Abstract
The essential oils of the flowers, leaves and stems of Senecio polyanthemoides Sch. Bip. Samples collected from two different localities within the city of uMhlathuze, KwaZulu-Natal Province (South Africa) were isolated by hydrodistillation and analyzed using GC and GC/MS. Twenty-six constituents were identified, representing an average of 86.0 - 99.6% of the total oil composition. The chemical profile reveals the dominance of monoterpenoid compounds, although some quantitative variance was noticed. The main constituents of the oils were limonene (3.1 – 43.0%), p-cymene (4.9-36.3%), β-selinene (1.3-32.7%), α-pinene (1.8-21.4%), β-pinene (7.6-16.5%) and 1,8-cineole (9.3-11.4%), caryophyllene oxide (4.1-13.4%) and humulene epoxide II (8.6-10.3%).
Keywords: Senecio polyanthemoides Sch. Bip., asteraceae, essential oil composition
Introduction
The genus Senecio (family Asteraceae; tribe Senecioneae) consists of more than 1,500 species of aromatic herbs and shrubby plants native to Southern Europe, but now spread all over the world. A few herbaceous species of the genus are grown as ornamental plants [1,2]. Literature reports on the phytochemistry of these species shows a large variety of pyrrolizidine alkaloids [3] and sesquiterpenoids [4], diterpenoids [5], triterpenoids [6], shikimic acid and cacalolide derivatives [7,8]. Furthermore, Biological activities such as antibacterial [9], molluscicidal [10], antimicrobial [11] and cytotoxic activities [12], and biosynthesis of algal pheromones [13] have been reported for these plants. In traditional medicine, the use of Senecio species for wound healing and treatment of coughs, bronchitis, asthma and eczema have been reported [14,15]. In the flora of South Africa, there are about 300 species of the genus Senecio with over 120 species found in KwaZulu-Natal Province [16,17]. The leaves are alternate in arrangement and the flowers are variously coloured (mostly yellow, but blue, purple or white forms are also found). Many species of the genus Senecio have been reportedly used by the Sotho, Xhosa and the Zulu tribes of South Africa as traditional remedies for colds and sore throats, coughs, burns and wounds, enemas in chest complaints, nausea and vomiting, stomach ache, hiccups, purgatives and also for anal protrusion in children, blood purifiers for skin eruptions and treatment of venereal diseases [18,19,20].
Senecio polyanthemoides Sch. Bip. is a bushy annual herb of about 1.8 m height with a woody stem at the base. The leaves (15 x 4 cm) are lanceolate, petiole-like and pinnately divided with smooth and thinly white-felted upper and lower surfaces respectively. Inflorescences are radiate, with many short bracteates arranged in spreading corymbose panicles and flowers mainly in September and October. S. polyanthemoides grows naturally on forests margins, farmlands and in scattered localities along roadsides in KwaZulu-Natal [16,17].
Figure 1.
Senecio polyanthemoides plants.
The chemical composition of the essential oils of some Senecio species have been reported [21,22,23,24,25,26,27,28,29,30,31,32]. The volatile oils from the aerial parts of S. nutans Sch. Bip collected from two different localities in Arequipa and in different seasons of the year from Luara region, both in Peru, Southern America, showed that monoterpene hydrocarbons predominated in all the oils. In the oils of Arequipa, sabinene and α-terpinene were the main constituents; while, the oils of the Luara region had α-phellandrene and p-cymene as the principal components. The leaf oil of S. squalidus L. from France was found to contain p-cymene (29.3%) and α-phellandrene (24.7%) as the major components. The essential oil of S. farfarifolius Boiss. Et Kotschy from Turkey was report to contain α-pinene (48.3%) and 1.8-cineole (10.3%) as the predominant constituents of the oil. The volatile constituents of S. glaucus subsp. coronopifloius from Belgium have myrcene (24.0%) and dehydrofukinone (21.0%) as the major components. The Indian species was found to be a potential source of α-thujone (84.17%). Furthermore, two Iranian Senecio species, S. leucostachys Baker essential oil has sabinene (20.7%), α-phellandrene (19.7%), germacrene D (10.8%) and β-caryophyllene (8.2%) ; while, S. vernalis Waldst. & Kit. had spathulenol (37.1%), 1,8-cineole (19.0%), m-cymene (16.6%), isobicyclogermacrenal (15.2%) and α-phellandrene (3.4%) as their major compounds. Also, the essential oils of S. aegyptius var. discoideus Boiss from Egypt have 1,10-epoxyfuranoeremophilane as the main component of the oils [9]. To the best of our knowledge, there are no reports on the essential oil profile of Senecio species growing in South Africa. Therefore, this paper reports for the first time the chemical composition of essential oils of flowers, leaves and stems of two Senecio polyanthemoides collected from two different locations in KwaZulu-Natal, South Africa.
