 A novel series of boron-containing compounds were designed, synthesized and evaluated as multi-target-directed ligands against Alzheimer's disease.
A novel series of boron-containing compounds were designed, synthesized and evaluated as multi-target-directed ligands against Alzheimer's disease.
Abstract
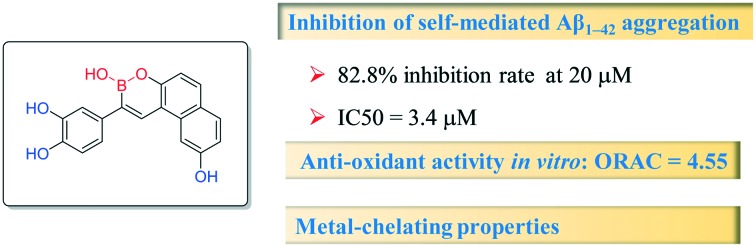
A novel series of boron-containing compounds were designed, synthesized and evaluated as multi-target-directed ligands against Alzheimer's disease. The biological activity results demonstrated that these compounds possessed a significant ability to inhibit self-induced Aβ aggregation (20.5–82.8%, 20 μM) and to act as potential antioxidants (oxygen radical absorbance capacity assay using fluorescein (ORAC-FL) values of 2.70–5.87). In particular, compound 17h is a potential lead compound for AD therapy (IC50 = 3.41 μM for self-induced Aβ aggregation; ORAC-FL value = 4.55). Compound 17h also functions as a metal chelator. These results indicated that boron-containing compounds could be new structural scaffolds for the treatment of AD.
1. Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by the progressive impairment of higher cognitive function, memory loss, and altered behavior.1 The pathological hallmarks of AD include low levels of acetylcholine (ACh), β-amyloid (Aβ) deposits, τ-protein aggregation, oxidative stress and biometal dyshomeostasis.2 Due to the complex pathogenesis of AD, there are only five drugs approved by the FDA for the treatment of AD to date, which include cholinesterase inhibitors (tacrine, donepezil, rivastigmine, and galantamine)3 and an NMDA receptor antagonist (memantine).4 These drugs give only a modest improvement in memory and cognitive function and do not prevent progressive neurodegeneration. Thus, the development of new drugs for the treatment of AD remains a challenge in the pharmaceutical community.
Senile plaques composed of extracellular amyloid beta (Aβ) peptide aggregates are a key pathological marker of AD. The “amyloid hypothesis” proposes that the production and accumulation of oligomeric aggregates of Aβ in the brain is a central event in the pathogenesis of AD and that these aggregates initiate the pathogenic cascade that ultimately leads to neuronal loss and dementia.5 Recent studies indicate that oxidative stress is one of the earliest events in AD pathogenesis.6 Oxidative damage present within the brain of AD patients can be observed within every class of biological macromolecules, including nucleic acids, proteins, lipids, and carbohydrates.7 The free-radical and oxidative stress theory of aging also suggests that oxidative damage is an important player in neuronal degeneration. Therefore, antioxidant protection is important for the treatment of AD as the endogenous antioxidant protection system rapidly declines. Indeed, several antioxidant compounds have demonstrated efficacy in a number of recent studies.8
Boron is an element that has potential for the development of pharmaceutical drugs. Bortezomib (Velcade),9 a proteasome inhibitor that has shown in vitro and in vivo activity against a variety of malignancies, has been used clinically for the treatment of cancers since 2003. Several cyclic boron-containing compounds have exhibited very good biological activity. For example, tavaborole (Kerydin)10,11 is a boron-containing small molecule antifungal agent that was approved by the FDA in 2014 for the topical treatment of onychomycosis. The benzoxaborole SCYX-7158 (ref. 12) is in clinical trials for the treatment of stage 2 human African trypanosomiasis. Some boron-containing compounds have also demonstrated inhibitory activity against the phosphodiesterase 4 enzyme (PDE4) and inflammation-related cytokine release,13 which have been shown to impact cognition enhancement in aging and Alzheimer's disease (AD).14
Inspired by the ‘multifunctional agent’ design strategy,15–18 our research group has a long-standing interest in the search for novel compounds with multifunctional effects and therapeutic potential in the treatment of AD.19–21 In this paper, we describe the design, synthesis and evaluation of a series of boron-containing compounds as Aβ aggregation inhibitors, antioxidants and metal-chelating agents for the treatment of AD.
2. Results and discussion
2.1. Chemistry
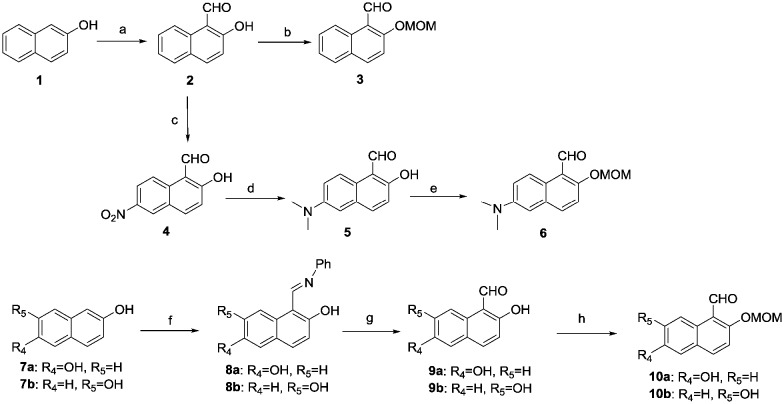
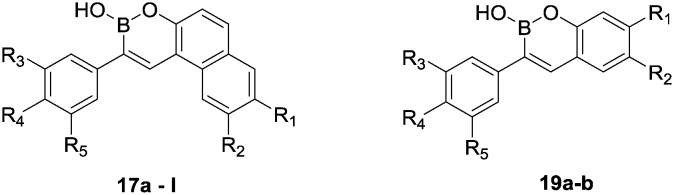
The synthetic routes for the new boron-containing compounds are shown in Schemes 1 and 2. Commercially available 2-naphthol (1) was treated with NaOH and chloroform to give 2-hydroxy-1-naphthaldehyde (2), which was then protected with a methoxymethyl (MOM) group through reaction with chloro(methoxy)methane in the presence of diisopropylethylamine to give 3. Meanwhile, the nitration, reduction and then protection of the hydroxyl group of 1 gave amine 6. Naphthalene-2,6-diol (7a) and naphthalene-2,7-diol (7b) were treated with N,N′-diphenylformamidine at 120 °C for 5 hours to provide 8a and 8b; these compounds were then treated with H2SO4 and then the hydroxyl groups were protected to afford compounds 10a and 10b (Scheme 1).
Scheme 1. Reagents and conditions: a. NaOH, CHCl3, EtOH, 80–90 °C, 6 h; b. CH2Cl2, MOMCl, i-Pr2NEt, 0 °C, 4 h; c. Fuming nitric acid, –5 °C, 20 min; d. Pt/C, formaldehyde, H2, EtOH; e. CH2Cl2, MOMCl, i-Pr2NEt, 0 °C, 4 h; f. N,N′-diphenylformamidine, Ar2, 120 °C, 5 h; g. Ether, H2SO4, H2O, 4d; h. CH2Cl2, MOMCl, i-Pr2NEt, 0 °C, 4 h.
