Abstract
The genus Aristolochia, an important source of physiologically active compounds that belong to different chemical classes, is the subject of research in numerous pharmacological and chemical studies. This genus contains a large number of terpenoid compounds, particularly diterpenes. This work presents a compilation of the 13C-NMR data of 57 diterpenoids described between 1981 and 2007 which were isolated from Aristolochia species. The compounds are arranged skeletonwise in each section, according to their structures, i.e., clerodane, labdane, and kaurane derivatives. A brief discussion on the 13C chemical shifts of these diterpenes is also included.
Keywords: Aristolochia, Aristolochiaceae, Clerodanes, Furanoditerpenes, Kauranes, Labdanes, 13C-NMR data.
Introduction
The genus Aristolochia (Aristolochiaceae) consists of about 500 species mostly distributed along tropical, subtropical, and Mediterranean regions of the world [1,2,3]. The Aristolochia species are cultivated as ornamentals [4] and popularly used as sources of abortifacient, emmenagogue [5,6], sedative [7], analgesic, anticancer, anti-inflammatory, antifeedant [8], muscle relaxant [9], antihistaminic, and antiallergic [10] drugs, for intestinal worms, in the treatment of cholera, stomach ache, abdominal pain, rheumatism [11], malaria [12], wounds and skin diseases [13], and also useful in treatment of different types of poisonous bites and stings [14,15]. Several other biological properties have been described [16]. On the other hand, consumption of many plants of the genus can lead to progressive nephrophathy and urothelial cancer in humans [17,18]. As a consequence, the distribution of herbal medicines containing Aristolochia extracts are prohibited in many countries due to their nephrotoxic, carcinogenic, and mutagenic properties [1].
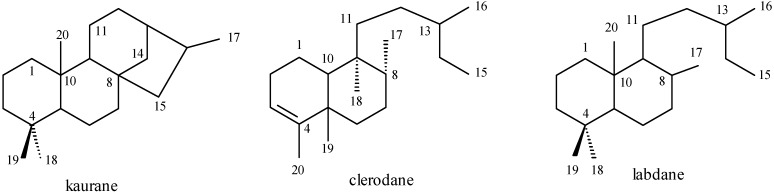
Aristolochic acids have been frequently found in Aristolochia species [19]. These compounds show toxic effects at the renal level and carcinogenic properties [20,21]. Phytochemical investigations of these species revealed both the presence of aporphinic, tetrahydroprotoberberinic, benzyltetra-hydroisoquinolinic, and bisbenzyltetrahydroisoquinolinic alkaloids [22] and other nitrogenated derivatives (phenantrenoids, aristolactams, and porphyrins) [23,24,25]. Quinones, coumarins, flavanoids, lignoids (phenylpropanoids, neolignans, and lignans), and fatty acids are frequently isolated from plants of the genus [26]. However, the most prominent compounds in Aristolochia are terpenoids, constituents of the essential oils isolated from the plant species. The majority of the identified terpenoids are kaurane, clerodane, and labdane diterpene derivatives (Figure 1).
Figure 1.
Diterpene classes present in Aristolochia species.
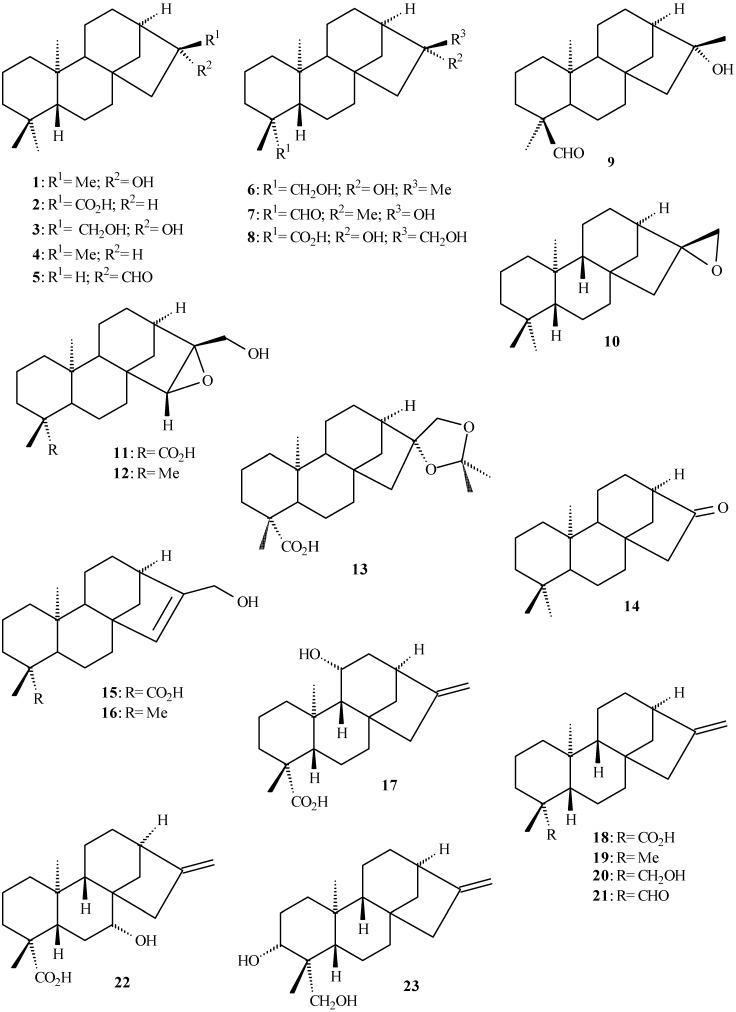
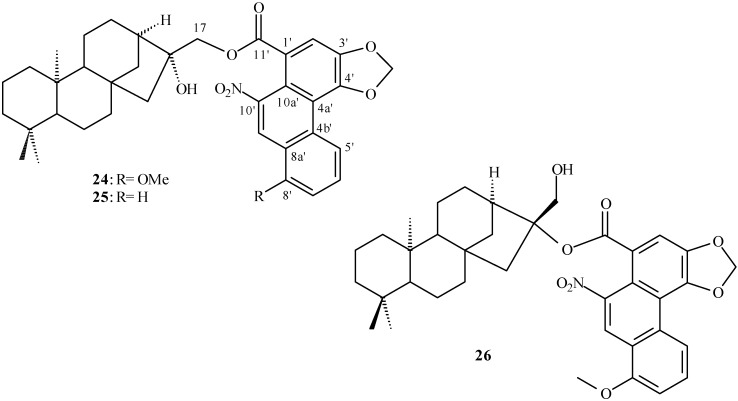
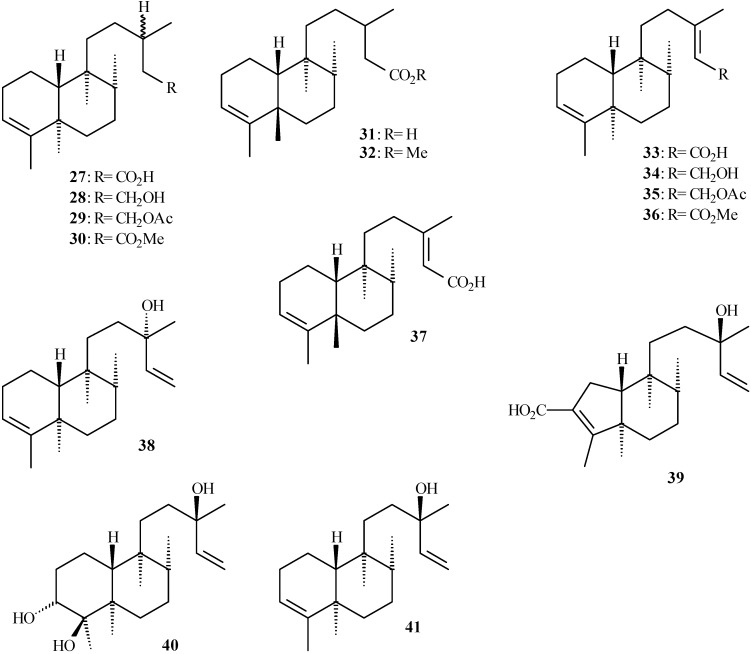
The review “Terpenoids of Aristolochia and their biological activities”, which covered the literature up to 2003, lists 52 diterpenoids isolated from the genus and their pharmacological properties [16]. In the present review a new comprehensive coverage of diterpenes isolated from Aristolochia species up to this moment (Table 1, Table 2 and Table 3) is described, broadly covering 26 kauranes (Figure 2 and Figure 3), 29 clerodanes (Figure 4 and Figure 5), one furanoditerpene derivative (Figure 6), and 9 labdanes (Figure 7). Moreover, the 13C-NMR data of these compounds are also compiled (Table 4 and Table 5). For some structures no 13C-NMR data was found in the investigated literature and there is disagreement concerning the 13C-NMR data of (–)-11-hydroxykaur-16-en-19-oic acid. Sometimes different structures were given the same names.
Table 1.
