Abstract
In this study, the antioxidant activities of 15 flavonoids against lard oil oxidation were determined by using the Rancimat test. Quercetin, dihydromyricetin, luteolin and kaempferol showed the strongest antioxidant activity, with protection factor values (PF) of 11.50, 11.29, 4.24 and 2.49, respectively. The role of conjugated hydroxyl groups of the B and C ring is discussed. By using the following descriptors: ΔHf (the difference in heat of formation between flavonoids and their free radicals resulted after hydrogen atom donation) and HBC (the number of conjugated hydroxyl groups of the B and C ring), the result obtained in the antioxidant Rancimat test could be successfully explained.
Keywords: Flavonoid, Structure-Activity Relationship, Quantum Chemistry, Antioxidant.
Introduction
A number of studies have shown that natural antioxidants from plant sources can effectively inhibit oxidation in food and reduce the risk of age-dependent diseases. Flavonoids, widespread in fruits, vegetables, teas and medicinal plants, have received the greatest attention, and have been studied extensively, since they are highly effective antioxidants, less toxic than synthetic antioxidants such as butylated hydroxyanisole (BHA) or butylated hydroxytoluene (BHT) [1,2]. It was found that many factors influence the antioxidant activity, such as the position of OH groups, the properties of substituent groups, and hydrogen bond formation. Theoretical calculations are helpful to investigate the activity differences of antioxidants. In fact, structure-activity relationship of flavonoids as antioxidants has been explained successfully by theoretical calculations [3,4]. However, it should be pointed out that most of the studies were obtained in the simple and hydrophilic tests as DPPH radical scavenging assay [5,6]. So it is necessary to determine whether the theoretical methods are still effective to characterize the antioxidant activity of flavonoids in a complex and hydrophobic system, e.g., in the experiments that show how antioxidants protect lipid. Therefore the goal of this paper is to measure the antioxidant activity of 15 natural flavonoids in lard oil by a Rancimat test and to present the relationships between molecular structure-derived parameters and the antioxidant activity of flavonoids.
Results and Discussion
Hydrogen-donation was thought to be the main mechanism of acton of phenolic antioxidants in oil, and the difference in heat of formation between the phenolic antioxidant and its free radical produced after H-abstraction (ΔHf), appeared to be a good index for measuring the scavenging activity of antioxidants [7]. The effectiveness of ΔHf can be understood easily, as it represents the strength of the O-H bond. The lower strength of the O-H bond corresponds to a higher scavenging activity. AM1 was selected because it was better than other semiempirical methods, such as Modified Neglect of Diatomic Differential Overlap (MNDO) and Parametric Method 3 (PM3), to calculate Hf [8]. The ΔHf values and the most active hydroxyl groups of 15 flavonoids were shown in Table 1, Table 2 and Table 3.
Table 1.
Chemical structures, descriptors and antioxidant activity of some flavones and flavonols.
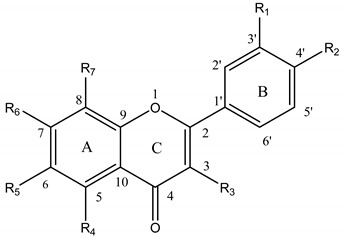
| No | Flavonoid | R1 | R2 | R3 | R4 | R5 | R6 | R7 | ΔHf (kcal/mol) | Most Active OH | HBC | PF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | luteolin | OH | OH | H | OH | H | OH | H | 22.09 | 4’-OH | 2 | 4.24 |
| 2 | kaempferol | H | OH | OH | OH | H | OH | H | 21.29 | 3-OH | 2 | 2.49 |
| 3 | nobiletin | OCH3 | OCH3 | H | OCH3 | OCH3 | OCH3 | OCH3 | — | — | 0 | 1.04 |
| 4 | chrysin | H | H | H | OH | H | OH | H | 30.92 | 7-OH | 0 | 0.98 |
| 5 | quercetin | OH | OH | OH | OH | H | OH | H | 20.84 | 4’-OH | 3 | 11.50 |
| 6 | apigenin | H | OH | H | OH | H | OH | H | 26.19 | 4’-OH | 1 | 0.99 |
| 7 | tangeretin | H | OCH3 | H | OCH3 | OCH3 | OCH3 | OCH3 | — | — | 0 | 0.99 |
| 8 | camellianin A | H | OH | H | O-[rham-6-O-acetyl –glu] | H | OH | H | 25.84 | 4’-OH | 1 | 1.01 |
Table 2.
