < 0.001, c-Fos: p < 0.001; figure 4c,f). However, IEG expression in the POA was similar in individuals exposed to regular mirrors and model opponents (all comparisons: p > 0.424) as well as non-reversing mirrors and live opponents (all comparisons: p > 0.479; figure 4c,f).
The results of analyses on actual RNA expression (i.e. values inferred from standard curve; electronic supplementary material, figure S12a–f) were similar to those obtained using ΔCt methods.
4. Discussion
(a). Natural and simulated social stimuli convey different information
Our results revealed differences in behavioural responses to standardized social stimuli and live opponents. Stationary models provide morphological information such as size but lack behavioural information. Studies in other fish species, such as cichlids (Pseudotropheus zebra) and damselfish (Dascyllus aruanus), demonstrate that fish can distinguish stationary from moving objects [30–32], and imply that stationary models convey relatively little information (e.g. morphological traits), insufficient for the brain to recognize models as conspecifics or threatening predators. In support of this, our results showed that stationary models elicit weak behavioural responses, but we cannot exclude the possibility that this was owing to the fact that our stationary model did not perfectly mimic real rivulus. Further studies using higher-quality decoys, like 3D printed scans of a real fish, might drive more pronounced behavioural responses.
Regular and non-reversing mirror images provide both morphological and behavioural information. This raises the question of how rivulus perceive differences between regular mirrors and real opponents but are apparently ‘tricked’ by non-reversing mirrors. One possible explanation centres around perceiving ‘motion types’, such as lateral display behaviour from the images. Animals can distinguish different species based on particular forms of locomotion or behaviour [30]. Some species prefer using head–head lateral/parallel display (e.g. fallow deer, Dama dama [33]), or head–tail lateral display (e.g. Beta splendens [34]), when first encountering social partners or competitors. Rivulus prefer head–tail lateral display [13]. Therefore, an individual may perceive regular mirror images as unusual because interactions can only occur in head–head posture. This, in turn, may be confusing and force animals to switch more often between orientations to adopt an unachievable display posture, consequently leading to decreased behavioural responsiveness. However, non-reversing mirror images might be good surrogates for live opponents because interactions can occur in familiar head-tail fashion. Our results suggest that non-reversing mirrors convey morphological and behavioural information that is accurate enough for the brain to recognize the image as a conspecific, and that promote behaviour similar to what would be exhibited against live opponents. On the other hand, the combination of accurate morphological information but inaccurate behavioural information, such as that provided by a regular mirror image, may cause individuals to react in atypical ways.
(b). Immediate early gene expression in fish encountering artificial stimuli and live opponents
The amygdala is strongly associated with fear responses in mammals [35,36]. While the hippocampus, which receives afferent input from the amygdala, is considered centrally important for spatial learning, studies on mammals and fishes also reveal that the hippocampus contributes in important ways to fear conditioning [37–39]. These specialized systems for fear learning are thought to have been conserved throughout vertebrate evolution [23]. The POA is associated with parental and sexual behaviour, and also with intruder-directed aggression [40]. All of these brain regions receive indirect input from multiple sensory systems [35]. In the present study, social information is communicated exclusively through the visual system. However, IEG expression patterns in each nucleus (Dl, Dm and POA) revealed that the brain may perceive very different types of information from these social stimuli. Elevated IEG expression levels suggest activation of short-term and long-term neural activity, such as an increase in neuronal firing rates and expression of late-acting genes that may trigger changes to physiology or behaviour [36].
Individuals facing stationary model opponents had low or even no IEG response in Dm, Dl and POA, suggesting that this stimulus does not initiate neural activity related to fear or aggressive behavioural responses. Comparing IEG patterns between model opponents and live opponents implies that morphological information is not sufficient for rivulus to recognize a novel, size-matched individual as a conspecific partner or formidable opponent. This is also supported by our behavioural data, which showed lower attack rates and less time interacting with stationary models. Our data also revealed that biologically relevant movement is essential to drive appropriate neurobiological responses during social interaction [41]; the brain cannot be easily deceived by a model stimulus that fails to deliver appropriate behavioural feedback. Alternatively, it also could be that IEG expression was higher in individuals facing live opponents compared with stationary models because of increased stress levels related to engaging with an opponent that reciprocates.