Kaurane diterpenoids isolated from Aristolochia species.
| Clerodane | Species |
|---|---|
| ent-Kauran-16β-ol [(–)-kauranol] (1) | A. rodriguesii [28] |
| ent-16β(H)-Kauran-17-oic acid (2) | A. elegans [7]; A. triangularis [13] |
| ent-Kauran-16β,17-diol (3) | A. elegans [7]; A. pubescens [31]; A. triangularis [13] |
| ent-16β(H)-Kaurane (4) | A. elegans [7]; A. triangularis [13] |
| ent-16α(H)-Kauran-17-al (5) | A. elegans [7] |
| ent-Kauran-16β,19-diol [ent-16β,19-dihydroxykaurane] (6) | A. rodriguesii [28] |
|
ent-16α-Hydroxy-kauran-19-al [16a-hydroxy-(–)-kauran-19-al] (7) |
A. rodriguesii [28]; A. triangularis [32] |
| ent-16β,17-Dihydroxy-(–)-kauran-19-oic acid (8) | A. rodriguesii [28] |
|
ent-16β-Hydroxy-kauran-18-al [(–)-kauran-16α-hydroxy-18-al] (9) |
A. triangularis [26] |
| ent-16β,17-Epoxykaurane (10) | A. elegans [7]; A. triangularis [32] |
| ent-15β,16β-Epoxy-17-hydroxy-kauran-19-oic acid (11) | A. rodriguesii [28] |
| ent-15β,16β-Epoxykauran-17-ol (12) | A. triangularis [13] |
| ent-16β,17-Isopropylidenedioxy-(–)-19-oic acid (13) | A. rodriguesii [28] |
| 17-nor-(–)-Kauran-16-one (14) | A. triangularis [13] |
| ent-17-Hydroxy-kaur-15-en-19-oic acid (15) | A. rodriguesii [28] |
| ent-Kaur-15-en-17-ol (16) |
A. elegans [7]; A. pubescens [31]; A. triangularis [13,32] |
|
ent-11β-Hydroxy-kaur-16-en-19-oic acid [(–)-11-hydroxy-kaur-16-en-19-oic acid] (17) |
A. anguicida [33] |
| ent-Kaur-16-en-19-oic acid [kaurenic acid] (18) | A. anguicida [33]; A. rodriguesii [28]; A. triangularis [13] |
| (–)-ent-Kaur-16-ene (19) | A. triangularis [13,32] |
| (–)-ent-Kaur-16-en-19-ol (20) | A. triangularis [13,32] |
| (–)-ent-Kaur-16-en-19-al (21) | A. triangularis [13,32] |
| ent-7β-Hydroxy-kaur-16-en-19-oic acid (22) | A. anguicida [34] |
|
ent-Kaur-16-en-3β,19-diol [ent-3β,18-dihydroxykaur-16-ene] (23) |
A. rodriguesii [28] |
|
ent-16β-Hydroxy-17-kauranyl aristolachate I [aristoloin I] (24) |
A. elegans [4] |
|
ent-16β-Hydroxy-17-kauranyl aristolachate II [aristoloin II] (25) |
A. pubescens [31] |
| ent-17-Hydroxy-16β-kauranyl aristolachate I [aristolin] (26) | A. elegans [4] |
Usual names are given in brackets
Table 2.
Clerodane diterpenoids isolated from Aristolochia species.
| Clerodane | Species |
| (5R,8R,9S,10R)-ent-3-Cleroden-15-oic acid [13,14-dihydrokolavenic acid; populifolic acid] (27) |
A. brasilienses [38]; A. cymbifera [39]; A. galeata [40] |
| (5R,8R,9S,10R)-ent-3-Cleroden-15-ol [dihydrokolavenol] (28) | A. galeata [40] |
| (5R,8R,9S,10R)-ent-15-Ethanoyl-3-clerodene [dihydrokolavenol acetate] (29) | A. galeata [40] |
| Methyl (5R,8R,9S,10R)-ent-3-cleroden-15-oate [methyl populifoloate]) (30) | A. esperanzae [38]; A. galeata [40] |
| (5S,8R,9S,10R)-ent-3-Cleroden-15-oic acid [epi-populifolic acid] (31) | A. cymbifera [39] |
| Methyl (5S,8R,9S,10R)-ent-3-cleroden-15-oate (32) | A. cymbifera [39] |
| (5R,8R,9S,10R)-ent-Clerod-3,13-dien-15-oic acid [Δ13,14-kolavenic acid] (33) | A. brasilienses [38]; A. galeata [40] |
| (5R,8R,9S,10R)-ent-Clerod-3,13-dien-15-ol [Δ13,14-kolavenol] (34) | A. galeata [40] |
| (5R,8R,9S,10R)-ent-15-Ethanoyl-clerod-3,13-diene [acetyl kolavenoate] (35) | A. galeata [40] |
| Methyl (5R,8R,9S,10R)-ent-clerod-3,13-dien-15-oate [methyl kolavenoate] (36) |
A. esperanzae [38]; A. galeata [40] |
| (5S,8R,9S,10R)-ent-Clerod-3,13-dien-15-oic acid (37) | A. brasilienses [38] |
| (5R,8R,9S,10R)-ent-Clerod-3,14-dien-13β-ol [(+)-kolavelool] (38) | A. galeata [40] |
| (5R,8R,9S,10R)-(4→2)-abeo-Clerod-13β-hydroxy-2,14-dien-3-oic acid [(+)-(4®2)-abeo-kolavelool-3-oic acid] (39) |
A. chamissonis [41] |
| (5R,8R,9S,10R)-ent-Clerod-14-en-3β,4α,13α-triol [(–)-3α,4β-dihydroxykolavelool] (40) | A. chamissonis [41] |
| (5R,8R,9S,10R)-ent-Clerod-3,14-dien-13α-ol [(–)-kolavelool] (41) |
A. chamissonis [41]; A. cymbifera [38]; A. galeata [38] |
| (5R,8R,9S,10R)-ent-Clerod-3,14-dien-2α,13α-diol [(–)-2β-hydroxykolavelool] (42) |
A. chamissonis [41] |
| (5R,8R,9S,10R)-ent-Clerod-3,14-dien-2β,13α-diol [(+)-13-epi-2α-hydroxykolavelool; 13-epi-roseostachenol] (43) |
A. chamissonis [41] |
| (5S,8R,9S,10R)-2-Oxo-ent-3-cleroden-15-oic acid (44) | A. brasilienses [38] |
| (5R,8R,9S,10R)-2-Oxo-ent-3-cleroden-15-oic acid [2-oxopopulifolic acid] (45) |
A. brasilienses [39]; A. cymbifera [39]; A. galeata [40] |
| (5R,8R,9S,10R)-2-Oxo-ent-15-ethanoyl-3-clerodene [2-oxodihydrokolavenol acetate] (46) |
A. galeata [40] |
| Methyl (5R,8R,9S,10R)-2-oxo-ent-3-cleroden-15-oate [methyl 2-oxopopulifoloate] (47) |
A. esperanzae [38] |
| (5R,8R,9S,10R)-2-Oxo-ent-clerod-3,14-dien-13α-ol [(–)-13-epi-2-oxokolavelool; 13-epi-roseostachenone] (48) |
A. chamissonis [41] |
| Methyl (5S,8R,9S,10R)-2-oxo-ent-clerod-3,13-dien-15-oate (49) | A. brasilienses [38] |
| (5R,8R,9S,10R)-2-Oxo-ent-clerod 3,13-dien-15-oic acid [Δ13,14-2-oxokolavenic acid] (50) |
A. brasilienses [38] |
| Methyl (5R,8R,9S,10R)-2-oxo-ent-clerod-3,13-dien-15-oate [methyl Δ13,14-2-oxokolavenoate] (51) |
A. esperanzae [38] |
| (5S,8S,9R,10S)-2-Oxo-ent-clerod-3,13-dien-15-oic acid (52) | A. brasilienses [38] |
| Methyl (5R,8R,9S,10R)-ent-2α-hydroperoxy-3-cleroden-15-oate (53) | A. esperanzae [38] |
| (5R,8R,9S,10R)-ent-2α-Hydroperoxy-clerod-3,14-dien-13α-ol [(–)-2β-hydroperoxykolavelool] (54) |
A. chamissonis [41] |
| Methyl (5R,8R,9S,10R)-ent-2α-hydroperoxy-clerod-3,13-dien-15-oate (55) | A. esperanzae [38] |
Usual names are given in brackets
Table 3.
