Abstract
Background
Clear cell renal cell carcinoma (ccRCC) is usually incurable once it progresses to metastatic stage. Hence, in-depth investigations to reveal the precise molecular mechanisms behind the metastasis of ccRCC are required to improve the therapeutic outcome of ccRCC.
Material/Methods
The level of astrocyte elevated gene 1 (AEG-1) in ccRCC tissues and cell lines was determined by quantitative real-time PCR (qRT-PCR) assay. The MTS, colony formation, wound-healing, and Transwell invasion assays were used to assess the role of AEG-1 in ccRCC cells growth, migration, and invasion in vitro, respectively. Xenograft model and lung metastasis models were constructed to analyze the functions of AEG-1 in ccRCC cells growth and metastasis in vivo.
Result
We found that AEG-1 was overexpressed in ccRCC and was associated with the progression of ccRCC. Knocked-down AEG-1 impaired the migration and invasion of ccRCC cells in vitro. Furthermore, under-expression of AEG-1 caused complete inhibition of ccRCC cells growth and metastasis in vivo. In contrast, overexpression of AEG-1 significantly increased the migration and invasion ability of ccRCC cells in vitro. Finally, we revealed that AEG-1 boosted the metastatic ability of ccRCC cells via regulating Notch homolog 1 (Notch1).
Conclusions
The AEG-1/Notch1 signaling axis plays a vital role in ccRCC cell growth and metastasis.
MeSH Keywords: Carcinoma, Renal Cell; Neoplasm Metastasis; Receptor, Notch1
Background
Clear cell renal cell carcinoma (ccRCC) is one of the most common and aggressive renal carcinomas worldwide [1,2]. Recently, great advances in functional genomics analysis has provided increased understanding of renal carcinoma tumorigenesis and development [2]. Nevertheless, the molecular pathogenesis of advanced ccRCC remains poorly investigated. Hence, development for more effective therapeutic strategy is urgently needed to combat ccRCC [3]. The aggressiveness of ccRCC is based on its high metastatic capacity, which is usually not efficiently targeted despite great advances in the treatment of metastatic ccRCC [4]. Metastasis is critical for the progression of ccRCC; it is one of the hallmarks of tumor progression and is the primary cause of poor over-all survival of ccRCC patients [5].
Astrocyte elevated gene1 (AEG-1) is cloned by rapid subtraction hybridization as a gene induced in primary human fetal astrocytes (PHFA) infected with HIV-1 or treated with tumor necrosis factor-α (TNF-α) [6]. Previous studies reveal that AEG-1 is overexpressed in breast carcinoma [7], melanoma [8], and glioma [9] compared to their normal counterparts. Overexpression of AEG-1 increases the anchorage-independent growth, migration, and invasion of HeLa cells and glioma cell lines [9]. Conversely, downregulation of AEG-1 remarkably suppresses the mobility and invasion of prostate cancer cells, glioma cells [10], and lung metastatic of breast cancer cells in vivo [7]. Inhibition of AEG-1 in prostate cancer cells inhibits protein kinase B (PKB) activation and increases the activity of forkhead box (FOXO) 3a [11]. Together, these results suggest that AEG-1 is a crucial gene regulating multiple signaling and biochemical pathways in cancer progression. Nevertheless, the level of AEG-1 in ccRCC and the underlying mechanism by which AEG-1 facilitates the metastasis of ccRCC cells have not yet been explored.
In this study we demonstrated that AEG-1 plays vital roles in growth and metastasis of ccRCC Caki-2 cells in vitro and in vivo. The functions of AEG-1 in the metastatic ability of ccRCC Caki-2 cells was revealed by knock-down and overexpression of AEG-1. Finally, we found that AEG-1 promoted ccRCC cells migration and invasion by regulating Notch1 expression. In conclusion, our results reveal the critical roles of AEG-1/Notch1 in the growth and metastasis of ccRCC.
Material and Methods
Cells cultures and tissue microarray
Three ccRCC cell lines (Caki-2, 786-O, and Caki-1) and an immortalized proximal tubule epithelial cell line (HK-2) were from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Caki-2, 786-O, and Caki-1 cells were cultured using DMEM supplemented with FBS (10%), 100 μg/ml streptomycin, and 100 μg/ml penicillin. HK-2 cells were cultured in 1640 supplemented with FBS (10%), 100 μg/ml streptomycin, and 100 μg/ml penicillin. The inhibitor of Notch, FLI-06, was purchased from Selleck (Houston, TX, USA). Fifty cases of human ccRCC tissues and paired surrounding tissues were histopathologically and clinically diagnosed at the First People’s Hospital of Jining City from 2002 to 20016. Prior patient consent and approval from the Institutional Research Ethics Committee were obtained. Tissues were probed using human AEG-1 antibody.
Oncomine analysis
Oncomine (https://www.oncomine.org/resource/login.html) is a powerful platform that contains cancer microarray data for bioinformatics analysis. The expression level of AEG-1 gene in ccRCC was analyzed using Oncomine [12]. For this, we compared clinical specimens of ccRCC versus normal tissues. To reduce our false discovery rate, we selected p<0.01 and fold change >1 as thresholds. We analyzed the results for their p-values and fold change. Oncomine analysis of neoplastic vs. normal tissue showed that AEG-1 was significantly overexpressed in the Jones Renal ccRCC dataset [13] and Gumz Renal ccRCC dataset [14].
