Abstract
Squalene is a triterpene that is an intermediate in the cholesterol biosynthesis pathway. It was so named because of its occurrence in shark liver oil, which contains large quantities and is considered its richest source. However, it is widely distributed in nature, with reasonable amounts found in olive oil, palm oil, wheat-germ oil, amaranth oil, and rice bran oil. Squalene, the main component of skin surface polyunsaturated lipids, shows some advantages for the skin as an emollient and antioxidant, and for hydration and its antitumor activities. It is also used as a material in topically applied vehicles such as lipid emulsions and nanostructured lipid carriers (NLCs). Substances related to squalene, including β-carotene, coenzyme Q10 (ubiquinone) and vitamins A, E, and K, are also included in this review article to introduce their benefits to skin physiology. We summarize investigations performed in previous reports from both in vitro and in vivo models.
Keywords: Squalene, skin surface lipid, skin, antioxidant, topical delivery
Introduction
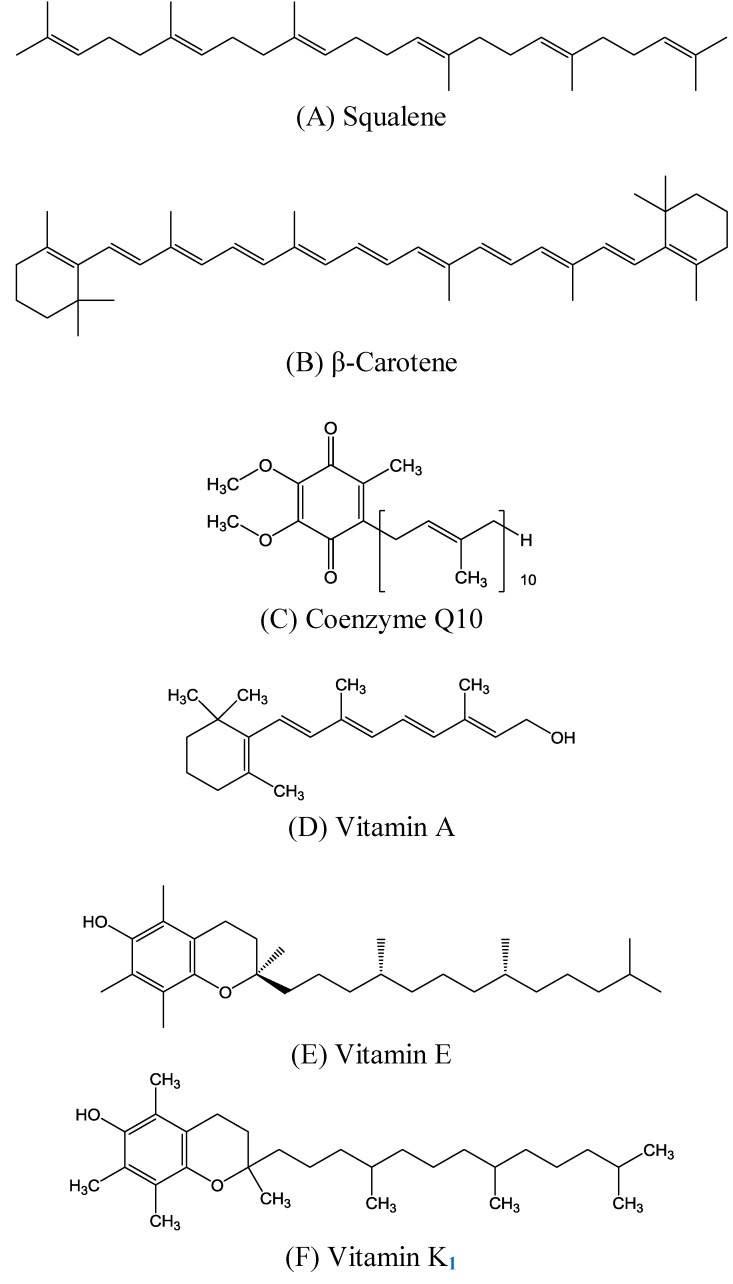
Human skin, covering the entire outer surface of the body, is the largest organ and is constantly exposed to sunlight stress, including ultraviolet (UV) light irradiation. The skin tissue is rich in lipids, which are thought to be vulnerable to oxidative stress from sunlight. Squalene (Figure 1A) is a structurally unique triterpene compound that is one of the main components (about 13%) of skin surface lipids [1]. It was so named because it was first isolated from shark (Squalus spp.) liver oil, which contains large quantities and is considered its richest source [2]. It is transported in serum generally in association with very low density lipoproteins and is distributed ubiquitously in human tissues, with the greatest concentration in the skin.
Figure 1.
Chemical structures of (A) squalene, (B) β-carotene, (C) coenzyme Q10, and (D) vitamins A, (E) E, and (F) K1.
Experimental studies have shown that squalene can effectively inhibit chemically induced skin, colon, and lung tumorigenesis in rodents [3]. The protective effect is observed when squalene is given before and/or during carcinogen treatment. The mechanisms involved in the chemopreventive activity of squalene may include inhibition of Ras farnesylation, modulation of carcinogen activation, and antioxidative activities [4]. However, several factors must be considered when the evidence for the inhibition of carcinogenesis by squalene is examined. These include the effective dose used and the time of exposure [5]. This type of information is obtained from animal bioassays, and the long-term effects of consuming increased levels of squalene are not known. Although animal studies have enhanced our understanding of the possible actions of squalene in decreasing carcinogenesis, one must apply caution in extrapolating the information obtained in animal studies to humans, because of possible differences among species.