Regular mirrors elicited higher levels of IEG expression than live opponents in Dm (basolateral amygdala) and Dl (hippocampus), suggesting that individuals may experience fear in this context. Different from stationary model opponents, regular mirrors attracted individuals' attention and elicited pronounced behavioural responses. However, regular mirrors clearly did not provide the same type of information to the brain as live opponents. Relative to neural responses to live opponents, IEG expression was higher in Dm and Dl, which corresponds to findings in cichlid fish A. burtoni [7]. Interestingly, similar to IEG expression following the model test, fish fighting against regular mirrors showed lower egr-1 and c-Fos expression levels in POA than those fighting against live opponents, which may suggest that regular mirrors do not potentiate neurobiological responses that drive appropriate aggressive responses. Differences in IEG expression patterns between regular mirrors and live opponents imply that, while regular mirrors can provide more information (morphological and behavioural) than model opponents (only morphological), the information content is inaccurate (unfamiliar display posture). The brain thus might be ‘confused’ and activate brain regions (like the amygdala and hippocampus) that specialize in responding to unfamiliar and fearful stimuli.
Surprisingly, there were no significant differences in IEG expression between fish interacting with non-reversing mirrors and live opponents, suggesting that these stimuli were perceived by the brain as being quite similar. Differences in IEG expression patterns between regular and non-reversing mirrors implies that, while both stimuli provide similar types of information (morphological and behavioural), only non-reversing mirrors are perceived as being similar to live opponents. This is likely because non-reversing mirrors enable transmission of familiar behavioural information (i.e. head-tail lateral display) for the brain to recognize conspecific opponents. This appropriate stimulus elicits a more pronounced aggressive response, and less pronounced neurobiological fear response, which are characteristic of interactions with a live conspecific opponent.
It is important to note that our data cannot speak to whether the standardized social stimuli elicited higher (or lower) brain IEG expression than baseline levels, because we did not assay IEG expression in animals that experienced no stimulus. With that said, our data concretely establish that behavioural and neural responses differ between fish interacting with artificial opponents and live opponents.
Overall, patterns of egr-1 and c-Fos expression were similar in each of the four treatments, and there was considerable among-individual variation in both IEG expression and behavioural responses to the standardized stimuli. Post hoc correlation analyses (electronic supplementary material, tables S13–S16) revealed that egr-1 and c-Fos were significantly positively correlated in Dl (p-value < 0.001) and POA (p-value < 0.001), but not in Dm (all p-values > 0.479) for all four standardized tests. Most IEGs are transcription factors, which drive distinct signal transduction cascades or activate expression of distinct genes that mediate neural plasticity and behaviour. Thus, levels of expression of different IEGs are not necessarily expected to be similar within a brain region at the individual level. For instance, in an African cichlid (A. burtoni), males ascending in social status (subordinate to dominant) showed higher expression levels of both egr-1 and c-Fos in nuclei of the brain social decision-making network compared with stable status controls [21]. However, in descending males (dominant to subordinate), only one of the IEGs (egr-1 or c-Fos) showed higher expression within specific brain nuclei compared to stable status controls [42].
We did not find any significant correlations between behaviour and IEGs (all p-value > 0.091). This also is not entirely unexpected. At the individual level, it might be difficult to predict behavioural responses solely based on IEG expression, because behavioural phenotypes probably result from a constellation of signal transduction cascades (which may or may not diverge further downstream from the IEGs). In addition, behavioural phenotypes are probably driven by coordination among the coordinated activity of multiple brain regions. Thus, because behavioural responses result from multitudes of cellular/molecular processes operating in many brain nuclei within a complex social decision-making network [43,44], expression of a single IEG in a single brain region may not be enough to predict the behavioural response.
The various standardized stimuli that we employed here are commonly used to examine social preferences, aggression, and cognition in vertebrates. Our study illustrates that care should be taken in interpreting responses towards different stimuli because they vary considerably in the degree to which they convey similar information as a live conspecific. Our study highlights that the brain of fishes is exquisitely sophisticated, in that it can perceive subtle differences during social interaction, such as specific display orientations, which allows for discrimination of real from fake opponents.
Supplementary Material
Acknowledgements
We thank Caitlin Curtis, Jacob Howell and Cassidy Reilly for their assistance collecting behavioural data.