Cells proliferation and colony formation assay
Cell proliferation was detected by MTS assay (Promega, Madison, WI, USA). First, Caki-2 cells were cultured into 96-well plates. After incubation for 1 day, 2 days, 3 days, or 4 days, 20 μl of MTS solution was added into 96-well plates and the cells were incubated for 4 h. Finally, the absorbance value was assessed at 490 nm. In colony formation analysis, cells (1000) were seeded into 6-well plates. After being cultured for a total of 3 weeks, cell colonies were stained using crystal violet (0.1%) and counted [15].
Plasmids and transfections
Short hairpin small interfering RNA (shRNA) specifically targeting AEG-1 was purchased from Santa Cruz (Santa Cruz, CA, USA). AEG-1 expression construct was produced by sub-cloning PCR-amplified full-length human AEG-1 cDNA into pMSCV retrovirus plasmid. The pCLEN-Notch1 plasmid (#17704, Addgene, Cambridge, MA, USA) was deposited by Dr. Nicholas Gaiano. Transfection of shRNA or plasmid was conducted using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA).
Immunoblotting
Total proteins were extracted using lysis buffer. We resolved 25 μg proteins by 8% SDS-PAGE and transferred them to a PVDF membrane (Millipore, USA). After blocking with blocking buffer, PVDF membranes were incubated with primary antibodies. After washing with TBST, PVDF membranes were incubated with horseradish peroxidase (HRP) secondary antibody. Signals were assessed using the ECL system (Millipore, Braunschweig, Germany).
Wound-healing and invasion assay
Cells were cultured in 6-well plates to form a confluent monolayer. A wound was scratched using a 100-μl pipette tip. The gap was photographed at 0 h and 24 h [16]. The invasion of Caki-2 cells was detected using a BioCoat™ Matrigel-coated Invasion Chamber (8.0-μm membrane, BD Biosciences, USA). We placed 1×105 Caki-2 cells into the upper chamber and 600 μl DMEM containing 25% serum was added to the lower chamber as a chemo-attractant. After 6 h, the invaded Caki-2 cells in the lower surface of the membrane were stained with crystal violet (0.1%) and were counted in 5 randomly selected fields [17].
Immunofluorescence
Cells on a glass coverslip were permeabilized using Triton X-100 and then incubated with 1% BSA in PBS to block non-specific binding. Then, Caki-2 cells were incubated with rabbit anti-AEG-1 antibody. The cells were washed with PBS 3 times and then were incubated with goat anti-rabbit FITC secondary antibody (1: 100, Boster Biological Technology, Wuhan, China). Cell nuclei were stained using DAPI (Boster Biological Technology).
Experimental pulmonary metastasis model
The BALB/c nude mice were bought from Shanghai Slack Laboratory Animal Co., LTD (Shanghai, China). The parental Caki-2 cells, AEG-1 OE, or Caki-2 transfected with AEG-1 shRNA plasmids were injected into nude mice via the tail vein. All nude mice were sacrificed after 4 weeks and lung tissue was fixed using 10% formalin and subjected to hematoxylin and eosin (H&E) staining.
Quantitative real-time PCR (qRT-PCR)
RNA was extracted using the RNEasy kit (Qiagen). We performed qRT-PCR using 1 μg RNA with the QuantiTect Reverse Transcription kit (Qiagen). The primers were as follows: GAPDH: Forward: 5′-TGGATTTGGACGCATTGGTC-3′, Reverse: 5′-TTTGCACTGGTACGTGTTGAT-3′; AEG-1: Forward: 5′-AAATGGG CGGACTGTTGAAGT-3′, Reverse: 5′-CTGTTTTGCACTGCTTTAGCAT-3′; Notch1: Forward: 5′-CCCTTGCTCTGCCTAACGC-3′, Reverse: 5′-GGAGTCCTGGCATCGTTGG-3′. The comparative cycle threshold (Ct) method was used to quantify the levels calculated using the 2(−ΔΔCt) method.
Xenografts
The nude mice were assigned to the following 2 groups: AEG-1 shRNA and shCon (control group). Then, 100 μl of Caki-2 (AEG-1shRNA/control-shRNA) cell suspension containing 1×106 cells was subcutaneously inoculated into nude mice. The tumor sizes were measured once a week. Five weeks later, the mice were sacrificed and the tumors were removed for further IHC staining. Experimental protocols involving animals were approved by the Institutional Animal Care and Use Committee of the First People’s Hospital of Jining City in Shandong Province.
Statistical analysis
The data are presented as mean ± standard deviation (SD). Differences were analyzed using either a 2-tailed t test or 1-way ANOVA followed by post hoc Dunnett’s test. P < 0.05 was considered to be statistically significant.