Many other polyprenyl compounds structurally similar to squalene exist in nature and perform critical biological functions. These include β-carotene (Figure 1B), coenzyme Q10 (Figure 1C), and vitamins A (Figure 1D), E (Figure 1E), and K1 (Figure 1F), which are introduced here because of their benefits to skin physiology. For example, animals utilize prenyl groups to form the side chain of coenzyme Q10. The coenzyme Q10 designation indicates that the molecule has 10 prenyl groups in its side-chain. Other well-known substances require prenyl groups for their synthesis, and therefore are structurally similar to squalene. In the present work, we attempted to introduce the activities and benefits of squalene and related molecules to skin tissue, the largest organ of the human body. Some important in vivo and clinical studies of squalene and its derivatives are also summarized and reviewed in this article.
Biological activities of squalene
Squalene appears to be critical for reducing free radical oxidative damage to the skin. Serum squalene originates partly from endogenous cholesterol synthesis and partly from dietary sources, especially in populations consuming large amounts of olive oil or shark liver [2]. The endogenous synthesis of squalene begins with the production of 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA). The initial reduction of HMG CoA (a niacin-dependent reaction) results in the formation of mevalonate [4].
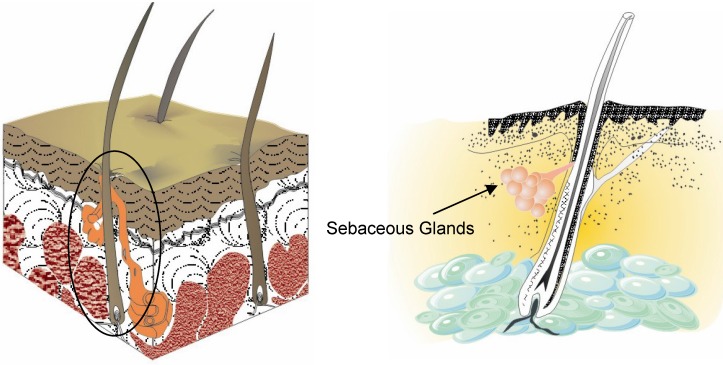
Sebaceous glands are small glands in the skin which secrete an oily matter (sebum) in the hair follicles to lubricate the skin and hair of animals (Figure 2). In humans, they are found in the greatest abundance on the face and scalp, although they are distributed throughout all skin sites except the palms and soles. Squalene is one of the predominant components (about 13%) of sebum (Table 1) [5].
Figure 2.
Sectional view of the skin with sebaceous glands.
Table 1.
Composition of sebum in humans.
| Substance | Composition (%) |
|---|---|
| Wax esters | 25 |
| Squalene | 13 |
| Cholesterol | 2 |
| Triglycerides, free fatty acids, and diglycerides | 57 |
| Other components | 3 |
Effects of squalene on the skin
Squalene is not very susceptible to peroxidation and appears to function in the skin as a quencher of singlet oxygen, protecting human skin surfaces from lipid peroxidation due to exposure to UV light and other sources of oxidative damage [6], as discussed here.
Emollient
Truly one of nature’s great emollients, squalene is quickly and efficiently absorbed deep into the skin, restoring healthy suppleness and flexibility without leaving an oily residue. New cosmetic emulsions with biomimetic molecules have been investigated using experimental designs [7]. That study determined the optimal composition of a squalene mixture in an oil-in-water emulsion, using a design of experiments to elaborate the experimental strategy. For this purpose, the stability, centrifugation, viscosity, and pH of squalene were measured, and a microscopic analysis was carried out. Results showed that the stability and viscosity of the emulsions exhibited the greatest influence on the percentage of squalene.
Skin hydration
In general, occlusion leads to increased skin hydration due to reduced water loss. Rissmann et al. [8] revealed that a vernix caseosa (VC) substitute can be an innovative barrier cream for barrier-deficient skin. This is because of the excellent properties of VC in facilitating stratum corneum hydration. Different lipid fractions were isolated from lanolin and subsequently mixed with squalene, triglycerides, cholesterol, ceramides, and fatty acids to generate semi-synthetic lipid mixtures that mimic the lipid composition of VC. The results showed that the rate of barrier recovery increased and was comparable to VC lipid treatment. Okuda et al. [9] also found that the elevated transepidermal water loss (TEWL) and riboflavin penetration in 5% sodium lauryl sulfate-treated rat and human skin were reversed by squalene supplementation (p < 0.05).
Antioxidation
Squalene has been reported to possess antioxidant properties. In vitro experimental evidence indicates that squalene is a highly effective oxygen-scavenging agent. Subsequent to oxidative stress such as sunlight exposure, squalene functions as an efficient quencher of singlet oxygen and prevents the corresponding lipid peroxidation at the human skin surface [10]. Kohno et al. [11] found that the rate constant for quenching singlet oxygen by squalene is much larger than those of other lipids on the human skin surface, and was comparable to that of 3,5-di-t-butyl-4-hydroxytoluene. They also reported that squalene is not particularly susceptible to peroxidation and is stable against attacks by peroxide radicals, suggesting that the chain reaction of lipid peroxidation is unlikely to be propagated with adequate levels of squalene present on the human skin surface. Aioi et al. [12] studied the effects of squalene on superoxide anion (O2-) generation in rats in order to elucidate the mechanism whereby this compound decreases erythema induced by 1% lauroylsarcosine (LS) ointment. LS (200~400 μg/mL) caused overt production of O2- from cultured keratinocytes and peritoneal exudate leukocytes. O2- was significantly reduced by the addition of squalene (100 μg/mL). These results suggest that a possible role of squalene for alleviating skin irritation is by suppression of O2- production, which is dependent on different mechanisms of action of superoxide dismutase.
Antitumor activities
During the past few years, squalene was found to show protective activities against several carcinogens [13]. Desai et al. [14] reported that skin tumors were initiated in 50 female CD-l mice with 7,12-dimethylbenz[a]anthracene and promoted with 12-O-tetradecanoylphorbol-13-acetate. The mice were treated with 5% squalene and at the end of the prevention study, there was a 26.67% reduction in the incidence of tumors in the squalene-treated group. In a related branch of research, a protective effect was observed when squalene was given before and/or during carcinogen treatment. Experimental studies have shown that squalene can effectively inhibit chemically induced skin tumorigenesis in rodents [15].