Ethics
All work was approved by the Institutional Animal Care and Use Committee of the University of Alabama (protocol no. 14-12-0091).
Data accessibility
Data are available from the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.mr2687q [45].
Authors' contributions
C.-Y.L and R.L.E. conceived of and designed the study, analysed data, and drafted the manuscript; C.-Y.L. collected behavioural and gene expression data; H.A.H. and M.L.H. participated in the study design and helped draft the manuscript.
Competing interests
We have no competing interests.
Funding
This research was supported by Sigma Xi Scientific Research Honor Society (G201510151660486) and Animal Behaviour Society.
References
- 1.Salva OR, Sovrano VA, Vallortigara G. 2014. What can fish brains tell us about visual perception? Front. Neural Circuits 8, 119 ( 10.3389/fncir.2014.00119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baragli P, Demuru E, Scopa C, Palagi E. 2017. Are horses capable of mirror self-recognition? A pilot study. PLoS ONE 12, e0176717 ( 10.1371/journal.pone.0176717) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliveira RF, Silva JF, Simões JM. 2011. Fighting zebrafish: characterization of aggressive behavior and winner-loser effects. Zebrafish 8, 73–81. ( 10.1089/zeb.2011.0690) [DOI] [PubMed] [Google Scholar]
- 4.Gallup GG. 1970. Chimpanzees: self recognition. Science 167, 86–87. ( 10.1126/science.167.3914.86) [DOI] [PubMed] [Google Scholar]
- 5.Tsubokawa T, Saito K, Kawano H, Kawamura K, Shinozuka K, Watanabe S. 2009. Pharmacological effects on mirror approaching behavior and neurochemical aspects of the telencephalon in the fish, medaka (Oryzias latipes). Soc. Neurosci. 4, 276–286. ( 10.1080/17470910802625215) [DOI] [PubMed] [Google Scholar]
- 6.Okuyama T, et al. 2014. A neural mechanism underlying mating preferences for familiar individuals in medaka fish. Science 343, 91–94. ( 10.1126/science.1244724) [DOI] [PubMed] [Google Scholar]
- 7.Desjardins JK, Fernald RD. 2010. What do fish make of mirror images? Biol. Lett. 6, 744–747. ( 10.1098/rsbl.2010.0247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dijkstra PD, Schaafsma SM, Hofmann HA, Groothuis TG. 2012. ‘Winner effect’ without winning: unresolved social conflicts increase the probability of winning a subsequent contest in a cichlid fish. Physiol. Behav. 105, 489–492. ( 10.1016/j.physbeh.2011.08.029) [DOI] [PubMed] [Google Scholar]
- 9.Oliveira RF, Carneiro LA, Canário AVM. 2005. Behavioural endocrinology: no hormonal response in tied fights. Nature 437, 207–208. ( 10.1038/437207a) [DOI] [PubMed] [Google Scholar]
- 10.Arnott G, Ashton C, Elwood RW. 2011. Lateralization of lateral displays in convict cichlids. Biol. Lett. 7, 683–685. ( 10.1098/rsbl.2011.0328) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Earley RL, Hsu Y, Wolf LL. 2000. The use of standard aggression testing methods to predict combat behaviour and contest outcome in Rivulus marmoratus dyads (Teleostei: Cyprinodontidae). Ethology 106, 743–761. ( 10.1046/j.1439-0310.2000.00586.x) [DOI] [Google Scholar]
- 12.Elwood RW, Stoilova V, Mcdonnell A, Earley RL, Arnott G. 2014. Do mirrors reflect reality in agonistic encounters? A test of mutual cooperation in displays. Anim. Behav. 97, 63–67. ( 10.1016/j.anbehav.2014.07.028) [DOI] [Google Scholar]
- 13.Li CY, Curtis C, Earley RL. 2018. Nonreversing mirrors elicit behaviour that more accurately predicts performance against live opponents. Anim. Behav. 137, 95–105. ( 10.1016/j.anbehav.2018.01.010) [DOI] [Google Scholar]
- 14.Ariyomo TO, Watt PJ. 2013. Aggression and sex differences in lateralization in the zebrafish. Anim. Behav. 86, 617–622. ( 10.1016/j.anbehav.2013.06.019) [DOI] [Google Scholar]
