Abstract
A new O-terpenoidal coumarin 1, named hekumarone, was isolated from the leaves and twigs of Clausena anisum-olens Merr. (Rutaceae) collected in Hekou County in Yunnan Province, P. R. China. Structure elucidation and unambiguous NMR assignments for the title compound was carried out on the basis of 1D and 2D NMR experiments.
Keywords: Rutaceae, Clausena anisum-olens Merr., Hekumarone, Monoterpenoid coumarins.
Introduction
The plants of the Rutaceae family are well-known to contain structurally diverse and biologically active coumarins [1,2,3,4,5,6]. Within this family, the plants of the genus Clausena are shrubs, widely distributed in South and Southeast Asia and many species are used in Chinese folk medicine for various indications [7]. Previous phytochemical studies have indicated that Clausena species are the rich sources of carbazole alkaloids and coumarins [4,5,6,8,9,10,6,8]. Clausena anisum-olens Merr. is a shrub that both grows wild and is cultivated from Philippines and South China through Southeast Asia. The aerial parts of this plant have been used for the treatment of dysentery and arthritis [7]. Our previous phytochemical studies on C. anisum-olens Merr., collected in Hekou County in Yunnan Province, P. R. China, resulted in the isolation of new cyclic peptides and monoterpenoid coumarins [11,12,13]. In our ongoing search for bioactive compounds from this medicinally important genus, a new O-terpenoidal coumarin 1, which we have named hekumarone, was isolated from C. anisum-olens Merr. In this report, we describe the isolation and structure elucidation of 1 using spectroscopic data analysis, including 1D and 2D NMR techniques (COSY, HSQC and HMBC).
Results and Discussion
The powdered plant material of Clausena anisum-olens Merr. (22.5 kg) was repeatedly extracted with EtOH at room temperature. The extract was then concentrated under reduced pressure to give a brown syrup, which was suspended in water and successively partitioned with petroleum ether, ethyl acetate (EtOAc) and n-butanol (n-BuOH). The EtOAc fraction was subjected to silica gel column chromatography, Pharmadex LH-20 and RP C-18 to yield compound 1, which was isolated as a light yellow oil, – 6.67 (c 0.75, CH3OH). The molecular formula C20H18O7 deduced from its 13C-NMR DEPT spectrum and HR-ESI-MS, which exhibited a molecular ion at m/z 369.1000 [M-1] +, indicated twelve degrees of unsaturation. Strong UV bands at λmax 256 and 319 and an IR band at 1,730 cm-1 indicated the presence of a 7, 8-dioxygenated coumarin [5].
The 1H-NMR spectrum (Table 1) showed resonances for two pairs of typical AB system characteristic doublets at δH 6.32 and 7.64 (each J = 9.5 Hz) and δH 6.98 and 7.20 (each J = 8.6 Hz), corresponding to H-3, H-4, H-5 and H-6 in the coumarin nucleus. Additional signals in the spectrum were consistent with three olefinic protons δ 6.37, 6.09 (each1H, s) and 7.01(1H, d, J =1.6 Hz), one oxymethylene group δ 5.57 (2H, s), two methylene protons δ 2.80, 2.62 and one olefinic methyl δ 1.77 could be seen.
Table 1.
The 1H- and 13C-NMR data for compounds 1-3 (δ in ppm, J in Hz)*.