Results
AEG-1 is overexpressed in ccRCC and positively correlates with its progression
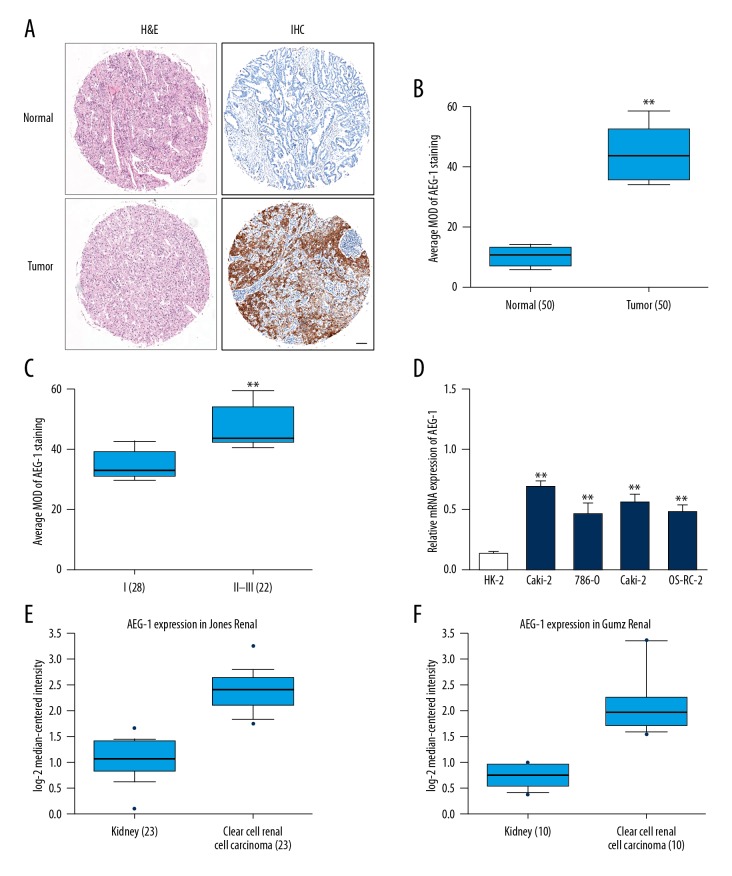
To determine the level of AEG-1 in ccRCC, the expression of AEG-1 was analyzed in ccRCC tissues and adjacent non-cancer tissues. Representative hematoxylin and eosin (H&E) staining of ccRCC tissues and representative results of immunohistochemistry (IHC) for AEG-1 in ccRCC tissues is shown (Figure 1A). As shown in Figure 1B, the level of AEG-1 was distinctly higher in ccRCC tissues than that in the surrounding normal tissues. Further analysis showed that AEG-1 was remarkably higher in ccRCC tissues at the late American Joint Committee on Cancer (AJCC) stages than that in the AJCC early stage (Figure 1C). To further test AEG-1 overexpression in kidney cancer, we utilized a panel of 4 ccRCC cell lines (Caki-2, 786-O, Caki-1, and OS-RC-2) and HK-2 cells (an immortalized proximal tubule epithelial cell line) to examine relative AEG-1 mRNA expression by qRT-PCR. A shown in Figure 1D, AEG-1 was overexpressed in ccRCC cell lines. Consistently, the mRNA level of AEG-1 was significantly increased in ccRCC compared to corresponding normal kidney tissues in the Jones Renal ccRCC dataset [13] and Gumz Renal ccRCC dataset [14] (Figure 1E, 1F). These results suggest that AEG-1 was aberrantly overexpressed in ccRCC and positively correlated with kidney cancer progression.
Figure 1.
Aberrant overexpression of AEG-1 in ccRCC. (A) Representative image of H&E staining of ccRCC tissues. Representative images of IHC staining with AEG-1 in ccRCC tissues. Scale bars: 100 μm. (B) The average of mean optical density (MOD) of AEG-1 staining in the carcinoma tissues (n=50) and adjacent tissues (n=50). ** P<0.01 compared with normal tissues. (C) AEG-1 IHC staining scores in the cancer tissues (n=50) in different AJCC stages. AJCC stages: I–III. ** P<0.01 compared with stage I. (D) The mRNA level of AEG-1 in ccRCC cell lines and an immortalized proximal tubule epithelial cell line, HK-2 was determined by qRT-PCR. The relative AEG-1 mRNA expression level was normalized to U6. ** P<0.01 compared with HK-2 cells. (E, F) Box plots show increased levels of AEG-1 in ccRCC (right) compared with normal kidney tissues (left).
Endogenous AEG-1 regulates ccRCC cells metastasis
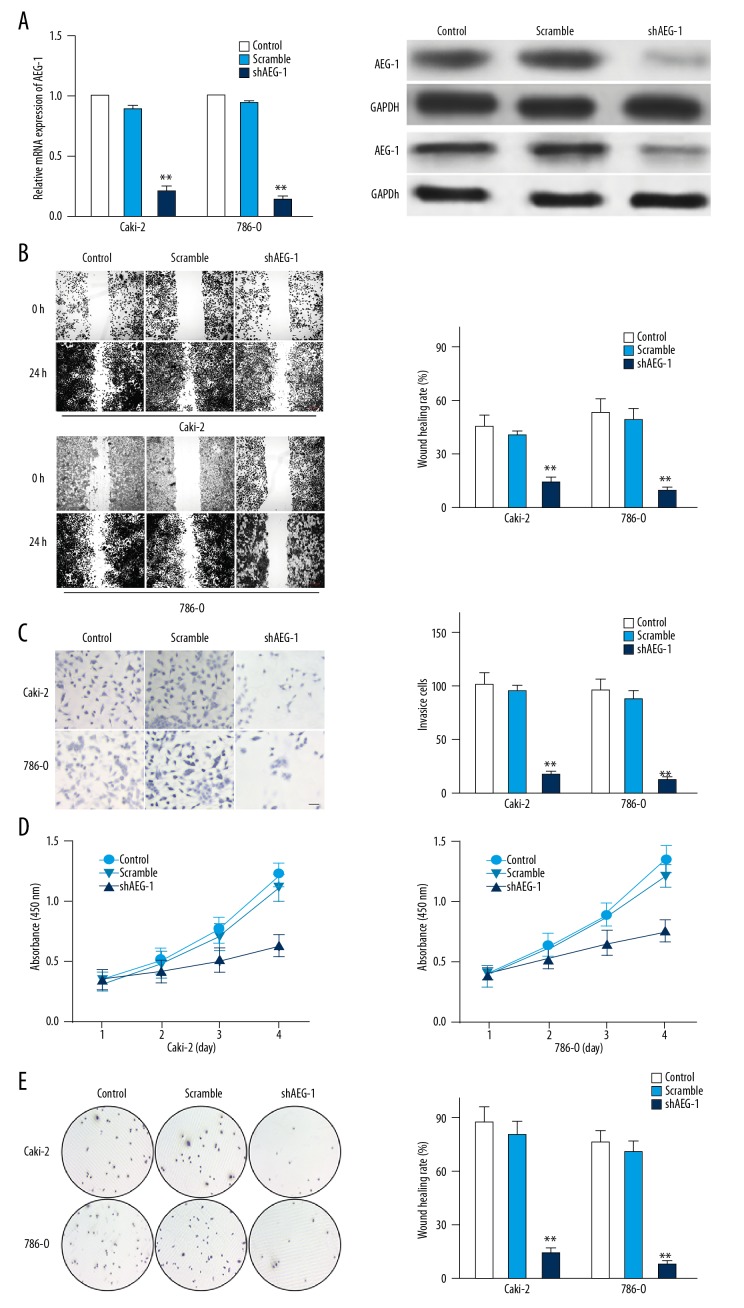