Results and Discussion
Table 1 lists the physical properties of the essential oils of S. polyanthemoides. As shown in the table, hydrodistillation of the flower, leaf and stem samples of S. polyanthemoides from two different locations gives pale yellow oils in yields of 0.10 – 0.23% for Sample A and 0.07 – 0.21% for Sample B. The percentage composition of the essential oils and the fragmentation pattern of the components identified are listed in order of their elution off a DB-5 column (Table 2).
Table 1.
Physical properties of essential oils of Senecio polyanthemoides from two different locations.
| Yield (%) | Color | Odour |
|
|
|---|---|---|---|---|
| Sample A | ||||
| Flower | 0.10 | Pale yellow | aromatic | 1.4726 |
| Leaf | 0.23 | Pale yellow | spicy | 1.4731 |
| Stem | 0.17 | Pale yellow | herbaceous | 1.4720 |
| Sample B | ||||
| Flower | 0.07 | Pale yellow | aromatic | 1.4347 |
| Leaf | 0.21 | Pale yellow | spicy | 1.4320 |
| Stem | 0.11 | Pale yellow | herbaceous | 1.4320 |
Table 2.
Chemical composition of essential oil of Senecio polyanthemoides Sch. Bip.
| (%) Composition | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample A | Sample B | |||||||
| Compounds | RRI | m/z | ||||||
| F | L | S | F | L | S | |||
| α-pinene | 938 | 7.4 | 11.8 | 21.4 | 1.8 | 7.0 | 4.2 | 93, 79, 41,136 |
| sabinene | 972 | 3.5 | 3.2 | 5.4 | tr | 4.0 | - | 93, 77, 41, 136 |
| β-pinene | 981 | 7.6 | 7.6 | 12.4 | 16.5 | 7.8 | tr | 93, 41, 79, 136 |
| myrcene | 991 | 15.7 | - | - | 14.3 | 31.7 | 4.3 | 41, 93, 69, 136 |
| α-phellandrene | 1003 | 2.7 | - | - | 7.8 | - | - | 93, 77, 41, 136 |
| p-cymene | 1022 | 24.7 | 5.3 | 18.7 | 36.3 | 4.9 | 20.1 | 119, 91, 77, 134 |
| limonene | 1029 | 18.3 | 8.7 | 18.1 | 3.1 | 19.7 | 43.0 | 68, 93, 79, 136 |
| 1,8-Cineole | 1031 | - | 11.4 | 9.3 | - | - | - | 43, 81, 55, 154 |
| Z-(β)-ocimene | 1027 | 4.4 | - | - | 3.8 | 15.9 | - | 93, 79, 41, 136 |
| E-(β)-ocimene | 1035 | 8.3 | - | - | 7.1 | tr | - | 91, 79, 41, 136 |
| γ-terpinene | 1056 | - | - | - | 0.3 | - | - | 93, 77, 121, 136 |
| terpinolene | 1083 | - | - | - | 0.3 | - | - | 93, 121, 79, 136 |
| linalool | 1096 | - | - | - | 0.6 | - | - | 43, 71, 55, 154 |
| terpinen-4-ol | 1176 | 4.4 | - | - | 0.1 | - | - | 71, 41, 93, 154 |
| α-copaene | 1373 | - | - | - | 0.1 | - | - | 119, 161, 105, 204 |
| β-caryophyllene | 1421 | - | - | - | 0.6 | - | - | 41, 91, 79, 204 |
| α-humulene | 1452 | - | - | - | 0.9 | 2.9 | - | 93, 80, 41 204 |
| (Z,E)-α-farnesene | 1457 | - | - | - | 0.1 | - | - | 93, 41, 135, 204 |
