Abstract
Background
Achieving stable closure of complex or contaminated abdominal wall incisions remains challenging. This study aimed to characterize the stage of innovation for biological mesh devices used during complex abdominal wall reconstruction and to evaluate the quality of current evidence.
Methods
A systematic review was performed of published and ongoing studies between January 2000 and September 2017. Eligible studies were those where a biological mesh was used to support fascial closure, either prophylactically after midline laparotomy, or for reinforcement after repair of incisional hernia with midline incision. The primary outcome measure was the IDEAL framework stage of innovation. The key secondary outcome measure was the GRADE criteria for study quality.
Results
Thirty‐five studies including 2681 patients were included. Four studies considered mesh prophylaxis, 23 considered hernia repair, and eight reported on both. There was one published randomized trial (IDEAL stage 3), none of which was of high quality; the others were non‐randomized studies (IDEAL stage 2a). A detailed description of surgical technique was provided in most studies (27 of 35); however, no study reported outcomes according to the European Hernia Society consensus statement and only two described quality control of surgical technique during the study. From 21 ongoing randomized trials and observational studies, 11 considered repair of incisional hernia and 10 considered prophylaxis (seven in elective settings).
Conclusion
The evidence base for biological mesh is limited, and better reporting and quality control of surgical techniques are needed. Although results of ongoing trials over the next decade will improve the evidence base, further study is required in the emergency and contaminated settings.
Introduction
Incisional hernias carry a significant burden for both patients and the health service1, 2, 3, 4. They prevent return to normal activities and can be painful. Elective repair can be challenging, and emergency repair carries significant clinical risks. Incisional hernia is common, occurring in up to 50 per cent of patients after laparotomy5 6, and with the growing number of emergency laparotomies performed in the UK, the number of affected patients is likely to increase7.
To limit the number of incisional hernias there has been a focus on the use of prophylactic mesh reinforcement. The cost of mesh is far less than that of major reoperations and emergency admissions3 8, 9, 10. Although synthetic meshes are accepted in many cases, they are not used in complex and contaminated settings owing to the risk of infection (as high as 50–90 per cent), pain, fistulation and need for explantation11, 12, 13, 14. Biological mesh has evolved to fill this gap, with expected reduced rates of infection leading to safer prophylaxis. Current guidelines, including the Ventral Hernia Working Group expert consensus, and several systematic reviews recommend against the use of synthetic mesh when the risk of wound complications is high, such as in the presence of gross contamination; instead they advocate the use of a biological absorbable mesh15, 16, 17.
Biological mesh has entered widespread clinical practice, but the quality and scope of the evidence base for use in complex and contaminated abdominal wounds are unclear. This review aimed to determine the quality and stage of innovation of the evidence supporting biological mesh placement during abdominal wall reconstruction with primary fascial closure. The hypothesis was that the evidence base supporting biological mesh use is currently too limited to support routine clinical use outside clinical trials.
Methods
Search strategy
A systematic search of PubMed, EMBASE and the Cochrane Library between 1 January 2000 and 27 September 2017 was performed by two independent investigators. The http://clinicaltrials.gov database was also queried for ongoing studies. The search terms used were ‘laparotomy’, ‘mesh’, ‘biologic material’, ‘abdominal wall’, ‘hernia’, and ‘complications’, ‘contamination’, ‘infection’ or ‘surgical site infection’, individually or in combination. The ‘related articles’ function was used to broaden the search, and all citations were considered for relevance. A manual search of reference lists in recent reviews and eligible studies was also undertaken. This paper is reported according to the PRISMA guidelines18.
Inclusion and exclusion criteria
Studies were included according to the following criteria: evaluation of the use of a xenograft biological mesh to support primary fascial closure of midline abdominal wounds or repair of incisional hernia with midline incision; study design was an RCT, prospective observational study, retrospective cohort study or case series; study included only patients aged 16 years or more.
The following exclusion criteria were employed: study design was a systematic review, meta‐analysis, letter, review, comment or conference abstract; fewer than five patients were included in the study; only synthetic mesh or composite meshes were evaluated; allograft or autograft meshes, including human‐derived acellular dermal matrix, were used (availability in Europe across the selected inclusion dates was low until recently, so reporting is likely to be incomplete); study reported bridging repairs (fascial closure not achieved), including studies where outcomes for fascial closure were not reported separately from bridging repair.
Study outcome measures
The primary outcome measure was the stage of innovation, according to the IDEAL framework19. The level of evidence in the IDEAL staging system were 1 (case series with high risk of bias), 2a (cohort study), 2b (feasibility RCT), 3 (RCT) and 4 (high‐quality prospective registry with long‐term monitoring and low risk of bias). All assessments in the present study were carried out independently by two authors; disagreement was resolved by re‐examining the relevant article until consensus was achieved.
Secondary outcome measures
The main secondary outcome measure was the quality of evidence assessed using the GRADE system20. In the GRADE approach, studies are categorized as of high (randomized trials or double‐upgraded observational studies), moderate (downgraded randomized trials or upgraded observational studies), low (double‐downgraded randomized trials or observational studies) and very low (triple‐downgraded randomized trials, downgraded observational studies or case series/case reports) quality. The other secondary outcome measures of interest were the numbers of studies reporting: outcomes according to the European Hernia Society consensus statement21, incidence of incisional hernia, surgical‐site infection (SSI) rate, and seroma.