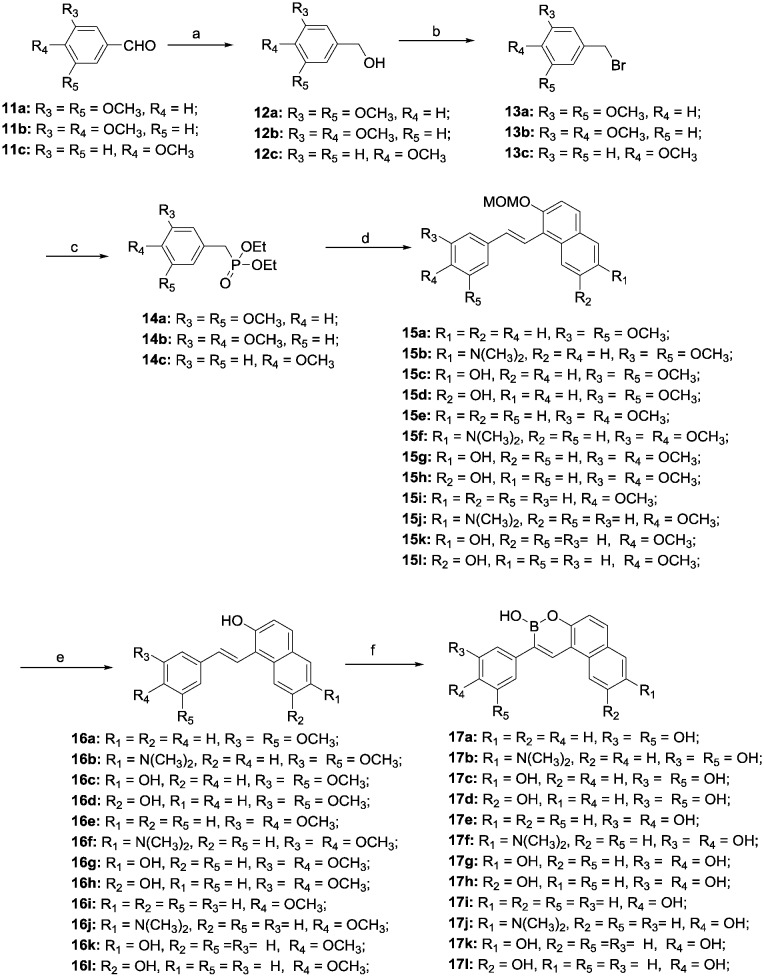
Scheme 2. Reagents and conditions: a. NaBH4, MeOH, 0 °C; b. PBr3, pyridine, 0 °C; c. Triethyl phosphite, 120 °C; d. 2, 6, 10a and b, CH3ONa, 0 °C, 1 h, rt, 12 h; e. HCl, CH3OH, 45 °C, 1 h; f. BBr3, CH2Cl2, –78 °C, 5 h.
The reduction of substituted benzaldehydes 11a–c with sodium borohydride in methanol provided alcohols 12a–c that were subsequently treated with PBr3 in the presence of pyridine to give bromides 13a–c, which were converted into Wittig reagents 14a–c in high yield. The Wittig olefination of 14a–c with the substituted benzaldehydes 2, 6 and 10a and b provided compounds 15a–l. Finally, target compounds 17a–l were obtained by removal of the protection groups on 15a–l with hydrochloric acid followed by demethylation and cyclization in the presence of boron tribromide at –78 °C (Scheme 2).
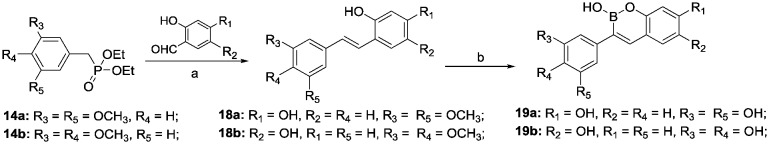
Resveratrol-boron derivatives 19a and b were also prepared according to Scheme 3. The Wittig olefination of 14a and b with 2,4-dihydroxybenzaldehyde or 2,5-dihydroxybenzaldehyde provided compounds 18a and b. Then, the resveratrol-boron derivatives 19a and b were obtained by treatment with BBr3.
Scheme 3. Reagents and conditions: a. 2, 6, 10a and b, CH3ONa, 0 °C, 1 h, rt, 12 h; b. BBr3, CH2Cl2, –78 °C, 5 h.
2.2. Inhibition of self-mediated Aβ1–42 aggregation
To evaluate the inhibitory activities of boron-containing compounds 17a–l against self-mediated Aβ1–42 aggregation, the thioflavin-T (ThT) fluorescence binding assay22 was performed. Curcumin, a known amyloid aggregation inhibitor, was used as a reference compound. The results shown in Table 1 indicate that almost half of the target compounds exhibited more potent Aβ aggregation inhibition (67.3%–82.8%, 20 μM) compared to curcumin (53.9%, 20 μM). Among them, compounds 17c, 17d, 17g and 17h, which feature two hydroxyl groups at the benzyl ring and one hydroxyl group on the naphthalene ring, exhibited the most potent inhibition activities (70.3%, 78.7%, 80.5% and 82.8%, 20 μM, respectively). However, compounds with only one hydroxyl group on the benzyl ring showed dramatically lower inhibition activities. For instance, compounds 17i and 17j, which possess one hydroxyl group at the benzyl ring and no substitution on the naphthalene ring, showed almost no inhibition of Aß aggregation at 20 μM. A simple analysis of the relationship between structure and activity suggests that two hydroxyl groups on the benzyl ring may play a key role in the inhibition of Aß1–42 aggregation. This result is consistent with previous studies that show that hydrogen bonds are crucial for interactions between polyphenols and proteins. The inhibition activity was also affected by substituent groups at the naphthalene ring. Hydroxyl-substituted naphthalene rings were better than unsubstituted or dimethylamino-substituted rings (the ability of compounds 17e, 17f, 17g and 17h to inhibit Aβ aggregation was 43.7%, 49.5% and 82.8% at 20 μM, respectively). The substituent position on the naphthalene ring showed a slight influence on inhibition activities. In order to compare the activities with the activity of resveratrol, resveratrol-boron compounds 19a and b were designed and synthesised. From the results, we can see that the inhibition activity of 19b was similar to that of resveratrol but was much lower than that of compound 17h.
Table 1. Inhibition of Aβ1–42 aggregation and oxygen radical absorbance capacity (ORAC, Trolox equivalents) of boron-containing compounds 17a–l, 19a and b, resveratrol, curcumin, ferulic acid and (E)-1,3-dimethoxy-5-(4-methoxystyryl)benzene.
| ||||||||
| Compd. | R1 | R2 | R3 | R4 | R5 | Inhibition of Aβ1–42 aggregation a (%) | Aβ1–42 IC50 d (μM) | ORAC b |
| 17a | H | H | OH | H | OH | 67.3 ± 4.6 | 12.6 ± 2.8 | 3.00 ± 0.15 |
| 17b | N(CH3)2 | H | OH | H | OH | 32.4 ± 2.4 | n.t.c | 5.27 ± 0.17 |
| 17c | OH | H | OH | H | OH | 70.3 ± 5.4 | 8.9 ± 1.8 | 5.87 ± 0.09 |
| 17d | H | OH | OH | H | OH | 78.7 ± 3.8 | 4.9 ± 0.7 | 5.34 ± 0.23 |
| 17e | H | H | OH | OH | H | 43.7 ± 3.6 | n.t. c | 2.70 ± 0.03 |
| 17f | N(CH3)2 | H | OH | OH | H | 49.5 ± 2.8 | n.t. c | 4.88 ±0.14 |
| 17g | OH | H | OH | OH | H | 80.5 ± 3.8 | 4.6 ± 1.1 | 4.05 ± 0.23 |
| 17h | H | OH | OH | OH | H | 82.8 ± 3.1 | 3.4 ± 0.5 | 4.55 ± 0.20 |
| 17i | H | H | H | OH | H | 0 | n.t. c | 2.06 ± 0.04 |
| 17j | N(CH3)2 | H | H | OH | H | 0 | n.t. c | 2.41 ± 0.11 |
| 17k | OH | H | H | OH | H | 12.3 ± 1.7 | n.t. c | 3.94 ± 0.20 |
| 17l | H | OH | H | OH | H | 20.5 ± 2.4 | n.t. c | 4.45 ± 0.12 |
| 19a | OH | H | OH | H | OH | 50.2 ± 3.5 | 21.7 ± 4.0 | 4.84 ± 0.31 |
| 19b | H | OH | OH | OH | H | 58.8 ± 2.9 | 17.5 ± 2.1 | 4.09 ± 0.27 |
| Resveratrol | — | — | — | — | — | 61.2 ± 5.2 | 16.8 ± 3.8 | 5.33 ± 0.17 |
| Curcumin | — | — | — | — | — | 53.9 ± 1.9 | 13.7 ± 1.9 | n.t. c |
| Ferulic acid | — | — | — | — | — | n.t. c | n.t. c | 3.24 ± 0.21 |
| (E)-1,3-Dimethoxy-5-(4-methoxystyryl)benzene | — | — | — | — | — | n.t. c | n.t. c | 0.21 ± 0.02 |
aThe thioflavin-T fluorescence method was used. The values are expressed as the mean ± SD of at least three independent measurements. All values were obtained at a compound concentration of 20 μM.
bThe mean ± SD of three independent experiments. The data are expressed as μmol of Trolox equivalents/μmol of the test compound.
cn.t. means not tested.
dMean ± SD of at least three independent measurements.
