Abstract
Three new trinor-cycloartane glycosides, 15α-hydroxy-16-dehydroxy-16(24)-en-foetidinol-3-O-β-d-xylopyranoside (1), 28-hydroxy-foetidinol-3-O-β-d-xylopyranoside (2) and foetidinol-3-O-β-d-xylopyranosyl-(1”→3’)-β-d-xylopyranoside (3) together with the known compound foetidinol-3-O-β-d-xylopyranoside (4) were isolated from the n-BuOH fraction of the roots of Cimicifuga foetida. Their structures were elucidated on the basis of spectroscopic and chemical reaction data.
Keywords: Cimicifuga foetida, Triterpenoids, Trinor-cycloartane, Glycosides
1. Introduction
The Traditional Chinese Medicine Rhizoma Cimicifugea is used to treat toothache, mouth ulcers, sore throats and to help erupt measles [1]. The Chinese Pharmacopoeia describes three Cimicifuga species (C. foetida, C. dahurica and C. heracleifolia) as the crude drug source [1], among which C. foetida is found widely spread throughout northwest Yunnan Province [2].
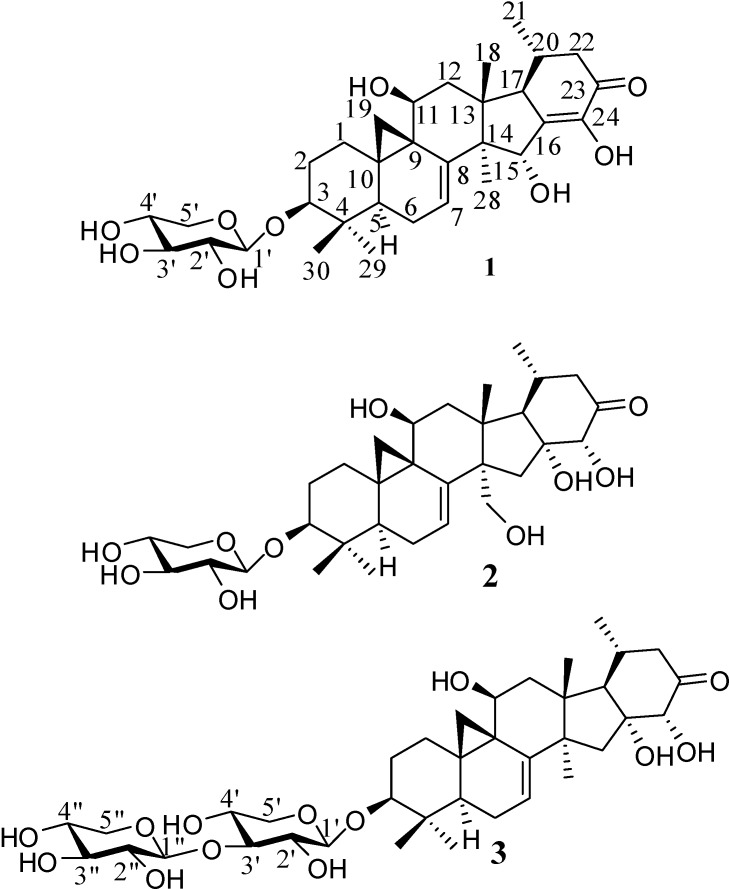
Ever since three trinor-cycloartanes were found in C. foetida by Asian research groups between 1994-96 [3,4,5,6,7], no additional compounds with such structure have been reported, as far as we know. While we were carrying out a phytochemical investigation on antitumor triterpenes of Cimiciguga species in Yunnan Province [8,9,10,11], three new trinor-cycloartane glycosides 1-3 were isolated from the roots of C. foetida (Figure 1).
Figure 1.
Structures of compounds 1, 2 and 3.