| germacrene D | 1479 | tr | - | - | - | 0.3 | 2.4 | 161, 105, 91, 204 |
| β-selinene | 1482 | - | 32.7 | - | - | 1.3 | - | 107, 93, 121, 204 |
| unknown | 1487 | tr | - | - | - | 1.9 | - | 41, 121, 93, 204 |
| (E,E)-α-farnesene | 1494 | - | - | - | 0.1 | - | - | 41, 93, 69, 204 |
| δ -cadinene | 1518 | - | - | - | 0.1 | - | - | 161, 119, 134, 204 |
| caryophyllene oxide | 1572 | tr | 13.4 | 5.7 | - | - | 4.1 | 41, 79, 93, 220 |
| humulene epoxide II | 1596 | - | - | 8.6 | - | - | 10.3 | 43, 67, 109, 220 |
| farnesol | 1719 | - | - | - | 0.1 | - | - | 41, 69, 81, 93 |
| Monoterpene hydrocarbons | 92.6 | 36.6 | 85.3 | 92.0 | 91.0 | 71.6 | ||
| Oxygenated monoterpenes | 4.4 | 11.4 | 9.3 | 0.1 | - | - | ||
| Sesquiterpene hydrocarbons | - | 32.7 | - | 2.3 | 8.5 | - | ||
| Oxygenated sesquiterpenes | - | 13.4 | 14.3 | 0.1 | - | 14.4 | ||
| Total identified | 97.0 | 94.1 | 99.6 | 95.3 | 99.5 | 86.0 | ||
RRI = Retention relative index to C9-C24 n-alkanes on DB-5 column; tr. = trace amount (< 0.05%); F = flowers; L = leaves; S = stems; Sample A = Owen Sithole College of Agriculture, Empangeni; Sample B = KwaDlangezwa road, off University of Zululand, KwaDlangezwa.
In sample A (Table 2), 13 constituents (97.0%) were identified in flower oil: nine monoterpenes (97.0%) and three sesquiterpenes (traces). p-Cymene (24.7%), limonene (18.3%) and myrcene (15.7%) were the major components. Eight constituents (94.1%) were identified in leaf oil: six monoterpenes (48.0%) and two sesquiterpenes (51.6%). β-Selinene (32.7%) was the most abundant constituent in this oil, followed by caryophyllene oxide (13.4%), α-pinene (11.8%) and 1,8-cineole (11.4%). In the stem oil, eight constituents (99.6%) were identified, with six being monoterpenes (85.3%) and two sesquiterpenes (14.3%). This oil was characterized by the presence of α-pinene (21.4%), p-cymene (18.7%), limonene (18.1%), β-pinene (12.4%) and 1,8-cineole (9.3%).
In sample B (Table 2), 21 constituents (94.3%) were identified in flower oil: 13 monoterpenes (92.0%) and eight sesquiterpenes (2.3%). p-Cymene (36.3%) was again the most abundant constituent; others were β-pinene (16.5%), myrcene (14.3%) and α-phellandrene (9.5%). The leaf oil has 12 identified constituents (99.5%): eight monoterpenes (91.0%) and four sesquiterpenes (8.5%). Myrcene (31.7%), limonene (19.7%) and Z-(β)-ocimene (15.9%) were the major components. In the stem oil, seven constituents (86.0%) were identified: Five monoterpenes (71.6%) and two sesquiterpenes (14.4%).
