Abstract
Background/Aims:
To compare water exchange (WE) method with conventional air insufflation (AI) method for colonoscopy, evaluating the technical quality, screening efficacy, and patients' acceptance.
Materials and Methods:
Electronic databases were systematically searched for randomized controlled trials comparing WE colonoscopy with AI colonoscopy. The pooled data of procedure-associated and patient-related outcomes were assessed, using the weighted mean difference (WMD) with 95% confidence interval (CI) for continuous variables and relative risk (RR) with 95% CI for dichotomous variables, respectively.
Results:
A total of 13 studies involving 7056 patients were included. The cecum intubation rate was similar between WE and AI methods (RR = 1.01, 95% CI = 0.99–1.02, P = 0.37); however, a significantly longer cecum intubation time was shown in WE group (WMD = 1.56, 95% CI = 0.75–2.37, P = 0.002). Compared with AI, WE was associated with a higher risk of adenoma detection rate (ADR) (RR = 1.28, 95% CI = 1.18–1.38, P < 0.00001) and polyp detection rate (PDR) (RR = 1.30, 95% CI = 1.21–1.39, P < 0.00001). Patients in WE group experienced significantly less maximum pain score (WMD = −1.99, 95% CI = −2.68 to −1.30, P < 0.00001) and less requested on-demand sedation (RR = 0.58, 95% CI = 0.44–0.77, P = 0.0002). Likewise, they also experienced less abdominal compression (RR = 0.62, 95% CI = 0.51-0.74, P < 0.00001) and reposition (RR = 0.74, 95% CI = 0.63-0.86, P = 0.0001). Moreover, patients' willingness to repeat colonoscopy was significantly greater for WE (RR = 1.14, 95% CI = 1.07–1.21, P < 0.0001).
Conclusion:
This meta-analysis confirmed that WE method could significantly increase ADR/PDR and improve patients' acceptance of colonoscopy, while reducing the degree of pain and minimize the need for on-demand sedation and adjunct maneuvers, despite requiring more cecal intubation time.
Keywords: Air insufflation, colonoscopy, meta-analysis, water exchange
INTRODUCTION
Colonoscopy remains the cornerstone examination for the diagnosis, surveillance, and treatment of colorectal diseases.[1] It is usually performed with standard air insufflation (AI) which distends the colonic lumen to permit visualization and passage of the instrument. Usually, AI colonoscopy is considered to be painful and poorly tolerated procedure for most patients without sedation. The undesirable outcomes are partly due to colon distension, angulations' exaggeration at flexures, and looping of the instrument. All these expose patients to potential risks (e.g., perforation, bleeding).[2]
Due to the relatively low cecal intubation rate and intolerable pain, sedation has generally been recommended for AI colonoscopy. However, sedated colonoscopy is associated with additional risk of sedatives, higher medical costs, and a longer recovery time compared with unsedated colonoscopy.[3] Therefore, any endoscopic technique that minimizes the sedation needs has the potential to improve the quality of the procedure.
Water infusion method is gaining increasing interest in recent years. Compared with AI method, the water infused opens colonic lumen and weighs down the left colon to straighten the sigmoid segment, thus facilitating the advancement of colonoscope and reducing insertion pain and discomfort.[4] Water infusion methods are mainly classified as water immersion (WI) and water exchange (WE). They are distinguished by removal of the infused water during withdrawal (WI) or insertion (WE).[5] When performed with WE, removal of the infused water in addition to suction residual feces and retained air may maximize the cleanliness of the colonic lumen, which is supposed to have a superior impact on colonoscopy than WI.[6]
Recently, considerable new data related to WE method instead of AI have been generated by investigators worldwide. These clinical studies have shown that WE is an attractive strategy used in colonoscopy as it can minimize sedation requirement, increase disease detection rate, and improve patients' acceptance of the procedure without compromising the success rate of cecal intubation.[7,8,9,10] Despite evidence in support of WE for improving the quality of colonoscopy, there are still some reservations among endoscopists regarding its true benefits.[11]
The aim of this meta-analysis was to investigate the effects of WE method compared with conventional AI method used in colonoscopy, in terms of procedure-associated and patient-related outcomes.