Squalene as a material in topical formulations
Squalene is also used as a material or additive in topically applied vehicles such as lipid emulsions and nanostructured lipid carriers (NLCs).
Lipid emulsions
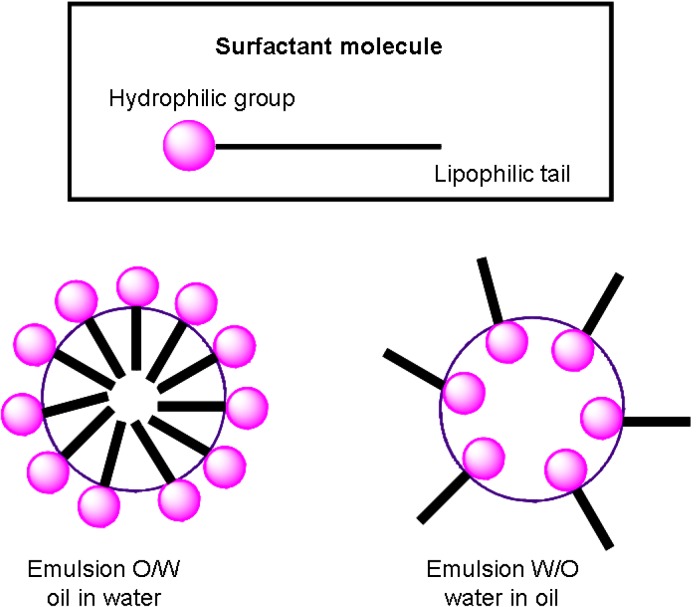
Lipid emulsions are potentially interesting drug delivery systems because of their ability to incorporate drugs with poor solubility within the dispersal phase (Figure 3). An emulsion is a mixture of two immiscible (unblendable) liquids. Lipid emulsions have been studied as parenteral drug carriers for sustained release and organ targeting. By using lipid emulsions, direct contact of the drug with the body fluid and tissues can also be avoided to minimize possible side effects [16]. Chung et al. [17] prepared oil-in-water type lipid emulsions to investigate the effects of different oils on emulsion particle size and stability. Squalene was shown to form stable emulsions when a lipophilic drug was loaded in the discontinuous oil phase. Even though the in vitro transfection activity of emulsions was lower than that of liposomes in the absence of serum, the activity of squalene emulsions, for instance, was approximately 30 times higher than that of liposome in the presence of 80% (v/v) serum (p < 0.05).
Figure 3.
A potent drug carrier vehicle: lipid emulsions.
Kim et al. [18] found that a squalene emulsion had the most potent transfection activity and showed the least cytotoxicity in a mouse model after intravenous administration. Squalene as the oil component can enhance the stability of cationic emulsions more effectively which might be useful for in vitro and in vivo gene transfer. In addition, Wang et al. [19] indicated that emulsions with squalene as the oil phase can act as a potential parenteral drug delivery system for nalbupline and its prodrugs. Squalene as the oil phase produced the smallest particle size compared to coconut oil. The in vivo analgesic activity of the emulsions was examined by a cold ethanol tail-flick test. The squalene system showed the ability to provide controlled delivery to prolong the analgesic duration in rats. The toxicity determined by erythrocyte hemolysis was also low for squalene emulsions.
Nanostructured lipid carriers (NLCs)
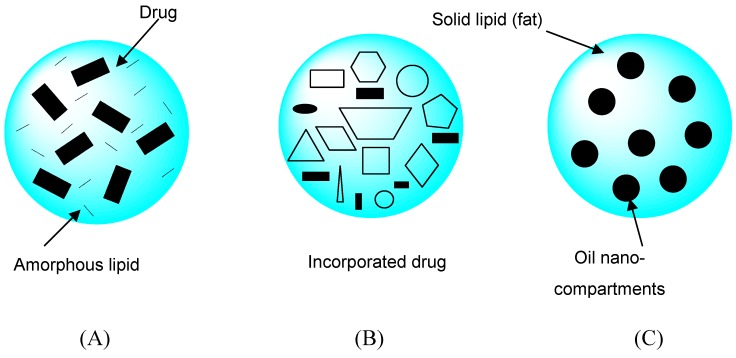
Solid lipid nanoparticles (SLNs) are a new generation of oil-in-water nanoparticulate systems and are attracting attention as novel colloidal drug carriers. Distinct advantages of SLNs are the solid state of the particle matrix, the ability to protect chemically labile ingredients, and the possibility of modulating and prolonging drug release. NLCs are a novel type of lipid nanoparticle with a solid particle matrix possessing structural specialties and improvements such as an increased loading capacity, long-term physical and chemical stability, triggered release, and potentially supersaturated topical formulations. NLCs are produced by mixing solid lipids with spatially incompatible lipids leading to a lipid matrix with a special structure. Depending on the method of production and composition of the lipid blend, different types of NLCs can be obtained (Figure 4). The basic idea is that by giving the lipid matrix a certain nanostructure, the payload of active compounds is increased and expulsion of the compound during storage is avoided [20,21,22,23,24].
Figure 4.
Three different types of nanostructured lipid carriers (NLCs) compared to the more or less highly ordered matrix of solid lipid nanoparticles (SLNs). The three types of NLC can be summarized as: (A) the amorphous type, (B) imperfect type, and (C) multiple type. This figure is modified from reference [24].