- 15.Hsu Y, Lee SP, Chen MH, Yang SY, Cheng KC. 2008. Switching assessment strategy during a contest: fighting in killifish Kryptolebias marmoratus. Anim. Behav. 75, 1641–1649. ( 10.1016/j.anbehav.2007.10.017) [DOI] [Google Scholar]
- 16.Garcia MJ, Ferro JM, Mattox T, Kopelic S, Marson K, Jones R, Svendsen JC, Earley RL. 2016. Phenotypic differences between the sexes in the sexually plastic mangrove rivulus fish (Kryptolebias marmoratus). J. Exp. Biol. 219, 988–997. ( 10.1242/jeb.124040) [DOI] [PubMed] [Google Scholar]
- 17.Clayton DF. 2000. The genomic action potential. Neurobiol. Learn. Mem. 74, 185–216. ( 10.1006/nlme.2000.3967) [DOI] [PubMed] [Google Scholar]
- 18.Weitekamp CA, Hofmann HA. 2017. Brain systems underlying social behavior. In Evolution of nervous systems, vol. 1, 2nd edn (ed. Kaas J.), pp. 327–334. Oxford, UK: Elsevier. [Google Scholar]
- 19.Davis S, Bozon B, Laroche S. 2003. How necessary is the activation of the immediate early gene zif268 in synaptic plasticity and learning? Behav. Brain Res. 142, 17–30. ( 10.1016/S0166-4328(02)00421-7) [DOI] [PubMed] [Google Scholar]
- 20.Okuno H. 2011. Regulation and function of immediate-early genes in the brain: beyond neuronal activity markers. Neurosci. Res. 69, 175–186. ( 10.1016/j.neures.2010.12.007) [DOI] [PubMed] [Google Scholar]
- 21.Maruska KP, Zhang A, Neboori A, Fernald RD. 2013. Social opportunity causes rapid transcriptional changes in the social behaviour network of the brain in an African cichlid fish. J. Neuroendocrinol. 25, 145–157. ( 10.1111/j.1365-2826.2012.02382.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karnik I, Gerlai R. 2012. Can zebrafish learn spatial tasks? An empirical analysis of place and single CS-US associative learning. Behav. Brain Res. 233, 415–421. ( 10.1016/j.bbr.2012.05.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lal P, et al. 2018. Identification of a neuronal population in the telencephalon essential for fear conditioning in zebrafish. BMC Biol. 16, 45 ( 10.1186/s12915-018-0502-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor DS. 2012. Twenty-four years in the mud: What have we learned about the natural history and ecology of the mangrove rivulus, Kryptolebias marmoratus? Integr. Comp. Biol. 52, 724–736. ( 10.1093/icb/ics062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avise JC, Tatarenkov A. 2015. Population genetics and evolution of the mangrove rivulus Kryptolebias marmoratus, the world's only self-fertilizing hermaphroditic vertebrate. J. Fish Biol. 87, 519–538. ( 10.1111/jfb.12741) [DOI] [PubMed] [Google Scholar]
- 26.Anken RH, Rahmann H. 1994. Brain atlas of the adult swordtail fish Xiphophorus helleri and of certain developmental stages. Stuttgart, Germany: G. Fisher. [Google Scholar]
- 27.Burmeister SS, Munshi RG, Fernald RD. 2009. Cytoarchitecture of a cichlid fish telencephalon. Brain. Behav. Evol. 74, 110–120. ( 10.1159/000235613) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Munchrath LA, Hofmann HA. 2010. Distribution of sex steroid hormone receptors in the brain of an African cichlid fish, Astatotilapia burtoni. J. Comp. Neurol. 518, 3302–3326. ( 10.1002/cne.22401) [DOI] [PubMed] [Google Scholar]
- 29.Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101–1108. ( 10.1038/nprot.2008.73) [DOI] [PubMed] [Google Scholar]
- 30.Schluessel V, Kortekamp N, Cortes JA, Klein A, Bleckmann H. 2015. Perception and discrimination of movement and biological motion patterns in fish. Anim. Cogn. 18, 1077–1091. ( 10.1007/s10071-015-0876-y) [DOI] [PubMed] [Google Scholar]