Labdane diterpenoids isolated from Aristolochia species.
| Clerodane | Species |
| (5R,8R,9S,10S)-ent-Labdan-8β-hydroxy-15-oic acid (57) | A. galeata [40] |
| (5R,8R,9S,10S)-ent-Labd-13-en-8β-hydroxy-15-oic acid [Δ13,14-ent-labd-8β-ol-15-oic acid] (58) |
A. galeata [40] |
| (5R,8R,9S,10S)-ent-Labd-14-en-8β-ol (60) | A. cymbifera [40] |
| (5S,8R,9R,10R)-ent-Labd-14-en-8β,13α-diol (61) | not isolated from Aristolochia species [54] |
| (5R,9S,10S)-ent-Labd-8(17)-en-15-oic acid (62) | A. ringens [55] |
| (5R,9S,10S)-ent-Labd-8(17),13-dien-15-oic acid [copalic acid] (63) | A. esperanzae [40]; A. galeata [40] |
| (5R,9S,10S)-ent-Labd-6β-hydroxy-8(17),13-dien-15-oic acid (64) | A. esperanzae [40] |
| Methyl (5R,8R,9S,10S)-ent-labd-8(17),13-dien-15-oate [methyl copalate] (65) | A. esperanzae [40] |
| Methyl (5R,9S,10S)-ent-labd-6β-hydroxy-8(17),13-dien-15-oate (66) | A. esperanzae [40] |
| (5R,10S)-ent-Labd-8,14-diene (67) | A. cymbifera [40] |
Usual names are given in brackets
Figure 2.
Kaurane diterpenoids isolated from Aristolochia species.
Figure 3.
Substituted kaurane diterpenoids isolated from Aristolochia species.
Figure 4.
Clerodane diterpenoids isolated from Aristolochia species.
Figure 5.
Clerodane diterpenoids isolated from Aristolochia species, showing oxygened C-2.
Figure 6.
Furanoditerpene isolated from Aristolochia species.
Figure 7.
Labdane diterpenoids isolated from Aristolochia species.
Table 4.
13C-NMR data (in CDCl3) of diterpenes from Aristolochia species.
| Carbon | Compound / δC (in ppm) | ||||||||||||||
| 1 [56] | 2 [13] | 3 [31] | 4 [13] | 6 [28] | 8 [57,58] | 10 [59] | 11 [28] | 12 [13] | 13 [28] | 14 [56] | 15 [28] | 16 [13] | 17 [33] | 18 [33] | |
| 1 | 42.0 | 39.2 | 40.3 | 40.9 | 40.35 | 41.1 | 40.4 | 39.82 | 40.4 | 40.66 | 41.0 | 40.73 | 42.0 | 39.10 | 41.13 |
| 2 | 18.6 | 18.2 | 18.6 | 18.6 | 17.97a | 19.8 | 18.6 | 18.39a | 18.7 | 19.06 | 18.5 | 19.05a | 18.6 | 20.07 | 19.52 |
| 3 | 42.0 | 42.0 | 41.9 | 42.0 | 35.55 | 38.7 | 42.0 | 34.33 | 42.1 | 38.39 | 41.9 | 38.03 | 43.8 | 35.00 | 38.23 |
| 4 | 33.2 | 33.1 | 33.2 | 33.6 | 38.50 | 43.9 | 33.2 | 48.47 | 33.3 | 43.66 | 33.2 | 43.55 | 33.2 | 43.60 | 44.66 |
| 5 | 56.2 | 56.1a | 56.2 | 56.1a | 56.90b | 57.0 | 56.2 | 56.68b | 55.9 | 56.96 | 56.1 | 56.65 | 55.8 | 49.80 | 57.49 |
| 6 | 20.4 | 20.6 | 20.4 | 20.7 | 20.49 | 23.0 | 20.2 | 20.10 | 19.3 | 22.00 | 19.2 | 20.70 | 19.2 | 19.51 | 22.28 |
| 7 | 40.3 | 40.3 | 42.0 | 40.4 | 42.31 | 42.8 | 41.1 | 42.02 | 32.5 | 41.52 | 40.3 | 39.24 | 39.2 | 40.84 | 41.71 |
| 8 | 45.3 | 45.3 | 44.7 | 45.1 | 45.17 | 45.0 | 45.4 | 45.20 | 43.4 | 44.51 | 42.5 | 48.81 | 48.8 | 47.63 | 44.17 |
| 9 | 56.8 | 56.0a | 56.7 | 56.0a | 56.70b | 56.3 | 55.9 | 55.20b | 50.8 | 55.40 | 55.0 | 47.57 | 48.3 | 47.89 | 55.55 |
| 10 | 39.3 | 38.0 | 39.4 | 39.2 | 39.15 | 40.1 | 39.3 | 39.44 | 39.2 | 39.64 | 39.4 | 39.72 | 39.4 | 40.00 | 40.09 |
| 11 | 18.0 | 18.5 | 18.3 | 18.3 | 17.93a | 19.0 | 19.3 | 18.13a | 18.2 | 19.06 | 18.5 | 18.75a | 18.6 | 77.52 | 18.43 |
| 12 | 26.9 | 31.2 | 26.3 | 31.3 | 26.06 | 26.8 | 29.2 | 26.90 | 27.0 | 27.06 | 29.7 | 25.31 | 25.6 | 33.97 | 33.53 |
| 13 | 49.0 | 44.7 | 45.5 | 41.4 | 48.64 | 45.9 | 42.7 | 48.93 | 36.0 | 45.64 | 47.9 | 40.92 | 41.1 | 44.41 | 44.28 |
| 14 | 37.7 | 40.8 | 37.3 | 38.1 | 37.36 | 37.8 | 38.6 | 37.81 | 36.0 | 37.88 | 37.5 | 43.71 | 40.4 | 39.79 | 40.13 |
| 15 | 58.0 | 45.0 | 53.4 | 44.7 | 57.66 | 53.9 | 48.9 | 57.80 | 65.7 | 55.54 | 55.2 | 135.39 | 135.7 | 47.41 | 49.39 |
| 16 | 79.4 | 55.9 | 81.9 | 45.9 | 79.16 | 81.7 | 66.4 | 79.29 | 69.5 | 89.16 | 222.5 | 145.82 | 145.6 | 155.24 | 156.32 |
| 17 | 24.5 | 182.5 | 66.4 | 14.8 | 24.05 | 66.5 | 50.4 | 24.47c | 59.9 | 70.05 | - | 60.63 | 61.1 | 104.09 | 103.69 |
| 18 | 33.5 | 33.5 | 33.5 | 33.2 | 26.89 | 29.3 | 33.6 | 24.26c | 33.6 | 28.93 | 33.6 | 28.87 | 35.5 | 30.08 | 29.38 |
| 19 | 21.6 | 21.5 | 21.5 | 21.6 | 64.98 | 180.1 | 21.6 | 205.80 | 21.6 | 182.64 | 21.7 | 180.90 | 21.5 | 184.05 | 184.00 |
| 20 | 18.0 | 17.3 | 17.8 | 17.4 | 18.11 | 16.0 | 17.8 | 16.40 | 17.5 | 15.74 | 18.0 | 15.25 | 17.6 | 15.93 | 16.02 |
| 1’ | 108.39 | ||||||||||||||
| 2’ | 26.81 | ||||||||||||||
| 3’ | 26.91 | ||||||||||||||
| Carbon | Compound / δC (in ppm) | ||||||||||||||
| 19 [56] | 20 [57](?) | 22 [34] | 22 [34] (P) | 22 [34](D) | 23 [60] | 27 [26] | 27 [34] | 29 [61] | 30 [38] | 30 [38] | 31 [39] | 32 [39] | 33 [38] | 33 [39] | |
| 1 | 41.3 | 40.5 | 39.79 | 40.9 | 40.3 | 38.7 | 18.3 | 17.4 | 17.3 | 17.5 | 18.2 | 17.7 | 17.6 | 17.3 | 18.3 |
| 2 | 18.7 | 18.3 | 20.07 | 19.7 | 19.0 | 27.6 | 26.8 | 27.6 | 27.5 | 27.1 | 26.8 | 24.1 | 24.0 | 27.5 | 26.9 |
| 3 | 42.0 | 35.7 | 40.84 | 38.5 | 37.9 | 80.6 | 120.5 | 120.6 | 120.4 | 120.0 | 120.4 | 123.2 | 123.1 | 120.5 | 120.4 |
| 4 | 33.3 | 39.3 | 43.60 | 43.7 | 42.5 | 42.7 | 144.5 | 144.4 | 144.4 | 143.7 | 144.4 | 139.9 | 139.9 | 144.5 | 144.4 |