Data extraction
Data extracted included patient demographics, indications and type of biological mesh used. Studies were grouped into those examining prophylactic placement in primary closure of laparotomy only (prophylaxis), repair of incisional hernia only (reinforcement), or both (mixed). Descriptions of procedures performed were collected, including surgical technique, number of procedures previously performed by the surgeon, and monitoring of technique. Degree of contamination (clean‐contaminated, contaminated or dirty surgery) was defined according to the US Centers for Disease Control and Prevention (CDC) surgical wounds classification22, and the location of biological mesh placement was also evaluated, as either intraperitoneal (intraperitoneal, intraperitoneal onlay mesh, underlay, intra‐abdominal) or extraperitoneal (sublay, onlay, inlay, retromuscular, retrorectus, prefascial)23.
Statistical analysis
Analysis was intended to be primarily descriptive in nature, with no need for modelling or multivariable analyses. Event rates are reported as percentages. Continuous variables were tested for normality.
Results
Of 1304 studies shortlisted, 35 full‐text articles24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58 met the inclusion criteria (Fig. 1). Of these, four examined biological mesh for prophylaxis, 23 reported on reinforcement after incisional hernia repair, and eight reported both prophylaxis and incisional hernia repair. Studies of biological mesh for prophylaxis included a total of 85 patients with a median follow‐up of 12 (i.q.r. 2–31) months; those used for reinforcement included 1744 patients with a median follow‐up of 16 (12–24) months, and those for mixed indications included 852 patients with a median follow‐up of 24 (17–48) months.
Figure 1.
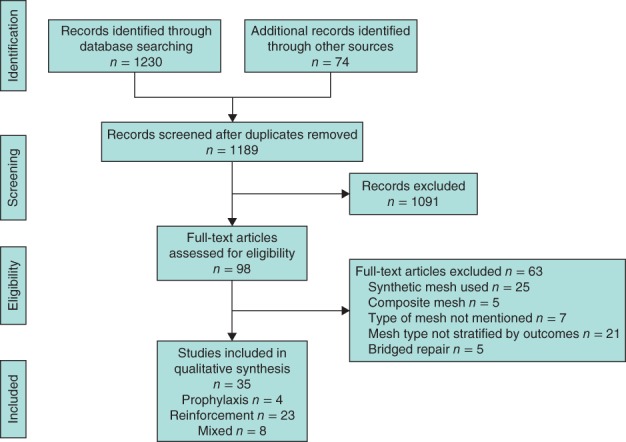
PRISMA diagram for the study
Mesh characteristics
Tables 1 and 2 summarize characteristics of the included studies. Strattice™ (KCI Medical, Dublin, Ireland) (2 studies), Surgisis® (Cook Biotech, West Lafayette, Indiana, USA) (1 study) and bovine pericardium (1) were used for prophylaxis in abdominal wound reconstruction. For reinforcement, Permacol™ (Tissue Science Laboratories, Andover, Massachusetts, USA) (9 studies) was the most commonly used mesh, followed by Strattice™ (4) and Surgisis® (3); a further seven studies each used different meshes. In papers reporting mixed indications, Permacol™ (5 studies) was the most commonly reported, followed by XenMatrix™ (Brennen Medical, St Paul, Minnesota, USA; Davol, Warwick, Rhode Island, USA) (1), Strattice™ (1) and SurgiMend™ (TEI Biosciences, Boston, Massachusetts, USA) (1).
Table 1.
Patient characteristics (arranged alphabetically by timing of surgery)
| Reference | No. of patients | Median age (years) | Mean BMI (kg/m2) | Timing of surgery* | Indication for surgery |
|---|---|---|---|---|---|
| Bali et al.26 | 40 | 75 | 25 | Elective | AAA repair |
| Bhangu et al.25 | 7 | 9 | n.a. | Elective | Stoma closure |
| Boules et al.49 | 45 | 57 | 33 | Elective | Incisional hernia repair |
| Boutros et al.27 | 8 | 60 | n.a. | Elective | AWR after HIPEC |
| Chamieh et al.31 | 58 | n.a. | n.a. | Elective | Incisional hernia repair |
| Chavarriaga et al.32 | 18 | 49 | n.a. | Elective | Incisional hernia repair |
| Cheng et al.28 | 270 | 60 | 32 | Elective | Incisional hernia repair |
| Cox et al.48 | 6 | 49 | 25 | Elective | Incisional hernia repair |
| Fayezizadeh et al.33 | 77 | 56 | 35 | Elective | Incisional hernia repair |
| Garvey et al.52 | 191 | 58 | 31 | Elective | AWR, incisional hernia repair |
| Giordano et al.47 | 109 | 64 | 30 | Elective | Incisional hernia repair |
| Giordano et al.53 | 484 | 59 | 31 | Elective | Data not available |
| Gnaneswaran et al.50 | 12 | 51 | 32 | Elective | Incisional hernia repair |
| Hicks et al.34 | 60 | 59 | 36 | Elective | Incisional hernia repair |
| Høyrup et al.57 | 10 | 66 | n.a. | Elective | Incisional hernia repair, stoma closure, left hemicolectomy, anterior resection, bowel obstruction |
| Hsu et al.35 | 28 | 55 | 34 | Elective | Incisional hernia repair |
| Itani et al.36 | 80 | 57 | n.a. | Elective | Incisional hernia repair |
| Limpert et al.37 | 26 | 54 | n.a. | Elective | Incisional hernia repair |
| Madani et al.38 | 46 | 58 | 28 | Elective | Incisional hernia repair |
| Maggiori et al.24 | 30 | 61 | 26 | Elective | Stoma closure |
| Majumder et al.39 | 126 | 59 | 37 | Elective | Incisional hernia repair |
| Nockolds et al.40 | 23 | 57 | n.a. | Elective | Incisional hernia repair |
| O'Halloran et al.41 | 85 | 56 | 33 | Elective | Incisional hernia repair |
| Patel et al.42 | 41 | 42 | 20 | Elective | Incisional hernia repair |
| Rosen et al.43 | 128 | 58 | 34 | Elective | Incisional hernia repair |
| Sbitany et al.44 | 41 | 66 | 25 | Elective | Incisional hernia repair |
| Shah et al.45 | 58 | 57 | 34 | Elective | Incisional hernia repair |
| Shaikh et al.56 | 20 | 51 | n.a. | Elective | Incisional hernia repair, re‐exploration laparotomy, multiple stab wounds, desmoid tumour resection |
| Ueno et al.29 | 20 | 60 | n.a. | Elective | Incisional hernia repair |
| Warwick et al.30 | 57 | 64 | 30 | Elective | Incisional hernia repair |
| Zerbib et al.46 | 14 | 60 | 35 | Elective | Incisional hernia repair |
| Abdelfatah et al.58 | 65 | 55 | 35 | Mixed | Incisional hernia repair, intestinal obstruction (bowel strangulation and resection), resection of large section abdominal wall, infected alloplastic mesh |
| Byrnes et al.51 | 57 | 49 | 32 | Mixed | Incisional hernia repair, trauma laparotomy |
| Parker et al.54 | 9 | 58 | n.a. | Mixed | Incisional hernia repair, AWR for abdominal wall tumour |
| Pomahac and Aflaki55 | 16 | 59 | 28 | Mixed | Incisional hernia repair, intra‐abdominal emergencies, extensive bowel resection, abdominal compartment syndrome secondary to necrotizing fasciitis |
Mixed indicates both elective and emergency surgery.