The complete dose–response curves of compounds with greater than 50% inhibition were evaluated. The IC50 values listed in Table 1 indicate that compound 17h demonstrated the most potent inhibition of self-mediated Aβ1–42 aggregation (IC50 = 3.4 μM), almost 4-fold greater than that of curcumin (IC50 = 13.7 μM).
2.3. Anti-oxidant activity in vitro
The antioxidant activity of the boron-containing compounds was determined using oxygen radical absorbance capacity assay using fluorescein (ORAC-FL) according to the method originally described by Ou et al.23 and modified by Dávalos.24 The vitamin E analog Trolox was used as a standard, and the antioxidant activity was expressed as Trolox equivalents (μmol of Trolox/μmol of the test compound). The results in Table 1 indicate that all the target compounds exhibited excellent antioxidant capacity with ORAC-FL values of 2.06–5.87 Trolox equivalents, which are similar to or more active than ferulic acid; compound 17c exhibited the best antioxidant activity with an ORAC-FL value of 5.87.
2.4. Metal-chelating properties of compound 17h
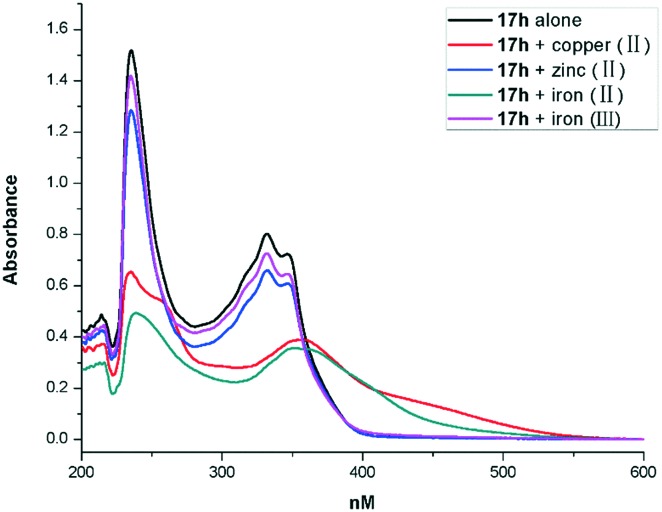
Based on the inhibition of self-mediated Aβ1–42 aggregation activity and anti-oxidant activity of compound 17h (Aβ1–42 aggregation activity: IC50 = 3.4 μM; ORAC-FL value of 4.55), we subjected this promising multi-functional inhibitor to further study. The metal-chelating ability of compound 17b toward biometals such as Cu(ii), Zn(ii), Fe(ii) and Fe(iii) was investigated using UV-vis spectrometry.25 The results in Fig. 1 show that 17b produces maximum absorbance peaks at 234 and 331 nm. When CuSO4 or FeSO4 was added, the maximum absorbance at 331 nm exhibited a red shift to 355 nm or 350 nm, which suggests the formation of a 17h–copper(ii) and a 17h–iron(ii) complex. However, with the addition of Fe2(SO4)3 and ZnCl2, there was no significant shift.
Fig. 1. UV spectra of compound 17h (40 μM) alone and in the presence of 20 μM CuSO4, ZnCl2, FeSO4 or Fe2(SO4)3. All solutions were prepared using a HEPES buffer solution (20 mM HEPES, 150 mM NaCl, pH 7.4).
3. Conclusion
In summary, we have developed a novel series of boron-containing compounds, which exhibited multifunctional activity as potential anti-AD drugs, including a significant ability to inhibit self-induced Aβ aggregation and to act as antioxidants and biometal chelators. Among the synthesized compounds, compound 17h gave the greatest inhibitory potency toward self-induced Aβ aggregation (82.8%, 20 μM, IC50 = 3.4 μM) and good antioxidant activity (ORAC = 4.55). In addition, 17h showed good metal-chelating ability toward biometals Cu(ii) and Fe(ii). These results indicate that boron-containing compounds could be new structural scaffolds for the treatment of AD. Further studies based on these results are in progress.
Experimental section
Chemistry
The 1H NMR and 13C NMR spectra were recorded using TMS as the internal standard on a Bruker BioSpin GmbH spectrometer at 400.132 MHz and 100.614 MHz, respectively. Coupling constants are given in Hz. LC-MS spectra were recorded on an Agilent LC-MS 6120 instrument with an ESI mass selective detector. High-resolution mass spectra were obtained using a Shimadzu LCMS-IT-TOF mass spectrometer. A high-performance liquid chromatography (HPLC) instrument, which was equipped with a TC-C18 column (4.6 × 250 mm, 5 μm), was used to determine the purity of the synthesised compounds. Flash column chromatography was performed using silica gel (200–300 mesh) purchased from Qingdao Haiyang Chemical Co. Ltd. All the reactions were monitored by thin layer chromatography using silica gel.
Synthesis procedure of 2-hydroxy-1-napthylaldehyde (2)
2-Hydroxy-1-napthylaldehyde (2) was synthesized according to a reported procedure.26 2-Naphthol (7.22 g, 50 mmol) was dissolved in EtOH at 80–90 °C, then NaOH (14.40 g, 360 mmol) in 100 mL water was added dropwise to the hot solution, and the solution became darker. After half an hour, CHCl3 (80 mmol) was added dropwise using a dropping funnel. The reaction mixture was stirred for six hours. Excess ethanol and chloroform were removed via the process of distillation. The dark oil produced was mixed with a considerable amount of sodium chloride. Sufficient water was added to dissolve the salt, and the oil was separated and washed with hot water. Then the solution was neutralized with dilute hydrochloric acid and extracted with chloroform. Finally, the product was purified using 60–120 mesh silica gel with 1–2% ethyl acetate in pet ether. The yield of the product was 3.78 g (44%). 1H NMR (400 MHz, DMSO-d6) δ 12.07 (s, 1H), 9.87 (s, 1H), 8.73 (d, J = 7.9 Hz, 1H), 8.02–8.09 (m, 2H), 7.76–7.81 (m, 2H), 7.08 (d, J = 7.2 Hz, 1H).
Synthesis procedure of 2-(methoxymethoxy)-1-naphthaldehyde (3)
The preparation was carried out according to our previously reported procedure.27 MOMCl (7.5 mmol) was added dropwise to an ice-cooled solution of diisopropylethylamine (10 mmol) and 2 (5 mmol) in dry CH2Cl2 (10 mL). After complete addition, the reaction mixture was allowed to warm to ambient temperature and stirred for 5 h. The reaction mixture was diluted with CH2Cl2. The organic layer was washed with saturated aqueous NaHCO3 and brine before being dried over Na2SO4 and evaporated. The residue was purified by flash chromatography on silica gel with petrol/ethyl acetate as the elution solvent to afford 2-(methoxymethoxy)-1-naphthaldehyde (3) as a light yellow solid, 0.93 g (yield: 86%). 1H NMR (400 MHz, DMSO-d6) δ 9.75 (s, 1H), 8.64 (d, J = 9.2 Hz, 1H), 8.02–8.08, 7.59–7.68 (m, 2H), 7.01 (d, J = 9.2 Hz, 1H).
Synthesis procedure of 2-hydroxy-6-nitro-1-naphthaldehyde (4)
Compound 3 (5 mmol, 0.86 g) was added to fuming nitric acid (5.0 mL) at –5 °C and the mixture was allowed to stir at –5 °C for 20 minutes. Then the reaction mixture was poured into ice-water (20 mL). The resulting precipitate was filtered off and recrystallized with ethyl acetate to give 2-hydroxy-6-nitro-1-naphthaldehyde (4) as a yellow powder in 58% yield (0.63 g). 1H NMR (400 MHz, DMSO-d6) δ 12.21(s, 1H), 10.71 (s, 1H), 9.11 (d, J = 9.1 Hz, 1H), 8.87 (s, 1H), 8.39 (d, J = 9.1 Hz, 1H), 8.29 (d, J = 9.1 Hz, 1H), 7.31 (d, J = 9.1 Hz, 1H).