| 5 | 56.1 | 56.9 | 49.80 | 49.5 | 46.3 | 55.8 | 38.2 | 38.3a | 38.3 | 38.0a | 38.4 | 38.5 | 38.2 | 38.3a | 38.2 |
| 6 | 20.3 | 20.5 | 35.00 | 30.4 | 29.3 | 20.1 | 36.9 | 36.5 | 35.9 | 36.4 | 36.8 | 37.8 | 37.7 | 36.4b | 36.8 |
| 7 | 40.4 | 41.7 | 77.52 | 76.2 | 75.0 | 41.3 | 27.6 | 27.0 | 26.8 | 26.4 | 27.5 | 28.8 | 28.7 | 26.9 | 27.5 |
| 8 | 44.2 | 44.0 | 47.63 | 48.9 | 48.0 | 43.9 | 36.2 | 36.3 | 36.1 | 36.0 | 36.1 | 37.3 | 37.2 | 36.4b | 36.3 |
| 9 | 56.1 | 56.2 | 47.89 | 47.4 | 48.6 | 55.8 | 40.0 | 38.7a | 38.1 | 38.3a | 39.9 | 39.9 | 39.9 | 38.4a | 38.3 |
| 10 | 39.3 | 38.7 | 40.00 | 39.5 | 38.8 | 39.6 | 46.4 | 46.6 | 46.4 | 46.1 | 46.3 | 44.5 | 44.5 | 46.6 | 46.5 |
| 11 | 18.1 | 18.2 | 19.51 | 18.5 | 17.7 | 18.3 | 35.5 | 35.1b | 35.5 | 35.0b | 35.4 | 35.1 | 35.0 | 35.0 | 36.3 |
| 12 | 33.3 | 33.2 | 33.97 | 34.0 | 33.3 | 33.0 | 29.5 | 35.6b | 35.4 | 35.8b | 29.4 | 29.4 | 29.3 | 36.9a | 35.0 |
| 13 | 44.2 | 44.2 | 44.41 | 44.3 | 43.4 | 43.9 | 30.9 | 31.0 | 30.6 | 30.6 | 31.0 | 30.9 | 31.1 | 164.4 | 164.6 |
| 14 | 39.9 | 39.7 | 39.10 | 39.1 | 38.4 | 38.5 | 41.6 | 41.7 | 36.5 | 41.0 | 41.5 | 41.6 | 41.5 | 114.9 | 114.8 |
| 15 | 49.2 | 49.1 | 47.41 | 46.6 | 45.6 | 48.8 | 179.4 | 179.8 | 62.8 | 172.8 | 173.8 | 179.4 | 173.8 | 172.0 | 172.1 |
| 16 | 156.0 | 155.9 | 155.24 | 156.2 | 155.4 | 155.4 | 19.9 | 19.9 | 19.6 | 19.5 | 19.9 | 19.8 | 19.8 | 19.5 | 19.5 |
| 17 | 102.8 | 103.0 | 104.09 | 103.6 | 103.2 | 103.1 | 16.0 | 16.1 | 15.7 | 15.5 | 15.9 | 16.0 | 15.9 | 15.9 | 16.0 |
| 18 | 33.7 | 27.1 | 30.08 | 28.6 | 28.5 | 22.8 | 19.9 | 18.4 | 18.3 | 18.0 | 19.9 | 33.0 | 33.0 | 18.3 | 20.0 |
| 19 | 21.7 | 65.6 | 184.05 | 178.2 | 179.0 | 64.3 | 18.0 | 20.0 | 19.3 | 19.5 | 18.0 | 20.1 | 19.9 | 20.0 | 18.0 |
| 20 | 17.6 | 18.1 | 15.93 | 15.7 | 15.4 | 18.3 | 18.5 | 18.1 | 17.8 | 17.8 | 18.4 | 17.4 | 17.3 | 17.9 | 18.3 |
| C=O | 178.6 | ||||||||||||||
| MeCO | 20.8 | ||||||||||||||
| OMe | 50.6 | 51.3 | 51.3 | ||||||||||||
| Carbon | Compound / δC (in ppm) | ||||||||||||||
| 34 [62] | 35 [63] | 36 [38] | 37 [38] | 38 [40] | 39 [41] | 40 [41] | 41 [41] | 42 [41] | 43 [41] | 44 [38] | 45 [40] | 46 [40] | 47 [38] | 48 [41] | |
| 1 | 18.37 | 18.40 | 17.5 | 18.6 | 18.1 | 29.4 | 16.2 | 18.2 | 27.3a | 28.9 | 35.1a | 35.6 | 35.6 | 35.6a | 34.3a |
| 2 | 26.98 | 26.92 | 27.1 | 24.1 | 27.7 | 125.6 | 30.3 | 27.4 | 65.6 | 69.5 | 199.1 | 201.2 | 200.2 | 200.0 | 200.5 |
| 3 | 120.52 | 120.46 | 120.0 | 123.3 | 120.3 | 171.0 | 76.2 | 120.4 | 122.1 | 124.4 | 128.5 | 125.5 | 125.5 | 125.5 | 127.4 |
| 4 | 144.60 | 144.50 | 143.7 | 139.8 | 144.4 | 168.7 | 76.5 | 144.5 | 150.1 | 147.9 | 168.6 | 173.4 | 171.0 | 172.6 | 172.6 |
| 5 | 38.28 | 38.30 | 38.0a | 37.9 | 38.3 | 50.6 | 38.3 | 38.1 | 38.0 | 38.0 | 38.6c | 40.0 | 38.8 | 39.9b | 39.7 |
| 6 | 36.94 | 36.99 | 36.4 | 37.6a | 36.1 | 34.4 | 32.4 | 36.8 | 36.4 | 36.5 | 36.8b | 36.0 | 35.5 | 34.8a | 35.5 |
| 7 | 27.61 | 27.63 | 26.4 | 28.9 | 26.8 | 28.3 | 26.4 | 26.8 | 27.2a | 27.2 | 29.0 | 27.0 | 26.9 | 26.9 | 26.8 |
| 8 | 36.36 | 36.41 | 36.0 | 37.5 | 36.8 | 37.1 | 36.0 | 36.1 | 36.3 | 35.9 | 37.3 | 36.1 | 36.1 | 36.1 | 35.8 |
| 9 | 38.72 | 38.72 | 38.3a | 38.9 | 38.1 | 37.5 | 41.2 | 38.3 | 38.9 | 38.6 | 39.3c | 38.6 | 38.6 | 38.8b | 38.3 |
| 10 | 46.53 | 46.60 | 46.0 | 44.9 | 46.3 | 53.9 | 40.7 | 46.3 | 40.4 | 45.2 | 45.7 | 45.7 | 45.7 | 45.6 | 45.5 |
| 11 | 36.63 | 36.72 | 34.2 | 35.0 | 31.8 | 33.5 | 32.2 | 31.8 | 31.0 | 31.8 | 35.4a | 34.9 | 34.9 | 34.9a | 31.1 |
| 12 | 32.95 | 32.97 | 37.7 | 37.0a | 35.2 | 35.5 | 35.4 | 35.3 | 36.4 | 35.2 | 36.2b | 36.0 | 34.7 | 35.9a | 34.7a |
| 13 | 140.93 | 143.22 | 160.8 | 164.5 | 73.3 | 73.0 | 73.5 | 73.4 | 73.2 | 73.3 | 30.7 | 30.8 | 30.4 | 30.9 | 73.0 |
| 14 | 123.13 | 118.03 | 114.5 | 115.1 | 145.2 | 144.9 | 145.0 | 145.1 | 146.4 | 145.0 | 41.4 | 41.5 | 36.9 | 41.3 | 144.8 |
| 15 | 59.51 | 61.44 | 166.5 | 172.4 | 111.6 | 111.9 | 111.6 | 111.8 | 110.9 | 111.9 | 178.7 | 178.9 | 63.0 | 173.4 | 111.9 |
| 16 | 16.52 | 16.70 | 18.5 | 19.6 | 27.4 | 27.8 | 27.4 | 27.7 | 26.3 | 27.7 | 19.9 | 19.9 | 19.9 | 19.8 | 27.7 |
| 17 | 16.07 | 15.98 | 15.5 | 16.1 | 15.8 | 15.0 | 15.9 | 15.9 | 15.8 | 15.9 | 16.0 | 15.7 | 15.8 | 15.7 | 15.6 |
| 18 | 18.45 | 17.92 | 18.0 | 20.0 | 18.3 | 11.7 | 21.3 | 18.0 | 17.9 | 17.7 | 20.5 | 18.4 | 18.5 | 18.4 | 18.9b |
| 19 | 20.03 | 19.99 | 19.5 | 33.2 | 19.8 | 16.9 | 17.2 | 19.8 | 18.3 | 19.9 | 32.1 | 18.9 | 18.7 | 18.9 | 18.2b |
| 20 | 18.07 | 18.34 | 17.8 | 17.9 | 17.8 | 18.2 | 18.5 | 18.4 | 18.3 | 18.5 | 18.0 | 17.9 | 18.0 | 18.0 | 17.9 |
| C=O | 170.94 | 178.9 | |||||||||||||
| MeCO | 20.94 | 20.8 | |||||||||||||
| OMe | 50.1 | 51.4 | |||||||||||||
| Compound / δC (in ppm) | |||||||||||||||
| Carbon | 49 [38] | 50 [38] | 51 [38] | 54 [41] | 56 [46] | 57 [40] | 58 [64] | 60 [40] | 61 [54]A | 62 [55] | 63 [65] | 65 (?) [66] | 66 [40] | 67 [40] | |
| 1 | 35.4a | 35.4a | 35.6a | 22.0 | 74.18 | 39.1 | 39.8 | 39.0 | 40.4 | 33.1 | 39.1 | 19.45 | 43.9 | 36.9 | |