2. Results and Discussion
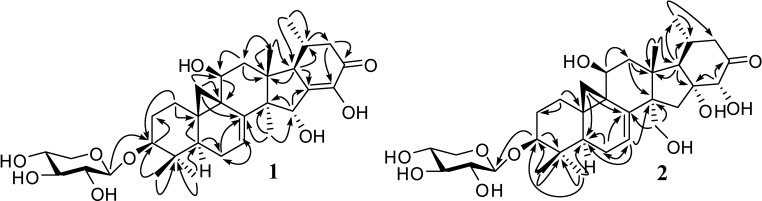
Compound 1: colorless needles, [α-25.6° (c=0.14, MeOH). The [M-1]- peak in the negative HRFABMS indicated a molecular formula of C32H46O9 (573.3014; calcd. for 573.3063). The IR spectrum displayed the presence of hydroxyl groups and a carbonyl group (νmax 3426 br and 1671 cm-1). The UV data [(MeOH) λmax(logε) nm: 282(3.4), 321(3.4)] suggested the presence of an α,β-unsaturated ketone group. The 13C-NMR (Table 1) and DEPT spectra of 1 displayed 32 signals, of which 27 were attributed to a trinor-triterpene skeleton and five to a β-d-xylopyranose moiety [δC 67.2 t (C-5’), 71.3 d (C-4’), 75.6 d (C-2’), 78.7 d (C-3’), 107.6 d (C-1’)]. The trinor-triterpene structure included characteristic signals of five methyls [δC 14.6 (C-30), 19.4 (C-21), 20.3 (C-28), 20.5 (C-18), 26.0 (C-29)], three oxygenated methines [δC 63.5 (C-11), 76.5 (C-15), 88.5 (C-3)], an isolated olefinic bond [δC 115.3 (C-7), 147.4 (C-8)] and an enone system [δC 141.1 (C-16), 146.9 (C-24), 195.8 (C-23)]. The 1H-NMR spectrum of 1 (Table 1) showed five methyl signals at δH 0.86 (d, 6.43 Hz, 3H-21), 1.12 (s, 3H-18), 1.14 (s, 3H-30), 1.40 (s, 3H-29) and 1.47 (s, 3H-28), cyclopropane methylene protons at δH 1.01 (d, 3.70 Hz, H-19) and 1.97 (d, 3.79 Hz, H-19), three oxygenated methine protons at δH 3.59 (dd, 12.11, 3.65 Hz, H-3), δH 4.63 (dd, 9.35, 2.95 Hz, H-11), δH 5.36 (d, 2.90 Hz, H-15) and an olefinic hydrogen at δH 6.17 (dd, 7.80, 1.44 Hz, H-7). The chemical shifts assignable to rings A, B and C were similar to those of foetidinol-3-O-β-d-xyloside [3], except that an olefinic bond between C-16 and C-24 in 1 formed an enone group with the carbonyl group at C-23. The assignments were confirmed by the HMBC correlations of H-15 with C-14, C-16, C-24 and C-28, of H-22 with C-17, C-20, C-21, C-23 and C-24, and of H-17 with C-13, C-16, C-18 and C-20 (Figure 2). An α-orientation for OH-15 was deduced from the ROESY correlations of H-15 with 3H-18. Therefore, the structure was established as 15α-hydroxy-16-dehydroxy-16(24)-en-foetidinol-3-O-β-d-xylopyranoside.
Table 1.
1H- and 13C-NMR Data for Compounds 1, 2 and 3.