Synthesis procedure of 6-(dimethylamino)-2-hydroxy-1-naphthaldehyde (5)
Compound 4 (0.54 g, 2.5 mmol) was dissolved in EtOH (25 mL). Formaldehyde (1.5 mL) and 5% Pd/C (100 mg) were added to the solution. The resulting mixture was stirred under hydrogen for 12 h. The reaction mixture was then filtered and evaporated. The residue was purified by flash chromatography on silica gel with petrol/ethyl acetate as the elution solvent to afford 6-(dimethylamino)-2-hydroxy-1-naphthaldehyde (5) as a light yellow oil in 72% yield (0.39 g). 1H NMR (400 MHz, DMSO-d6) δ 11.92 (s, 1H), 10.75 (s, 1H), 8.91 (d, J = 9.1 Hz, 1H), 8.05 (d, J = 9.1 Hz, 1H), 7.67 (d, J = 9.0 Hz, 1H), 7.48 (d, J = 9.1 Hz, 1H), 7.22 (d, J = 9.1 Hz, 1H), 3.09 (s, 6H).
Synthesis procedure of 6-(dimethylamino)-2-(methoxymethoxy)-1-naphthaldehyde (6)
MOMCl (6 mmol) was added dropwise to an ice-cooled solution of diisopropylethylamine (8 mmol) and 5 (4 mmol) in dry CH2Cl2 (10 mL). After complete addition, the reaction mixture was allowed to warm to ambient temperature and stirred for 5 h. The reaction mixture was diluted with CH2Cl2. The organic layer was washed with saturated aqueous NaHCO3 and brine before being dried over Na2SO4 and evaporated. The residue was purified by flash chromatography on silica gel with petrol/ethyl acetate as the elution solvent to afford 2-(methoxymethoxy)-1-naphthaldehyde (6) as a light yellow solid, 0.86 g (yield: 83%). 1H NMR (400 MHz, DMSO-d6) δ 10.81 (s, 1H), 8.78 (d, J = 9.2 Hz, 1H), 8.25 (d, J = 9.2 Hz, 1H), 7.47 (d, J = 9.2 Hz, 1H), 7.22 (d, J = 9.1 Hz, 1H), 7.08 (d, J = 9.1 Hz, 1H), 6.17 (s, 3H), 3.31 (s, 3H), 3.09 (s, 6H).
General synthesis procedure of 8
The preparation was carried out according to our previously reported procedure.28 A mixture of 2,6-dihydroxynaphtalene (7a, 30 mmol) or 2,7-dihydroxynaphtalene (7b, 30 mmol) and diphenyl formamidine (45 mmol) was stirred at 120 °C under argon for 5 h. The progress of the reaction was monitored by TLC. The resulting mixture was cooled to room temperature and 30 ml of acetone were added; the resulting precipitate was filtered off and dried to give 8a (5.67 g; 72%) and 8b (6.14 g; 78%) as a red powder. Compounds 8a and 8b were used in the next step without further purification.
General synthesis procedure of 9
A solution of 8a (20 mmol) or 8b (20 mmol) in 5 ml of water and 4 ml of concentrated H2SO4 (96%) was subjected to liquid–liquid extraction by upward displacement with ether for 4 days. Removal of the solvent under reduced pressure gave compounds 9a and 9b as a yellow solid.
2,6-Dihydroxy-1-naphthaldehyde (9a)
Yellow solid, yield: 67%. 1H NMR (400 MHz, DMSO-d6) δ 12.00 (s, 1H), 11.64 (s, 1H), 10.84 (s, 1H), 8.35 (s, 1H), 7.94 (d, J = 9.2 Hz, 1H), 7.61 (d, J = 8.8 Hz, 1H), 7.32 (d, J1 = 9.2 Hz, 1H), 7.22 (d, J = 8.8 Hz, 1H).
2,7-Dihydroxy-1-naphthaldehyde (9b)
Yellow solid, yield: 72%. 1H NMR (400 MHz, DMSO-d6) δ 12.05 (s, 1H), 11.90 (s, 1H), 10.79 (s, 1H), 8.86 (d, J = 2.2 Hz, 1H), 8.05 (d, J = 9.0 Hz, 1H), 7.38 (d, J = 8.8 Hz, 1H), 7.24 (dd, J1 = 9.0 Hz, J2 = 2.2 Hz, 1H), 7.18 (d, J = 8.8 Hz, 1H).
General synthesis procedure of 10
MOMCl (15 mmol) was added dropwise to an ice-cooled solution of diisopropylethylamine (20 mmol) and 5 (10 mmol) in dry CH2Cl2 (20 mL). After complete addition, the reaction mixture was allowed to warm to ambient temperature and stirred for 5 h. The reaction mixture was diluted with CH2Cl2. The organic layer was washed with saturated aqueous NaHCO3 and brine before being dried over Na2SO4 and evaporated. The residue was purified by flash chromatography on silica gel with petrol/ethyl acetate as the elution solvent to afford 10a and 10b as a light yellow solid.
6-Hydroxy-2-(methoxymethoxy)-1-naphthaldehyde (10a)
Light yellow solid, yield: 59%. 1H NMR (400 MHz, DMSO-d6) δ 11.57 (s, 1H), 10.40 (s, 1H), 8.44 (d, J = 2.4 Hz, 1H), 8.01 (d, J = 9.0 Hz, 1H), 7.49 (d, J = 8.8 Hz, 1H), 7.28 (d, J1 = 9.0 Hz, 1H), 7.20 (d, J = 8.4 Hz, 1H), 6.10 (s, 3H), 3.25 (s, 3H).
7-Hydroxy-2-(methoxymethoxy)-1-naphthaldehyde (10b)
Light yellow solid, yield: 71%. 1H NMR (400 MHz, DMSO-d6) δ 11.88 (s, 1H), 10.68 (s, 1H), 8.80 (d, J = 2.0 Hz, 1H), 8.11 (d, J = 9.2 Hz, 1H), 7.69 (d, J = 8.8 Hz, 1H), 7.48 (d, J1 = 9.2 Hz, 1H), 7.27 (d, J = 8.8 Hz, 1H),6.14 (s, 3H), 3.28 (s, 3H).
General synthesis procedure of 12
The preparation was carried out according to our previously reported procedure.29 To a stirred solution of 3,5-dimethoxybenzaldehyde (11a) and 3,4-dimethoxybenzaldehyde or 4-methoxybenzaldehyde in methanol, NaBH4 was added in batches at 0 °C. The reaction mixture was slowly warmed to room temperature and stirred for another 2 hours. The solvent was evaporated under vacuum and the residue was extracted with EtOAc, washed with water, dried over Na2SO4, and concentrated to give 12a, 12b or 12c as a white solid. The product was used directly in the next step without further purification.
General synthesis procedure of 13
The preparation was carried out according to our previously reported procedure.29 PBr3 was added dropwise to a solution of 12a, 12b or 12c and pyridine in CH2Cl2 at 0 °C. After the mixture was slowly warmed to room temperature and stirred for 4 h, the reaction was quenched by the slow addition of ice water, extracted with CH2Cl2, washed with brine, dried over Na2SO4, and concentrated to provide 13a, 13b or 13c as a white solid.
General synthesis procedure of 14
The preparation was carried out according to our previously reported procedure.4 A mixture of 13a, 13b or 13c and triethyl phosphate was heated at 120 °C for 10 h. The excess triethyl phosphate was removed under vacuum and the crude product was purified by silica gel column chromatography (EtOAc/petroleum ether) to give compound 14a, 14b or 14c as oil.
General procedure for the preparation of 15
Sodium methoxide (3 mmol) was added to a solution of phosphonic acid diethyl ester 14a, 14b or 14c (1 mmol) in dry DMF (2 mL). The resulting mixture was stirred at room temperature for 5 min, and compound 2, 6, 10a or 10b (1.2 mmol) was added at 0 °C. The mixture was stirred at room temperature for 0.5 h and then for 12 h at 80 °C. The reaction was quenched by pouring ice-water with stirring. Solids obtained from the reactions were filtered and dried. Oils from the reactions were extracted with ethyl acetate, and the ethyl acetate layer was washed with water and brine and then dried over Na2SO4. Filtration and evaporation of the solvent afforded the oils. The crude solids or oils were purified by flash chromatography on silica gel with petrol/ethyl acetate as the elution solvent to afford the desired products 15a–15l.
(E)-1-(3,5-Dimethoxystyryl)-2-(methoxymethoxy)naphthalene (15a)
Yellow solid, yield: 88%. 1H NMR (400 MHz, DMSO-d6) δ 8.09 (d, J = 9.1 Hz, 1H), 7.92 (d, J = 8.6 Hz, 1H), 7.44–7.65 (m, 3H), 7.20 (d, J = 8.6 Hz, 1H), 7.08 (d, J = 8.3 Hz, 1H), 6.92 (s, 1H), 6.87 (s, 1H), 6.72 (d, J = 8.6 Hz, 1H), 6.21 (s, 1H), 4.61 (t, 2H), 3.85 (s, 6H), 3.68 (t, 2H), 3.31 (s, 3H).