| 2 | 200.3 | 200.0 | 200.2 | 79.2 | 128.68 | 18.2 | - | 18.2 | 19.0 | 21.7 | 19.4 | 22.36 | 19.5 | 19.0 | |
| 3 | 128.5 | 125.3 | 125.4 | 116.7 | 136.84 | 42.1 | 41.9 | 41.9 | 42.7 | 35.4 | 42.1 | 24.51 | 42.0 | 41.2 | |
| 4 | 167.5 | 172.3 | 172.7 | 155.0 | 80.48 | 33.1 | - | 33.3 | 33.7 | 39.1 | 33.6 | 33.64 | 34.5 | 33.2 | |
| 5 | 38.6b | 39.8b | 39.9b | 37.8 | 37.16 | 55.9 | 56.1 | 55.8 | 56.9 | 36.6 | 55.5 | 55.59 | 57.5 | 51.8 | |
| 6 | 36.7a | 34.8a | 34.9a | 33.2 | 25.59 | 18.1 | 23.5 | 18.1 | 21.1 | 28.6 | 24.5 | 32.75 | 69.4 | 19.0 | |
| 7 | 28.9 | 26.7 | 26.9 | 27.1 | 17.33 | 41.3 | 44.7 | 40.5 | 45.1 | 37.4 | 38.3 | 38.41 | 47.7 | 33.5 | |
| 8 | 36.6 | 36.1 | 36.1 | 36.4 | 47.58 | 73.3 | 74.4 | 73.3 | 73.9 | 160.6 | 148.3 | 148.17 | 144.0 | 125.4 | |
| 9 | 39.9b | 38.7 | 38.7b | 39.1 | 35.28 | 59.3 | 61.3 | 58.2 | 62.3 | 48.6 | 56.2 | 57.20 | 56.7 | 140.4 | |
| 10 | 45.7 | 45.6 | 45.8 | 40.4 | 44.49 | 38.9 | 39.2 | 38.8 | 39.8 | 40.0 | 39.7 | 39.80 | 40.9 | 37.2 | |
| 11 | 34.0 | 34.2a | 34.0a | 30.7 | 41.90 | 22.3 | 20.5 | 22.4 | 20.0 | 27.5 | 21.5 | 38.96 | 21.6 | 25.3 | |
| 12 | 36.8a | 35.9a | 35.9a | 36.1 | 70.66 | 42.1 | 44.5 | 41.9 | 46.2 | 29.8 | 40.1 | 42.21 | 39.5 | 41.7 | |
| 13 | 160.3 | 162.6 | 160.3 | 73.9 | 124.79 | 30.9 | 163.9 | 30.9 | 73.3 | 30.8 | 164.3 | 160.69 | 160.7 | 31.1 | |
| 14 | 115.2 | 115.1 | 115.3 | 146.3 | 108.40 | 40.5 | 114.7 | 144.7 | 147.5 | 41.4 | 114.6 | 115.73 | 115.2 | 145.9 | |
| 15 | 167.0 | 171.2 | 167.1 | 111.2 | 139.66 | 177.9 | 171.5 | 111.8 | 110.7 | 179.8 | 171.8 | 166.40 | 167.3 | 112.0 | |
| 16 | 19.1 | 19.3 | 19.1 | 25.0 | 143.96 | 19.5 | 19.4 | 19.5 | 27.8 | 19.8 | 19.2 | 14.51 | 19.0 | 20.0 | |
| 17 | 15.9 | 15.5 | 15.7 | 15.6 | 175.48 | 30.5 | 24.0 | 30.4 | 24.5 | 102.5 | 106.4 | 21.73 | 110.3 | 19.4 | |
| 18 | 20.5 | 18.2 | 18.4 | 18.3 | 172.37 | 21.5 | 21.5 | 21.5 | 33.7 | 18.2 | 33.6 | 25.26 | 23.6 | 21.5 | |
| 19 | 32.1 | 18.7 | 18.9 | 18.1 | 27.00 | 33.3 | 33.4 | 33.1 | 21.8 | 20.8 | 21.7 | 33.64 | 33.7 | 33.2 | |
| 20 | 17.8 | 17.6 | 17.9 | 18.4 | 24.31 | - | 15.4 | - | 15.8 | 15.9 | 14.5 | 106.53 | 17.1 | 19.3 | |
| 1’ | 50.8 | ||||||||||||||
| 2’ | |||||||||||||||
| 3’ | 50.7 | 50.56 | 50.8 | ||||||||||||
a, b, and c may be interchanged for the same structure; (*) reassigned 13C-NMR data in CDCl3; (P) 13C-NMR data in C5D5N; (D) 13C-NMR data in DMSO-d6; (A) 13C-NMR data in acetone-d6; (?) solvent not given
Table 5.
13C-NMR data (in CDCl3) of substituted diterpenes isolated from Aristolochia species.
| Carbon | Compound / δC (in ppm) | Carbon | Compound / δC (in ppm) | ||||
|---|---|---|---|---|---|---|---|
| 24 [ 31] | 25 [ 31] | 26 [ 31] | 24 | 25 | 26 | ||
| 1 | 40.3 | 40.4 | 40.4 | 1’ | - | - | - |
| 2 | 18.6 | - | - | 2’ | 112.7 | 112.8 | 112.5 |
| 3 | 41.9 | 42.0 | 42.0 | 3’ | 143.1 | - | - |
| 4 | 33.3 | - | - | 4’ | 147.5 | 146.8 | 146.2 |
| 5 | 56.0 | 56.1 | 56.2 | 4a’ | - | - | - |
| 6 | 20.5 | - | 20.5 | 4b’ | 131.0 | - | 131.0 |
| 7 | 42.1 | 42.0 | 42.0 | 5’ | 119.2 | 127.4 | 119.2 |
| 8 | 44.9 | - | - | 6’ | 131.0 | 130.5 | - |
| 9 | 56.7 | 56.6 | 56.5 | 7’ | 108.0 | - | 108.0 |
| 10 | 39.4 | - | - | 8’ | 156.9 | 130.2 | 156.9 |
| 11 | 18.3 | - | - | 8a’ | 120.2 | 128.5 | 120.2 |
| 12 | 26.3 | 27.1 | 27.4 | 9’ | 121.2 | 126.5 | 121.1 |
| 13 | 46.3 | 46.5 | 43.5 | 10’ | 145.9 | - | - |
| 14 | 37.2 | 37.5 | 38.1 | 10a’ | - | 118.3 | 119.2 |
| 15 | 53.3 | 53.4 | 51.0 | 11’ | 167.2 | 167.5 | 167.2 |
| 16 | 80.1 | 80.0 | - | OCH2O | 102.4 | 103.0 | 102.4 |
| 17 | 69.6 | 70.2 | 63.7 | OMe | 56.2 | 56.2 | |
| 18 | 33.6 | 33.6 | 33.6 | ||||
| 19 | 21.5 | 22.0 | 21.6 | ||||
| 20 | 17.8 | 18.0 | 17.8 | ||||
(-) Data not observed
Kaurane derivates isolated from Aristolochia species
Kaurane diterpenoids show several biological properties such as antioxidative, antityrosinase [27], abortifacient, and anti-inflammatory activities, they are used against snake bite poisoning [28], and present cytotoxicity against tumor cells of human prostate, colon, and breast cancer [29]. Table 1 lists the kaurane derivates isolated from Aristolochia species (1 to 26 in Figure 2 and Figure 3) and their respective plant sources. Acetonide 13 and kaurane derivative 14 were isolated from A. rodriguesii and A. triangularis, respectively. Both compounds were also prepared from 3 [30].
Clerodane derivatives isolated from Aristolochia species
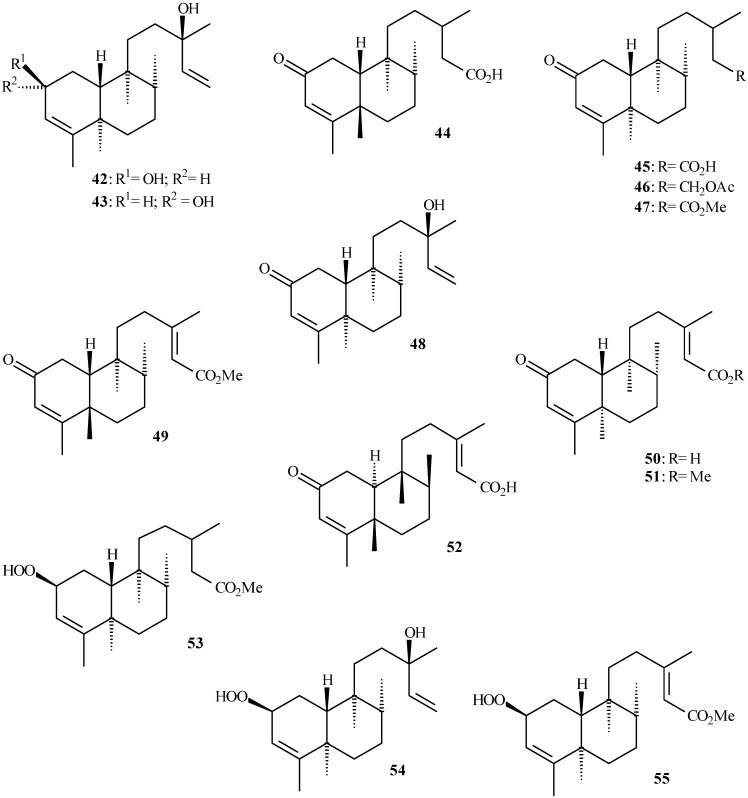
Clerodane diterpenoids show a broad spectrum of biological properties [35,36] including insecticidal activity [37]. Table 2 shows the clerodane diterpenoids isolated from the genus Aristolochia (27 to 55 in Figure 4 and Figure 5) and their respective plant sources. Structure 52 has been also named as 2-oxokolavenic acid (50). The corresponding acid of 49 has been described by Wu et al. [16].