| 1 | 2 | 3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | δC | δH, mult. (J in Hz) | δC, mult. | δH, mult. (J in Hz) | δC, mult. | δH, mult. (J in Hz) | |||||||
| C5D5N | |||||||||||||
| 1 | 27.6 t | 1.72m | 27.5 t | 1.61 t (12.2) | 27.5 t | 1.67 m | |||||||
| 2.81 m | 2.62 d (12.9) | 2.72 dt (13.7, 3.6) | |||||||||||
| 2 | 30.0 t | 2.10 m | 29.9 t | 2.03 m | 29.9 t | 2.05 m | |||||||
| 2.41 m | 2.35 m | 2.33 m | |||||||||||
| 3 | 88.5 d | 3.59 dd (12.1, 3.7) | 88.4 d | 3.47 dd (11.1, 3.0) | 88.8 d | 3.55 dd (11.9, 4.1) | |||||||
| 4 | 40.8 s | 40.7 s | 40.8 s | ||||||||||
| 5 | 44.0 d | 1.43 m | 43.8 d | 1.40 m | 44.1 d | 1.35 dd (12.6, 4.9) | |||||||
| 6 | 22.1 t | 1.76 m | 22.2 t | 1.76 dd (27.0, 13.5) | 22.1 t | 1.76 m | |||||||
| 2.03 m | 1.92 dd (16.4, 6.1) | 1.94 m | |||||||||||
| 7 | 115.3 d | 6.17 dd (7.8, 1.4) | 117.5 d | 5.26 d (7.0) | 113.8 d | 5.20 dd (7.8, 1.6) | |||||||
| 8 | 147.4 s | 144.4 s | 149.4 s | ||||||||||
| 9 | 27.8 s | 27.6 s | 27.5 s | ||||||||||
| 10 | 29.1 s | 29.3 s | 29.2 s | ||||||||||
| 11 | 63.5 d | 4.63 dd (9.4, 3.0) | 63.5 d | 4.57 m | 63.6 d | 4.59 m | |||||||
| 12 | 48.1 t | 2.02 m | 44.0 t | 2.46 m | 49.0 t | 2.06 m | |||||||
| 2.84 m | 2.80 d (13.8) | 2.84 dd (14.0, 9.6) | |||||||||||
| 13 | 43.6 s | 46.5 s | 46.4 s | ||||||||||
| 14 | 53.2 s | 56.8 s | 50.9 s | ||||||||||
| 15 | 76.5 d | 5.36 d (2.9) | 47.9 t | 2.17 m | 48.6 t | 2.25 m | |||||||
| 3.00 dd (13.3, 9.4) | 2.53 m | ||||||||||||
| 16 | 141.1 s | 82.4 s | 82.1 s | ||||||||||
| 17 | 54.9 d | 2.55 dd (10.1, 2.9) | 64.1 d | 2.40 m | 63.7 d | 2.21 m | |||||||
| 18 | 20.5 q | 1.12 s | 21.7 q | 1.34 s | 21.3 q | 1.26 s | |||||||
| 19 | 18.6 t | 1.01 d (3.7) | 19.2 t | 1.02 d (2.8) | 18.8 t | 1.01 d (3.5) | |||||||
| 1.97 d (3.8) | 2.02 d (3.6) | 1.98 d (3.8) | |||||||||||
| 20 | 34.2 d | 1.90 ddt (9.7, 6.6, 3.3) | 26.0 d | 2.20 m | 25.9 d | 2.17 m | |||||||
| 21 | 19.4 q | 0.86 d (6.4) | 20.7 q | 0.88 d (5.9) | 20.8 q | 0.91 d (6.1) | |||||||
| 22 | 47.1 t | 2.20 dd (15.8, 2.2) | 45.0 t | 2.46 m | 45.0 t | 2.41 dd (18.8, 3.2) | |||||||
| 2.51 dd (16.4, 3.6) | 2.46 m | 2.48 d (12.3) | |||||||||||
| 23 | 195.8 s | 211.3 s | 211.5 s | ||||||||||
| 24 | 146.9 s | 82.3 d | 4.58 s | 82.5 d | 4.49 s | ||||||||
| 28 | 20.3 q | 1.47 s | 67.4 t | 3.77 d (6.7) | 28.2 q | 1.59 s | |||||||
| 4.47 d (10.8) | |||||||||||||
| 29 | 26.0 q | 1.40 s | 25.9 q | 1.33 s | 25.9 q | 1.39 s | |||||||
| 30 | 14.6 q | 1.14 s | 14.6 q | 1.12 s | 14.7 q | 1.14 s | |||||||
| 1’ | 107.6 d | 4.88 d (7.5) | 107.6 d | 4.85 d (7.4) | 107.2 d | 4.82 d (7.5) | |||||||
| 2’ | 75.6 d | 4.03 t (8.1) | 75.6 d | 4.02 t (7.8) | 74.5 d | 4.03 m | |||||||
| 3’ | 78.7 d | 4.16 t (8.7) | 78.7 d | 4.17 dd (8.2, 16.8) | 87.4 d | 4.11 m | |||||||
| 4’ | 71.3 d | 4.20 | 71.3 d | 4.22 dd (11.7, 6.7) | 69.4 d | 4.08 m | |||||||
| 5’ | 67.2 t | 3.73 dd (10.9,10.1) | 67.2 t | 3.73 dd (10.2, 6.2) | 66.6 t | 3.70 m | |||||||
| 4.34 dd (11.3, 5.0) | 4.35 dd (11.2, 4.7) | 4.29 m | |||||||||||
| 1” | 106.3 d | 5.27 d (7.7) | |||||||||||
| 2” | 75.4 d | 4.01 m | |||||||||||
| 3” | 78.3 d | 4.14 m | |||||||||||
| 4” | 71.0 d | 4.15 m | |||||||||||
| 5” | 67.5 t | 3.66 m | |||||||||||
| 4.29 m | |||||||||||||
Figure 2.