(E)-5-(3,5-Dimethoxystyryl)-6-(2-methoxymethoxy)-N,N-dimethylnaphthalen-2-amine (15b)
Yellow solid, yield: 61%. 1H NMR (400 MHz, DMSO-d6) δ 7.82 (d, J = 8.6 Hz, 1H), 7.44–7.50 (m, 2H), 7.18 (d, J = 8.7 Hz, 1H), 7.04 (d, J = 8.3 Hz, 1H), 6.90 (s, 1H), 6.85 (s, 1H), 6.67 (d, J = 8.6 Hz, 1H), 6.78 (s, 1H), 6.19 (s, 1H), 4.60 (t, 2H), 3.83 (s, 6H), 3.65 (t, 2H), 3.29 (s, 3H), 3.13 (s, 6H).
(E)-5-(3,5-Dimethoxystyryl)-6-(2-methoxymethoxy)naphthalen-2-ol (15c)
Yellow solid, yield: 73%. 1H NMR (400 MHz, DMSO-d6) δ 8.01 (d, J = 9.2 Hz, 1H), 7.65 (d, J = 8.3 Hz, 1H), 7.19 (d, J = 8.8 Hz, 1H), 7.12 (s, 1H), 7.08 (d, J = 8.3 Hz, 1H), 7.02 (s, 1H), 6.95 (s, 1H), 6.84 (s, 1H), 6.71 (d, J = 8.8 Hz, 1H), 6.24 (s, 1H), 4.59 (t, 2H), 3.84 (s, 6H), 3.62 (t, 2H), 3.30 (s, 3H).
(E)-8-(3,5-Dimethoxystyryl)-7-(2-methoxymethoxy)naphthalen-2-ol (15d)
Yellow solid, yield: 51%. 1H NMR (400 MHz, DMSO-d6) δ 7.80 (d, J = 8.4 Hz, 1H), 7.60 (d, J = 8.6 Hz, 1H), 7.20 (d, J = 8.8 Hz, 1H), 7.01–7.12 (m, 3H), 6.90 (s, 1H), 6.85 (s, 1H), 6.74 (d, J = 8.8 Hz, 1H), 6.18 (s, 1H), 4.60 (t, 2H), 3.85 (s, 6H), 3.65 (t, 2H), 3.28 (s, 3H).
(E)-1-(3,4-Dimethoxystyryl)-2-(2-methoxymethoxy)naphthalene (15e)
Yellow solid, yield: 76%. 1H NMR (400 MHz, DMSO-d6) δ 8.05 (d, J = 7.6 Hz, 1H), 7.86 (d, J = 8.8 Hz, 1H), 7.48–7.66 (m, 3H), 7.36 (s, 1H), 7.32 (d, J = 8.4 Hz, 1H), 7.22 (d, J = 8.8 Hz, 1H), 7.08 (d, J = 8.4 Hz, 1H), 6.99 (d, J = 8.4 Hz, 1H), 6.72 (d, J = 8.8 Hz, 1H), 4.50 (t, 2H), 3.83 (s, 6H), 3.71 (t, 2H), 3.29 (s, 3H).
(E)-5-(3,4-Dimethoxystyryl)-6-(2-methoxymethoxy)-N,N-dimethylnaphthalen-2-amine (15f)
Yellow solid, yield: 61%. 1H NMR (400 MHz, DMSO-d6) δ 7.88 (d, J = 8.7 Hz, 1H), 7.48–7.50 (m, 2H), 7.34 (s, 1H), 7.30 (d, J = 8.6 Hz, 1H), 7.20 (d, J = 8.8 Hz, 1H), 6.79–6.99 (m, 3H), 6.70 (d, J = 8.8 Hz, 1H), 4.52 (t, 2H), 3.82 (s, 6H), 3.74 (t, 2H), 3.30 (s, 3H), 3.06 (s, 6H).
(E)-5-(3,4-Dimethoxystyryl)-6-(2-methoxymethoxy)naphthalen-2-ol (15g)
Yellow solid, yield: 68%. 1H NMR (400 MHz, DMSO-d6) δ 7.96 (d, J = 9.2 Hz, 1H), 7.52 (d, J = 8.6 Hz, 1H), 7.34 (s, 1H), 7.32 (d, J = 8.4 Hz, 1H), 7.22 (d, J = 8.8 Hz, 1H), 7.19 (s, 1H), 7.03 (d, J = 8.6 Hz, 1H), 6.94–6.99 (m, 2H), 6.77 (d, J = 8.8 Hz, 1H), 4.42 (t, 2H), 3.82 (s, 6H), 3.75 (t, 2H), 3.31 (s, 3H).
(E)-8-(3,4-Dimethoxystyryl)-7-(2-methoxymethoxy)naphthalen-2-ol (15h)
Yellow solid, yield: 64%. 1H NMR (400 MHz, DMSO-d6) δ 7.79 (d, J = 9.2 Hz, 1H), 7.62 (d, J = 8.6 Hz, 1H), 7.32–7.35 (m, 2H), 7.21 (d, J = 8.8 Hz, 1H), 7.15 (d, J = 8.6 Hz, 1H), 6.87–6.96 (m, 3H), 6.74 (d, J = 8.8 Hz, 1H), 4.50 (t, 2H), 3.83 (s, 6H), 3.71 (t, 2H), 3.29 (s, 3H).
(E)-2-(2-Methoxymethoxy)-1-(4-methoxystyryl)naphthalene (15i)
Yellow solid, yield: 64%. 1H NMR (400 MHz, DMSO-d6) δ 7.75–7.85 (m, 5H), 7.58–7.54 (m, 1H), 7.24–7.38 (m, 3H), 7.20 (d, J = 8.6 Hz, 1H), 7.03 (d, J = 8.6 Hz, 1H), 6.79 (d, J = 8.2 Hz, 1H), 4.43 (t, 2H), 3.82 (s, 3H), 3.70 (t, 2H), 3.39 (s, 3H).
(E)-6-(2-Methoxymethoxy)-5-(4-methoxystyryl)-N,N-dimethylnaphthalen-2-amine (15j)
Yellow solid, yield: 51%. 1H NMR (400 MHz, DMSO-d6) δ 7.68–7.74 (m, 3H), 7.54–7.57 (m, 2H), 7.23 (d, J = 9.2 Hz, 1H), 7.11–7.15 (m, 2H), 6.55–6.59 (m, 2H), 6.78 (d, J = 9.2 Hz, 1H), 4.41 (t, 2H), 3.82 (s, 3H), 3.74 (t, 2H), 3.40 (s, 3H), 2.99 (s, 6H).
(E)-6-(2-Methoxymethoxy)-5-(4-methoxystyryl)naphthalen-2-ol (15k)
Yellow solid, yield: 45%. 1H NMR (400 MHz, DMSO-d6) δ 7.72–7.79 (m, 3H), 7.67 (d, J = 8.6 Hz, 1H), 7.43 (s, 1H), 7.25 (d, J = 9.2 Hz, 1H), 7.14–7.19 (m, 2H), 6.87–6.92 (m, 2H), 6.80 (d, J = 9.2 Hz, 1H), 4.44 (t, 2H), 3.83 (s, 3H), 3.76 (t, 2H), 3.38 (s, 3H).
(E)-7-(2-Methoxymethoxy)-8-(4-methoxystyryl)naphthalen-2-ol (15l)
Yellow solid, yield: 49%. 1H NMR (400 MHz, DMSO-d6) δ 7.74–7.80 (m, 3H), 7.69 (d, J = 9.0 Hz, 1H), 7.49 (s, 1H), 7.22 (d, J = 9.2 Hz, 1H), 7.15–7.19 (m, 2H), 6.94–7.00 (m, 2H), 6.78 (d, J = 9.2 Hz, 1H), 4.40 (t, 2H), 3.83 (s, 3H), 3.75 (t, 2H), 3.39 (s, 3H).