Fang et al. [25] found that NLCs consisting of Precirol® and squalene (12% w/v) showed respective mean particle sizes of 200 nm. The lipophilicity of NLCs decreased with an increase in the squalene content in the formulations. Psoralen derivatives (i.e. 8-methoxypsoralen) for psoriasis treatment were loaded in NLCs to examine their ability to permeate via the skin. Enhanced permeation and controlled release of psoralen were both achieved using NLCs with squalene. The in vitro permeation results showed that NLCs stabilized with Tween® 80 increased the 8-methoxypsoralen flux 2.8 times over that of a conventional emulsion.
Production of toxins by squalene oxidation
Squalene monohydroperoxide (SQOOH) is a primary oxidized lipid produced from squalene by solar UV. It is produced at the human skin surface due to natural exposure to sunlight during daily activities. Recent studies demonstrated that repeated application of SQOOH to the skin can induce skin roughness and wrinkles in the hairless mouse [26]. Uchino et al. [27] also reported that SQOOH induced skin damage in hairless mice. Furthermore, a few papers have also described the cytotoxicity of SQOOH, which is a primary oxidized form of epidermal lipids produced by solar UV rays. SQOOH is produced on human forehead skin, and it was suggested that skin squalene may be the principal target lipid for oxidative stress (e.g., sunlight exposure). Nakagawa et al. [28] demonstrated that SQOOH accumulation may be involved in inflammatory skin disorders such as skin cancer, cutaneous autoimmune disease, and skin aging.
Chiba et al. [29] studied the effects of SQOOH, i.e., the initial product of UV-peroxidated squalene, on the skin of hairless mice. Repeated topical application of 10 mM SQOOH to hairless mice for 15 weeks induced definite skin wrinkling. Those results clearly suggested that wrinkling and changes in dermal collagen content induced by SQOOH qualitatively differ from those induced by UVB exposure (p < 0.001). This may provide a useful model for studying skin aging, particularly with regard to the collagen content.
Biological activities of squalene analogs
β-Carotene
Many other polyprenyl compounds structurally similar to squalene exist in nature and perform critical biological functions. β-Carotene is well known to be a potent 1O2 quencher and unique free radical scavenger [30]. This pro-vitamin A, carotenoid, can accumulate in the human skin through oral intake. Stahl et al. [31] demonstrated that oral supplementation of carotenoid from sea algae Dunaliella salina (94% β-carotene and small amounts of α-carotene, cryptoxanthin, zeaxanthin, and lutein) protected human skin from UV light-induced erythema. Bando et al. [30] found that dietary β-carotene acts as a photoprotective agent in the skin. That in vivo study focused on determining the mechanism of action of β-carotene against UVA-induced skin damage by characterizing β-carotene oxidation products. BALB/c mice were fed a β-carotene-supplemented diet, and homogenates from the dorsal skin were prepared after three weeks for UVA irradiation. The results indicated that dietary β-carotene accumulated in the skin and acted as a protective agent against UVA-induced oxidative damage, by quenching 1O2.
A number of studies have demonstrated that dietary β-carotene protects human skin from UV light-induced erythema, but little is known about the protective effect of dietary β-carotene on UVA-induced skin photoaging [32]. Antille et al. [33] showed that topical β-carotene penetrated well into the human and mouse epidermis and induced 10- (human) and a 3-fold (mouse) increases, respectively, in epidermal retinyl esters, which demonstrates that topical β-carotene is converted into retinyl esters by human and mouse epidermis and thus appears as a precursor of epidermal vitamin A.
β-Carotene was partially successful in treating a photosensitivity disorder, erythropoietic protoporphyria, of which singlet oxygen is believed to be an important mediator. Several studies were performed to examine whether β-carotene protects against UV-induced erythema in healthy humans, with widely differing reported effects. The incidence of nonmelanoma skin cancer was reported to be inversely related to serum β-carotene concentrations, and earlier experimental UV-carcinogenesis studies found β-carotene to be photoprotective [34]. However, the role of β-carotene as an anticancer agent was questioned as a result of randomized intervention studies in which supplementation did not reduce the incidence of nonmelanoma skin cancers in humans. Some concern exists over the long-term (eight weeks) use of high-dose β-carotene because supplements of this substance can have deleterious effects at higher doses (15 mg/day) in human skin (p < 0.05).
Coenzyme Q10
Coenzyme Q10 is an important lipophilic antioxidant synthesized by the body [34]. Topical coenzyme Q10 treatment may therefore be proposed as a good pharmacological tool in dermatology and cosmetology [35]. The amount of coenzyme Q10 in skin decreases with age. Topical application of coenzyme Q10 to human skin was found to be effective in reducing the depth of wrinkles. Coenzyme Q10 (0, 1, and 100 mg/kg oral administration) was administered daily for two weeks. Supplementation with 100 mg/kg coenzyme Q10 significantly increased serum and epidermal coenzyme Q10 levels, but did not increase coenzyme Q10 levels in the dermis or other organs. This indicates that coenzyme Q10 intake elevates the epidermal coenzyme Q10 level, which may be a prerequisite to reducing wrinkles and for other benefits related to the potent antioxidant and energizing effects of coenzyme Q10 in the skin [36].
Coenzyme Q10 is a popular antioxidant used in many skin care products to protect the skin from free radical damage. The effects of coenzyme Q10 and colorless carotenoids on the production of inflammatory mediators in human dermal fibroblasts treated with UV radiation and possible synergistic effects of these two antioxidants were evaluated by Fuller et al. [36]. Treatment of fibroblasts with 10 µM coenzyme Q10 suppressed the UV- and interleukin (IL)-1-induced increases in prostaglandin E2, IL-6, and matrix metalloproteinase (MMP)-1. The results suggested that the combination of carotenoids and coenzyme Q10 in topical skin care products may provide enhanced protection from inflammation and premature aging caused by sun exposure.