HMBC correlations of compounds 1 and 2.
Compound 2: white powder, [α-62.0° (c=0.12, MeOH). The IR spectrum indicated the presence of hydroxyl groups, a carbonyl group and an olefinic bond (νmax 3460 br, 1724 and 1636 cm-1). The [M-1]- peak in the negative HRFABMS inferred a molecular formula of C32H48O10 (591.3131; calcd. for 591.3169). It has the same molecular formula as 15α-hydroxy-foetidinol-3-O-β-d-xyloside [3]. Comparison of its 13C-NMR data (Table 1) with those of 15α-hydroxy-foetidinol-3-O-β-d-xyloside, a methyl in the known compound was hydroxylated into a methylene at δC 67.4 in 2, which was located at C-28 by the HMBC associations of H-28 with C-8, C-12 and C-14, H-12 with C-13, C-14, C-17 and C-28. In addition, the other four methyls had no possibility to be hydroxylated due to 3H-21 and 3H-18 showed HMBC correlations with C-17 while 3H-29 and 3H-30 correlated with C-3, C-4 and C-5. Thus, the structure of 2 was elucidated as 28-hydroxy-foetidinol-3-O-β-d- xylopyranoside.
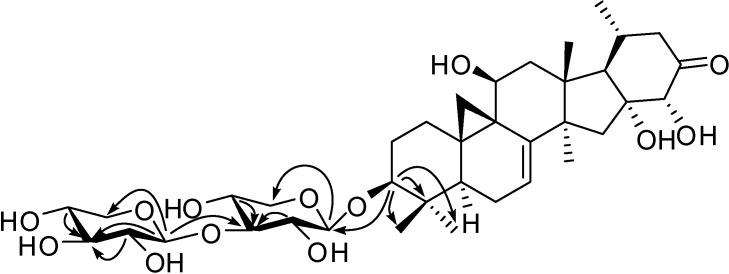
Compound 3: white powder, [α-46.2° (c=0.12, C5H5N). The IR spectrum indicated the presence of hydroxyl groups, a carbonyl group and an olefinic bond (νmax 3410 br, 1724 and 1633 cm-1). The [M+Cl]- peak in the negative HRESIMS corresponds to a molecular formula of C37H56O13 (743.3393; calcd. for 743.3409). The NMR data (Table 1) of the aglycon moiety were in good agreement with those of foetidinol aglycone [3], however, the ten additional resonances in the 13C-NMR spectrum at δC 66.6, 67.5, 69.4, 71.0, 74.5, 75.4, 78.3, 87.4, 106.3 and 107.2 and the signals of two anomeric protons at δH 4.82 (d, 7.49 Hz, H-1’) and 5.27 (d, 7.66 Hz, H-1”) in 1H-NMR spectrum indicated the existence of two pentoses in 3. The 13C-NMR data of the pentoses revealed the presence of xylose, and in the ROESY spectrum, H-1’ showed associations with H-3’ and H-5’ (δH 3.70 m) while H-1” showed associations with H-3” and H-5”. This was confirmed by acid hydrolysis and TLC comparison with an authentic sample. A linkage of the diglycoside of xylosyl-(1”→3’)-xylosyl to C-3 of the aglycone was proved by the HMBC correlations of H-3 with C-1’, C-4, C-29 and C-30; of H-1’ with C-3 and C-5’, of H-1” with C-3’, C-3” and C-5”; of H-2’, H-4’, 2H-5’ and H-1” with C-3’ and of H-2”, H-4” and 2H-5” with C-3” (Figure 3). In sum, compound 3 was established as foetidinol-3-O-β-d-xylopyranosyl-(1”→3’)-β-d-xylopyranoside.