SUBJECTS AND METHODS
Study identification
This study was performed according to the standard guidelines for meta-analyses and systematic reviews of randomized controlled trials (RCTs). Medline, Embase, Cochrane Library, and ISI Web of Knowledge were searched systematically for RCTs published up to December 2017 concerning the water method in colonoscopy, using the terms ”water” and ”colonoscopy.” The reference lists were also inspected for relevant studies.
Study selection and quality assessment
Eligible studies were included in the meta-analysis if they met the following criteria: (1) full-text RCTs published in English; (2) patients undergoing colonoscopy; (3) using WE or AI method; (4) outcome measures including procedure-associated or patient-related data; and (5) allowing no AI any time during insertion in the water group.
Exclusion criteria were as follows: (1) any comment, review, or guidelines were excluded; (2) colonoscopy procedures conducted by trainees; (3) using carbon dioxide or other gas insufflations; (4) using WI technique; and (5) using water infusion technique combined with AIs.
Study quality was assessed using Cochrane Risk of Bias Tool (based on a description of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective report and other bias).
Data extraction and synthesis
Two of the authors (Y.L. and Q.-K.H.) performed study selections independently and reached consensus on all items. Data were extracted as follows:first author, publication year, country, number of patients, water temperature, water volume, cecal intubation time, cecal intubation rate, ADR/PDR, maximum pain score during the procedure, on-demand sedation rate, need for abdominal compression or position change, and willingness to undergo a repeat colonoscopy. For summary statistics in meta-analyses, we calculated the pooled relative risk (RR) for dichotomous data using intention-to-treat analysis and weighted mean difference (WMD) for continuous data using per-protocol analysis, respectively. Pooled estimates were presented with a 95% confidence interval (CI). Summary estimates of the RRs/WMDs were derived using a fixed-effects model (Mantel–Haenszel method) or random-effects model (DerSimonian and Laird method).[12] Heterogeneity between studies was evaluated by I2 statistic. Publication bias was assessed by funnel plot, using Begg–Mazumdar indicator[13] and Harbord–Egger indicator.[14] P < 0.1 indicated the presence of publication bias. For continuous data, if the study reported only the median and interquartile range (IQR) while sample size is larger than 100, then the median was regarded as mean and standard deviation (SD) was calculated as IQR/1.35.[15] All meta-analyses were performed using Review Manager (version 5.3; Cochrane Collaboration, Copenhagen, Denmark) and Stata Software (version 12.0; Stata Corporation, College Station, TX, USA).
RESULTS
Eligible studies and quality assessment
A total of 13 studies involving 7056 patients were included in the final analysis.[7,8,9,10,16,17,18,19,20,21,22,23,24] Figure 1 presents the selection procedure. Table 1 describes the main details of the included studies. Methodological quality of the RCTs was adequate except blinding of endoscopist, as it could not be carried out in clinical practice [Figure 2].
Figure 1.
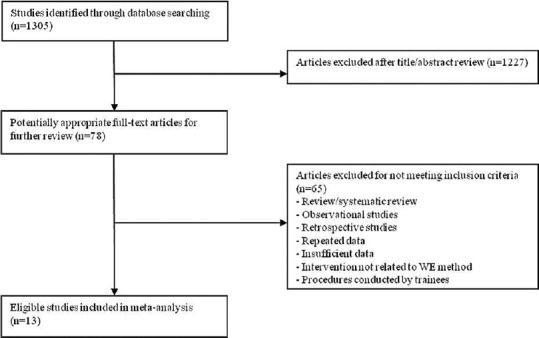
Flow diagram of the selection of trials
Table 1.
Characteristics of the included studies
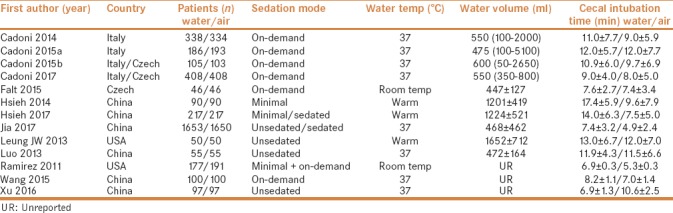
Figure 2.