General procedure for the preparation of 16
A solution of compounds 15a–15l (0.5 mmol) in methanol (5 mL) was treated with 6 M HCl (0.5 mL), and the mixture was refluxed for 3 h. The solvent was removed by evaporation, and the residue was neutralised with saturated aqueous NaHCO3 and extracted with ethyl acetate. The ethyl acetate layer was washed with water and brine and dried over Na2SO4. The solvent was removed by evaporation, and the residue was purified by flash chromatography on silica gel with petrol/ethyl acetate as the elution solvent to afford the desired products 16a–16l.
General procedure for the preparation of 17
BBr3 (5–7.5 eq.) was added dropwise at –78 °C under nitrogen to a solution of dried CH2Cl2 containing compounds 16a–16l (0.5 mmol). The resulting solution was slowly warmed to room temperature and stirred overnight. After monitoring the reaction progress by TLC, water was added slowly. The mixture was neutralised with saturated aqueous NaHCO3 and extracted with ethyl acetate. The ethyl acetate layer was washed with water and then dried over Na2SO4. The solvent was removed by evaporation, and the residue was purified by flash chromatography on silica gel with CH2Cl2/methanol as the elution solvent to afford the desired products 17a–17l.
5-(3-Hydroxy-3H-naphtho[1,2-e][1,2]oxaborinin-2-yl)benzene-1,3-diol (17a)
Red solid, yield: 80%. 1H NMR (400 MHz, MeOD) δ: 8.56 (s, 1H), 8.30 (d, J = 8.4 Hz, 1H), 7.79 (dd, J = 12.2, 8.6 Hz, 2H), 7.54 (t, J = 7.2 Hz, 1H), 7.46–7.27 (m, 2H), 6.76 (d, J = 2.1 Hz, 2H), 6.44–5.99 (m, 1H). 13C NMR (101 MHz, MeOD) δ: 159.3, 151.4, 144.0, 138.7, 131.9, 131.4, 130.9, 129.7, 128.0, 125.6, 122.6, 119.9, 118.4, 107.6, 102.5, 101.4. HRMS (ESI) m/z [M – H]– for C18H13BO4 pred. 303.0834, meas. 303.0827. HPLC purity: 99.3%.
5-(8-(Dimethylamino)-3-hydroxy-3H-naphtho[1,2-e][1,2]oxaborinin-2-yl)benzene-1,3-diol (17b)
Red solid, yield: 60%. 1H NMR (400 MHz, MeOD) δ: 8.46 (s, 1H), 8.09 (d, J = 9.3 Hz, 1H), 7.58 (d, J = 8.9 Hz, 1H), 7.28–7.11 (m, 2H), 7.00–6.90 (m, 1H), 6.71 (d, J = 2.2 Hz, 2H), 6.23 (t, J = 2.2 Hz, 1H), 2.98–2.82 (m, 6H). 13C NMR (101 MHz, MeOD) δ: 159.2, 149.3, 144.3, 139.1, 132.8, 129.7, 124.6, 123.4, 119.9, 118.4, 109.5, 107.6, 102.4, 41.2. HRMS (ESI) m/z [M – H]– for C20H18BNO4 pred. 346.1256, meas. 346.1250. HPLC purity: 96.2%.
2-(3,5-Dihydroxyphenyl)-3H-naphtho[1,2-e][1,2]oxaborinine-3,8-diol (17c)
Red solid, yield: 73%. 1H NMR (400 MHz, MeOD) δ: 8.58 (s, 1H), 8.26 (d, J = 8.6 Hz, 1H), 7.65 (d, J = 8.7 Hz, 1H), 7.37 (d, J = 8.6 Hz, 1H), 7.18 (d, J = 13.8 Hz, 2H), 6.75 (d, J = 2.1 Hz, 2H), 6.26 (d, J = 2.0 Hz, 1H). 13C NMR (101 MHz, MeOD) δ: 159.2, 157.8, 152.1, 144.2, 139.1, 133.8, 131.4, 130.9, 126.1, 117.3, 117.2, 116.8, 107.5, 105.1, 102.3. HRMS (ESI) m/z [M – H]– for C18H13BO5 pred. 319.0783, meas. 319.0780. HPLC purity: 97.7%.
2-(3,5-Dihydroxyphenyl)-3H-naphtho[1,2-e][1,2]oxaborinine-3,9-diol (17d)
Red solid, yield: 78%. 1H NMR (400 MHz, MeOD) δ: 8.48 (s, 1H), 7.70 (dd, J = 8.8, 3.3 Hz, 2H), 7.63 (d, J = 2.1 Hz, 1H), 7.20 (d, J = 8.8 Hz, 1H), 7.02 (dd, J = 8.8, 2.3 Hz, 1H), 6.73 (d, J = 2.2 Hz, 2H), 6.26 (t, J = 2.2 Hz, 1H). 13C NMR (101 MHz, MeOD) δ: 159.2, 157.8, 152.1, 144.2, 139.1, 133.8, 131.4, 130.9, 126.1, 117.3, 117.2, 116.8, 107.5, 105.1, 102.3. HRMS (ESI) m/z [M – H]– for C18H13BO5 pred. 319.0783, meas. 319.0775. HPLC purity: 98.0%.
4-(3-Hydroxy-3H-naphtho[1,2-e][1,2]oxaborinin-2-yl)benzene-1,2-diol (17e)
Red solid, yield: 80%. 1H NMR (400 MHz, MeOD) δ: 8.56 (s, 1H), 8.37 (d, J = 8.5 Hz, 1H), 7.80 (dd, J = 16.1, 8.5 Hz, 2H), 7.52–7.56 (m, 1H), 7.45–7.35 (m, 2H), 7.27 (d, J = 2.1 Hz, 1H), 7.13 (dt, J = 24.2, 12.1 Hz, 1H), 6.78 (d, J = 8.2 Hz, 1H). 13C NMR (101 MHz, MeOD) δ: 151.3, 146.0, 137.0, 134.0, 131.9, 131.5, 130.4, 129.7, 127.2, 125.6, 122.8, 120.7, 119.9, 118.7, 116.2, 116.1. HRMS (ESI) m/z [M – H]– for C18H13BO4 pred. 303.0834, meas. 303.0829. HPLC purity: 96.8%.
4-(8-(Dimethylamino)-3-hydroxy-3H-naphtho[1,2-e][1,2]oxaborinin-2-yl)benzene-1,2-diol (17f)
Red solid, yield: 70%. 1H NMR (400 MHz, MeOD) δ: 8.47 (s, 1H), 8.19 (d, J = 9.3 Hz, 1H), 7.61 (d, J = 8.9 Hz, 1H), 7.32–7.23 (m, 3H), 7.15 (dd, J = 8.2, 2.1 Hz, 1H), 7.00 (d, J = 2.5 Hz, 1H), 6.82 (d, J = 8.2 Hz, 1H). 13C NMR (101 MHz, MeOD) δ: 149.3, 148.9, 145.9, 145.8, 137.3, 134.2, 132.9, 129.2, 124.7, 123.5, 120.6, 119.9, 118.7, 118.3, 116.2, 116.1, 109.5, 41.3. HRMS (ESI) m/z [M – H]– for C20H18BNO4 pred. 346.1256, meas. 346.1248. HPLC purity: 96.3%.
2-(3,4-Dihydroxyphenyl)-3H-naphtho[1,2-e][1,2]oxaborinine-3,8-diol (17g)
Red solid, yield: 68%. 1H NMR (400 MHz, MeOD) δ: 8.57 (s, 1H), 8.32 (d, J = 9.2 Hz, 1H), 7.65 (d, J = 8.9 Hz, 1H), 7.39 (d, J = 8.9 Hz, 1H), 7.30 (d, J = 2.1 Hz, 1H), 7.24–7.15 (m, 3H), 6.82 (d, J = 8.2 Hz, 1H). 13C NMR (101 MHz, MeOD) δ: 158.9, 153.0, 145.4, 139.4, 138.0, 133.2, 132.0, 129.9, 127.0, 118.5, 116.8, 114.2, 107.0, 104.9, 101.8. HRMS (ESI) m/z [M – H]– for C18H13BO5 pred. 319.0783, meas. 319.0774. HPLC purity: 97.7%.