Figure 3.
HMBC correlations of the disaccharide in compound 3.
3. Experimental
3.1. General
Optical rotations: Horiba SEPA-300 polarimeter. UV spectra: Shimadzu UV-2401A spectrophotometer. IR spectra: Bio-Rad FTS-135 infrared spectrophotometer. 1H-, 13C-NMR and 2D-NMR spectra: Bruker AM-400 MHz or DRX-500 spectrometers with TMS as internal standard. MS: VG Autospec-300, Finnigan MAT-90 and API Qstar-Plusar-1 spectrometers. The pentose authentic samples were purchased from Acros Organics.
3.2. Plant material
The roots of C. foetida were collected in Daju Village of Lijiang County, Yunnan Province in July 2004 and identified by Prof. Pei Shengji (Kunming Institute of Botany, the Chinese Academy of Sciences). The voucher specimen (KIB 04072601) has been deposited at the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences.
3.3. Extraction and Isolation
Air-dried and powdered roots of C. foetida (10 kg) were extracted three times with MeOH (25 L) under reflux. After removal of the solvent by evaporation, the residue (950 g) was suspended in H2O and partitioned sequentially with CHCl3 and n-BuOH. The n-BuOH fraction (250 g) was subjected to silica gel chromatography and eluted with CHCl3-MeOH (20:1, 10:1, 8:1, 5:1) to give four fractions (Fr. A - D). Fr. B (20 g) was chromatographed repeatedly over RP-18 (45-70% MeOH-H2O) to successively yield compounds 2 (8 mg), 4 (200 mg), 1 (20 mg) and 3 (25 mg).
15 α-Hydroxy-16-dehydroxy-16(24)-en-foetidinol-3-O-β-d-xylopyranoside (1): colorless needles; [α - 25.6° (c=0.14, MeOH); HRFABMS (573.3014; calcd for C32H45O9, 573.3063). IR (KBr) cm-1: 3426 (br, OH), 1671 (C=O); UV (MeOH) λmax (logε) nm: 282 (3.4), 321 (3.4); 1H- and 13C-NMR data, see Table 1.
28-Hydroxyfoetidinol-3-O-β-d-xylopyranoside (2): white powder, [α - 62.0° (c=0.12, MeOH); HRFABMS (573.3131; calcd for C32H48O10, 573.3169). IR (KBr) cm-1: 3460 (br, OH), 1724 (C=O), 1636 (C=C); 1H- and 13C-NMR data, see Table 1.
Foetidinol-3-O-β-d-xylopyranosyl-(1”→3’)-β-d-xylopyranoside (3): white powder, [α - 46.2° (c=0.12, C5H5N); HRESIMS (743.3393; calcd for C37H56O13Cl, 743.3409). IR (KBr) cm-1: 3410 (br, OH), 1724 (C=O), 1633 (C=C); 1H- and 13C-NMR data, see Table 1.
3.4. Acid hydrolysis of compounds 1-3
Compounds 1-3 (2 mg of each) were refluxed with 6% HCl-MeOH-n-BuOH (20 mL, 2:1:1 v/v/v) for 1 h at 90 °C, then neutralized with 12 M NaOH. The concentrated methanol soluble part showed a TLC spot (n-BuOH-acetone-H2O, 4:3:1, Rf = 0.7) matching that of an authentic sample of d-xylose.