Chemical structures, descriptors and antioxidant activity of some flavanones and flavanonols
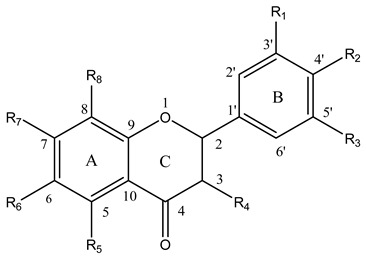
| No | Flavonoid | R1 | R2 | R3 | R4 | R5 | R6 | R7 | R8 | ΔHf (kcal/mol) | Most Active OH | HBC | PF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | naringenin | H | OH | H | H | OH | H | OH | H | 27.49 | 4’-OH | 1 | 1.09 |
| 2 | hesperetin | OH | OCH3 | H | H | OH | H | OH | H | 23.15 | 3’-OH | 1 | 1.28 |
| 3 | dihydromyricetin | OH | OH | OH | OH | OH | H | OH | H | 23.14 | 4’-OH | 3 | 11.29 |
Table 3.
Chemical structures, descriptors and antioxidant activity of some isoflavones
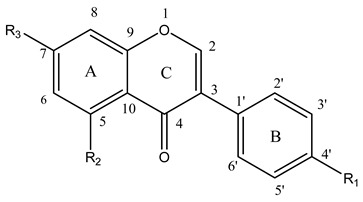
| No | Flavonoid | R1 | R2 | R3 | ΔHf (kcal/mol) | Most Active OH | HBC | PF |
|---|---|---|---|---|---|---|---|---|
| 1 | genistein | OH | OH | OH | 24.65 | 4’-OH | 1 | 1.13 |
| 2 | sophoricoside | O-glu | OH | OH | 49.71 | 5 -OH | 0 | 0.99 |
| 3 | daidzein | OH | H | OH | 24.78 | 4’-OH | 1 | 1.14 |
| 4 | formononetin | OCH3 | H | OH | 29.79 | 7 -OH | 0 | 1 |
It could be found through the analysis of these data that:
-
(1)
In flavonoids with a C2 -C3 double bond and a C-3 hydroxyl group, the most active hydroxyl groups for H-donating are the ones attached to C4’ or C3.
-
(2)
In flavonoids with a C2 -C3 double bond, but without a C-3 hydroxyl group, the most active hydroxyl groups for H-donating are the ones attached to C4’.
-
(3)
In flavonoids with a C2 -C3 single bond and a hydroxyl group linked to C3 or not, the most active H-donating hydroxyl groups are the ones attached to C4’.
-
(4)
In flavonoids with an ortho-dihydroxyl group on B ring, the ΔHf values of these adjacent hydroxyl groups correspond to the values of the most active hydroxyl group for hydrogen-donating. This indicates that the substitution enhances radical scavenging ability.
-
(5)
The ΔHf value of the C-5 and C-7 hydroxyl group is significantly higher than that of the hydroxyl group on B ring. This agrees with the conclusion that C ring deactivates A ring, reported by Zhang [8].
The above analysis fully showed that considering from the antioxidation mechanism, the hydroxyl groups on B ring of flavonoids make a great contribution to the flavonoid antioxidant activity, in certain circumstances, the hydroxyl groups on C ring also contributed. Therefore, a new descriptor, the number of conjugated hydroxyl groups of the B and C ring (HBC), was put forward in this study.