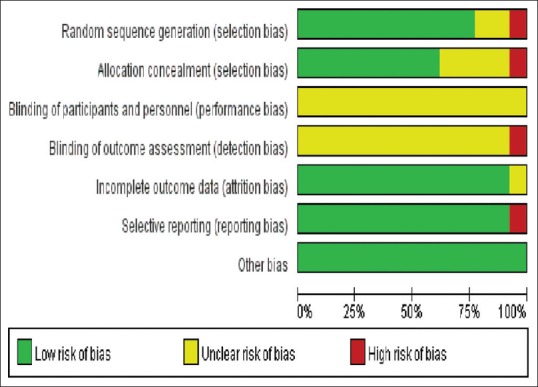
Summary of the risk of bias assessment
Cecal intubation rate
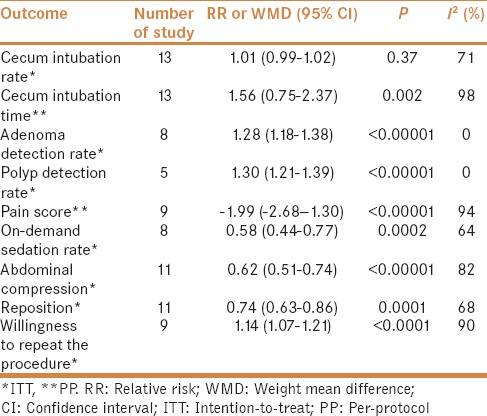
A total of 13 studies were eligible for evaluation of the cecal intubation rate, which was almost identical for WE and AI (RR = 1.01, 95% CI = 0.99–1.02, P = 0.37, I2 = 71%) [Table 2].
Table 2.
Subgroup analysis of studies comparing water exchange with air insufflation for colonoscopy
Cecal intubation time
A total of 13 studies were eligible for evaluation of mean cecal incubation time, which was demonstrated to be relatively longer in WE group (WMD = 1.57, 95% CI = 0.75–2.37, P = 0.002, I2 = 98%) [Table 2].
Adenoma/polyp detection rate
As for ADR, eight studies involving a total of 6067 colonoscopies were included, and in 1586 of them at least one adenoma was detected. ADR was significantly higher in WE group (RR = 1.28, 95% CI = 1.18–1.38, P < 0.00001, I2 = 0%) [Table 2]. As for PDR, five studies involving a total of 3907 colonoscopies were included, and in 1768 of them at least one polyp was detected. Similarly, PDR was also higher in WE group (RR = 1.30, 95% CI = 1.21–1.39, P < 0.00001, I2 = 0%) [Table 2].
Pain score
A total of nine studies investigating the difference in the maximum pain score during colonoscopy procedure were carried out using the visual analog scale from 0 to 10 to grade patients' pain (0 = no pain, 10 = most severe pain). Pooled estimates showed that the maximum pain score was significantly lower in WE group (WMD = −1.99, 95% CI = −2.68 to −1.30, P < 0.00001, I2 = 94%) [Table 2].
On-demand sedation rate
A total of eight trials investigating on-demand sedation rate were eligible to assess if lesser sedation was required with WE (RR = 0.58, 95% CI = 0.44–0.77, P = 0.0002, I2 = 64%) [Table 2].
Adjunct maneuvers
Pooled estimates of 11 trials showed that less abdominal compression (RR = 0.62, 95% CI = 0.51–0.74, P < 0.00001, I2 = 82%) and less reposition (RR = 0.74, 95% CI = 0.63–0.86, P = 0.0001, I2 = 68%) were needed during WE colonoscopy [Table 2].
Willingness to repeat colonoscopy
Pooled estimates of nine studies revealed that 91% of the patients (2499/2744) undergoing WE colonoscopy would repeat the procedure, when compared with 85% of those who (2330/2746) underwent AI colonoscopy (RR = 1.14, 95% CI = 1.07–1.21, P < 0.0001, I2 = 90%) [Table 2].