2-(3,4-Dihydroxyphenyl)-3H-naphtho[1,2-e][1,2]oxaborinine-3,9-diol (17h)
Red solid, yield: 75%. 1H NMR (400 MHz, MeOD) δ: 8.49 (s, 1H), 7.74 (d, J = 8.8 Hz, 2H), 7.69 (d, J = 2.1 Hz, 1H), 7.27 (dd, J = 10.7, 5.5 Hz, 2H), 7.19–7.11 (m, 1H), 7.04 (dd, J = 8.8, 2.3 Hz, 1H), 6.81 (d, J = 8.2 Hz, 1H). δ: 159.1, 158.0, 151.8, 144.2, 138.8, 134.0, 132.1, 131.2, 127.4, 116.7, 115.0, 107.0, 105.1, 103.1. HRMS (ESI) m/z [M – H]– for C18H13BO5 pred. 319.0783, meas. 319.0776. HPLC purity: 98.0%.
2-(4-Hydroxyphenyl)-3H-naphtho[1,2-e][1,2]oxaborinin-3-ol (17i)
Red solid, yield: 80%. 1H NMR (400 MHz, MeOD) δ: 8.48 (s, 1H), 8.30 (d, J = 8.5 Hz, 1H), 7.79 (d, J = 7.9 Hz, 1H), 7.73 (d, J = 8.9 Hz, 1H), 7.62–7.56 (m, 2H), 7.50–7.54 (m, 1H), 7.44–7.37 (m, 1H), 7.32 (d, J = 8.9 Hz, 1H), 6.87–6.76 (m, 2H). 13C NMR (101 MHz, MeOD) δ: 157.9, 150.9, 136.9, 133.3, 131.8, 131.4, 130.3, 130.0, 129.6, 127.8, 125.5, 122.8, 119.9, 118.7, 116.1. HRMS (ESI) m/z [M – H]– for C18H13BO3 pred. 287.0885, meas. 287.0882. HPLC purity: 96.2%.
8-(Dimethylamino)-2-(4-hydroxyphenyl)-3H-naphtho[1,2-e][1,2]oxaborinin-3-ol (17j)
Red solid, yield: 80%. 1H NMR (400 MHz, DMSO-d6) δ 9.40 (s, 1H), 9.06 (s, 1H), 8.60 (s, 1H), 8.42 (d, J = 9.4 Hz, 1H), 7.71 (dd, J = 8.6, 6.3 Hz, 2H), 7.29 (dd, J = 9.1, 4.1 Hz, 2H), 7.04 (d, J = 2.5 Hz, 1H), 6.80 (d, J = 8.6 Hz, 1H), 2.99 (s, 4H). 13C NMR (101 MHz, DMSO-d6) δ: 159.9, 148.8, 144.0, 138.4, 133.0, 131.7, 128.7, 125.0, 122.9, 119.4, 118.9, 108.2, 107.0, 101.7, 41.2. HRMS (ESI) m/z [M – H]– for C20H18BNO3 pred. 330.1307, meas. 330.1299. HPLC purity: 96.3%.
2-(4-Hydroxyphenyl)-3H-naphtho[1,2-e][1,2]oxaborinine-3,8-diol (17k)
Red solid, yield: 74%. 1H NMR (400 MHz, MeOD) δ: 8.50 (s, 1H), 8.24 (d, J = 9.0 Hz, 1H), 7.62–7.53 (m, 3H), 7.31 (d, J = 8.9 Hz, 1H), 7.17–7.08 (m, 2H), 6.81–6.76 (m, 2H). 13C NMR (101 MHz, MeOD) δ: 158.0, 153.8, 150.4, 134.9, 133.1, 131.9, 130.8, 130.0, 129.3, 126.9, 125.1, 123.0, 120.2, 118.1, 115.3. HRMS (ESI) m/z [M – H]– for C18H13BO4 pred. 303.0834, meas. 303.0825. HPLC purity: 97.7%.
2-(4-Hydroxyphenyl)-3H-naphtho[1,2-e][1,2]oxaborinine-3,9-diol (17l)
Red solid, yield: 80%. 1H NMR (400 MHz, MeOD) δ: 8.50 (s, 1H), 8.24 (d, J = 9.0 Hz, 1H), 7.62–7.53 (m, 3H), 7.31 (d, J = 8.9 Hz, 1H), 7.17–7.08 (m, 2H), 6.81–6.76 (m, 2H). 13C NMR (101 MHz, MeOD) δ: 158.2, 152.1, 149.8, 134.7, 133.5, 132.0, 130.7, 130.2, 129.0, 127.0, 125.7, 122.8, 120.1, 117.9, 114.8. HRMS (ESI) m/z [M – H]– for C18H13BO4 pred. 303.0834, meas. 303.0824. HPLC purity: 97.1%.
Biological assays
ThT assay
Aβ1–42 (Millipore, counter ion: NaOH) was dissolved in ammonium hydroxide (1% v/v) to give a stock solution (2000 μM), which was aliquoted into small samples and stored at –80 °C.
For the experiment on inhibition of self-mediated Aβ1–42 aggregation, the Aβ stock solution was diluted with 50 mM phosphate buffer (pH 7.4) to 50 μM before use. A mixture of the peptide (10 μL; 25 μM, final concentration) with or without the test compound (10 μL; 20 μM, final concentration) was incubated at 37 °C for 48 h. Blanks using 50 mM phosphate buffer (pH 7.4) instead of Aβ with or without inhibitors were also analyzed. The sample was diluted to a final volume of 200 μL with 50 mM glycine–NaOH buffer (pH 8.0) containing thioflavin T (5 μM). Then the fluorescence intensities were recorded five minutes later (excitation, 450 nm; emission, 485 nm).24 The percent inhibition of aggregation was calculated using the expression (1-IFi/IFc) × 100%, in which IFi and IFc are the fluorescence intensities obtained for Aβ in the presence and absence of inhibitors after subtracting the background, respectively.
For the experiment on inhibition of copper-mediated Aβ1–42 aggregation, the Aβ stock solution was diluted in 20 μM HEPES (pH 6.6) with 150 μM NaCl. A mixture of the peptide (10 μL; 25 μM, final concentration) with or without copper (10 μL; 25 μM, final concentration) and the test compound (10 μL; 50 μM, final concentration) was incubated at 37 °C for 24 h. Then 20 μL of sample was diluted to a final volume of 200 μL with 50 mM glycine–NaOH buffer (pH 8.0) containing thioflavin T (5 μM). The detection method was the same as that of the self-mediated Aβ1–42 aggregation experiment.
For the experiment on disaggregation of self-induced Aβ fibrils, the Aβ stock solution was diluted with 10 mM phosphate buffer (pH 7.4). The peptide (15 μL, 50 μM) was incubated at 37 °C for 24 h. The test compound (15 μL, 50 μM) was then added and incubated at 37 °C for another 24 h. Then 20 μL of sample was diluted to a final volume of 200 μL with 50 mM glycine–NaOH buffer (pH 8.0) containing thioflavin T (5 μM). The detection method was the same as above.
For the experiment on disaggregation of copper-induced Aβ fibrils, the Aβ stock solution was diluted in 20 μM HEPES (pH 6.6) with 150 μM NaCl. A mixture of the peptide (10 μL; 25 μM, final concentration) and copper (10 μL; 25 μM, final concentration) was incubated at 37 °C for 24 h. The test compound (10 μL; 50 μM, final concentration) was then added and the mixture was incubated at 37 °C for another 24 h. Then 20 μL of sample was diluted to a final volume of 200 μL with 50 mM glycine–NaOH buffer (pH 8.0) containing thioflavin T (5 μM). The detection method was the same as above.
Oxygen radical absorbance capacity (ORAC-FL) assay
The test compound and the fluorescein (FL) stock solution were diluted with 75 mM phosphate buffer (pH 7.4) to 10 μM and 0.117 μM, respectively. A solution of (±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was diluted with the same buffer to 100, 80, 60, 50, 40, 20, and 10 μM. A solution of 2,2′-azobis-(amidinopropane)dihydrochloride (AAPH) was prepared before the experiment by dissolving 108.4 mg AAPH in 10 mL 75 mM phosphate buffer (pH 7.4) to a final concentration of 40 mM. A mixture of the test compound (20 μL) and FL (120 μL; 70 nM, final concentration) was pre-incubated for 10 min at 37 °C, and then 60 μL of the AAPH solution was added. The fluorescence was recorded every minute for 120 min (excitation, 485 nm; emission, 520 nm). A blank using phosphate buffer instead of the test compound was also analyzed. All reaction mixtures were prepared in triplicate and at least three independent runs were performed for each sample. The antioxidant curves (fluorescence versus time) were normalized to the curve of the blank. The area under the fluorescence decay curve (AUC) was calculated using the following equation: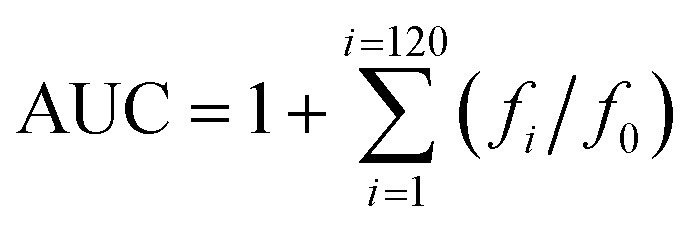 where f0 is the initial fluorescence reading at 0 min and fi is the fluorescence reading at time i. The net AUC was calculated using the expression AUCsample–AUCblank. Regression equations between net AUC and Trolox concentrations were calculated. The ORAC-FL value for each sample was calculated by using the standard curve which indicates the ORAC-FL value of the test compound expressed as Trolox equivalents.
where f0 is the initial fluorescence reading at 0 min and fi is the fluorescence reading at time i. The net AUC was calculated using the expression AUCsample–AUCblank. Regression equations between net AUC and Trolox concentrations were calculated. The ORAC-FL value for each sample was calculated by using the standard curve which indicates the ORAC-FL value of the test compound expressed as Trolox equivalents.