Furanoditerpene isolated from Aristolochia species
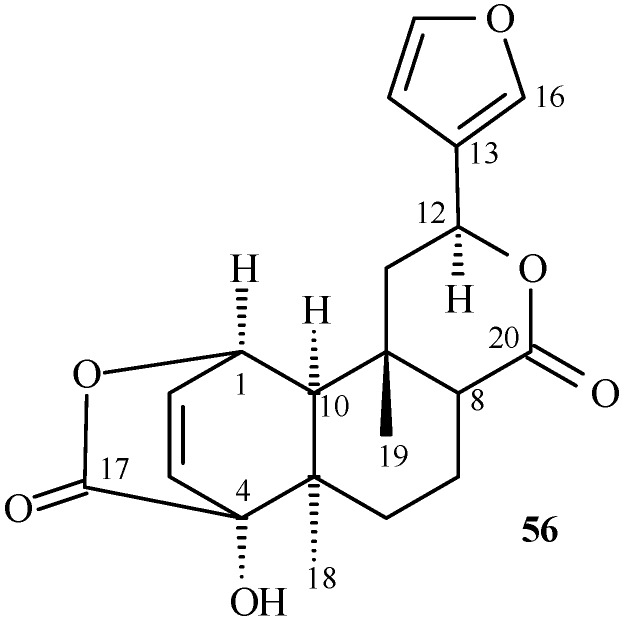
Analgesic and anti-inflammatory activities have been observed for furanoditerpenes [42,43], and their derivatives also show sedative [42], anticonvulsant [44], and plant growth regulatory activities [45]. Columbin (56) was isolated from A. albida [46] (Figure 6). The furan moiety is not fused to other rings, as is commonly found in several furanoditerpenes from natural products or synthesized [47]. It is the only furanoditerpene found in the genus Aristolochia.
Labdane derivatives isolated from Aristolochia species
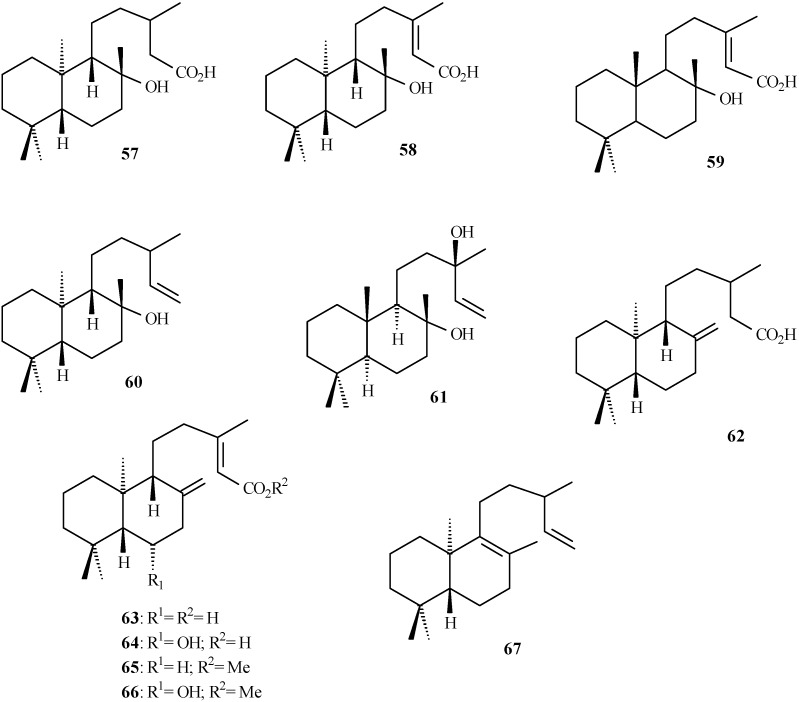
Labdane diterpenoids are fungal growth regulator and plant growth inhibitor [48,49,50], showing high antibacterial activity [51]. Commercially, labdanes are used as natural fixatives, modifiers, and lotions by the perfume industry, and as a flavouring agent in the tobacco industry [52]. Table 3 shows the labdane diterpenoids isolated from Aristolochia species (57 to 67 in Figure 7). Structures 58 and 59 have both been named as ent-labd-13-en-8β-ol-15-oic acid [53].
13C-NMR data of diterpenes
Table 4 and Table 5 show the 13C-NMR data of the diterpenoids 1 to 67. In Table 4 the 13C-NMR data of 17 (in CDCl3) were reassigned and a new structure 22 was proposed according to 13C-NMR data in CDCl3, C5D5N, and DMSO-d6 [34] (see Figure 2). The carbon chemical shifts of the kaurane diterpenoids 1 to 23 are characteristic only at region between δC 38.0 and 42.0 assigned to C-1 and C-10. The other carbon chemical shifts do not show any characteristic features for this skeleton type. In diterpenes 15 and 16 containing a double bond between C-15 and C-16, the carbon chemical shifts are registered near δC 135.0 and 145.0, respectively. On the other hand, the double bond is located between C-16 and C-17 of 17 to 23 and their carbon chemical shifts are registered near δC 156.0 and 103.0, respectively.
The characteristic carbon chemical shifts for clerodane derivatives (except for 39 and 40) are observed around δC 120.0-123.0 and 139.0-145.0, which are assigned to C-3 and C-4, respectively, as shown in Table 4 (see Figure 4 and Figure 5). However, the carbon chemical shift ranges show higher values when C-2 is oxygened, as is the case of 42 to 55 (Figure 5). The carbon chemical shifts around δC 38.0-40.0 (assigned to C-5 and C-9) and δC 36.0-38.0 (assigned to C-6 and C-8) are registered in the 13C- NMR spectra of these compounds. Values close to δC 145.0 and 112.0 are assigned to the double bond between C-14 and C-15 in clerodane diterpenoids, as shown in 38-43, 48, and 54. Besides, the values close to δC 160.0 and 115.0 were assigned to double bond between C-13 and C-14, respectively, and δC 73.0 for hydroxylated C-13 of these compounds.
Structure of furanoditerpenes (Figure 6) can be confirmed by carbon chemical shifts at δC 124.79, 108.40, 139.66, and 143.96 assigned to C-13, C-14, C-15, and C-16, respectively for 56.
Despite the fact that 61 was not isolated from an Aristolochia species, its stereochemistry is close to that of 59 (see Figure 7). Thus, 13C-NMR data of 61 were included in Table 4 to provide insights about the corresponding data of 59. The carbon chemical shifts close to δC 145.0 and 112.0 are assigned to double bond between C-14 and C-15 in labdanes, as shown in Table 4 for 60, 61, and 67. Values close to δC 160.0 and 115.0 can be assigned to double bond between C-13 and C-14 in 58 and 63-66.
Table 5 shows the 13C-NMR data of the substituted kaurane diterpenoids 24 to 26 (see Figure 3). These compounds present an aristolochic acid derivative bound to a kaurane diterpenoids at O-16 (for 24 and 25) and O-17 (for 26). Some carbon chemical shifts were not observed in the 13C NMR data of 25 and 26. As it would be expected only the carbon chemical shifts of C-13 to C-17 of 26 are different when comparing to 24 and 25.
Acknowledgements
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for their financial support.
Footnotes
Sample Availability: Samples of the compounds 27 and 45 are available from the authors.