AAA, abdominal aortic aneurysm; AWR, abdominal wall reconstruction; HIPEC, hyperthermic intraperitoneal chemotherapy.
Table 2.
Summary of surgery and mesh characteristics (arranged chronologically by indication)
| Reference | Year | Country | Indication | Type of mesh | Median follow‐up (months) |
|---|---|---|---|---|---|
| Boutros et al.27 | 2010 | USA | Prophylaxis | Surgisis® | 6 |
| Bhangu et al.25 | 2014 | UK | Prophylaxis | Strattice™ | 1 |
| Bali et al.26 | 2015 | Greece | Prophylaxis | Bovine pericardium | 36 |
| Maggiori et al.24 | 2015 | France | Prophylaxis | Strattice™ | 17 |
| Ueno et al.29 | 2004 | USA | Reinforcement | Surgisis® | 16 |
| Limpert et al.37 | 2009 | USA | Reinforcement | Bovine pericardium | 22 |
| Hsu et al.35 | 2009 | USA | Reinforcement | Permacol™ | 16 |
| Chavarriaga et al.32 | 2010 | USA | Reinforcement | Permacol™ | 7 |
| Cox et al.48 | 2010 | USA | Reinforcement | Surgisis® | 10 |
| Shah et al.45 | 2011 | USA | Reinforcement | XenMatrix™ | 12 |
| Patel et al.42 | 2012 | USA | Reinforcement | Strattice™ | 16 |
| Itani et al.36 | 2012 | USA | Reinforcement | Strattice™ | 24 |
| Rosen et al.43 | 2013 | USA | Reinforcement | Strattice™ | 22 |
| Nockolds et al.40 | 2014 | UK | Reinforcement | Permacol™ | 17 |
| Cheng et al.28 | 2014 | USA | Reinforcement | Permacol™/Strattice™ | 25 |
| O'Halloran et al.41 | 2014 | USA | Reinforcement | Unknown | 14 |
| Zerbib et al.46 | 2015 | France | Reinforcement | Permacol™ | 13 |
| Giordano et al.47 | 2015 | UK | Reinforcement | Permacol™ | 24 |
| Sbitany et al.44 | 2015 | USA | Reinforcement | Strattice™ | 5 |
| Gnaneswaran et al.50 | 2016 | Australia | Reinforcement | BioDesign® | 14 |
| Fayezizadeh et al.33 | 2016 | USA | Reinforcement | Permacol™ | 28 |
| Majumder et al.39 | 2016 | USA | Reinforcement | Permacol™ | 22 |
| Hicks et al.34 | 2016 | USA | Reinforcement | SurgiMend™ | 12 |
| Warwick et al.30 | 2017 | UK | Reinforcement | Permacol™ | 18 |
| Madani et al.38 | 2017 | Canada | Reinforcement | Surgisis® | 47 |
| Chamieh et al.31 | 2017 | USA | Reinforcement | Mixed | 11 |
| Boules et al.49 | 2018 | USA | Reinforcement | Permacol™ | 72 |
| Parker et al.54 | 2006 | USA | Mixed | Permacol™ | 18 |
| Shaikh et al.56 | 2007 | Ireland | Mixed | Permacol™ | 18 |
| Pomahac and Aflaki55 | 2010 | USA | Mixed | Permacol™ | 17 |
| Byrnes et al.51 | 2011 | USA | Mixed | XenMatrix™ | 31 |
| Høyrup et al.57 | 2012 | Denmark | Mixed | Permacol™ | 8 |
| Abdelfatah et al.58 | 2015 | USA | Mixed | Permacol™ | 60 |
| Garvey et al.52 | 2017 | USA | Mixed | Strattice™ | 53 |
| Giordano et al.53 | 2017 | USA | Mixed | SurgiMend™ | 31 |
IDEAL stage of innovation and GRADE quality of evidence
Distribution of IDEAL stage and GRADE quality of included studies are presented in Tables 3 and 4 respectively. Of the four prophylaxis studies, two24 25 evaluated biological mesh at the time of stoma closure, one26 following midline laparotomy after abdominal aortic aneurysm (AAA) repair, and one27 after cytoreduction and hyperthermic intraperitoneal chemotherapy. All four studies included only elective patients and the degrees of contamination were clean‐contaminated (2) and contaminated (2). Strattice™ was used in two studies24 25 with an intraperitoneal placement; the others used bovine pericardium in an extraperitoneal position (1)26 or Surgisis® in an intraperitoneal position (1)27. One study24 was IDEAL stage 2a (low quality) and the other26 was IDEAL stage 3 (moderate quality). Two studies25 27 reported only outcomes of patients with biological mesh; both studies were IDEAL stage 2a (very low quality).