Analyses of the oils shows that they were predominantly monoterpenoid in nature, like some other species in the genus Senecio. The oils were characterized by large amount of monoterpenes (48.0 – 97.0%), except for the leaf oil of sample A with a higher amount of sesquiterpenes (46.1%).
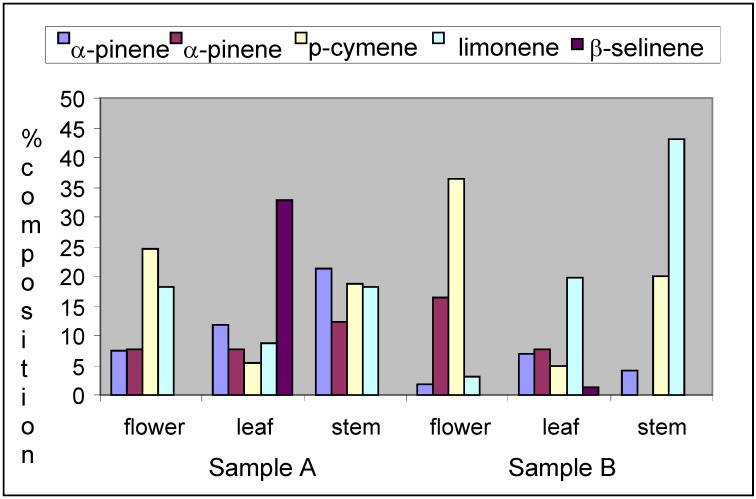
In terms of the organs studied, that is the flower, leaves and stem, the flowers of sample B had β-pinene (16.5%) α-phellandrene (7.8%) and p-cymene (36.3%) in susbtantial quantity, compared to sample A with β-pinene (7.6%) α-phellandrene (2.7%) and p-cymene (24.7%). Other compounds identified in sample B such as linalool, γ-terpinene, α-humulene, β-caryophyllene were completely absent in the flower oil of sample A. The flower oil of sample A on the other hand could be a good source of limonene, considering the compositional concentration (18.3%) when compared to 3.1% in sample B. This effect was noticeable in the odour of the flowers as that of sample A was stronger than that of sample B. The leaves of sample B had myrcene (31.7%), α-humulene (2.9%) and germacrene D (2.4%) as major or significant compounds present in the oil and not found in the Sample A leaf oil. In contrast, sample A had β-selinene (32.7%) and 1,8-cineole (11.4%) as major components. Variation in the compositional concentration of some compounds was also observed in the two leaf oils. The concentration of limonene (19.7%) in sample B was double the amount identified in the leaf oil of sample A (8.7%). Noteworthy is the concentration of limonene also in the stem oil of sample B which accounts for the major component of the oil. Considering the compositional variation, it could be said that the leaves and stem sample A would be of more medicinal value due to the presence of 1,8-cineole when compared to sample B, that did not have this chemical compound present in the oils. However, the stems of Sample B can be a very good source for limonene, which suggests its uses in flavoring applications. Figure 2 shows the major components of the oils of Senecio polyanthemoides from two different locations. In our own opinion, the two samples are completely different thus leading us to suspect that they are likely to be different chemotypes.
Figure 2.
Major components of the flower, leaf and stem oils of Senecio polyanthemoides from two different locations.
Comparing the present data (Table 1) with those previously reported in literature, the studied essential oils displayed different chemical profiles, although monoterpene hydrocarbons have been reported as the main constituents of the essential oils of several species of the genus Senecio [25,27,28,30,31], which are similar to the South Africa species. However, some species of this genus are characterized by high percentage of oxygenated compounds and mainly furanoeremophilan, as was found in Senecio chrysanthemoides, Senecio vernalis and Senecio aegyptius var. discoideus [22,32,9], and were not detected in the present study. Interestingly, limonene, which was found to be a major component in our study, has not been reported as the main constituent in any Senecio species. Many reports have shown how plant growth and development are affected by genetic and environmental factors, and how these factors contributes to differences in chemical variation of essential oils of plants with different chemotypes [33,34,35,36,37,38,39]. The chemical variation of essential oils of different chemotypes of Thymus species from different locations or growing in the same habitat have been attributed to different in environmental and genetic factors [33,34,35,36,37]. Furthermore, ecological factors, particularly, light and temperature have also been reported to influences the production of essential oils as well as other active agents in plants [38,39].