| No. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δ H | δ C | δ H | δ C | δ H | δ C | |
| 2 | / | 159.2 (s) | / | 160.4 (s) | / | 160.4 (s) |
| 3 | 6.32 (d,9.5) | 112.7 (d) | 6.33 (d, 9.8) | 113.6 (d) | 6.33 (d, 9.8 ) | 113.6 (d) |
| 4 | 7.64 (d,9.5) | 142.9 (d) | 7.71 (d, 9.8) | 143.6 (d) | 7.71 (d, 9.8 ) | 143.6 (d) |
| 5 | 7.20 (d,8.6) | 122.3 (d) | 7.36(d, 8.6) | 123.0 (d) | 7.36 (d, 8.6 ) | 123.0 (d) |
| 6 | 6.98 (d,8.6) | 109.3 (d) | 6.97 (d, 8.6) | 110.4 (d) | 6.97 (d, 8.6) | 110.4 (d) |
| 7 | / | 153.5 (s) | / | 154.3 (s) | / | 154.3 (s) |
| 8 | / | 135.6 (s) | / | 136.3 (s) | / | 136.3 (s) |
| 9 | / | 113.4 (s) | / | 114.1 (s) | / | 114.1(s) |
| 10 | / | 147.7 (s) | / | 147.9 (s) | / | 147.9 (s) |
| 1'a | 5.57 (s) | 69.6 (t) | 4.23 (m) | 72.8 (t) | 4.24 (m) | 72.8 (t) |
| 1'b | 4.14 (m) | 72.8 (t) | 4.17 (m) | 72.8 (t) | ||
| 2' | / | 193.8 (s) | 4.66 (m) | 72.7 (d) | 4.68 (m) | 72.6 (d) |
| 3' | / | 139.6 (s) | / | 142.0 (s) | / | 142.3 (s) |
| 4'a | 2.80 (dd,14.1, 4.8) | 33.5 (t) | 2.63 (dd, 14.6, 7.3) | 36.4 (t) | 2.69 (dd, 14.6, 5.1) | 36.4 (t) |
| 4'b | 2.62 (dd, 14.1, 9.4) | 2.54 (dd, 14.6, 6.4 ) | 2.45 (dd, 14.6, 8.1) | |||
| 5' | 5.11 (m) | 78.2 (d) | 5.25 (m) | 79.8 (d) | 5.25 (m) | 80.0 (d) |
| 6' | 7.01(d,1.6) | 147.6 (d) | 7.22 (d, 1.7 ) | 148.7 (d) | 7.22 (d, 1.7 ) | 148.7 (d) |
| 7' | / | 128.8 (s) | / | 130.0 (s) | / | 130.0 (s) |
| 8' | / | 172.7 (s) | / | 174.0 (s) | / | 174.0 (s) |
| 9' | 1.77 (s) | 9.3 (q) | 1.98 (s) | 10.5 (q) | 1.98 (s) | 10.5 (q) |
| 10'a | 6.37 (s) | 128.3 (t) | 5.43 (d, 6.8 ) | 116.2 (t) | 5.45 (d, 6.8 ) | 115.9 (t) |
| 10'b | 6.09 (s) | 5.28 (d, 6.8 ) | 5.28 (d, 6.8 ) | |||
| OMe | 4.05 (s) | 60.0 (q) | 3.94 (s) | 61.5 (q) | 3.94 (s) | 61.5 (q) |
* Compound 1 recorded in pyridine-d5, 2/3 in CDCl3
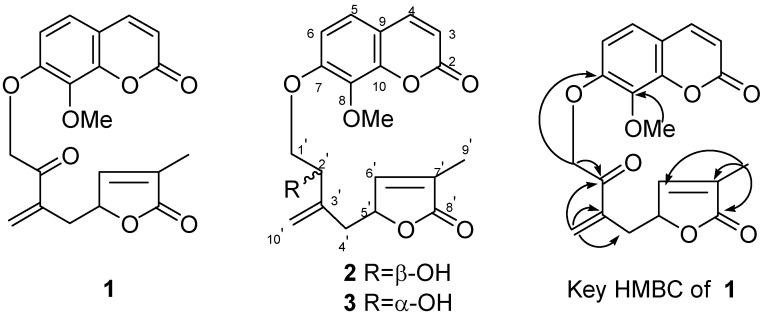
The 13C-NMR spectrum (Table 1) showed, in addition to the resonances of the carbons belonging to the coumarin nucleus, 10 other signals arising from one methyl, one oxymethylene, one methylene, one oxygen-bearing methine, four olefinic and two carbonyl carbons that could only be located on the side-chain. Joint analysis of HSQC spectra and HMBC correlations (Figure 1) confirmed the presence of a C10 terpenoid side-chain attached to the coumarin skeleton. The connection of the side chain to C-7 on the coumarin nucleus was revealed by observation of a three-bond correlation between the oxymethylene proton signals (H-1') and carbon C-7 at δC 153.5s. The presence of only one methoxyl signal at δH 4.05 and a significant HMBC correlation with a quaternary carbon signal at δC 135.6s revealed that the methoxyl group must be unequivocally located on C-8. Meanwhile, the EI-MS spectra also showed characteristic 8-OMe coumarin fragment ion at m/z 193 corresponding to loss of side chain [10].
Figure 1.
Structures of compounds 1- 3 and key HMBC correlations of 1.