In the Rancimat method, the sample is exposed to a stream of air at temperatures ranging from 50-220 °C. The volatile oxidation products (chiefly formic acid) are transferred to a measuring vessel by the air stream and absorbed in the measuring solution (distilled water). When the conductivity of this measuring solution is recorded continuously an oxidation curve is obtained, whose point of inflection is known as the induction time; this provides a good characteristic value for the oxidation stability. The protection factor values (PF) of 15 flavonoids were shown in Table 1, Table 2 and Table 3. As shown in Figure 1, quercetin, dihydromyricetin, luteolin and kaempferol showed the strongest antioxidant activity. The performance of quercetin (PF, 11.5) and dihydromyricetin (PF, 11.29) were superior to that of TBHQ (PF, 4.75).
Figure 1.
Antioxidant activity of luteolin, dihydromyricetin, quercetin and TBHQ in the Rancimat test.
By using HBC and ΔHf, the results of this study could be successfully explained as follows:
-
(1)
Flavonoids with HBC of 0, such as chrysin, formononetin, sophoricoside, tangeretin and nobiletin, failed to show any antioxidant activity against lard oil oxidation. This is attributable to the fact that they could not easily donate hydrogen atoms.
-
(2)
Flavonoids with HBC of 1, such as genistein, naringenin, daidzein, apigenin, hesperetin and camellianin A, showed the poor antioxidant activity, with PFs of 1.11±0.01.
-
(3)
Flavonoids with HBC of 2, such as luteolin and kaempferol, showed strong antioxidant activity. The PF values of luteolin and kaempferol were 4.24 and 2.49. The performance of luteolin was superior to that of kaempferol, as the former has a structure containing ortho-diphenolic hydroxyl groups.
-
(4)
Flavonoids with HBC of 3, such as quercetin and dihydromyricetin, showed the strongest antioxidant activity, with PF values of 11.50 and 11.29, respectively. The ΔHf value of quercetin (20.84 kcal/mol) was less than that of dihyromyricetin (23.14 kcal/mol), which meant the capacity of H-donating of quercetin was stronger than that of dihydromyricetin. As a result, the antioxidant performance of quercetin was superior to that of dihydromyricetin.
In conclusion, flavonoids inhibit oil oxidation by H-donation. The antioxidant performance of flavonoids could be explained by both ΔHf and the number of hydroxyl groups in the conjugated B and C ring system.
Experimental
Materials and Chemicals
Apigenin and quercetin were purchased from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, P.R. China). The following flavonoids were purchased from Shanxi Huike Botanical Development Co., LTD (Xi’an, P.R. China) and their purities (about 98 %) were confirmed by HPLC: luteolin, kaempferol, nobiletin, chrysin, tangeretin, naringenin, hesperetin, genistein, sophoricoside, daidzein, and formononetin. Camellian A and dihydromyricetin with purity of 99 % came from our previous studies [9,10]. Other chemicals were of analytical grade and were used as received.
Rancimat Test
The antioxidant activities of the samples were performed on a 743 Rancimat analyzer according to the method of Proestos et al. [11]. Samples of lard oil (3 g) containing flavonoids (1 μM) were subjected to oxidation at 110 °C (air flow 20 L/h). Tertiary butylhydroquinone (TBHQ) was used as the positive control. A control containing ethanol instead of flavonoids or TBHQ was also used. Induction periods (IP, h), were recorded automatically. The protection factors (PF) were calculated according to the following formula: (PF = IPsample/IPcontrol).