References
- 1.Neinhuis C, Wanke S., Hilu K.W., Müller K., Borsch T. Phylogeny of Aristolochiaceae based on parsimony, likelihood and Bayesian analyses of trnL-trnF sequences. Plant Syst. Evol. 2005;250:7–26. [Google Scholar]
- 2.Wanke S., Gonzales F., Neinhuis C. Systematics of Pipevines: Combining morphological and fast-evolving molecular characters to investigate the relationships within subfamily Aristolochioideae (Aristolochiaceae) Inter. J. Plant Sci. 2006;167:1215–1227. [Google Scholar]
- 3.Wanke S., Jaramillo M.A., Borsch T., Samain M.-S., Quandt D., Neinhuis C. Evolution of Piperales – matK gene and trnK intron sequence data reveal lineage specific resolution contrast. Mol. Phylogenet. Evol. 2007;42:477–497. doi: 10.1016/j.ympev.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Wu T.S., Tsai Y.L., Damu A.G., Kuo P.C., Wu P.L. Constituents from the root and stem of Aristolochia elegans. J. Nat. Prod. 2002;65:1522–1525. doi: 10.1021/np020218u. [DOI] [PubMed] [Google Scholar]
- 5.Che C.-T., Almed M.S., Kang S.S., Waller D.P., Bengel A.S., Martin A., Rajamahendran P., Bunyapraphatsara J., Lankin D.C., Cordell G.A., Soejarto D.D., Wijesekera R.O.B., Fong H.H.S. Studies on Aristolochia III. Isolation and biological evaluation of constituents of Aristolochia indica roots for fertility-regulationg activity. J. Nat. Prod. 1984;47:331–341. doi: 10.1021/np50032a017. [DOI] [PubMed] [Google Scholar]
- 6.Corrêa M.P. Dicionário das Plantas Úteis do Brasil e das Exóticas Cultivadas. Imprensa Nacional; Rio de Janeiro: 1978. [Google Scholar]
- 7.Lopes L.M.X., Bolzani V.S., Trevisan L.M.V., Luiz V. ent-Cauranos e lignanas de Aristolochia elegans. Quím. Nova. 1990;13:250–251. [Google Scholar]
- 8.Lajide L., Escoubas P., Mizutani J. Antifeedant activity of metabolites of Aristolochia albida against the Tobacco cutworm Spodoptera litura. J. Agric. Food Chem. 1993;41:669–673. doi: 10.1021/jf00028a031. [DOI] [Google Scholar]
- 9.Lemos V.S., Thomas G., Barbosa Filho J.M. Pharmacological studies on Aristolochia papillaris Mast (Aristolochiaceae) J. Ethnopharmacol. 1993;40:141–145. doi: 10.1016/0378-8741(93)90060-I. [DOI] [PubMed] [Google Scholar]
- 10.Terada S., Motomiya T., Yoshioka K., Narita T., Yasui S., Takase M. Antiallergic substance from Assarum sagittarioides and synthesis of some analogs. Chem. Pharm. Bull. 1987;35:2437–2442. doi: 10.1248/cpb.35.2437. [DOI] [PubMed] [Google Scholar]
- 11.Yu J.Q., Liao Z.X., Cai X.Q., Lei J.C., Zouc G.L. Composition, antimicrobial activity and citotoxicity of essential oils from Aristolochia mollissima. Environm. Toxic. Pharm. 2007;23:162–167. doi: 10.1016/j.etap.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Kubmarawa D., Ajoku G.A., Enwerem N.M., Okorie D.A. Preliminary phytochemical and antimicrobial screening of 50 medicinal plants from Nigeria. Afric. J. Biotech. 2007;6:1690–1696. [Google Scholar]
- 13.Lopes L.M.X., Bolzani V.S., Trevisan L.M.V., Grigolli T.M. Terpenes from Aristolochia triangularis. Phytochemistry. 1990;29:660–662. [Google Scholar]
- 14.Shafi P.M., Rosamma M.K., Jamil K., Reddy P.S. Antibacterial activity of the essential oil from Aristolochia indica. Fitoterapia. 2002;73:439–441. doi: 10.1016/s0367-326x(02)00130-2. [DOI] [PubMed] [Google Scholar]
- 15.Nok A.J. A novel nonhemorrhagic protease from the Africa puff adder (Bitis arietans) venom. J. Biochem. Molec. Toxic. 2001;15:215–220. doi: 10.1002/jbt.19. [DOI] [PubMed] [Google Scholar]
- 16.Wu T.-S., Damu A.G., Su C.-R., Kuo P.-C. Terpenoids of Aristolochia and their biological activities. Nat. Prod. Rep. 2004;21:594–624. doi: 10.1039/b401950d. [DOI] [PubMed] [Google Scholar]
- 17.Meinl W., Pabel U., Osterloh-Quiroz M., Hengstler J.G., Glatt H. Human sulphotransferases are involved in the activation of aristolochic acids and are expressed in renal target tissue. Intern. J. Cancer. 2006;118:1090–1097. doi: 10.1002/ijc.21480. [DOI] [PubMed] [Google Scholar]
- 18.Martinez M.-C. M., Nortier J., Vereerstraeten P., Vanherweghem J.-L. Progression rate of Chinese herb nephropathy: impact of Aristolochia fangchi ingested dose. Nephrol Dial. Transplant. 2002;17:408–412. doi: 10.1093/ndt/17.3.408. [DOI] [PubMed] [Google Scholar]
- 19.Mix D.B., Guinaudeau H., Shamma M. The aristolochic acid and aristolactams. J. Nat Prod. 1982;45:657–666. [Google Scholar]
- 20.Stiborova M., Frei E., Breuer A., Bieler C.A., Schmeiser H.H. Aristolactam I a metabolite of aristolochic acid I upon activation forms and adduct found in DNA of patients with chinese herts nephropathy. Experim. Toxic. Pathol. 1999;51:421–427. doi: 10.1016/S0940-2993(99)80033-5. [DOI] [PubMed] [Google Scholar]
- 21.Grollman A.P., Shibutani S., Moriya M., Miller F., Wu L., Moll U., Suzuki N., Fernandes A., Rosenquist T., Medverec Z., Jakovina K., Brdar B., Slade N., Turesky R.J., Goodenough A.K., Rieger R., Vukelić M., Jelaković B. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. PNAS. 2007;104:12129–12134. doi: 10.1073/pnas.0701248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rastrelli L., Capasso A., Pizza C., De Tommasi N., Sorrentino L. New protopine and benzyltetrahydroprotoberberine alkaloids from Aristolochia constricta and their activity on isolated Guinea-Pig ileum. J. Nat. Prod. 1997;60:1065–1069. doi: 10.1021/np960710b. [DOI] [PubMed] [Google Scholar]
- 23.Wu T.S., Chan Y.Y., Leu Y.L. The constituents of the root and stem of Aristolochia cucurbitifolia hayata and their biological activity. Chem. Pharm. Bull. 2000;48:1006–1009. doi: 10.1248/cpb.48.1006. [DOI] [PubMed] [Google Scholar]
- 24.Wu T.S., Chan Y.Y., Wu P.L., Li C.Y., Mori Y. Four aristolochic acid esters of rearranged ent-elemane sesquiterpenes from Aristolochia heterophylla. J. Nat. Prod. 1999;62:348–351. doi: 10.1021/np980354s. [DOI] [PubMed] [Google Scholar]
- 25.Wu T.S., Tsai Y.L., Wu P.L., Leu Y.L., Lin F.W., Lin J.K. Constituents from the leaves of Aristolochia elegans. J. Nat. Prod. 2000;63:692–693. doi: 10.1021/np990483o. [DOI] [PubMed] [Google Scholar]
- 26.Leitão G.G., Kaplan M.A.C. Chemistry of the genus Aristolochia. Rev. Bras. Farm. 1992;73:65–75. [Google Scholar]
- 27.Shi L.-S., Kuo P.-C., Tsai Y.-L., Damu A.G., Wu T.-S. The alkaloids and other constituents from the root and stem of Aristolochia elegans. Bioorg. Med. Chem. 2004;12:439–446. doi: 10.1016/j.bmc.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 28.Correa M.S., Guilhon G.M.S.P., Conserva L.M. Kauranoids from Aristolochia rodriguesii. Fitoterapia. 1998;69:277–278. [Google Scholar]
- 29.Henry G.E., Adams L.S., Rosales J.C., Jacobs H., Heber D., Seeram N.P. Kaurene diterpenes from Laetia thamnia inhibit the growth of human cancer cells in vitro. Cancer Lett. 2006;244:190–194. doi: 10.1016/j.canlet.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 30.Habib A.A.M., El-Sebakhy N.A. ent-Kaurane-16α,17-diol and (–)-cubebin as natural products from Aristolochia elegans. Pharmazie. 1981;36:291–294. [Google Scholar]
- 31.Lopes L.M.X., Nascimento I.R. Diterpene esters of aristolochic acids from Aristolochia pubescens. Phytochemistry. 2003;63:953–957. doi: 10.1016/S0031-9422(03)00335-2. [DOI] [PubMed] [Google Scholar]
- 32.Rucker G., Langmann B., Siqueira N.S. Constituents of Aristolochia triangularis. Planta Med. 1981;41:143–149. doi: 10.1055/s-2007-971691. [DOI] [PubMed] [Google Scholar]