Publication bias
Publication bias was evaluated by Begg's funnel plot and Egger's test. The shape of Begg's funnel plot did not reveal any evidence of asymmetry [Figure 3]. Egger's test did not suggest any evidence of publication bias (P = 0.30).
Figure 3.
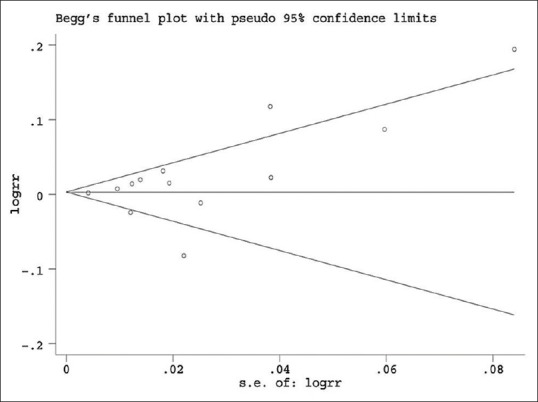
Begg's funnel plot of publication bias test
DISCUSSION
In this meta-analysis, although evidence was not adequate to prove that WE method was better for increasing cecal intubation rate, the use of WE seemed to have a higher ADR/PDR and patients' acceptance of colonoscopy. Moreover, pooled data showed that WE relieved patients' pain and minimized the need for on-demand sedation and adjunct maneuvers. However, WE was demonstrated to be more time-consuming during the insertion phase.
ADR was considered to be the leading parameter of our study as the quality of endoscopist's performance of colonoscopy is mainly defined by the capacity of detecting precancerous lesions.[25] Currently, the recommended ADR target for a mixed male–female population is at least 25%.[26] However, previous studies have reported an approximately 22% adenoma missing rate undergoing tandem colonoscopy.[27] Interval cancers from these missed lesions would occur in 0.3% of the screened persons.[28] Thus, the recognition of interval cancers in patients with low ADR calls for meticulous examination. In our study, pooled data suggested that WE was superior to AI with respect to ADR (29.4% vs. 22.9%). The increase was significant (6.5%). Similar trend was observed for PDR (50.2% vs. 39.3%), with an increase of 10.9%. There are several possible mechanisms. The most important factor is the improved bowel preparation, which allows the colonoscopists focusing on searching for lesions. Besides, with water infusion, colon topography may not be changed and small polyps floating underwater can be more easily visible. Moreover, the magnifying effect of water can make the change in vasculature of neoplasm more obvious.
Minimizing pain and discomfort are of greatest importance for patients to accept colonoscopy. For this purpose, routine sedation, as one of the assistive techniques, has been commonly implemented into colonoscopy. However, with WE method, we found that there was a major reduction in pain score (by more than 1 unit compared with AI), thus the proportion of patients asking for sedation was significantly lower. Besides, our study also showed less need for abdominal compression or position change in WE group, since water reduces angulations during intubation and facilitates instrument passage with less looping, especially in the left colon. All these contributed to a significantly higher proportion of patients willing to repeat the procedure with WE than AI (91% vs. 85%).
The time efficiency of performance is another important factor in adoption of a new method for colonoscopy. With regard to WE, more time has been spent for water infusion and suction during cecal intubation. In RCTs included in our study, the mean cecal insertion time for water method ranged from 6.9 to 17.4 min, suggesting a small but significant increase when compared with air method (4.9–12.0 min). However, the longer time (about 2 min) required by WE may play an important role in revealing more lesions during insertion, as confirmed in our study. We believe that this disadvantage might be overcome by more standardized technique training.
However, WE method has its own limitations. First, it is more prone to interference by suboptimal bowel preparation. Second, the prolonged insertion time will discourage its widespread application in current busy clinical practice. Third, it could not significantly improve an already high cecal intubation rate for most endoscopists at present. Fourth, it is more difficult for endoscopists to master this technique. However, it is noteworthy that there was no report of perforation or bleeding in patients undergoing WE colonoscopy examination. Severe procedure-related complications are not likely to be increased with WE method.