Experimental
Plant Material
Senecio polyanthemoides plants growing wild on the campus of Owen Sithole College of Agriculture, Empangeni and along KwaDlangezwa road, opposite University of Zululand, KwaDlangezwa, in the city of uMhlathuze, KwaZulu-Natal Province, South Africa, at a distance of about 120 Km from each other were randomly collected at flowering stage in September, 2006. The taxonomic identification of the plant materials was confirmed by a senior plant taxonomist, Dr S.J. Siebert of the Department of Botany, University of Zululand, KwaDlangezwa. Voucher specimens [Lawal, OA 23 & 24 (ZULU)] were deposited at the University of Zululand, Herbarium.
Oil isolation
Fresh flowers (115 g), leaves (300 g) and stems (500 g) of each sample were separately subjected to hydrodistillation in a Clevenger-type apparatus for 3h in accordance with the British Pharmacopoeia specification [40,41,42]. Briefly, sample was added to 750-1.5 mL of distilled, deionized water in a 2-5 L round-bottomed flask and heated to boiling, after which the essential oil was evaporated together with water vapour and finally collected in a condenser. The upper phase that contained the essential oil was separated from the lower one and the distillate isolated was preserved in a sealed sample tube and stored under refrigeration until analysis.
GC analyses
GC analyses was carried out on a Hewlett Packard HP 6820 Gas Chromatograph equipped with a FID detector and HP-5 MS column (60 m x 0.25 mm id, 0.25 µm film thickness) and split ratio of 1:25. Column temperature was initially kept at 50 oC for 2 min, then gradually increased to 240 oC at a 5 oC/min rate, and was held for 10 min. Injection and detector temperatures were 200 oC and 240 oC respectively. Hydrogen was the carrier gas, at a flow rate of 1 mL/min. A 1.0 µL aliquot of the diluted oil was injected into the GC. Peaks were measured by electronic integration. n-Alkanes were run at the same condition for Kováts indices determination.
GC-MS analyses
GC-MS analyses of the oils were performed using a Hewlett Packard Gas Chromatography HP 6890 equipped with a HP-5 MS capillary column (30 m x 0.25 mm id, film thickness 0.25 µm) interfaced with a Hewlett Packard 5973 mass spectrometer system. The oven temperature was programmed from 70 - 240 oC at the rate of 5 oC /min. The ion source was set at 240 oC and electron ionization at 70eV. Helium was used as the carrier gas at a flow rate of 1 mL/min, with a 1:25 split ratio. Scanning range was 35 to 425 amu. A sample (1.0 µL) of diluted oil in hexane was manually injected into the GC/MS.
Identification of compounds
The components of the oils were identified base on the comparison of their retention indices and mass spectra with those standards, Wiley Library mass spectra database of the GC/MS system and published data [43,44,45].
Acknowledgments
The authors would like to acknowledge the financial support by National Research Fund, South Africa, University of Zululand Research committee and Lagos state University, Ojo, Nigeria, and Dr S.J. Siebert for his help in identifying the plant materials.
Footnotes
Sample Availability: Samples of the plants are available at the University of Zululand Herbarium on request from the authors.