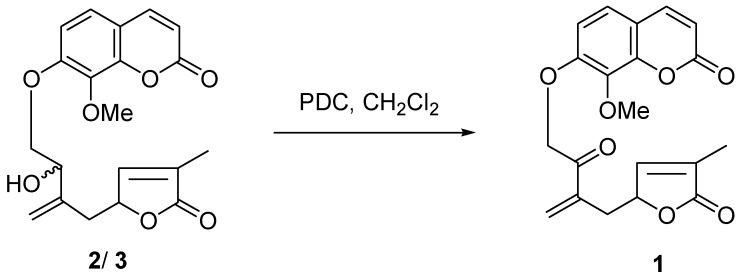
Carefully analysis of the 1H and 13C NMR data of 1 (Table 1), showed that the signal patterns were in good agreement with those of previously isolated compounds 2/3 [13], except for some chemical shift differences. IR bands at 1,753, 1,748 and 1,730 cm-1 were indicative of the presence of three carbonyl groups in 1, suggesting a ketonic group and a lactone in the side chain. The major difference between the 13C-NMR spectra of 1 and 2/3 was the appearance of a characteristic keto quaternary carbon signal at δC 193.8s (Table 1) in 1 and disappearance of oxygen-bearing carbons at δC 72.7d/72.6d compared to 2/3. The proton signal at δ 5.57 (2H, H-1′) appeared as a singlet, revealing the ketone (δC 193.8s) was located at C-2′. In the HMBC spectrum (Figure 1), an informative correlation between the oxymethylene proton signal at δH 5.57 (2H, H-1′) and keto quaternary carbon signal at δC 193.8s confirmed it. Compared to 2/3, because the C-2′ chiral center disappeared, there were no NMR signals pairs for C-2′, C-5′ and C-10′ in 1. All these suggested that 1 was the C-2′ oxygenation product of 2/3. To confirm the assigned structure, compound pair 2/3 was oxygenated to afford 1 with H2Cr2O7-2Pyr. (PDC) in CH2Cl2 (Figure 2). On the basis of these results, the structure of 1 as shown in Figure 1 is proposed.
Figure 2.
Oxidation of 2/3.
Conclusions
As a continuation of our phytochemical investigation on Clausena, a new O-terpenoidal coumarin 1, named hekumarone, was isolated from the leaves and twigs of Clausena anisum-olens Merr., collected in Hekou County in Yunnan Province, P. R. China. It is documented that coumarins are one of the major constituents in plants belonging to the genus Clausena [4,5,6,9]. The result of the present study supports the conclusion that coumarins are characteristic and distinguishable chemical markers for the Rutaceae family, especially, the genus Clausena.
Experimental
General
Commercial silica-gel plates (Qing Dao Marine Chemical Group Co.) were used for TLC analyses. UV/VIS Spectra was measured on a Shimadzu UV-2401PC spectrophotometer; λmax in nm. IR spectra were obtained on a Bio-Rad FTS-135 infrared spectrophotometer, νmax in cm-1. 1H- and 13C-NMR as well as 2D-NMR spectra were recordered on a Bruker DRX-500 spectrometer with TMS as internal standard, coupling constant J are expressed in Hz. MS spectra were recorded on a VG Autospec-3000mass spectrometer.
Plant material
The leaves and twigs of Clausena anisum-olens Merr. were collected in Hekou County of Yunnan province, P. R. China, in May 2003 and identified by Professor De-Ding Tao of the Kunming Institute of Botany. A voucher specimen (No. 02041705) is deposited in the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences.
Extraction and isolation
The powdered leaves and twigs of Clausena anisum-olens Merr. (22.5 kg) was repeatedly extracted with EtOH at room temperature. The extract was then concentrated under reduced pressure to give brown syrup, which was partitioned in H2O and extracted with solvents into petroleum ether-fraction, EtOAc-fraction and n-BuOH-fraction fractions. The EtOAc extracts (110.5g) were subjected to silica gel column chromatography eluting with PE-EtOAc (4:1, 2:1, 1:1, 2:3), EtOAc, EtOAc–MeOH (8:2, 7:3, 6:4, 1:1), MeOH, by which nine fractions (I-IX) were obtained. Fraction II was resubmitted to silica gel column chromatography, Pharmadex LH-20 (MeOH) and RP C-18 to yield compound 1 (4 mg).
Hekumarone (1). Light yellow oil; – 6.67 (c 0.75, CH3OH); IR (KBr): IR νKBrmax cm-1: 3,429, 2,923, 2,852, 1,753, 1,730, 1,606; UV (MeOH) λmax nm: 319, 256, 208; 1H-NMR (δ ppm, pyridine-d5) and 13C-NMR data are shown in Table 1; EI-MS m/z: 370 ([M]+, 85), 273 (7), 204 (35), 193 (100), 161 (45); HRESI-MS m/z 369.1000 [M-1]+ (calcd. for C20H18O7 369.0974).