Vitamin A
Squalene and some of its related substances, including vitamin A, were examined in an animal model to determine the existence of chemopreventive effects. Varani et al. [37] pointed out that topical vitamin A (retinol) stimulated new collagen deposition in sun-protected aged skin, as it did in photoaged skin. In a separate group of 53 individuals (80 years of age), topical application of 1% vitamin A for seven days increased fibroblast growth and collagen synthesis, and concomitantly reduced the levels of matrix-degrading MMPs. In addition, vitamin A treatment reduced MMP expression in aged, sun-protected skin. Retinyl esters, a storage form of vitamin A, are concentrated in the epidermis and absorb UV radiation with a maximum at 325 nm. Antille et al. [38] applied topical retinyl palmitate onto the back of hairless mice before exposing them to 1 J/cm2 UVB, and assayed the levels of thymine dimers produced in epidermal DNA 2 h following UVB exposure. The results demonstrated that epidermal retinyl esters have a biologically relevant filter activity and suggest, besides their pleomorphic biologic actions, a new role for vitamin A that is concentrated in the epidermis (p < 0.05). Moreover, Alberts et al. [39] concluded that vitamin A doses of 50,000 and 75,000 IU/day for one year proved safe, were equally more efficacious than a 25,000 IU/day dose, and could be recommended for future skin cancer chemoprevention studies.
Vitamin A-derived agents still continue to be used to treat acne. Furthermore, retinoids act as chemopreventive and/or chemotherapeutic agents for several types of cancer. They have major effects on the growth and differentiation of normal, premalignant, and malignant epithelial cells both in vitro and in vivo [40]. Retinol (vitamin A) is widely used in the cosmetics industry as an anti-wrinkle agent. However, its photoinstability and skin irritation potential make it challenging to use in general cosmetic formulations [41]. Retinol was applied to skin in either an oil-in-water emulsion or a gel vehicle. Because of the substantial skin reservoir for retinol (0.3%) found at the end of 24 h in human skin studies, additional studies were conducted to compare the in vitro and in vivo skin absorption levels of retinol in the fuzzy rat. Results from those studies were used to help interpret the significance of the in vitro retinol human skin reservoir in determining systemic absorption (p < 0.001) [42]. Jenning et al. [43] evaluated the potential use of solid lipid nanoparticles (SLNs) in dermatology and cosmetics. The influence on drug penetration into porcine skin of glyceryl behenate SLNs loaded with vitamin A (retinol and retinyl palmitate) and incorporated in a hydrogel and oil-in-water cream was tested. Excised full-thickness skin was mounted in an in vitro Franz diffusion assembly, and formulations were applied for 6 and 24 h. Vitamin A concentrations in the skin tissue suggested a certain drug localizing effect. High retinol concentrations were found in the upper skin layers following application of SLN preparations, whereas the deeper regions showed only very low vitamin A levels (p < 0.05).
Vitamin E
Vitamin E is the most potent lipid-soluble antioxidant in vivo. It is thought to play an important role in skin protection [44]. Uddin et al. [45] examined the protective efficacy of vitamin E against tumors induced by UV and arsenite. Hairless mice were exposed to UV plus sodium arsenite (5 mg/L in drinking water) and fed laboratory chow supplemented with vitamin E (R,R,R-a-tocopheryl acetate, 62.5 IU/kg diet) for 26 weeks. Vitamin E reduced the tumor yield in mice given UV and arsenite by 2.1-fold (p < 0.001). Those results show that vitamin E can strongly protect against arsenite-induced enhancement of UV-caused carcinogenesis.
Vitamin E is a group of eight different compounds, but only two of the forms, α-tocopherol and γ-tocopherol, are commonly found in the human body [44]. Lopez-Torres et al. [46] investigated the effects of topical α-tocopherol application on epidermal and dermal tissues and its ability to prevent UV-induced oxidative damage. Hairless mice received a topical application of α-tocopherol 24 h before a single acute exposure to UV irradiation (10 × minimal erythemal dose). α-Tocopherol treatment significantly reduced the formation of epidermal lipid hydroperoxides after UV irradiation (p < 0.05). Those results demonstrated that topical administration of α-tocopherol protects cutaneous tissues against oxidative damage induced by UV irradiation in vivo.
Yoshida et al. [47] investigated whether the topical application of a novel, water-soluble γ-tocopherol derivative, γ-tocopherol-N,N-dimethylglycinate hydrochloride (γ-TDMG), could protect against UV-induced skin damage in hairless mice. Topical pre- or post-application of a 5% (93 mM) γ-TDMG solution in water/propylene glycol/ethanol (2:1:2) significantly prevented sunburned cell formation, lipid peroxidation, and edema/inflammation, which were induced by exposure to a single dose of UV irradiation at 5 kJ/m2 (290~380 nm, maximum 312 nm). Those results suggest that the topical application of γ-TDMG may be efficacious in preventing and reducing UV-induced inflammation.
Ekanayake-Mudiyanselage et al. [48] recently demonstrated that even the use of rinsing products containing α-tocopherol in concentrations of < 0.2% can lead to significantly increased levels of vitamin E in the stratum corneum of human skin and protects against lipid peroxidation in vivo. Therefore, topical formulations containing α-tocopherol at concentrations ranging from 0.1% to 1% are likely to be effective skin care measures to enhance antioxidative protection of the skin barrier. According to the antioxidant network theory, combinations with co-antioxidants such as vitamin C may help enhance the antioxidant effects and stability of vitamin E [49]. A better knowledge of the unique skin-specific physiology of vitamin E, including its percutaneous penetration, skin barrier interactions, bioconversion of vitamin E esters, and cutaneous delivery pathways of oral vitamin E could help in developing more-efficacious skin care products and better evaluating indications and dosage regimens for preventing and treating acute and chronic skin disorders.
Vitamin K
Vitamin K is another squalene-related substance that exhibits benefits to skin physiology. Lou et al. [50] examined the safety and efficacy of a topical vitamin K cream for shortening the duration of laser-induced purpura. From the results of that work, a combination of 1% vitamin K and 0.3% retinol in an acrylate copolymer cream hastened the resolution of laser-induced purpura.