References
- 1.Nordenstam B. Senecioneae and Liabeae-systematic review. In: Heywood V.H., Harborne J.B., Turner B.L., editors. The Biology and Chemistry of the Compositae. Volume 2. Academic Press; London, UK: 1977. pp. 799–830. [Google Scholar]
- 2.Joffe P. Creative gardening with indigenous plants: A South African guide. Briza Publications; Pretoria, South Africa: 2001. pp. 16–32. [Google Scholar]
- 3.Bohlmann F., Zdero C., Jakupovic J., Grenz M., Castro V., King R.M., Robinson H., Vincent L.P.D. Further pyrrolizidine alkaloids and furoeremophitane from Senecio species. Phytochemistry. 1986;25:1151–1159. doi: 10.1016/S0031-9422(00)81571-X. [DOI] [Google Scholar]
- 4.Bohlmann F., Ziesche J. Sesquiterpenes from three Senecio species. Phytochemistry. 1981;20:469–472. doi: 10.1016/S0031-9422(00)84168-0. [DOI] [Google Scholar]
- 5.Rucker G., Manns D., Schenkel E.P., Hartmann R., Heinzmann B.M. Triterpenes with a new 9-epi-cucurbitan skeletol from Senecio selloi. Phytochemistry. 1999;52:1587–1591. doi: 10.1016/S0031-9422(99)00276-9. [DOI] [Google Scholar]
- 6.Cheng D., Cao X., Cheng J., Roedei E. Diterpene glycosides from Senecio rufus. Phytochemistry. 1993;32:151–153. doi: 10.1016/0031-9422(92)80122-U. [DOI] [Google Scholar]
- 7.Ndom J.C., Mbafor J.T., Azebaze A.G.B., Vardamides J.C., Kakam Z., Kamdem A.F.W., Deville A., Ngando T.M., Fomum Z.T. Secondary metabolites from Senecio burtonii (Compositae) Phytochemistry. 2006;67:838–842. doi: 10.1016/j.phytochem.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Bohlmann F., Bapuji M. Cacalol derivatives from Senecio lydenburgensis. Phytochemistry. 1982;21:681–683. doi: 10.1016/0031-9422(82)83165-8. [DOI] [Google Scholar]
- 9.El-Shazly A., Doral G., Wink M. Chemical Composition and Biological Activity of the Essential oils of Senecio aegyptius var. discoideus Boiss. Z. Naturforsch., C Biosci. 2002;57:434–415. doi: 10.1515/znc-2002-5-605. [DOI] [PubMed] [Google Scholar]
- 10.Grace M.H., Khattab A.M. Chemical constituents and molluscicidal activity of Senecio cineraria. D. C. Egypt. J. Pharm. Sci. 1998;39:253–266. [Google Scholar]
- 11.Perez C., Agnese A.M., Cabrera J.L. The essential oil of Senecio graveolens (Compositae): Chemical composition and antimicrobial activity test. J. Ethnopharmacol. 1999;66:91–96. doi: 10.1016/S0378-8741(98)00204-9. [DOI] [PubMed] [Google Scholar]
- 12.Loizzo M.R., Tundis R., Statti G.A., Menichini F. Jacaranone: A cytotoxic constituent from Senecio ambiguus subsp. ambiguus (Biv.) DC. against renal adenocarcinoma aCHN and prostate carcinoma LNCaP cells. Arch. Pharm. Res. 2007;30:701–707. doi: 10.1007/BF02977631. [DOI] [PubMed] [Google Scholar]
- 13.Boland W., Mertes K. Biosyntheses of algal pheromones. A model study with the composite Senecio isatideus. Eur. J. Biochem. 1985;147:83–91. doi: 10.1111/j.1432-1033.1985.tb08722.x. [DOI] [PubMed] [Google Scholar]
- 14.Hammond G.B., Fernandez I.D., Villegas L.F, Vaisberg A.J. A survey of traditional medicinal plants from the Callejon de Huaylas, Department of Ancash, Peru. J. Ethnopharmacol. 1998;61:17–30. doi: 10.1016/S0378-8741(98)00009-9. [DOI] [PubMed] [Google Scholar]