To future confirm that AEG-1 is responsible for the metastasis of the ccRCC cells, AEG-1 shRNA was transfected into Caki-2 or 786-O cells to establish AEG-1-under-expressing cells (Figure 2A). We next explored whether the metastasis of Caki-2 or 786-O cells could be inhibited by shAEG-1 transfection. In the wound-healing analysis, shAEG-1 Caki-2 or 786-O cells exhibited decreased migration ability compared to parental cells (Figure 2B). Consistently, the invasion of Caki-2-shAEG-1 or 786-O-shAEG-1 cells was also remarkably inhibited (Figure 2C). To examine whether AEG-1 knock-down regulated Caki-2 or 786-O cells proliferation in vitro, we investigated the effect of AEG-1 knocked-down on the growth and colony formation ability of Caki-2 or 786-O cells. Both assays suggested the growth (Figure 2D) as well as colony formation (Figure 2E) of AEG-1 knocked-down ccRCC cells were markedly reduced compared the control cells. Under-expression of AEG-1 specificity inhibited the growth and invasion capability of ccRCC cells in vitro.
Figure 2.
The effect of shAEG-1 on ccRCC cells metastasis. (A) The level of AEG-1 in Caki-2 or 786-O cells and cells transfected with shAEG-1 was detected by qPCR and immunoblotting. (B) Wound-healing assay was used to determine the migration of cells. Scale bar represents 200 μm. (C) Caki-2 or 786-O cells were transfected with shAEG-1 and were subjected to Transwell invasion assay. Scale bar represents 100 μm. (D) MTS assay was applied to analyze cell proliferation. (E) Mean number of tumor colonies formed by AEG-1-shRNA-transfected cells and control ccRCC cells. ** P<0.01 compared with control cells.
Endogenous AEG-1 promotes ccRCC cell metastasis
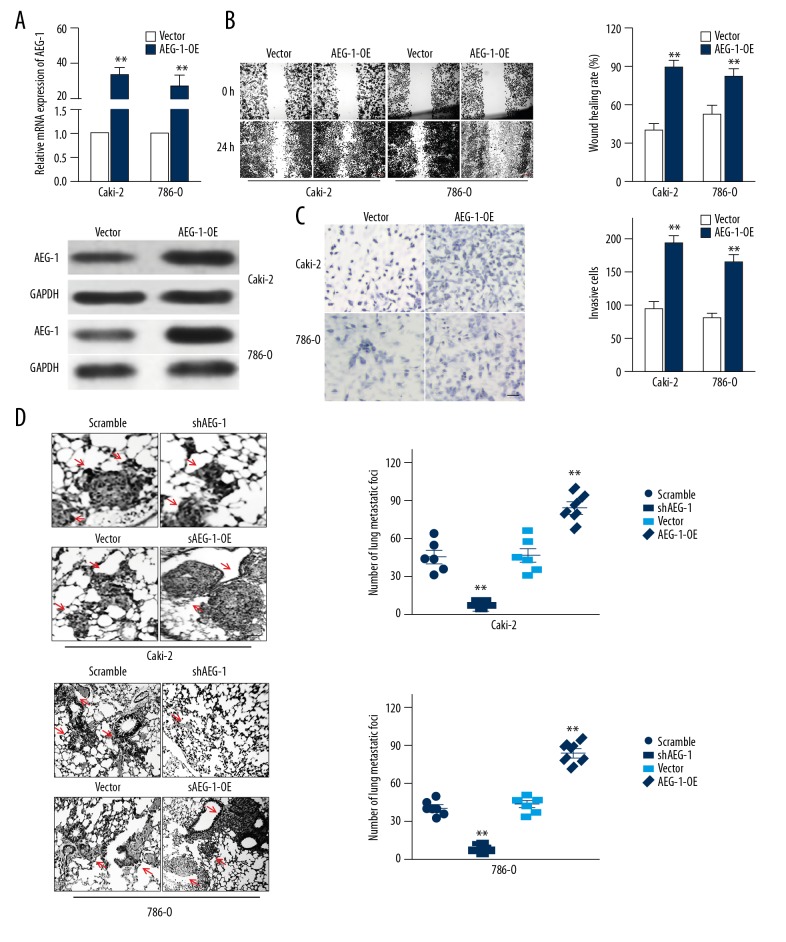
To further verify the roles of AEG-1 in ccRCC cells metastasis, Caki-2 or 786-O cells that stably overexpressed AEG-1 (AEG-1-OE) were established (Figure 3A). Ectopic expression of AEG-1 dramatically increased the metastasis of ccRCC cells as evidenced by accelerated migration and invasion (Figure 3B, 3C). Finally, to explore whether AEG-1 regulated ccRCC cell metastasis in vivo, the control cells (AEG-1-OE) or shAEG-1 ccRCC cells were injected into the tail vein. As shown in Figure 3D, the metastatic foci formed by AEG-1-OE Caki-2 or AEG-1-OE 786-O cells were remarkably increased; conversely, AEG-1 knocked-down cells resulted in less lung metastasis lesions. These results proved the crucial role of AEG-1 in ccRCC cells metastasis.