Oxidation of 2/3
PDC (60 mg) was resolved in dry CH2Cl2 (2 mL) under N2. The mixture was stirred 5 min at room temperature. Then a soln. of 2/3 (6 mg) in CH2Cl2 (1 mL) was added. The reaction mixture was stirred overnight. The mixture was filtered and H2O (2 ml) was added, and then extracted with CH2Cl2. The org. phase dried (MgSO4) and evaporated to afford 1 (3 mg) as a light yellow oil.
Acknowledgements
This work was supported by National Nature Science Foundation of China (No. 20862018), Science Foundation of Yunnan University (Grant No. 2004Q004A and 2005Z001A) and Science Foundation of Yunnan (Grant No. 2006B0003Q and 2007PY01-23).
Footnotes
Sample Availability: Available from the authors.
References
- 1.Phuwapraisirisan P., Surapinit S., Sombund S., Siripong P., Tip-pyang S. Feroniellins A–C, novel cytotoxic furanocoumarins with highly oxygenated C10 moieties from Feroniella lucida. Tetrahedron Lett. 2006;47:3685–3688. doi: 10.1016/j.tetlet.2006.03.129. [DOI] [Google Scholar]
- 2.Chlouchi A., Muyard F., Girard C., Waterman P. G., Bévalot F. Coumarins from the twigs of Diplolaena mollis P. G. Wilson (Rutaceae) Biochem. Syst. Ecol. 2005;33:967–969. doi: 10.1016/j.bse.2005.03.002. [DOI] [Google Scholar]
- 3.Nájera C., Yus M. Natural products with polyene amide structures. Stud. Nat. Prod. Chem. 2000;21:373–455. doi: 10.1016/S1572-5995(00)80011-3. [DOI] [Google Scholar]
- 4.He H., Shen Y., He Y., Yang X., Zhu W., Hao X. Six new O-terpenoidal coumarins, excavacoumarins B-G from Clausena excavata. Heterocycles. 2000;53:2067–2070. doi: 10.3987/COM-00-8977. [DOI] [Google Scholar]
- 5.Ito C., Itoigawa M., Katsuno S., Omura M., Tokuda H., Nishino H., Furukawa H. Chemical constituents of Clausena excavata: isolation and structure elucidation of novel furanone-coumarins with inhibitory effects for tumor-promotion. J. Nat. Prod. 2000;63:1218–1224. doi: 10.1021/np990619i. [DOI] [PubMed] [Google Scholar]
- 6.Huang S.C., Wu P.L., Wu T.S. Two coumarins from the root bark of Clausena excavata. Phytochemistry. 1997;44:179–181. doi: 10.1016/S0031-9422(96)00532-8. [DOI] [Google Scholar]
- 7.Institutum Botanicum Kunmingenge Academiae Sinicae . In: Flora Yunnanica (Spermatophyta) Wu C.Y., editor. Science Press; Beijing: 2001. p. 767. Tomus 6. (in Chinese) [Google Scholar]
- 8.Chakraborty A., Chowdhury B.K., Bhattacharyya P. Clausenol and clausenine-two carbazole alkaloids from Clausena anisata. Phytochemistry. 1995;40:295–298. doi: 10.1016/0031-9422(95)00047-B. [DOI] [PubMed] [Google Scholar]
- 9.Wu T.S., Huang S.C., Wu P.L. Carbazole-pyranocoumarin dimer and binary carbazole alkaloids from Clausena excavata. Tetrahedron Lett. 1996;37:7819–7822. doi: 10.1016/0040-4039(96)01741-8. [DOI] [Google Scholar]
- 10.Takemura Y., Nakamura K., Hirusawa T., Ju-ichi M., Ito C., Furukawa H. Four new furanone-coumarins from Clausena excavata. Chem. Pharm. Bull. 2000;48:582–584. doi: 10.1248/cpb.48.582. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y.S., He H.P., Yang J.H., Shen Y.M., Zhou J., HAO X.J. A new cyclopeptide from Clausena anisum-olens Merr. Helv. Chim. Acta. 2005;88:2345–2348. doi: 10.1002/hlca.200590169. [DOI] [Google Scholar]
- 12.Wang Y.S., He H.P., Yang J.H., Di Y.T., HAO X.J. New monoterpenoid coumarins from Clausena anisum-olens Merr. Molecules. 2008;13:931–937. doi: 10.3390/molecules13040931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Y.S., Huang R., Li L., Zhang H.B., Yang J.H. O-terpenoidal coumarins from Clausena anisum-olens Merr. Biochem. Syst. Ecol. 2008;36:801–803. doi: 10.1016/j.bse.2008.06.005. [DOI] [Google Scholar]