- 31.Rhoad KD, Kalat JW, Klopfer PH. 1975. Aggression and avoidance by Betta splendens toward natural and artificial stimuli. Anim. Learn. Behav. 3, 271 ( 10.3758/BF03213443) [DOI] [Google Scholar]
- 32.Näslund J, Pettersson L, Johnsson JI. 2016. Behavioural reactions of three-spined sticklebacks to simulated risk of predation—effects of predator distance and movement. FACETS 1, 55–66. ( 10.1139/facets-2015-0015) [DOI] [Google Scholar]
- 33.Jennings DJ, Gammell MP, Carlin CM, Hayden TJ. 2003. Is the parallel walk between competing male fallow deer, Dama dama, a lateral display of individual quality? Anim. Behav. 65, 1005–1012. ( 10.1006/anbe.2003.2124) [DOI] [Google Scholar]
- 34.Cantalupo C, Bisazza A, Vallortigara G. 1996. Lateralization of displays during aggressive and courtship behaviour in the Siamese fighting fish (Betta splendens). Physiol. Behav. 60, 249–252. ( 10.1016/0031-9384(96)00015-7) [DOI] [PubMed] [Google Scholar]
- 35.Northcutt RG. 2006. Connections of the lateral and medial divisions of the goldfish telencephalic pallium. J. Comp. Neurol. 494, 903–943. ( 10.1002/cne.20853) [DOI] [PubMed] [Google Scholar]
- 36.Korb E, Finkbeiner S. 2011. Arc in synaptic plasticity: from gene to behavior. Trends Neurosci. 34, 591–598. ( 10.1016/j.tins.2011.08.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanchard DC, Blanchard RJ, Lee EMC, Fukunaga KK. 1977. Movement arrest and the hippocampus. Physiol. Psychol. 5, 331–335. ( 10.3758/BF03335340) [DOI] [Google Scholar]
- 38.Portavella M, Torres B, Salas C. 2004. Avoidance response in goldfish: emotional and temporal involvement of medial and lateral telencephalic pallium. J. Neurosci. 24, 2335–2342. ( 10.1523/JNEUROSCI.4930-03.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Phillips RG, LeDoux JE. 1992. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 106, 274–285. ( 10.1037/0735-7044.106.2.274) [DOI] [PubMed] [Google Scholar]
- 40.Weitekamp CA, Nguyen J, Hofmann HA. 2017. Social context affects behavior, preoptic area gene expression, and response to D2 receptor manipulation during territorial defense in a cichlid fish. Genes Brain Behav. 16, 601–611. ( 10.1111/gbb.12389) [DOI] [PubMed] [Google Scholar]
- 41.Woo KL, Rieucau G. 2011. From dummies to animations: a review of computer-animated stimuli used in animal behavior studies. Behav. Ecol. Sociobiol. 65, 1671–1685. ( 10.1007/s00265-011-1226-y) [DOI] [Google Scholar]
- 42.Maruska KP, Becker L, Neboori A, Fernald RD. 2013. Social descent with territory loss causes rapid behavioral, endocrine and transcriptional changes in the brain. J. Exp. Biol. 216, 3656–3666. ( 10.1242/jeb.088617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Connell LA, Hofmann HA. 2011. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 519, 3599–3639. ( 10.1002/cne.22735) [DOI] [PubMed] [Google Scholar]
- 44.O'Connell LA, Hofmann HA. 2012. Evolution of a vertebrate social decision-making network. Science 336, 1154–1157. ( 10.1126/science.1218889) [DOI] [PubMed] [Google Scholar]
- 45.Li CY, Hofmann HA, Harris ML, Earley RL. 2018. Data from: Real or fake? Natural and artificial social stimuli elicit divergent behavioral and neural responses in the mangrove rivulus, Kryptolebias marmoratus Dryad Digital Repository. ( 10.5061/dryad.mr2687q) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Li CY, Hofmann HA, Harris ML, Earley RL. 2018. Data from: Real or fake? Natural and artificial social stimuli elicit divergent behavioral and neural responses in the mangrove rivulus, Kryptolebias marmoratus Dryad Digital Repository. ( 10.5061/dryad.mr2687q) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository at: http://dx.doi.org/10.5061/dryad.mr2687q [45].