Figure 3.
Confirmation the role of AEG-1 in ccRCC metastasis. (A) The levels of AEG-1 in vector-control transfected cells or AEG-1-overexpressing ccRCC cells were examined by qRT-PCR and Western blotting. (B) The cells migration was determined using wound-healing assay. Scale bar represents 200 μm. (C) Cell invasion was analyzed using Transwell invasion assay. Scale bar represents 100 μm. (D) Representative images of lung from nude mice after 4 weeks of injection with AEG-1-overexpressing ccRCC cells or shAEG-1 ccRCC cells. Numbers of lung metastasis lesions were quantified. ** P<0.01, compared to control.
AEG-1 induces ccRCC cells metastasis via regulating Notch1
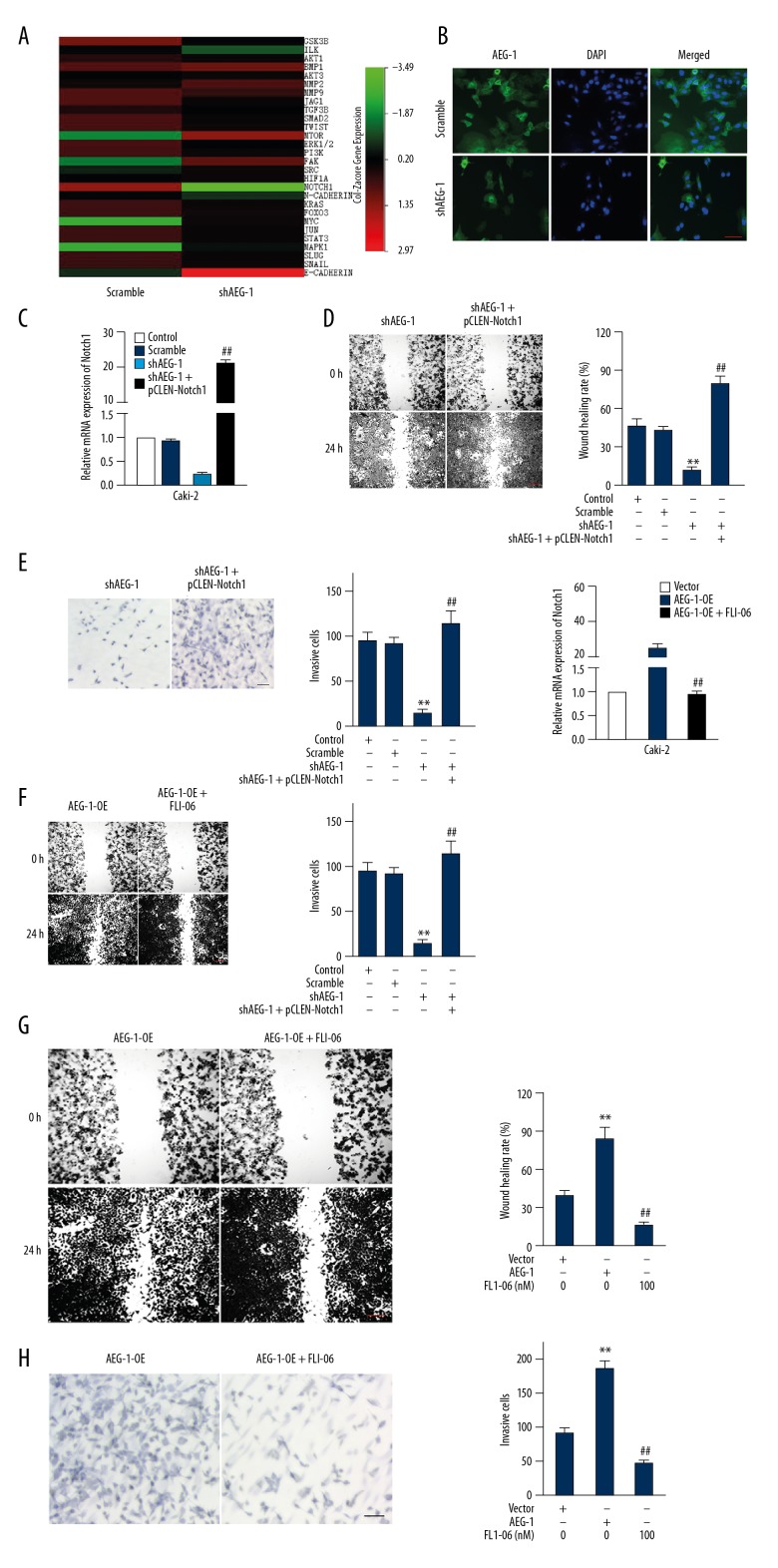
To further reveal the mechanisms by which AEG-1-regulated ccRCC cells metastasize, we performed qRT-PCR analysis in the scramble cells and AEG-1 under-expression Caki-2 cells. Twenty-eight cancer metastasis-associated genes were analyzed and we found the most downregulated gene in AEG-1-depleted Caki-2 cells was Notch1 (Figure 4A). Importantly, we confirmed that AEG-1 knock-down inhibited the protein level of Notch1 in Caki-2 cells (Figure 4B). To further explore the function of Notch1 in AEG-1-induced metastasis of ccRCC cells, pCLEN-Notch1 (a constitutively active form of Notch1) was transfected into AEG-1-silenced Caki-2 cells (Figure 4C). The migration and invasion of Caki-2 cells was analyzed using the wound-healing and Transwell assays, respectively. As expected, pCLEN-Notch1 transfection rescued the impaired migration and invasion ability in the AEG-1-silenced Caki-2 cells (Figure 4D, 4E). Furthermore, the Notch1 inhibitor (FLI-06) was also applied to assess the function of Notch1 in the migration and invasion of ccRCC cells [18]. As shown in Figure 4F, the expression of Notch1 in AEG-1-overexpressing Caki-2 cells was decreased by FLI-06. Consistently, the migration and invasion activity of Caki-2 cells promoted by AEG-1 overexpression was also suppressed by FLI-06 (Figure 4G, 4H). Altogether, these results suggest that AEG-1/Notch1 signaling plays a vital role in the metastasis of ccRCC cells.