The application of vitamin K to the skin has also been used to suppress pigmentation and resolve bruising. Lopes et al. [51] investigated the in vitro skin penetration and transdermal delivery of vitamin K (2.5%, w/w), and whether these parameters were enhanced by lipid-based drug delivery systems. The experimental results demonstrated that the topical delivery of vitamin K incorporated in a lipophilic vehicle was low. It could be enhanced (~3-fold increase) by monoolein-based systems, which may be useful in increasing the effectiveness of topical vitamin K therapy.
Conclusions
Squalene appears to be critical in reducing free radical oxidative damage to the skin. Although epidemiological, experimental, and animal evidence suggests antitumor properties, few human trials have been conducted to date to verify the role of squalene in cancer therapy. Further studies are needed to explore the usefulness of squalene for treating skin. Several implications can be drawn from this review. Squalene shows several advantages for skin tissues. It is also useful as a material in topically applied vehicles. Substances related to squalene such as β-carotene, coenzyme Q10, and vitamins A, E, and K also exhibit their benefits for skin physiology. Topical administration via the skin is an important route to supplement these compounds within skin tissues. The present success of squalene and its analogs shows the promise of further clinical trials for skin use.
Footnotes
Sample availability: Not available.
References and Notes
- 1.Passi S., De Pità O., Puddu P., Littarru G.P. Lipophilic antioxidants in human sebum and aging. Free Radic. Res. 2002;36:471–477. doi: 10.1080/10715760290021342. [DOI] [PubMed] [Google Scholar]
- 2.Gershbein L.L., Singh E.J. Hydrocarbons of dogfish and cod liver and herring oil. J. Am Oil Chem. Soc. 1969;46:554–557. doi: 10.1007/BF02633182. [DOI] [PubMed] [Google Scholar]
- 3.Auffray B. Protection against singlet oxygen, the main actor of sebum squalene peroxidation during sun exposure, using Commiphora myrrha essential oil. Int. J. Cosmet. Sci. 2007;29:23–29. doi: 10.1111/j.1467-2494.2007.00360.x. [DOI] [PubMed] [Google Scholar]
- 4.Charlton-Menys V., Durrington P.N. Squalene synthase inhibitors: clinical pharmacology and cholesterol-lowering potential. Drugs. 2007;67:11–16. doi: 10.2165/00003495-200767010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Pragst F., Auwärter V., Kiessling B., Dyes C. Wipe-test and patch-test for alcohol misuse based on the concentration ratio of fatty acid ethyl esters and squalene CFAEE/CSQ in skin surface lipids. Forensic Sci. Int. 2004;143:77–86. doi: 10.1016/j.forsciint.2004.02.041. [DOI] [PubMed] [Google Scholar]
- 6.Kelly G.S. Squalene and its potential clinical uses. Altern. Med. Rev. 1999;4:29–36. [PubMed] [Google Scholar]
- 7.Blasco L., Duracher L., Forestier J.P. Skin constituents as cosmetic ingredients: part I: a study of bio-mimetic monoglycerides behavior at the squalene-water interface by the "pendant drop" method in a static mode. J. Dispers. Sci. Technol. 2006;27:799–810. doi: 10.1080/01932690500482137. [DOI] [Google Scholar]
- 8.Rissmann R., Oudshoorn M.H., Kocks E., Hennink W.E., Ponec M., Bouwstra J.A. Lanolin-derived lipid mixtures mimic closely the lipid composition and organization of vernix caseosa lipids. Biochim. Biophys. Acta. 2008;1778:2350–2360. doi: 10.1016/j.bbamem.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Okuda M., Yoshiike T., Ogawa H. Detergent-induced epidermal barrier dysfunction and its prevention. J. Dermatol. Sci. 2002;30:173–179. doi: 10.1016/S0923-1811(02)00106-8. [DOI] [PubMed] [Google Scholar]
- 10.Saint-Leger D., Bague A., Cohen E., Chivot M. A possible role for squalene in the pathogenesis of acne. I. In vitro study of squalene oxidation. Br. J. Dermatol. 1986;114:535–542. doi: 10.1111/j.1365-2133.1986.tb04060.x. [DOI] [PubMed] [Google Scholar]
- 11.Kohno Y., Egawa Y., Itoh S., Nagaoka S., Takahashi M., Mukai K. Kinetic study of quenching reaction of singlet oxygen and scavenging reaction of free radical by squalene in n-butanol. Biochim. Biophys. Acta. 1995;1256:52–56. doi: 10.1016/0005-2760(95)00005-w. [DOI] [PubMed] [Google Scholar]
- 12.Aioi A., Shimizu T., Kuriyama K. Effect of squalene on superoxide anion generation induced by a skin irritant, lauroylsarcosine. Int. J. Pharm. 1995;113:159–164. doi: 10.1016/0378-5173(94)00190-G. [DOI] [Google Scholar]
- 13.Senthilkumar S., Devaki T., Manohar B.M., Babu M.S. Effect of squalene on cyclophosphamide-induced toxicity. Clin. Chim. Acta. 2006;364:335–342. doi: 10.1016/j.cca.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 14.Desai K.N., Wei H., Lamartiniere C.A. The preventive and therapeutic potential of the squalene-containing compound, Roidex, on tumor promotion and regression. Cancer Lett. 1996;19:93–96. doi: 10.1016/0304-3835(96)04122-5. [DOI] [PubMed] [Google Scholar]
- 15.Smith T.J. Squalene: potential chemopreventive agent. Expert Opin. Invest. Drugs. 2000;9:1841–1848. doi: 10.1517/13543784.9.8.1841. [DOI] [PubMed] [Google Scholar]