- 15.Uzun E., Sariyar G., Adsersen A., Karakoc B., Otuk G., Oktayoglu E., Pirildar S. Traditional medicine in Sakarya Province (Turkey) and antimicrobial activities of selected species. J. Ethnopharmacol. 2004;95:287–296. doi: 10.1016/j.jep.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Hilliard O.M. Compositae in Natal. University of Natal Press; Pietermaritzburg, South Africa: 1997. pp. 385–502. [Google Scholar]
- 17.Hutchings A., Scott A.H., Lewis G., Cunningham A B. Zulu Medicinal Plants: An Inventory. University of Natal Press; Scottsville, South Africa: 1996. pp. 328–330. [Google Scholar]
- 18.Hutchings A. A survey and analysis of traditional medicinal plants as used by the Zulu, Xhosa and Sotho. Bothalia. 1989;19:111–123. [Google Scholar]
- 19.Watt J.M., Breyer-Brandwijk M.J. The medicinal and Poisonous Plants of Southern and Eastern Africa. 2nd ed. Livingstone Ltd; Edinburgh, UK: 1962. p. 257. [Google Scholar]
- 20.Rose E.F. Senecio species: toxic plants used as food and medicine in Transkei. S. Afr. Med. J. 1972:1039–1043. [PubMed] [Google Scholar]
- 21.Vera R.R., Laurent S.J., Fraisse D.J. Chemical composition of the essential oil of Senecio ambavilla (Bory) Pers. From Reunion Island. J. Essent. Oil Res. 1994;6:21–25. doi: 10.1080/10412905.1994.9698319. [DOI] [Google Scholar]
- 22.Mengi N., Garg S.N., Agarwal S.K., Mathela C.S. Occurrence of β-thujone and a new p-menthane derivative in Senecio chrysanthemoides leaf oil. J. Essent. Oil Res. 1995;7:511–514. doi: 10.1080/10412905.1995.9698575. [DOI] [Google Scholar]
- 23.De Pooter V., De Buyck L.F., Schamp N.M., Aboutabi E., De Bruyn A., Husain S.Z. The volatile fraction of Senecio glaucus subsp. coronopifolius. Flavour Frag. J. 2006;1:159–163. [Google Scholar]
- 24.El-Shazly A.M. Essential oil composition of Senecio desfontainei Druce (Compositae) Zagazig J. Pharm. Sci. 1999;8:1–8. [Google Scholar]
- 25.Baser K.H.C., Demirci B. The essential oil of Senecio farfarifolius Boiss. et Kotschy growing in Turkey. J. Essent. Oil Res. 2004;16:558–559. doi: 10.1080/10412905.2004.9698797. [DOI] [Google Scholar]
- 26.Balzaretti V.T., Arancibia A., Marchiaro A.M.E., Feijoo M.S. Variation in the composition of the essential oil of Senecio filaginoides DC. Molecules. 2000;5:459–461. doi: 10.3390/50300459. [DOI] [Google Scholar]
- 27.Mirza M., Baher N.Z. Chemical composition of essential oil of Senecio leucostachys Baker. J. Essent. Oil Bearing Plants. 2008;11:179–183. doi: 10.1080/0972060X.2008.10643616. [DOI] [Google Scholar]
- 28.Belaunde A.J., Sandoval J.G., De Martino L., Senatore F., De Feo V. Chemical composition of essential oils of Senecio nutans Sch. Bip. (Asteraceae) J. Essent. Oil Bearing Plants. 2007;10:332–338. doi: 10.1080/0972060X.2007.10643564. [DOI] [Google Scholar]
- 29.Fernández-Zúñiga G., Fernández-Valderrama I., Hammond G.B. Investigation of the essential oils of Senecio tephrosioides and Salvia oppositiflora. Rev. Latinoam. Quim. 1996;25:14–16. [Google Scholar]
- 30.De Feo V., Soria E.U., Soria R.U., Senatore F. Chemical composition of essential oils of Senecio nutans Sch.-Bip. (Asteraceae) Flavour Fragr. J. 2003;18:234–236. doi: 10.1002/ffj.1204. [DOI] [Google Scholar]