Figure 4.
AEG-1 increases the metastasis of ccRCC cells via regulating Ntoch1. (A) AEG-1 knock-down altered the expression of pro-metastasis factors in shAEG-1-transfected Caki-2 cells compared to control cells. Gene expression analysis was performed using qRT-PCR. (B) The levels of Notch1 in Caki-2 cells were detected by immunofluorescence. (C) The level of Notch1 was assessed by qRT-PCR. (D) Wound-healing assay was used to analyze the migration of cells that transfected shAEG-1 alone or shAEG-1 combination with pCLEN-Notch1 plasmid. Scale bar represents 200 μm. (E) Transwell invasion was used to identify the invasion of cells. ** P<0.01 compared to control cells, ## P<0.01 compared to cells transfected with shAEG-1 alone. Scale bar represents 100 μm. (F) After being treated with FLI-06, the level of Notch1 was analyzed by qRT-PCR assay. (G) Wound-healing assay was used to evaluate the migration of AEG-1 overexpressing cells that were treated with FLI-06. Scale bar represents 200 μm. (H) Transwell invasion assay was used to evaluate the invasion ability of AEG-1-overexpressing cells treated with FLI-06. Scale bar represents 100 μm. ** P<0.01 compared to cells transfected with vector, ## P<0.01 compared to cells transfected with AEG-1.
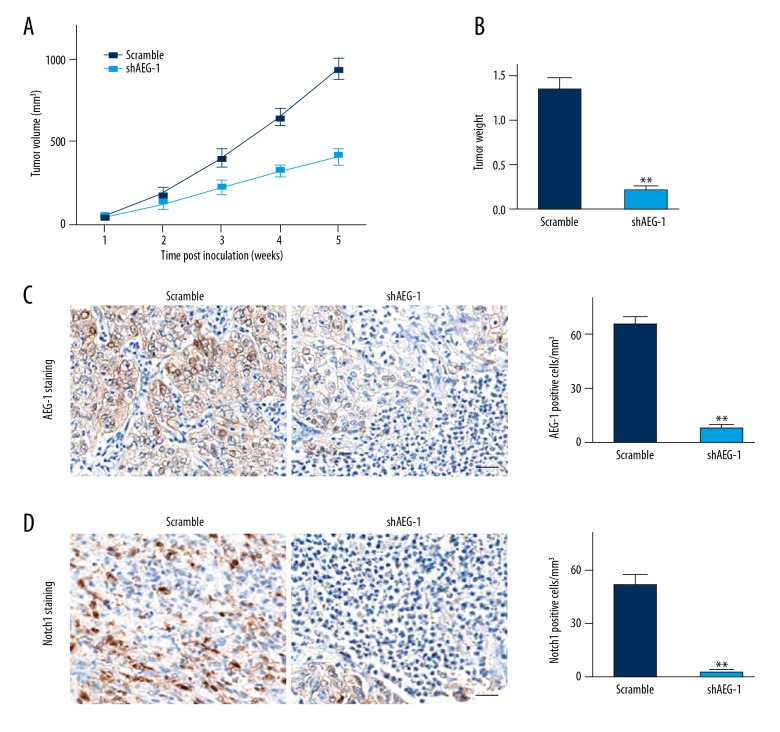
Oncogenic activity of AEG-1 in the xenografted model
To finally explore the impact of AEG-1 on ccRCC cells growth in vivo, an athymic nude mouse xenografted model was examined. Photographs of the tumors at necropsy and the measured volumes of the tumors indicated that AEG-1-depleated Caki-2 cells grew much more slowly than control cells (Figure 5A). The weights of the tumors from these mice were remarkably lower than those from the control mice (Figure 5B). We performed IHC to further assess the expression of AEG-1 and Notch1 in tumor tissues. As shown in Figure 5C, 5D, the expression of AEG-1 and Notch1 in the AEG-1 shRNA group was significantly decreased compared with that in the control group. Collectively, these results suggested that AEG-1 acted as a tumor promoter in ccRCC and that depletion of AEG-1 dramatically attenuated ccRCC oncogenic properties in vivo.