- 33.Gaitan R., Gómez H.A., Tapia S., Villadiego A.M., Méndez D. (–)-Acido-II-hidroxikaur-16-en-19-óico, um nuevo kaureno aislado de Aristolochia anguicida Jacq. Rev. Latinoamer. Quim. 2002;30:83–86. [Google Scholar]
- 34.Fraga B.M. On the structure of an ent-kaurene diterpene from Aristolochia anguicida. Rev. Latinoamer. Quim. 2004;32:76–79. [Google Scholar]
- 35.Lajide L., Escoubas P., Mizutani J. Termite antifeedant activity in Detarium microcarpum. Phytochemistry. 1995;40:1101–1104. doi: 10.1016/0031-9422(95)00153-X. [DOI] [Google Scholar]
- 36.Salah M.A., Bedir E., Toyang N.J., Khan I.A., Harries M.D., Wedge D.E. Antifungal clerodane diterpenes from Macaranga monandra (L.) Muell et Arg. (Euphorbiaceae) J. Agric. Food Chem. 2003;51:7607–7610. doi: 10.1021/jf034682w. [DOI] [PubMed] [Google Scholar]
- 37.Messiano G.B., Vieira L., Machado M.B., Lopes L.M.X., Bortoli S.A., Zukerman-Schpector J. Evaluation of insecticidal activity of diterpenes and lignans from Aristolochia malmeana against Anticarsia gemmatalis. J. Agric. Food Chem. 2008;56:2655–2659. doi: 10.1021/jf703594z. [DOI] [PubMed] [Google Scholar]
- 38.Lopes L.M.X., Bolzani V.S., Trevisan L.M.V. Clerodane diterpenes from Aristolochia species. Phytochemistry. 1987;26:2781–2784. doi: 10.1016/S0031-9422(00)83590-6. [DOI] [Google Scholar]
- 39.Leitão G.G., Kaplan M.A.C., Galeffi C. epi-Populifolic acid from Aristolochia cymbifera. Phytochemistry. 1992;31:3277–3279. doi: 10.1016/0031-9422(92)83495-K. [DOI] [Google Scholar]
- 40.Lopes L.M.X., Bolzani V.S. Lignans and diterpenes of three Aristolochia species. Phytochemistry. 1988;27:2265–2268. doi: 10.1016/0031-9422(88)80139-0. [DOI] [Google Scholar]
- 41.Bomm M.D., Zukerman-Schpector J., Lopes L.M.X. Rearranged (4→2) –abeo-clerodane and clerodane diterpenes from Aristolochia chamissonis. Phytochemistry. 1999;50:455–461. doi: 10.1016/S0031-9422(98)00583-4. [DOI] [Google Scholar]
- 42.Duarte I.D., Ferreira-Alves D.L., Piló-Veloso D., Nakamura-Craig M. Evidence of the involvement of biologic amines in the antinoceptive effect of a vouacapan extracted from Pterodon polygalaeflorus Benth. J. Ethnopharm. 1996;55:13–18. doi: 10.1016/S0378-8741(96)01465-1. [DOI] [PubMed] [Google Scholar]
- 43.Ruggiero S.G., Rodrigues B.L., Fernandes N.G., Stefani G.M., Piló-Veloso D. Structure of 6α,7β-dihydroxyvouacapan-17β-oic acid. Acta Crystallogr. 1997;C53:982–984. [Google Scholar]
- 44.Belinelo V.J., Reis G.T., Stefani G.M., Ferreira-Alves D.L., Piló-Veloso D. Synthesis of 6α,7β-dihydroxyvouacapan-17β-oic acid derivatives. Part IV: Mannich base derivatives and its activities on the electrically stimulated guinea-pig ileum preparation. J. Braz. Chem. Soc. 2002;13:830–836. doi: 10.1590/S0103-50532002000600016. [DOI] [Google Scholar]
- 45.King-Díaz B., Pérez-Reyes A., Santos F.J.L., Ferreira-Alves D.L., Piló-Veloso D., Carvajal S. U., Lotina-Hennsen B. Natural diterpene derivative β-lactone as photosystem II inhibitor on spinach chloroplast. Pest. Biochem. Physiol. 2006;84:109–115. doi: 10.1016/j.pestbp.2005.05.007. [DOI] [Google Scholar]
- 46.Choudhury M.K., Haruna A.K., Johnson E.C., Houghton P.J. Structural elucidation of columbin, a diterpene isolated from the rhizomes of Aristolochia albida. Indian J. Pharm. Sci. 1997;59:34–37. [Google Scholar]
- 47.Santos F.J.L., Alcântara A.F.C., Ferreira-Alves D.L., Piló-Veloso D. Theoretical and experimental NMR studies of the Swern oxidation of methyl 6α,7β-dihydroxyvouacapan-17β-oate. Struct. Chem. 2008;19:624–631. [Google Scholar]
- 48.Bailey J.A., Vincent G.G., Burden R.S. Diterpenes from Nicotiana glutinosa and their effect on fungal growth. J. Gen. Microbiol. 1974;85:57–64. doi: 10.1099/00221287-85-1-57. [DOI] [PubMed] [Google Scholar]
- 49.Cutler H.G., Reid W.W., Deletang J. Plant growth inhibiting properties of diterpenes from tobacco. Plant Cell Physiol. 1977;18:711–714. [Google Scholar]
- 50.Bailey J.A., Carter G.A., Burden R.S., Wain R.L. Control of rust diseases by diterpenes from Nicotiana glutinosa. Nature. 1975;255:328–329. doi: 10.1038/255328a0. [DOI] [Google Scholar]
- 51.Ulubelen A., Miski M., Johansson C., Lee E., Mabry T.J., Matlin S.A. Terpenoids from Salvia palaestina. Phytochemistry. 1985;24:1386–1387. doi: 10.1016/S0031-9422(00)81143-7. [DOI] [Google Scholar]
- 52.Decorzant R., Vial C., Naef F., Whitesides G.M. A short synthesis of ambrox from sclareol. Tetrahedron. 1987;43:1871–1879. doi: 10.1016/S0040-4020(01)81499-X. [DOI] [Google Scholar]
- 53.Baratta M.T., Ruberto G., Tringali C. Constituents of the pods of Piliostigma thonningii. Fitoterapia. 1999;70:205–208. doi: 10.1016/S0367-326X(98)00035-5. [DOI] [Google Scholar]
- 54.Kouzi S.A., McChesney J.D. Microbial models of mammalian metabolism: fungal metabolism of the diterpene sclareol by Cunninghamella species. J. Nat. Prod. 1991;54:483–490. doi: 10.1021/np50074a021. [DOI] [PubMed] [Google Scholar]
- 55.Larrahondo J.E., Acevedo C. Terpenoides de Aristolochia ringens. An. Asoc. Quim. Argent. 1990;78:355–358. [Google Scholar]
- 56.Hanson J.R., Siverns M., Piozzi F., Savona G. The 13C Nuclear Magnetic Resonance Spectra of kauranoid diterpenes. J. Chem. Soc., Perkin Trans. I. 1976:114–117. [Google Scholar]
- 57.Wu Y.-C., Hung Y.-C., Chang F.-R., Cosentino M., Wang H.-K., Lee K.-H. Identification of ent-16β,17-dihydroxykauran-19-oic acid as an anti-HIV principle and isolation of the new diterpenoids Annosquamosins A and B from Annona squamosa. J. Nat. Prod. 1996;59:635–637. doi: 10.1021/np960416j. [DOI] [PubMed] [Google Scholar]
- 58.Etse J.T., Gray A.I., Waterman P.G. Chemistry in the Annonaceae - XXIV. Kaurane and kaur-16-ene diterpenes from the stem bark of Annona riticulata. J. Nat. Prod. 1987;50:979–983. doi: 10.1021/np50053a043. [DOI] [Google Scholar]
- 59.Aljančić I., Macura S., Juranić N., Andjelković S., Randjelović N., Milosavljević S. Diterpenes from Achillea clypeolata. Phytochemistry. 1996;43:169–171. doi: 10.1016/0031-9422(96)00271-3. [DOI] [Google Scholar]
- 60.Piozzi F., Savona G., Hanson J.R. Kaurenoid diterpenes from Satchys lanata. Phytochemistry. 1980;19:1237–1238. doi: 10.1016/0031-9422(80)83095-0. [DOI] [Google Scholar]
- 61.Misra R., Pandey R.C., Dev S. Higher isoprenoids - IX. Diterpenoids from the oleoresin of Hardwickia pinnata Part 2: Kolavic, kolavenic, kolavenolic and kolavonic acids. Tetrahedron. 1979;35:979–984. doi: 10.1016/S0040-4020(01)93712-3. [DOI] [Google Scholar]
- 62.Monti H., Tiliacos N., Faure R. Copaiba oil: isolation and characterization of a new diterpenoid with the dinorlabdane skeleton. Phytochemistry. 1999;51:1013–1015. doi: 10.1016/S0031-9422(98)00711-0. [DOI] [Google Scholar]
- 63.Urones J.G., Marcos I.S., Cubillo L., Monje V.A., Hernández J.M., Basade P. Derivatives of malonic acid in Parentucellia latifolia. Phytochemistry. 1989;28:651–653. doi: 10.1016/0031-9422(89)80077-9. [DOI] [Google Scholar]
- 64.Caputo R., Mangoni L. Diterpenes from Araucaria bidwilli. Phytochemistry. 1974;13:467–470. doi: 10.1016/S0031-9422(00)91234-2. [DOI] [Google Scholar]
- 65.Cavin A.-L., Hay A.-E., Marston A., Stoeckli-Evans H., Scopelliti R., Diallo D., Hostettmann K. Bioactive diterpenes from the fruits of Detarium microcarpum. J. Nat. Prod. 2006;69:768–773. doi: 10.1021/np058123q. [DOI] [PubMed] [Google Scholar]
- 66.Dey A.K., Wolf H.R. Synthesis of ethers of the enantio-14,15-dinorlabdane series from eperuic acid. Helv. Chim. Acta. 1978;61:1004–1010. doi: 10.1002/hlca.19780610309. [DOI] [Google Scholar]