Table 3.
Distribution of IDEAL stage of innovation, by indication
| IDEAL stage | |||||
|---|---|---|---|---|---|
| Indication | 1 (case report) | 2a (cohort study) | 2b (feasibility RCT) | 3 (RCT) | 4 (registry) |
| Total (n = 35) | 0 | 34 | 0 | 1 | 0 |
| Prophylaxis (n = 4) | 0 | 3 | 0 | 1 | 0 |
| Reinforcement (n = 23) | 0 | 23 | 0 | 0 | 0 |
| Mixed (n = 8) | 0 | 8 | 0 | 0 | 0 |
Table 4.
Distribution of GRADE study quality, by indication
| GRADE quality | ||||
|---|---|---|---|---|
| Indication | High | Moderate | Low | Very low |
| Total (n = 35) | 0 | 5 | 18 | 12 |
| Prophylaxis (n = 4) | 0 | 1 | 1 | 2 |
| Reinforcement (n = 23) | 0 | 2 | 14 | 7 |
| Mixed (n = 8) | 0 | 2 | 3 | 3 |
Of the 23 studies28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 using biological mesh for reinforcement, all reported only elective patients undergoing repair of incisional hernia. The degree of contamination in all studies was clean‐contaminated. Mesh placement was reported as intraperitoneal in five studies34 35, 42 44, 46, extraperitoneal in seven30 32, 33 37, 40 48, 50 and a combination in ten studies29 31, 36 38, 39 41, 43 45, 47 49. One study28 did not report the location of mesh placement. Four30 31, 39 41 of the 23 studies compared biological versus synthetic mesh. All 23 studies were IDEAL stage 2a (cohort studies). Seven29 31, 38 40, 48, 49, 50 were of very low quality, 1430 32, 33, 34, 35, 36, 37 39, 42, 43, 44, 45, 46, 47 of low quality, and two28 41 of moderate quality. None reported standardization of technique or location of biological mesh placement; the choice of mesh type was based on the preference of operating surgeon.
Of the eight studies evaluating biological mesh for mixed indications, four included patients undergoing elective surgery and the remaining four studies included both elective and emergency operations. The eight studies involved a mixture of procedures, with degree of contamination ranging from clean‐contaminated to dirty. Mesh placement was intraperitoneal in six studies51, 52, 53, 54, 55, 56, extraperitoneal in one study57, and a combination in one study58. All were IDEAL stage 2a (cohort studies). Evidence was of very low quality in three studies54 56, 57, low quality in three51 52, 55, and moderate quality in two53 58. The evidence in one study58 of abdominal wall reconstruction with porcine acellular dermal matrix (Permacol™) was of moderate quality owing to reporting of long‐term outcomes of at least 5 years.
Outcome reporting
None of the studies in this review reported outcomes according to the European Hernia Society consensus statement21, and none reported ‘free from hernia’ survival times. All four studies24, 25, 26, 27 in the prophylaxis group reported a definition for detection of incisional hernia, which included a combination of clinical examination and radiological assessment. In the reinforcement group, 1329 30, 32 33, 35 36, 38 39, 41, 42, 43, 44 47 of the 23 studies gave a definition for recurrence of hernia (6 clinical, 7 radiological, none patient‐reported). SSI rates were reported in one25 of the four studies in the prophylaxis group, and in 21 of the 23 studies in the reinforcement group. The incidence of seroma was reported in three prophylaxis and 19 reinforcement studies.
Reporting of surgical technique
Of the 35 studies, 27 provided details of surgical procedures: all four studies in the prophylaxis group, 16 in the reinforcement group, and seven in the mixed group (Table 5). Only one paper46 reported the minimum number of procedures performed by the operating surgeons as a requirement.
Table 5.
Reporting of surgical technique
| All indications* | Prophylaxis only | Reinforcement only | |
|---|---|---|---|
| No. of studies | 35 | 4 | 23 |
| Total no. of patients | 2681 | 85 | 1744 |
| Mesh type | |||
| BioDesign® | 1 | 0 | 1 |
| Bovine pericardium | 2 | 1 | 1 |
| Mixed | 1 | 0 | 1 |
| Permacol™ | 12 | 0 | 9 |
| Permacol™/Strattice™ | 1 | 0 | 1 |
| Strattice™ | 7 | 2 | 4 |
| SurgiMend™ | 2 | 0 | 1 |
| Surgisis® | 4 | 1 | 3 |
| XenMatrix™ | 3 | 0 | 2 |
| n.r. | 1 | 0 | 1 |
| Location of mesh placement | |||
| Intraperitoneal (intraperitoneal underlay) | 14 | 3 | 5 |
| Extraperitoneal (sublay, onlay, inlay) | 9 | 1 | 7 |
| Mixed (intraperitoneal and extraperitoneal) | 11 | 0 | 10 |
| n.r. | 1 | 0 | 1 |
| Description of procedure | |||
| Detailed surgical technique provided | 27 | 4 | 16 |
| Surgeon's no. of previous procedures provided | 1 | 0 | 1 |
| Monitoring of technique | 2 | 0 | 2 |
Includes prophylaxis only, reinforcement only, and mixed. n.r., Not reported.