Figure 5.
Tumorigenicity of AEG-1 in vivo. (A) Following subcutaneous injections of Caki-2 cells in athymic nude mice and tumor growth for 35 days, photographs of the tumors were obtained at necropsy. The volumes of the generated tumors were measured once a week. (B) Mice were killed 35 days after the subcutaneous injection. Scatter plot analysis of the mouse tumor weights. (C, D) IHC staining of AEG-1 and Notch1 in the tumor mass from each group. ** P<0.01 compared to scramble.
Discussion
Clear cell renal cell carcinoma (ccRCC) is a malignant cancer with high mortality owing to its high metastatic potential [14,19]. Hence, there is urgent need to explore the underlying mechanisms of metastasis in ccRCC [20]. In the present study, we revealed that AEG-1 promoted the metastasis of ccRCC cells via regulating Notch1. These results suggest that AEG-1 can serve as a potential target for inhibiting ccRCC metastasis.
AEG-1 is overexpressed in various cancers and is involved into tumor metastasis [21]. In papillary thyroid cancer, AEG-1 is closely related to the progression and metastasis of disease [22]. Knock-down of AEG-1 reduces the abilities of migration and invasion through downregulation of MMP-2/9 in thyroid cancer cells. The mRNA level of AEG-1 is markedly higher in lung cancer cells than that that in the normal bronchial epithelial cell line and the higher mRNA level of AEG-1 is correlated with clinical stages and lymph node metastasis of NSCLC [23]. In addition, overexpression of AEG-1 is associated with metastasis in patients with gastric cancer, and AEG-1 promotes gastric cancer cells metastasis via upregulation of eIF4E-mediated Twist and MMP-9 [24]. Altogether, these findings prove the vital roles of AEG-1 in tumorigenesis. Nevertheless, the functions of AEG-1 in ccRCC metastasis have not yet been explored. In this research, we revealed the precise role of AEG-1 in ccRCC metastasis. We found that the migration and invasion of Caki-2 cells was remarkably suppressed when the AEG-1 gene was knocked-down by shRNA, as demonstrated by wound-healing analysis and Transwell assay. In contrast, overexpression of AEG-1 greatly increased cells mobility in vitro and the metastasis of ccRCC cells in vivo. Consistently, under-expression of AEG-1 suppressed the pulmonary metastasis of ccRCC Caki-2 cells in vivo.
In vertebrates there are 4 Notch genes (Notch1, Notch2, Notch3, and Notch4), which encode receptors for at least 5 different DSL Notch ligands, including Jagged1, Jagged2, Delta1, Delta3, and Delta4 [25]. In cancer progression, Notch acts as either an oncogene or tumor suppressor gene depending on the cancer type [26]. A previous study demonstrated that overexpression of Notch signaling increases the metastasis of T1 stage ccRCC through stimulating the growth and migration of tumor cells [27]. In our study, the molecular mechanism of AEG-1-regulated ccRCC cells metastasis was proved to be associated with Ntoch1. This study demonstrates that AEG-1 knocked-down decreased the expression of Notch1 in ccRCC Caki-2 cells. Furthermore, restoration of Notch1 in AEG-1 knocked-down Caki-2 via transfection with an active form of Notch1 rescued the decreased migration and invasion of Caki-2 cells caused by AEG-1 silencing. Additional, in the presence of the Notch inhibitor, FLI-06, the migration and invasion of Caki-2 cells promoted by AEG-1 overexpression were markedly inhibited, which suggests the vital role of Notch1 in AEG-1-induced ccRCC cell metastasis.
Conclusions
To identify novel targets for inhibiting ccRCC metastasis, it is necessary to identify effective biomarkers and key genes that mediate the metastasis of ccRCC. Our study revealed the crucial roles of AEG-1 in the metastasis of ccRCC cells, which suggests AEG-1 as a potential target for treating ccRCC metastasis.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Jingushi K, Kashiwagi Y, Ueda Y, et al. High miR-122 expression promotes malignant phenotypes in ccRCC by targeting occludin. Int J Oncol. 2017;51:289–97. doi: 10.3892/ijo.2017.4016. [DOI] [PubMed] [Google Scholar]
- 2.He L, Zhao X, Wang H, et al. RUNX3 mediates suppression of tumor growth and metastasis of human CCRCC by regulating cyclin related proteins and TIMP-1. PLoS One. 2012;7:e32961. doi: 10.1371/journal.pone.0032961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xie W, Wang L, Sheng H, et al. Metformin induces growth inhibition and cell cycle arrest by upregulating MicroRNA34a in renal cancer cells. Med Sci Monit. 2017;23:29–37. doi: 10.12659/MSM.898710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroeger N, Seligson DB, Signoretti S, et al. Poor prognosis and advanced clinicopathological features of clear cell renal cell carcinoma (ccRCC) are associated with cytoplasmic subcellular localisation of Hypoxia inducible factor-2alpha. Eur J Cancer. 2014;50:1531–40. doi: 10.1016/j.ejca.