- 16.Nicolaos G., Crauste-Manciet S., Farinotti R., Brossard D. Improvement of cefpodoxime proxetil oral absorption in rats by an oil-in-water submicron emulsion. Int. J. Pharm. 2003;16:165–171. doi: 10.1016/s0378-5173(03)00365-x. [DOI] [PubMed] [Google Scholar]
- 17.Chung H., Kim T.W., Kwon M., Kwon I.C., Jeong S.Y. Oil components modulate physical characteristics and function of the natural oil emulsions as drug or gene delivery system. J. Control. Release. 2001;71:339–350. doi: 10.1016/S0168-3659(00)00363-1. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y.J., Kim T.W., Chung H., Kwon I.C., Sung H.C., Jeong S.Y. The effects of serum on the stability and the transfection activity of the cationic lipid emulsion with various oils. Int. J. Pharm. 2003;252:241–252. doi: 10.1016/S0378-5173(02)00676-2. [DOI] [PubMed] [Google Scholar]
- 19.Wang J.J., Sung K.C., Hu O.Y., Yeh C.H., Fang J.Y. Submicron lipid emulsion as a drug delivery system for nalbuphine and its prodrugs. J. Control. Release. 2006;115:140–149. doi: 10.1016/j.jconrel.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 20.Müller R.H., Radtke M., Wissing S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002;54:S131–S155. doi: 10.1016/S0169-409X(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 21.Müller R.H., Petersen R.D., Hommoss A., Pardeike J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007;59:522–530. doi: 10.1016/j.addr.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Lombardi Borgia S., Regehly M., Sivaramakrishnan R., Mehnert W., Korting H.C., Danker K., Röder B., Kramer K.D., Schäfer-Korting M. Lipid nanoparticles for skin penetration enhancement-correlation to drug localization within the particle matrix as determined by fluorescence and parelectric spectroscopy. J. Control. Release. 2005;110:151–163. doi: 10.1016/j.jconrel.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 23.Schäfer-Korting M., Mehnert W., Korting H.C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007;59:427–443. doi: 10.1016/j.addr.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Müller R.H., Radtke M., Wissing S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Adv. Drug Deliv. Rev. 2002;242:121–128. doi: 10.1016/s0378-5173(02)00180-1. [DOI] [PubMed] [Google Scholar]
- 25.Fang J.Y., Fang C.L., Liu C.H., Su Y.H. Lipid nanoparticles as vehicles for topical psoralen delivery: solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC) Eur. J. Pharm. Biopharm. 2008;70:633–640. doi: 10.1016/j.ejpb.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Chiba K., Yoshizawa K., Makino I., Kawakami K., Onoue M. Changes in the levels of glutathione after cellular and cutaneous damage induced by squalene monohydroperoxide. J. Biochem. Mol. Toxicol. 2001;15:150–158. doi: 10.1002/jbt.12. [DOI] [PubMed] [Google Scholar]
- 27.Uchino T., Kawahara N., Sekita S., Satake M., Saito Y., Tokunaga H., Ando M. Potent protecting effects of catuaba (Anemopaegma mirandum) extracts against hydroperoxide-induced cytotoxicity. Toxicol. In Vitro. 2004;18:255–263. doi: 10.1016/j.tiv.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa K., Ibusuki D., Suzuki Y., Yamashita S., Higuchi O., Oikawa S., Miyazawa T. Ion-trap tandem mass spectrometric analysis of squalene monohydroperoxide isomers in sunlight-exposed human skin. J. Lipid Res. 2007;48:2779–2787. doi: 10.1194/jlr.D700016-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Chiba K., Kawakami K., Sone T., Onoue M. Characteristics of skin wrinkling and dermal changes induced by repeated application of squalene monohydroperoxide to hairless mouse skin. Skin Pharmacol. Appl. Skin Physiol. 2003;16:242–251. doi: 10.1159/000070847. [DOI] [PubMed] [Google Scholar]
- 30.Bando N., Hayashi H., Wakamatsu S., Inakuma T., Miyoshi M., Nagao A., Yamauchi R., Terao J. Participation of singlet oxygen in ultraviolet-a-induced lipid peroxidation in mouse skin and its inhibition by dietary beta-carotene: an ex vivo study. Free Radic. Biol. Med. 2004;37:1854–1863. doi: 10.1016/j.freeradbiomed.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Stahl W., Heinrich U., Jungmann H., Sies H., Tronnier H. Carotenoids and carotenoids plus vitamin E protect against ultraviolet light-induced erythema in humans. Am. J. Clin. Nutr. 2000;71:795–798. doi: 10.1093/ajcn/71.3.795. [DOI] [PubMed] [Google Scholar]
- 32.Minami Y., Kawabata K., Kubo Y., Arase S., Hirasaka K., Nikawa T., Bando N., Kawai Y., Terao J. Peroxidized cholesterol-induced matrix metalloproteinase-9 activation and its suppression by dietary beta-carotene in photoaging of hairless mouse skin. J. Nutr. Biochem. 2008 doi: 10.1016/j.jnutbio.2008.04.010. in press. [DOI] [PubMed] [Google Scholar]
- 33.Antille C., Tran C., Sorg O., Saurat J.H. Topical beta-carotene is converted to retinyl esters in human skin ex vivo and mouse skin in vivo. Exp. Dermatol. 2004;13:558–561. doi: 10.1111/j.0906-6705.2004.00194.x. [DOI] [PubMed] [Google Scholar]