Ongoing studies
Twenty‐one ongoing studies were identified from http://clinicaltrials.gov, of which ten were for prophylaxis and 11 for reinforcement. In the prophylaxis group, all were RCTs; four had completed data collection, five were still recruiting, and one had terminated early. Patient groups being studied included emergency midline laparotomy (1 study), elective patients for AAA repair (1), midline laparotomy (1), contaminated abdominal wall defect (1, terminated), abdominoperineal resection (1) and stoma closure (5). Of these ten, the majority studied Strattice™ (4), followed by Permacol™ (1) and Surgisis® (1). The type of biological mesh was not mentioned in the remaining four studies. In the 11 ongoing trials of reinforcement, nine were RCTs and two were cohort studies. Two studies (1 cohort study of Permacol™ and 1 RCT of XenMatrix™) were in follow‐up phase; the remainder were still recruiting patients.
Discussion
This review identified that the evidence base for biological mesh in complex and contaminated settings is still evolving, and highlighted areas for improvement. At present, the quality of the evidence base is generally low, with a few exceptions. The majority of studies included in this review were IDEAL stage 1 or 2 (case series or cohort studies) with a low or very low GRADE quality of evidence, indicating that biological meshes remain in the early stages of evaluation and adoption. This is compounded by a wide variation in mesh types and mesh placement, with little control for surgical technique, making synthesis of evidence ineffective.
There are two key recommendations from the present study. First, the evidence base needs to be improved by testing the efficacy of biological mesh in randomized trials. This should include standardization of techniques and reporting, and inclusion of more emergency cases to establish the limits of indication. Second, future studies should allow consistent reporting of mesh type and exact placement to enable high‐quality recommendations to help standardize practice. Until such data are available, use in selected higher‐risk patients (such as prophylaxis during abdominal wall closure in contaminated cases at high risk of incisional hernia) should be supported by data capture within controlled trials or registries. Routine clinical use in low‐risk patients is not yet justified.
Surgeons and patients will benefit from knowing about mesh performance based on the specific type of mesh, the position it is placed in, and the expected long‐term outcome. The present study identified variation in outcome reporting for recurrence rates, SSI and seroma. This variation precludes reliable assessment of outcomes and formation of recommendations. Recently, Blencowe and colleagues59 proposed a standard approach for the description, standardization and monitoring of the intervention to enable reliable assessment of outcome from this type of study and, importantly, reproducibility of an intervention by surgeons in their clinical practice. In this review, only one study46 had monitoring of technique by a senior surgeon to allow consistency of mesh placement.
It is plausible that different biological meshes may have varying failure rates, degrees of immunogenicity, biocompatibility and risk profiles60. In a rat study61 of 85 laparoscopic ventral hernia repairs, Strattice™ and Parietex™ (Covidien Surgical, Dublin, Ireland) were seen to grow a new mesothelial layer on their visceral side, whereas microscopic degradation and new collagen formation were seen in the Surgisis® group. In a mouse model of 135 mice with peritonitis, XCM BIOLOGIC® (LifeCell, KCI, Branchburg, New Jersey, USA) and Permacol™ showed better incorporation than Strattice™, whereas Strattice™ had fewer strong adhesions62. More accurate information from human studies may allow improved selection of mesh for patients in future clinical practice.
The direct advantages of biological mesh remain unproven in widespread practice. First, the long‐term durability of biological grafts used for complex abdominal wall reconstruction has been disappointing36 43. Rosen and co‐workers43 reported the overall hernia recurrence rate as 31 per cent over a mean follow‐up of 21·7 (range 1–74) months, and estimated the 3‐year recurrence‐free survival rate to be 51 per cent. Second, implementation and use of biological mesh in clinical practice depend on the cost, as biological meshes can be up to ten times more expensive than synthetic ones17 63. Totten et al.64 demonstrated that use of biological mesh for hernia repair can cost $21 000 (€17 100; exchange rate 20 April 2018) in comparison with synthetic mesh, which costs $7100 (€5780) for minimal improvement in surgical outcomes such as SSI.
With high costs of abdominal wall reconstruction using biological meshes and limited long‐term data, there has been emerging interest in the use of long‐term absorbable synthetic materials. These biosynthetic meshes are a clinical alternative to biological meshes and are significantly cheaper. A prospective longitudinal study by Rosen and colleagues65, evaluating the use of GORE® BIO‐A® (W. L. Gore, Newark, Delaware, USA) biosynthetic mesh in CDC class II–IV wounds, demonstrated an SSI rate of 18 per cent and a hernia recurrence rate of 17 per cent at 24 months. In contrast, the RICH trial36, which evaluated CDC II–IV wounds with biological mesh, had an SSI rate of 66 per cent and recurrence rate of 28 per cent at 24 months. Although this evidence with biosynthetic meshes is promising, any superiority over biological mesh in clean, clean‐contaminated, contaminated or infected wounds remains to be tested in RCTs.
Several ongoing cohort studies and RCTs will improve the evidence base, although they predominantly involve elective patients. Only three studies include both elective and emergency patients for prophylaxis. Future studies in high‐risk patients (such as those undergoing emergency surgery, with active sepsis or high BMI) will establish new indications for biological mesh, with potentially greater benefit in these patients. Preventing the need for reoperation in high‐risk groups is likely to provide even greater cost savings to health services.
There are weaknesses to this study. Assessment of quality using the GRADE tool is subjective, although this was overcome by discussion between the two authors involved in assessing grade of evidence, and resolving disagreement by re‐examining the relevant article until consensus had been achieved. Nevertheless, this scoring system is used widely for assessing strength of evidence in the literature20. Biosynthetic resorbable meshes and patients undergoing bridged repairs were not included in the study, as they represent a clinically separate group and are likely to have a different stage of innovation due to timing of introduction.