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira RC, Ivanovic RF, Leite KRM, et al. Expression of micro-RNAs and genes related to angiogenesis in ccRCC and associations with tumor characteristics. BMC Urol. 2017;17:113. doi: 10.1186/s12894-017-0306-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng S, Yao J, Zhang Z, et al. miR96 inhibits EMT by targeting AEG1 in glioblastoma cancer cells. Mol Med Rep. 2018;17:2964–72. doi: 10.3892/mmr.2017.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M, Dai Y, Wang L, et al. Astrocyte elevated gene-1 promotes the proliferation and invasion of breast cancer cells by activating the Wnt/beta-catenin signaling pathway. Oncol Lett. 2017;13:2385–90. doi: 10.3892/ol.2017.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Long J, Menggen Q, Wuren Q, et al. Long noncoding RNA taurine-upregulated Gene1 (TUG1) promotes tumor growth and metastasis through TUG1/Mir-129-5p/astrocyte-elevated Gene-1 (AEG-1) axis in malignant melanoma. Med Sci Monit. 2018;24:1547–59. doi: 10.12659/MSM.906616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SY, Choi M, Park D, et al. AEG-1 promotes mesenchymal transition through the activation of Rho GTPases in human glioblastoma cells. Oncol Rep. 2016;36:2641–46. doi: 10.3892/or.2016.5106. [DOI] [PubMed] [Google Scholar]
- 10.Hu G, Wei Y, Kang Y. The multifaceted role of MTDH/AEG-1 in cancer progression. Clin Cancer Res. 2009;15:5615–20. doi: 10.1158/1078-0432.CCR-09-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wan L, Hu G, Wei Y, et al. Genetic ablation of metadherin inhibits autochthonous prostate cancer progression and metastasis. Cancer Res. 2014;74:5336–47. doi: 10.1158/0008-5472.CAN-14-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rhodes DR, Kalyana-Sundaram S, Mahavisno V, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–80. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones J, Otu H, Spentzos D, et al. Gene signatures of progression and metastasis in renal cell cancer. Clin Cancer Res. 2005;11:5730–39. doi: 10.1158/1078-0432.CCR-04-2225. [DOI] [PubMed] [Google Scholar]
- 14.Gumz ML, Zou H, Kreinest PA, et al. Secreted frizzled-related protein 1 loss contributes to tumor phenotype of clear cell renal cell carcinoma. Clin Cancer Res. 2007;13:4740–49. doi: 10.1158/1078-0432.CCR-07-0143. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Bo L, Lu W, et al. MicroRNA-148b targets Rho-associated protein kinase 1 to inhibit cell proliferation, migration and invasion in hepatocellular carcinoma. Mol Med Rep. 2016;13:477–82. doi: 10.3892/mmr.2015.4500. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Ren H, Jiang J, et al. KIAA0247 inhibits growth, migration, invasion of non-small-cell lung cancer through regulating the Notch pathway. Cancer Sci. 2018;109:1055–65. doi: 10.1111/cas.13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qu F, Cui X, Hong Y, et al. MicroRNA-185 suppresses proliferation, invasion, migration, and tumorigenicity of human prostate cancer cells through targeting androgen receptor. Mol Cell Biochem. 2013;377:121–30. doi: 10.1007/s11010-013-1576-z. [DOI] [PubMed] [Google Scholar]
- 18.Kramer A, Mentrup T, Kleizen B, et al. Small molecules intercept Notch signaling and the early secretory pathway. Nat Chem Biol. 2013;9:731–38. doi: 10.1038/nchembio.1356. [DOI] [PubMed] [Google Scholar]
- 19.Lin YL, Wang YP, Li HZ, et al. Aberrant promoter methylation of PCDH17 (Protocadherin 17) in serum and its clinical significance in renal cell carcinoma. Med Sci Monit. 2017;23:3318–23. doi: 10.12659/MSM.902077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.HIF2alpha antagonism has antitumor activity in advanced ccRCC. Cancer Discov. 2018;8:OF6. doi: 10.1158/2159-8290.CD-RW2018-007. [DOI] [PubMed] [Google Scholar]
- 21.Emdad L, Lee SG, Su ZZ, et al. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc Natl Acad Sci USA. 2009;106:21300–5. doi: 10.1073/pnas.0910936106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Wu J, Ying Z, et al. Astrocyte elevated gene-1 upregulates matrix metalloproteinase-9 and induces human glioma invasion. Cancer Res. 2010;70:3750–59. doi: 10.1158/0008-5472.CAN-09-3838. [DOI] [PubMed] [Google Scholar]
- 23.Zhu R, Tian Y. Astrocyte elevated gene-1 increases invasiveness of NSCLC through up-regulating MMP7. Cell Physiol Biochem. 2015;37:1187–95. doi: 10.1159/000430242. [DOI] [PubMed] [Google Scholar]
- 24.Jian-bo X, Hui W, Yu-long H, et al. Astrocyte-elevated gene-1 overexpression is associated with poor prognosis in gastric cancer. Med Oncol. 2011;28:455–62. doi: 10.1007/s12032-010-9475-6. [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Chen T, Zhang J, et al. Notch1 promotes glioma cell migration and invasion by stimulating beta-catenin and NF-kappaB signaling via AKT activation. Cancer Sci. 2012;103:181–90. doi: 10.1111/j.1349-7006.2011.02154.x. [DOI] [PubMed] [Google Scholar]
- 26.Sun Z, Zhou C, Liu F, et al. Inhibition of breast cancer cell survival by Xanthohumol via modulation of the Notch signaling pathway in vivo and in vitro. Oncol Lett. 2018;15:908–16. doi: 10.3892/ol.2017.7434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braune EB, Lendahl U. Notch – a goldilocks signaling pathway in disease and cancer therapy. Discov Med. 2016;21:189–96. [PubMed] [Google Scholar]