- 34.McArdle F., Rhodes L.E., Parslew R.A., Close G.L., Jack C.I., Friedmann P.S., Jackson M.J. Effects of oral vitamin E and beta-carotene supplementation on ultraviolet radiation-induced oxidative stress in human skin. Am. J. Clin. Nutr. 2004;80:1270–1275. doi: 10.1093/ajcn/80.5.1270. [DOI] [PubMed] [Google Scholar]
- 35.Ashida Y., Yamanishi H., Terada T., Oota N., Sekine K., Watabe K. CoQ10 supplementation elevates the epidermal CoQ10 level in adult hairless mice. Biofactors. 2005;25:175–178. doi: 10.1002/biof.5520250120. [DOI] [PubMed] [Google Scholar]
- 36.Fuller B., Smith D., Howerton A., Kern D. Anti-inflammatory effects of CoQ10 and colorless carotenoids. J. Cosmet. Dermatol. 2005;5:30–38. doi: 10.1111/j.1473-2165.2006.00220.x. [DOI] [PubMed] [Google Scholar]
- 37.Varani J., Warner R.L., Gharaee-Kermani M., Phan S.H., Kang S., Chung J.H., Wang Z.Q., Datta S.C., Fisher G.J., Voorhees J.J. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J. Invest. Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- 38.Antille C., Tran C., Sorg O., Carraux P., Didierjean L., Saurat J.H. Vitamin A exerts a photoprotective action in skin by absorbing ultraviolet B radiation. J. Invest. Dermatol. 2003;121:1163–1167. doi: 10.1046/j.1523-1747.2003.12519.x. [DOI] [PubMed] [Google Scholar]
- 39.Alberts D., Ranger-Moore J., Einspahr J., Saboda K., Bozzo P., Liu Y., Xu X.C., Lotan R., Warneke J., Salasche S., Stratton S., Levine N., Goldman R., Islas M., Duckett L., Thompson D., Bartels P., Foote J. Safety and efficacy of dose-intensive oral vitamin A in subjects with sun-damaged skin. Clin. Cancer Res. 2004;10:1875–1880. doi: 10.1158/1078-0432.CCR-03-0188. [DOI] [PubMed] [Google Scholar]
- 40.Lee M.S., Lee K.H., Sin H.S., Um S.J., Kim J.W., Koh B.K. A newly synthesized photostable retinol derivative (retinyl N-formyl aspartamate) for photodamaged skin: profilometric evaluation of 24-week study. J. Am. Acad. Dermatol. 2006;55:220–224. doi: 10.1016/j.jaad.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Carlotti M.E., Rossatto V., Gallarate M. Vitamin A and vitamin A palmitate stability over time and under UVA and UVB radiation. Int. J. Pharm. 2002;240:85–94. doi: 10.1016/S0378-5173(02)00128-X. [DOI] [PubMed] [Google Scholar]
- 42.Guo X., Ruiz A., Rando R.R., Bok D., Gudas L.J. Esterification of all-trans-retinol in normal human epithelial cell strains and carcinoma lines from oral cavity, skin and breast: reduced expression of lecithin: retinol acyltransferase in carcinoma lines. Carcinogenesis. 2000;21:1925–1933. doi: 10.1093/carcin/21.11.1925. [DOI] [PubMed] [Google Scholar]
- 43.Jenning V., Gysler A., Schäfer-Korting M., Gohla S.H. Vitamin A loaded solid lipid nanoparticles for topical use: occlusive properties and drug targeting to the upper skin. Eur. J. Pharm Biopharm. 2000;49:211–218. doi: 10.1016/S0939-6411(99)00075-2. [DOI] [PubMed] [Google Scholar]
- 44.Mitchel R.E., McCann R.A. Skin tumor promotion by vitamin E in mice: amplification by ionizing radiation and vitamin C. Cancer Detect. Prev. 2003;27:102–108. doi: 10.1016/S0361-090X(03)00028-X. [DOI] [PubMed] [Google Scholar]
- 45.Uddin A.N., Burns F.J., Rossman T.G. Vitamin E and organoselenium prevent the cocarcinogenic activity of arsenite with solar UVR in mouse skin. Carcinogenesis. 2005;26:2179–2186. doi: 10.1093/carcin/bgi180. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Torres M., Thiele J.J., Shindo Y., Han D., Packer L. Topical application of alpha-tocopherol modulates the antioxidant network and diminishes ultraviolet-induced oxidative damage in murine skin. Br. J. Dermatol. 1998;138:207–215. doi: 10.1046/j.1365-2133.1998.02062.x. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida E., Watanabe T., Takata J., Yamazaki A., Karube Y., Kobayashi S. Topical application of a novel, hydrophilic gamma-tocopherol derivative reduces photo-inflammation in mice skin. J. Invest. Dermatol. 2006;126:1633–1640. doi: 10.1038/sj.jid.5700236. [DOI] [PubMed] [Google Scholar]
- 48.Ekanayake-Mudiyanselage S., Tavakkol A., Polefka T.G., Nabi Z., Elsner P., Thiele J.J. Vitamin E delivery to human skin by a rinse-off product: penetration of alpha-tocopherol versus wash-out effects of skin surface lipids. Skin. Pharmacol. Physiol. 2005;18:20–26. doi: 10.1159/000081682. [DOI] [PubMed] [Google Scholar]
- 49.Thiele J.J., Ekanayake-Mudiyanselage S. Vitamin E in human skin: organ-specific physiology and considerations for its use in dermatology. Mol. Aspects Med. 2007;28:646–667. doi: 10.1016/j.mam.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Lou W.W., Quintana A.T., Geronemus R.G., Grossman M.C. Effects of topical vitamin K and retinol on laser-induced purpura on nonlesional skin. Dermatol. Surg. 1999;25:942–944. doi: 10.1046/j.1524-4725.1999.99145.x. [DOI] [PubMed] [Google Scholar]
- 51.Lopes L.B., Speretta F.F., Bentley M.V. Enhancement of skin penetration of vitamin K using monoolein-based liquid crystalline systems. Eur. J. Pharm. Sci. 2007;32:209–215. doi: 10.1016/j.ejps.2007.07.006. [DOI] [PubMed] [Google Scholar]