The evidence base for biological mesh in this clinical context is limited and evolving. Better reporting and quality control of surgical techniques is needed and, although new trial results over the next decade will improve the evidence base, more trials in emergency and contaminated settings are required.
Disclosure
The authors declare no conflict of interest.
Funding information.
No funding
References
- 1. Deerenberg EB, Harlaar JJ, Steyerberg EW, Lont HE, van Doorn HC, Heisterkamp J et al Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double‐blind, multicentre, randomised controlled trial. Lancet 2015; 386: 1254–1260. [DOI] [PubMed] [Google Scholar]
- 2. Bosanquet DC, Ansell J, Abdelrahman T, Cornish J, Harries R, Stimpson A et al Systematic review and meta‐regression of factors affecting midline incisional hernia rates: analysis of 14 618 patients. PLoS One 2015; 10: e0138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fischer JP, Basta MN, Mirzabeigi MN, Bauder AR, Fox JP, Drebin JA et al A risk model and cost analysis of incisional hernia after elective, abdominal surgery based upon 12 373 cases: the case for targeted prophylactic intervention. Ann Surg 2016; 263: 1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Ramshorst GH, Eker HH, Hop WC, Jeekel J, Lange JF. Impact of incisional hernia on health‐related quality of life and body image: a prospective cohort study. Am J Surg 2012; 204: 144–150. [DOI] [PubMed] [Google Scholar]
- 5. Chand B, Indeck M, Needleman B, Finnegan M, Van Sickle KR, Ystgaard B et al A retrospective study evaluating the use of Permacol™ surgical implant in incisional and ventral hernia repair. Int J Surg 2014; 12: 296–303. [DOI] [PubMed] [Google Scholar]
- 6. Iacco A, Adeyemo A, Riggs T, Janczyk R. Single institutional experience using biological mesh for abdominal wall reconstruction. Am J Surg 2014; 208: 480–484. [DOI] [PubMed] [Google Scholar]
- 7. Saunders DI, Murray D, Pichel AC, Varley S, Peden CJ; UK Emergency Laparotomy Network . Variations in mortality after emergency laparotomy: the first report of the UK Emergency Laparotomy Network. Br J Anaesth 2012; 109: 368–375. [DOI] [PubMed] [Google Scholar]
- 8. Burger JW, Luijendijk RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long‐term follow‐up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg 2004; 240: 578–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Luijendijk RW, Hop WC, van den Tol MP, de Lange DC, Braaksma MM, IJzermans JN et al A comparison of suture repair with mesh repair for incisional hernia. N Engl J Med 2000; 343: 392–398. [DOI] [PubMed] [Google Scholar]
- 10. Bhangu A, Fitzgerald JE, Singh P, Battersby N, Marriott P, Pinkney T. Systematic review and meta‐analysis of prophylactic mesh placement for prevention of incisional hernia following midline laparotomy. Hernia 2013; 17: 445–455. [DOI] [PubMed] [Google Scholar]
- 11. Cevasco M, Itani KM. Ventral hernia repair with synthetic, composite, and biologic mesh: characteristics, indications, and infection profile. Surg Infect (Larchmt) 2012; 13: 209–215. [DOI] [PubMed] [Google Scholar]
- 12. Pérez‐Köhler B, Bayon Y, Bellón JM. Mesh infection and hernia repair: a review. Surg Infect (Larchmt) 2016; 17: 124–137. [DOI] [PubMed] [Google Scholar]
- 13. Jones JW, Jurkovich GJ. Polypropylene mesh closure of infected abdominal wounds. Am Surg 1989; 55: 73–76. [PubMed] [Google Scholar]
- 14. Voyles CR, Richardson JD, Bland KI, Tobin GR, Flint LM, Polk HC Jr. Emergency abdominal wall reconstruction with polypropylene mesh: short‐term benefits versus long‐term complications. Ann Surg 1981; 194: 219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ventral Hernia Working Group , Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF et al Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery 2010; 148: 544–558. [DOI] [PubMed] [Google Scholar]
- 16. Shankaran V, Weber DJ, Reed RL 2nd, Luchette FA. A review of available prosthetics for ventral hernia repair. Ann Surg 2011; 253: 16–26. [DOI] [PubMed] [Google Scholar]
- 17. Bachman S, Ramshaw B. Prosthetic material in ventral hernia repair: how do I choose? Surg Clin North Am 2008; 88: 101–112, ix. [DOI] [PubMed] [Google Scholar]
- 18. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McCulloch P, Altman DG, Campbell WB, Flum DR, Glasziou P, Marshall JC et al No surgical innovation without evaluation: the IDEAL recommendations. Lancet 2009; 374: 1105–1112. [DOI] [PubMed] [Google Scholar]
- 20. Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S et al; GRADE Working Group . Grading quality of evidence and strength of recommendations. BMJ 2004; 328: 1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muysoms FE, Deerenberg EB, Peeters E, Agresta F, Berrevoet F, Campanelli G et al Recommendations for reporting outcome results in abdominal wall repair: results of a Consensus meeting in Palermo, Italy, 28–30 June 2012. Hernia 2013; 17: 423–433. [DOI] [PubMed] [Google Scholar]
- 22. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999; 27: 97–132. [PubMed] [Google Scholar]