Metal-chelation study
The chelation studies were performed with a UV-Vis spectrophotometer. The absorption spectra of each compound (20 μM, final concentration) alone or in the presence of CuSO4, FeSO4, or ZnCl2 (40 μM, final concentration) for 30 min in 20% (v/v) ethanol/buffer (20 mM HEPES, 150 mM NaCl, pH 7.4) were recorded at room temperature.
Conflicts of interest
The author declare no conflict of interests.
Acknowledgments
We thank the National Natural Science Foundation of China (No. 21402175 and 21772241), the Guangdong Natural Science Foundation (2014A030313124), the Guangdong High-level Personnel of Special Support Program-Young Top-notch Talent Project (2015TQ01R244), the Guangzhou Pearl River New Star Fund Science and Technology Planning Project (201610010111) and the Fundamental Research Funds for the Central Universities (15ykpy04) for financial support of this study.
References
- Goedert M., Spillantini M. G. Science. 2006;314:777. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P., Kovari E., Gold G., von Gunten A., Hof P. R., Bouras C. Front. Neurosci. 2009;24:20. doi: 10.1159/000197881. [DOI] [PubMed] [Google Scholar]
- Pepeu G., Giovannini M. G. Curr. Alzheimer Res. 2009;6:86. doi: 10.2174/156720509787602861. [DOI] [PubMed] [Google Scholar]
- Tayeb H. O., Yang H. D., Price B. H., Tarazi F. I. Pharmacol. Ther. 2012;134:8. doi: 10.1016/j.pharmthera.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D. J. Science. 2002;297:353. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Bonda D. J., Wang X., Perry G., Nunomura A., Tabaton M., Zhu X., Smith M. A. Neuropharmacology. 2010;59:290. doi: 10.1016/j.neuropharm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Sultana R., Perluigi M., Butterfield D. A. Antioxid. Redox Signaling. 2006;8:2021. doi: 10.1089/ars.2006.8.2021. [DOI] [PubMed] [Google Scholar]
- Smith M. A., Rottkamp C. A., Nunomura A., Raina A. K., Perry G. Biochim. Biophys. Acta, Mol. Basis Dis. 2000;1502:139. doi: 10.1016/s0925-4439(00)00040-5. [DOI] [PubMed] [Google Scholar]
- Adams J., Behnke M., Chen S. W., Cruickshank A. A., Dick L. R., Grenier L., Klunder J. M., Ma Y. T., Plamondon L., Stein R. L. Bioorg. Med. Chem. Lett. 1998;8:333. doi: 10.1016/s0960-894x(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Rock F. L., Mao W., Yaremchuk A., Tukalo M., Crepin T., Zhou H., Zhang Y. K., Hernandez V., Akama T., Baker S. J., Plattner J. J., Shapiro L., Martinis S. A., Benkovic S. J., Cusack S., Alley M. R. Science. 2007;316:1759. doi: 10.1126/science.1142189. [DOI] [PubMed] [Google Scholar]
- Baker S. J., Zhang Y.-K., Akama T., Lau A., Zhou H., Hernandez V., Mao W., Alley M. R. K., Sanders V., Plattner J. J. Med. Chem. 2006;49:4448. doi: 10.1021/jm0603724. [DOI] [PubMed] [Google Scholar]
- Wring S., Gaukel E., Nare B., Jacobs R., Beaudet B., Bowling T., Mercer L., Bacchi C., Yarlett N., Randolph R., Parham R., Rewerts C., Platner J., Don R. Parasitology. 2014;141:104. doi: 10.1017/S003118201300098X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger G. B. Cell. Signalling. 2016;28:706. doi: 10.1016/j.cellsig.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman P. R. A., Wouters C., Prickaerts J. Curr. Pharm. Des. 2015;21:317. doi: 10.2174/1381612820666140826114601. [DOI] [PubMed] [Google Scholar]
- Fernández-Bachiller M. I., Pérez C., Monjas L., Rademann J., Rodríguez-Franco M. I. J. Med. Chem. 2012;55:1303. doi: 10.1021/jm201460y. [DOI] [PubMed] [Google Scholar]
- León R., Garcia A. G., Marco-Contelles J. Med. Res. Rev. 2013;33:139. doi: 10.1002/med.20248. [DOI] [PubMed] [Google Scholar]
- Zha X., Lamba D., Zhang L., Lou Y., Xu C., Kang D., Chen L., Xu Y., Zhang L., De Simone A., Samez S., Pesaresi A., Stojan J., Lopez M. G., Egea J., Andrisano V., Bartolini M. J. Med. Chem. 2016;59:114. doi: 10.1021/acs.jmedchem.5b01119. [DOI] [PubMed] [Google Scholar]
- Huang L., Lu C., Sun Y., Mao F., Luo Z., Su T., Jiang H., Shan W., Li X. J. Med. Chem. 2012;55:8483. doi: 10.1021/jm300978h. [DOI] [PubMed] [Google Scholar]
- Lu C., Guo Y., Yan J., Luo Z., Luo H.-B., Yan M., Huang L., Li X. J. Med. Chem. 2013;56:5843. doi: 10.1021/jm400567s. [DOI] [PubMed] [Google Scholar]
- Luo Z., Sheng J., Sun Y., Lu C., Yan J., Liu A., Luo H. B., Huang L., Li X. J. Med. Chem. 2013;56:9089. doi: 10.1021/jm401047q. [DOI] [PubMed] [Google Scholar]
- Wang Z., Wang Y., Wang B., Li W., Huang L., Li X. J. Med. Chem. 2015;58:8616. doi: 10.1021/acs.jmedchem.5b01222. [DOI] [PubMed] [Google Scholar]
- Lindgren M., Sorgjerd K., Hammarstrom P. Biophys. J. 2005;88:4200. doi: 10.1529/biophysj.104.049700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou B., Hampsch-Woodill M., Prior R. L. J. Agric. Food Chem. 2001;49:4619. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- Dávalos A., Gómez-Cordovés C., Bartolomé B. J. Agric. Food Chem. 2004;52:48. doi: 10.1021/jf0305231. [DOI] [PubMed] [Google Scholar]
- Sharma A. K., Pavlova S. T., Kim J., Finkelstein D., Hawco N. J., Rath N. P., Kim J., Mirica L. M. J. Am. Chem. Soc. 2012;134:6625. doi: 10.1021/ja210588m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami S., Maity S., Das A. K., Maity A. C. Tetrahedron Lett. 2013;54:6631–6634. [Google Scholar]
- Mao F., Yan J., Li J., Jia X., Miao H., Sun Y., Huang L., Li X. Org. Biomol. Chem. 2014;12:5936–5944. doi: 10.1039/c4ob00998c. [DOI] [PubMed] [Google Scholar]
- Dax C., Duffieux F., Chabot N., Coincon M., Sygusch J., Michels P. A. M., Blonski C. J. Med. Chem. 2006;49:1499–1502. doi: 10.1021/jm050237b. [DOI] [PubMed] [Google Scholar]
- Lu C., Guo Y., Li J., Yao M., Liao Q., Xie Z., Li X. Bioorg. Med. Chem. Lett. 2012;22:7683–7687. doi: 10.1016/j.bmcl.2012.09.105. [DOI] [PubMed] [Google Scholar]