Several limitations of this study should be mentioned. First, significant heterogeneity was observed for some outcomes. The characteristics of study protocols, patient cohorts, endoscopists' experience, and other variabilities across studies may partly contribute to some heterogeneity within our analysis. Second, the blinding of outcome assessors was not possible for all variables. The lack of method concealment may contribute to a high Hawthorne effect which could not be completely ruled out and the final results might be affected to some extent. Third, raw data from some studies could not be compared directly. For example, pain score was not provided in the form of mean and SD in all studies.
CONCLUSION
In summary, WE rather than AI during the insertion phase of colonoscopy could significantly lower maximum abdominal pain score and minimize the need for adjunct maneuvers, allow less sedative use without affecting the efficiency of the examination, and improve patients' acceptance of the procedure. This novel technique should be considered as an option to reduce pain and discomfort in patients who prefer to undergo an unsedated colonoscopy, especially in those who have additional comorbidities with increased sedation risk. More importantly, it has shown to increase ADR/PDR in the colorectum. We postulate that the use of WE could assist colonoscopists with low ADR/PDR to improve their performance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors acknowledge support of all the colleagues in the Department of Gastroenterology and Hepatology of the first Affiliated Hospital of Wenzhou Medical University.
REFERENCES
- 1.Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW, Jr, et al. US Preventive Services Task Force. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2016;315:2564–75. doi: 10.1001/jama.2016.5989. [DOI] [PubMed] [Google Scholar]
- 2.de Groen PC. Editorial: Polyps, pain, and propofol: Is water exchange the panacea for all? Am J Gastroenterol. 2017;112:578–80. doi: 10.1038/ajg.2017.29. [DOI] [PubMed] [Google Scholar]
- 3.Garborg K, Kaminski MF. Water-aided insertion and CO2-assisted withdrawal: Do we still need routine sedation for colonoscopy? Gastrointest Endosc. 2017;85:219–20. doi: 10.1016/j.gie.2016.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Baumann UA. Water intubation of the sigmoid colon: Water instillation speeds up left-sided colonoscopy. Endoscopy. 1999;31:314–7. doi: 10.1055/s-1999-23. [DOI] [PubMed] [Google Scholar]
- 5.Leung FW, Amato A, Ell C, Friedland S, Harker JO, Hsieh YH, et al. Water-aided colonoscopy: A systematic review. Gastrointest Endosc. 2012;76:657–66. doi: 10.1016/j.gie.2012.04.467. [DOI] [PubMed] [Google Scholar]
- 6.Leung FW. Water exchange may be superior to water immersion for colonoscopy. Clin Gastroenterol Hepatol. 2011;9:1012–4. doi: 10.1016/j.cgh.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Cadoni S, Falt P, Rondonotti E, Radaelli F, Fojtik P, Gallittu P, et al. Water exchange for screening colonoscopy increases adenoma detection rate: A multicenter, double-blinded, randomized controlled trial. Endoscopy. 2017;49:456–67. doi: 10.1055/s-0043-101229. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh YH, Tseng CW, Hu CT, Koo M, Leung FW. Prospective multicenter randomized controlled trial comparing adenoma detection rate in colonoscopy using water exchange, water immersion, and air insufflation. Gastrointest Endosc. 2017;86:192–201. doi: 10.1016/j.gie.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Jia H, Pan Y, Guo X, Zhao L, Wang X, Zhang L, et al. Water exchange method significantly improves adenoma detection rate: A multicenter, randomized controlled trial. Am J Gastroenterol. 2017;112:568–76. doi: 10.1038/ajg.2016.501. [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Zhu H, Chen D, Fan L, Lu T, Shen Q, et al. Carbon dioxide insufflation or warm-water infusion for unsedated colonoscopy: A randomized controlled trial in patients with chronic constipation in China. Saudi J Gastroenterol. 2016;22:18–24. doi: 10.4103/1319-3767.173754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishaq S, Neumann H. Water assisted colonoscopy: A promising new technique. Dig Liver Dis. 2016;48:569–70. doi: 10.1016/j.dld.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 13.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 14.Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443–57. doi: 10.1002/sim.2380. [DOI] [PubMed] [Google Scholar]