Calculation of ΔHf
The heat of formation differences between flavonoids and their free radicals produced after H-abstraction (ΔHf) were calculated from computational calculations on the 3D molecular structure using the program Hyperchem 6.0 (HyperCube, Inc., Gainesville, FL, USA). The structures were drawn as 2D structures and the Molecular Mechanics force field (MM+) was selected for geometry optimization using the Polak-Ribiere algorithm with a maximum cycle (450) and a convergence limit of 0.1 kcal/mol. The obtained three-dimensional geometries were submitted to a final geometry optimization by the AM1 method using the Polak-Ribiere algorithm with a maximum cycle (450) and a convergence limit of 0.1 kcal/mol. Heat of formation of the parent molecules (Hfm) and the free radicals (Hff) were obtained to calculate the index: ΔHf= Hff – Hfm. To make a comparison, parent molecules and free radicals were all calculated in Unrestricted Hartree Fock (UHF) approximation. In the calculations, only the most stable conformations of flavonoids and their free radicals were taken into consideration. And the corresponding hydroxyl group donating H in the most stable free radical was determined as most active hydroxyl group in a flavonoid molecule.
Acknowledgements
The financial support provided by the National Natural Science Foundation of China (No.20806029) and the Science and Technology Plan of Henan Institute of Science and Technology (No.7040) is greatly appreciated.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- 1.Burda S., Oleszek W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001;49:2774–2779. doi: 10.1021/jf001413m. [DOI] [PubMed] [Google Scholar]
- 2.Zou Y., Lu Y., Wei D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004;52:5032–5039. doi: 10.1021/jf049571r. [DOI] [PubMed] [Google Scholar]
- 3.Ghiotto R.C.T., Lavarda F.C., Ferreira F.J.B. Antioxidant activity of flavonols. Int. J. Quantum Chem. 2004;97:949–952. doi: 10.1002/qua.10798. [DOI] [Google Scholar]
- 4.Teixeira S., Siquet C., Alves C., Boal I., Marques M.P., Borges F., Lima J.L.F.C., Reis S. Structure-property studies on the antioxidant activity of flavonoids present in diet. Free Radical Bio. Med. 2005;39:1099–1108. doi: 10.1016/j.freeradbiomed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Butkoić V., Klasinc L., Bors W. Kinetic Study of flavonoid reaction with stable radicals. J. Agric. Food Chem. 2004;52:2816–2820. doi: 10.1021/jf049880h. [DOI] [PubMed] [Google Scholar]
- 6.Om A., Kim J.H. A quantitative structure-activity relationship model for radical scavenging activity of flavonoids. J. Med. Food. 2008;11:29–37. doi: 10.1089/jmf.2007.048. [DOI] [PubMed] [Google Scholar]
- 7.Khlebnikov A.I., Schepetkin I.A., Domina N.G., Kirpotina L.N., Quinn M.T. Improved quantitative structure-activity relationship models to predict antioxidant activity of flavonoids in chemical, enzymatic, and cellular systems. Bioorgan. Med. Chem. 2007;15:1749–1770. doi: 10.1016/j.bmc.2006.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H. Theoretical methods used in elucidating activity differences of phenolic antioxidants. J Am. Oil Chem. Soc. 1999;76:645–748. [Google Scholar]
- 9.Liu B., Ning Z., Zhan Y., Xu K., Gao J. Characterization and 1,1-diphenyl-2-picrylhydrazyl radical scavenging activity of methanol and supercritical carbon dioxide extracts from leaves of Adinandra nitida. J. Food Biochem. 2008;32:431–442. doi: 10.1111/j.1745-4514.2008.00159.x. [DOI] [Google Scholar]
- 10.Zhang Y., Ning Z., Yang S., Wu H. Antioxidant properties and mechanism of action of dihydromyricetin from Ampelopsis grossedentata. Acta Pharm. Sin. 2003;38:241–244. [PubMed] [Google Scholar]
- 11.Proestos C., Boziaris I.S., Nychas G.J.E., Komaitis M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006;95:664–671. doi: 10.1016/j.foodchem.2005.01.049. [DOI] [PubMed] [Google Scholar]