- 23. Muysoms F, Jacob B. International Hernia Collaboration consensus on nomenclature of abdominal wall hernia repair. World J Surg 2018; 42: 302–304. [DOI] [PubMed] [Google Scholar]
- 24. Maggiori L, Moszkowicz D, Zappa M, Mongin C, Panis Y. Bioprosthetic mesh reinforcement during temporary stoma closure decreases the rate of incisional hernia: a blinded, case‐matched study in 94 patients with rectal cancer. Surgery 2015; 158: 1651–1657. [DOI] [PubMed] [Google Scholar]
- 25. Bhangu A, Futaba K, Patel A, Pinkney T, Morton D. Reinforcement of closure of stoma site using a biological mesh. Tech Coloproctol 2014; 18: 305–308. [DOI] [PubMed] [Google Scholar]
- 26. Bali C, Papakostas J, Georgiou G, Kouvelos G, Avgos S, Arnaoutoglou E et al A comparative study of sutured versus bovine pericardium mesh abdominal closure after open abdominal aortic aneurysm repair. Hernia 2015; 19: 267–271. [DOI] [PubMed] [Google Scholar]
- 27. Boutros C, Somasundar P, Espat NJ. Early results on the use of biomaterials as adjuvant to abdominal wall closure following cytoreduction and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 2010; 8: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cheng AW, Abbas MA, Tejirian T. Outcome of abdominal wall hernia repair with biologic mesh: Permacol™ versus Strattice™. Am Surg 2014; 80: 999–1002. [PubMed] [Google Scholar]
- 29. Ueno T, Pickett LC, de la Fuente SG, Lawson DC, Pappas TN. Clinical application of porcine small intestinal submucosa in the management of infected or potentially contaminated abdominal defects. J Gastrointest Surg 2004; 8: 109–112. [DOI] [PubMed] [Google Scholar]
- 30. Warwick AM, Smart NJ, Daniels IR. Retro‐rectus repair of complex incisional hernia leads to low recurrence rate. ANZ J Surg 2017; 87: 591–594. [DOI] [PubMed] [Google Scholar]
- 31. Chamieh J, Tan WH, Ramirez R, Nohra E, Apakama C, Symons W. Synthetic versus biologic mesh in single‐stage repair of complex abdominal wall defects in a contaminated field. Surg Infect (Larchmt) 2017; 18: 112–118. [DOI] [PubMed] [Google Scholar]
- 32. Chavarriaga LF, Lin E, Losken A, Cook MW, Jeansonne LO, White BC et al Management of complex abdominal wall defects using acellular porcine dermal collagen. Am Surg 2010; 76: 96–100. [PubMed] [Google Scholar]
- 33. Fayezizadeh M, Majumder A, Belyansky I, Novitsky YW. Outcomes of retromuscular porcine biologic mesh repairs using transversus abdominis release reconstruction. J Am Coll Surg 2016; 223: 461–468. [DOI] [PubMed] [Google Scholar]
- 34. Hicks CW, Poruk KE, Baltodano PA, Soares KC, Azoury SC, Cooney CM et al Long‐term outcomes of sandwich ventral hernia repair paired with hybrid vacuum‐assisted closure. J Surg Res 2016; 204: 282–287. [DOI] [PubMed] [Google Scholar]
- 35. Hsu PW, Salgado CJ, Kent K, Finnegan M, Pello M, Simons R et al Evaluation of porcine dermal collagen (Permacol) used in abdominal wall reconstruction. J Plast Reconstr Aesthet Surg 2009; 62: 1484–1489. [DOI] [PubMed] [Google Scholar]
- 36. Itani KM, Rosen M, Vargo D, Awad SS, Denoto G III, Butler CE; RICH Study Group . Prospective study of single‐stage repair of contaminated hernias using a biologic porcine tissue matrix: the RICH Study. Surgery 2012; 152: 498–505. [DOI] [PubMed] [Google Scholar]
- 37. Limpert JN, Desai AR, Kumpf AL, Fallucco MA, Aridge DL. Repair of abdominal wall defects with bovine pericardium. Am J Surg 2009; 198: e60–e65. [DOI] [PubMed] [Google Scholar]
- 38. Madani A, Niculiseanu P, Marini W, Kaneva PA, Mappin‐Kasirer B, Vassiliou MC et al Biologic mesh for repair of ventral hernias in contaminated fields: long‐term clinical and patient‐reported outcomes. Surg Endosc 2017; 31: 861–871. [DOI] [PubMed] [Google Scholar]
- 39. Majumder A, Winder JS, Wen Y, Pauli EM, Belyansky I, Novitsky YW. Comparative analysis of biologic versus synthetic mesh outcomes in contaminated hernia repairs. Surgery 2016; 160: 828–838. [DOI] [PubMed] [Google Scholar]
- 40. Nockolds CL, Hodde JP, Rooney PS. Abdominal wall reconstruction with components separation and mesh reinforcement in complex hernia repair. BMC Surg 2014; 14: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. O'Halloran EB, Barwegen CJ, Dombrowski JM, Vandevender DK, Luchette FA. Can't have one without the other: component separation plus mesh for repairing difficult incisional hernias. Surgery 2014; 156: 894–899. [DOI] [PubMed] [Google Scholar]
- 42. Patel KM, Nahabedian MY, Gatti M, Bhanot P. Indications and outcomes following complex abdominal reconstruction with component separation combined with porcine acellular dermal matrix reinforcement. Ann Plast Surg 2012; 69: 394–398. [DOI] [PubMed] [Google Scholar]
- 43. Rosen MJ, Krpata DM, Ermlich B, Blatnik JA. A 5‐year clinical experience with single‐staged repairs of infected and contaminated abdominal wall defects utilizing biologic mesh. Ann Surg 2013; 257: 991–996. [DOI] [PubMed] [Google Scholar]