- 31.Chalchat J.C., Maksimovic Z.A., Petrovic S.D., Gorunovic M.S. Essential oil of Senecio squalidus L., Asteraceae. J. Essent. Oil Res. 2004;16:227–228. doi: 10.1080/10412905.2004.9698704. [DOI] [Google Scholar]
- 32.Nori-Shargh D., Raftari S., Deyhimi F. Analysis of the essential oil of Senecio vernalis waldst. & kit. from Iran. Flavour Frag. J. 2008;23:357–359. doi: 10.1002/ffj.1860. [DOI] [Google Scholar]
- 33.Loziene K., Venskutonis P.R. Influence of environmental and genetic factors on the stability of essential oil composition of Thymus pulegioides. Bio. Syst. Ecol. 2005;33:517–525. doi: 10.1016/j.bse.2004.10.004. [DOI] [Google Scholar]
- 34.Boira H., Blanquer A. Environmental factors affecting chemical variability of essential oils in Thymus piperella L. Bio. Syst. Ecol. 1998;26:811–822. doi: 10.1016/S0305-1978(98)00047-7. [DOI] [Google Scholar]
- 35.Salgueiro L.R., Vila R., Tomas X., Tomi F., Canigueral S., Casanova J., Proenca da Cunha A., Adzet T. Chemical polymorphism of the essential oil of Thymus carnosus from Portugal. Phytochemistry. 1995;38:391–396. doi: 10.1016/0031-9422(94)00657-F. [DOI] [Google Scholar]
- 36.Curado M.A., Oliveira C.B.A., Jesus J.G., Santos S.C., Seraphin J.C., Ferri P.H. Environmental factors influence on chemical polymorphism of the essential oils of Lychnophora ericoides. Phytochemistry. 2006;67:2363–2367. doi: 10.1016/j.phytochem.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Robles C., Garzino S. Infraspecific variability in the essential oil composition of cistus monspeliensis leaves. Phytochemistry. 2000;53:71–75. doi: 10.1016/S0031-9422(99)00460-4. [DOI] [PubMed] [Google Scholar]
- 38.Burbott A.J., Loomis W.D. Effects of light and temperature on the monoterpenes of peppermint. Plant Physiol. 2000;42:20–28. doi: 10.1104/pp.42.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark R.J., Menary R.C. Environmental effects on peppermint (Mentha piperita L.). II. Effects of temperature on photosynthesis, photorespiration and dark respiration in peppermint with reference to oil composition. Aust. J. Plant Physiol. 1980b;7:693–697. doi: 10.1071/PP9800693. [DOI] [Google Scholar]
- 40.British Pharmacopoeia Part II. HMSO; London, UK: 1988. pp. 109–110. [Google Scholar]
- 41.Oyedeji O.A., Ekundayo O., Koenig W.A. Constituents of the essential oils from the leaves of Lenontis nepetaefolia (L.) Ait. F. J. Essent. Oil Res. 1999;11:716–718. doi: 10.1080/10412905.1999.9712003. [DOI] [Google Scholar]
- 42.Oyedeji O.A., Ekundayo O., Olawore O.N., Koenig W.A. Essential oil composition of two varieties of Eucalyptus camaldulensis from Nigeria. J. Essent. Oil Res. 2000;12:102–104. doi: 10.1080/10412905.2000.9712053. [DOI] [Google Scholar]
- 43.Adams R.P. Identification of Essential Oil Components by ion trap mass spectroscopy. Academic Press; New York. U.S.A.: 1989. [Google Scholar]
- 44.Joulain D., Koenig W.A. The atlas of spectral data of sesquiterpenes hydrocarbons. E.B-Verlag; Hamburg, Germany: 1998. [Google Scholar]
- 45.The complete database of essential oils B.A.C.I.S. The Netherlands: 1999. ESO 2000. [Google Scholar]