- 15.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions [updated March 2011]. Ver. 510. Cambridge, UK: The Cochrane Collaboration; 2011. [Google Scholar]
- 16.Cadoni S, Falt P, Gallittu P, Liggi M, Mura D, Smajstrla V, et al. Water exchange is the least painful colonoscope insertion technique and increases completion of unsedated colonoscopy. Clin Gastroenterol Hepatol. 2015;13:1972–800. doi: 10.1016/j.cgh.2015.04.178. [DOI] [PubMed] [Google Scholar]
- 17.Cadoni S, Gallittu P, Sanna S, Fanari V, Porcedda ML, Erriu M, et al. A two-center randomized controlled trial of water-aided colonoscopy versus air insufflation colonoscopy. Endoscopy. 2014;46:212–8. doi: 10.1055/s-0033-1353604. [DOI] [PubMed] [Google Scholar]
- 18.Cadoni S, Sanna S, Gallittu P, Argiolas M, Fanari V, Porcedda ML, et al. A randomized, controlled trial comparing real-time insertion pain during colonoscopy confirmed water exchange to be superior to water immersion in enhancing patient comfort. Gastrointest Endosc. 2015;81:557–66. doi: 10.1016/j.gie.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 19.Falt P, Šmajstrla V, Fojtík P, Urban O, Hill M. Water-aided colonoscopy in inflammatory bowel disease patients-A randomised, single-centre trial. J Crohns Colitis. 2015;9:720–4. doi: 10.1093/ecco-jcc/jjv093. [DOI] [PubMed] [Google Scholar]
- 20.Hsieh YH, Koo M, Leung FW. A patient-blinded randomized, controlled trial comparing air insufflation, water immersion, and water exchange during minimally sedated colonoscopy. Am J Gastroenterol. 2014;109:1390–400. doi: 10.1038/ajg.2014.126. [DOI] [PubMed] [Google Scholar]
- 21.Leung JW, Mann S, Siao-Salera R, Canete W, Prather D, Leung FW. The established and time-tested water exchange method in scheduled unsedated colonoscopy significantly enhanced patient-centered outcomes without prolonging procedural times – A randomized controlled trial. J Interv Gastroenterol. 2013;3:7–11. [Google Scholar]
- 22.Luo H, Zhang L, Liu X, Leung FW, Liu Z, Wang X, et al. Water exchange enhanced cecal intubation in potentially difficult colonoscopy. Unsedated patients with prior abdominal or pelvic surgery: A prospective, randomized, controlled trial. Gastrointest Endosc. 2013;77:767–73. doi: 10.1016/j.gie.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Ramirez FC, Leung FW. A head-to-head comparison of the water vs.air method in patients undergoing screening colonoscopy. J Interv Gastroenterol. 2011;1:130–5. doi: 10.4161/jig.1.3.18512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Luo H, Xiang Y, Leung FW, Wang L, Zhang L, et al. Left-colon water exchange preserves the benefits of whole colon water exchange at reduced cecal intubation time conferring significant advantage in diagnostic colonoscopy – A prospective, randomized controlled trial. Scand J Gastroenterol. 2015;50:916–23. doi: 10.3109/00365521.2015.1010569. [DOI] [PubMed] [Google Scholar]
- 25.Kaminski MF, Wieszczy P, Rupinski M, Wojciechowska U, Didkowska J, Kraszewska E, et al. Increased rate of adenoma detection associates with reduced risk of colorectal cancer and death. Gastroenterology. 2017;153:98–105. doi: 10.1053/j.gastro.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Rex DK, Schoenfeld PS, Cohen J, Pike IM, Adler DG, Fennerty MB, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2015;81:31–53. doi: 10.1016/j.gie.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 27.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E, et al. Polyp miss rate determined by tandem colonoscopy: A systematic review. Am J Gastroenterol. 2006;101:343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 28.Pohl H, Robertson DJ. Colorectal cancers detected after colonoscopy frequently result from missed lesions. Clin Gastroenterol Hepatol. 2010;8:858–64. doi: 10.1016/j.cgh.2010.06.028. [DOI] [PubMed] [Google Scholar]