- 44. Sbitany H, Kwon E, Chern H, Finlayson E, Varma MG, Hansen SL. Outcomes analysis of biologic mesh use for abdominal wall reconstruction in clean‐contaminated and contaminated ventral hernia repair. Ann Plast Surg 2015; 75: 201–204. [DOI] [PubMed] [Google Scholar]
- 45. Shah BC, Tiwari MM, Goede MR, Eichler MJ, Hollins RR, McBride CL et al Not all biologics are equal! Hernia 2011; 15: 165–171. [DOI] [PubMed] [Google Scholar]
- 46. Zerbib P, Caiazzo R, Piessen G, Rogosnitzky M, Séquier C, Koriche D et al Outcome in porcine acellular dermal matrix reinforcement of infected abdominal wall defects: a prospective study. Hernia 2015; 19: 253–257. [DOI] [PubMed] [Google Scholar]
- 47. Giordano P, Pullan RD, Ystgaard B, Gossetti F, Bradburn M, McKinley AJ et al The use of an acellular porcine dermal collagen implant in the repair of complex abdominal wall defects: a European multicentre retrospective study. Tech Coloproctol 2015; 19: 411–417. [DOI] [PubMed] [Google Scholar]
- 48. Cox TC, Pearl JP, Ritter EM. Rives–Stoppa incisional hernia repair combined with laparoscopic separation of abdominal wall components: a novel approach to complex abdominal wall closure. Hernia 2010; 14: 561–567. [DOI] [PubMed] [Google Scholar]
- 49. Boules M, Strong AT, Corcelles R, Haskins IN, Ilie R, Wathen C et al Single‐center ventral hernia repair with porcine dermis collagen implant. Surg Endosc 2018; 32: 1820–1827. [DOI] [PubMed] [Google Scholar]
- 50. Gnaneswaran N, Perera M, Jenkin A, Lau H, Presley R. Ventral hernia repair with lateral component separation and onlay Biodesign graft. Eur J Plast Surg 2016; 39: 279–286. [Google Scholar]
- 51. Byrnes MC, Irwin E, Carlson D, Campeau A, Gipson JC, Beal A et al Repair of high‐risk incisional hernias and traumatic abdominal wall defects with porcine mesh. Am Surg 2011; 77: 144–150. [PubMed] [Google Scholar]
- 52. Garvey PB, Giordano SA, Baumann DP, Liu J, Butler CE. Long‐term outcomes after abdominal wall reconstruction with acellular dermal matrix. J Am Coll Surg 2017; 224: 341–350. [DOI] [PubMed] [Google Scholar]
- 53. Giordano S, Garvey PB, Baumann DP, Liu J, Butler CE. Primary fascial closure with biologic mesh reinforcement results in lesser complication and recurrence rates than bridged biologic mesh repair for abdominal wall reconstruction: a propensity score analysis. Surgery 2017; 161: 499–508. [DOI] [PubMed] [Google Scholar]
- 54. Parker DM, Armstrong PJ, Frizzi JD, North JH Jr. Porcine dermal collagen (Permacol) for abdominal wall reconstruction. Curr Surg 2006; 63: 255–258. [DOI] [PubMed] [Google Scholar]
- 55. Pomahac B, Aflaki P. Use of a non‐cross‐linked porcine dermal scaffold in abdominal wall reconstruction. Am J Surg 2010; 199: 22–27. [DOI] [PubMed] [Google Scholar]
- 56. Shaikh FM, Giri SK, Durrani S, Waldron D, Grace PA. Experience with porcine acellular dermal collagen implant in one‐stage tension‐free reconstruction of acute and chronic abdominal wall defects. World J Surg 2007; 31: 1966–1972. [DOI] [PubMed] [Google Scholar]
- 57. Høyrup S, Bruun J, Bertelsen CA. Use of biological mesh in facilitation of early closure in potentially infected abdominal wall defects. Dan Med J 2012; 59: A4389. [PubMed] [Google Scholar]
- 58. Abdelfatah MM, Rostambeigi N, Podgaetz E, Sarr MG. Long‐term outcomes (> 5‐year follow‐up) with porcine acellular dermal matrix (Permacol) in incisional hernias at risk for infection. Hernia 2015; 19: 135–140. [DOI] [PubMed] [Google Scholar]
- 59. Blencowe NS, Mills N, Cook JA, Donovan JL, Rogers CA, Whiting P et al Standardizing and monitoring the delivery of surgical interventions in randomized clinical trials. Br J Surg 2016; 103: 1377–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hammond TM, Chin‐Aleong J, Navsaria H, Williams NS. Human in vivo cellular response to a cross‐linked acellular collagen implant. Br J Surg 2008; 95: 438–446. [DOI] [PubMed] [Google Scholar]
- 61. Ditzel M, Deerenberg EB, Grotenhuis N, Harlaar JJ, Monkhorst K, Bastiaansen‐Jenniskens YM et al Biologic meshes are not superior to synthetic meshes in ventral hernia repair: an experimental study with long‐term follow‐up evaluation. Surg Endosc 2013; 27: 3654–3662. [DOI] [PubMed] [Google Scholar]
- 62. Kaufmann R, Jairam AP, Mulder IM, Wu Z, Verhelst J, Vennix S et al Characteristics of different mesh types for abdominal wall repair in an experimental model of peritonitis. Br J Surg 2017; 104: 1884–1893. [DOI] [PubMed] [Google Scholar]
- 63. Primus FE, Harris HW. A critical review of biologic mesh use in ventral hernia repairs under contaminated conditions. Hernia 2013; 17: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Totten CF, Davenport DL, Ward ND, Roth JS. Cost of ventral hernia repair using biologic or synthetic mesh. J Surg Res 2016; 203: 459–465. [DOI] [PubMed] [Google Scholar]
- 65. Rosen MJ, Bauer JJ, Harmaty M, Carbonell AM, Cobb WS, Matthews B et al Multicenter, prospective, longitudinal study of the recurrence, surgical site infection, and quality of life after contaminated ventral hernia repair using biosynthetic absorbable mesh: the COBRA study. Ann Surg 2017; 265: 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]