4. Conclusions
Since three trinor-cycloartanes were found for the first time in C. foetida between 1994 and 1996, no such type of structure have been reported in past ten years. The new compounds found were trace constituents compared with foetidinol-3-O-β-d-xylopyranoside, and presumably are formed as side products or intermediates due to elimination, oxygenation or glycosylation reactions during the biosynthesis of this compound [12]. They suggest a diversity of trinor-cycloartane structures and the high-content of foetidinol-related structures found in Daju village of Lijiang County made the C. foetida growing in this area a new resource of novel trinor-cycloartane structures.
Acknowledgements
We are grateful to the Natural Science Foundation of Yunnan (No. 2005C0010Z) and Natural Science Foundation of China (No. 30772636), as well as NKIP Foundation of the CAS (No. KSCX2-YW-G-038 and KSCX2-YW-G-027), High-Tech Special Project of Yunnan Province (2007), Foundation of Key State Lab. of Phytochemistry and Plant Resources in West China for financial support.
Footnotes
Sample Availability: Samples of all the four compounds are available from the authors.
References
- 1.Pharmacopoeial Commission of the People’s Republic of China . The Pharmacopoeia of Chinese People’s Republic. The People’s Health Publishing House & the Chemical Industry Publishing House; Beijing, P.R. China: 2005. p. 50. [Google Scholar]
- 2.Institutum Botanicum Kunmingense Acadimiae Sinicae . Index florae yunnanensis, Tomus 1. The People’s Publishing House; Yunnan, P.R. China: 1984. p. 105. [Google Scholar]
- 3.Kadota S., Li J.X., Tanaka K., Namba T. Constituents of Cimicifugae rhizoma. II. Isolation and structures of new cycloartenol triterpenoids and related compounds from Cimicifuga foetida L. Tetrahedron. 1995;51:1143–1166. doi: 10.1016/0040-4020(94)01015-R. [DOI] [Google Scholar]
- 4.Koeda M., Aoki Y., Sakurai N., Kawai K., Nagai M. Three novel cyclolanostanol xylosides from Cimicifuga rhizome. Chem. Pharm. Bull. 1994;42:2205–2207. doi: 10.1248/cpb.42.2205. [DOI] [PubMed] [Google Scholar]
- 5.Sakurai N., Koeda M., Aoki Y., Nagai M. Studies on the Chinese crude drug "Shoma." X. Three new trinor-9,19-cyclolanostanol xylosides, cimicifugosides H-3, H-4 and H-6, from Cimicifuga Rhizome and transformation of cimicifugoside H-1 into cimicifugosides H-2, H-3 and H-4. Chem. Pharm. Bull. 1995;43:1475–1482. doi: 10.1248/cpb.43.1475. [DOI] [PubMed] [Google Scholar]
- 6.Li J.X., Kadota S., Pu X.F., Namba T. Foetidinol, a new trinor-triterpenoid with a novel carbon skeleton, from a Chinese crude drug "Shengma" (Cimicifuga foetida L.) Tetrahedron Lett. 1994;35:4575–4576. doi: 10.1016/S0040-4039(00)60732-3. [DOI] [Google Scholar]
- 7.Li C.J., Li Y.H., Xiao P.G., Mabry T.J., Watson W.H., Krawiec M. An unusual cycloartane triterpenoid from Cimicifuga foetida. Phytochemistry. 1996;42:489–494. doi: 10.1016/0031-9422(95)00895-0. [DOI] [PubMed] [Google Scholar]
- 8.Qiu M.H., Kim J.H., Lee H.K., Min B.S. Anticomplement activity of cycloartane glycosides from the rhizome of Cimicifuga foetida. Phytother. Res. 2006;20:945–948. doi: 10.1002/ptr.1982. [DOI] [PubMed] [Google Scholar]
- 9.Sun L.R., Yan J., Pei S.J., Qiu M.H. A new cycloartane triterpenoid from the rhizome of Cimicifuga foetida collected in Dali. Acta. Bot. Yunnan. 2005;27:331–336. [Google Scholar]
- 10.Sun L.R., Qing C., Zhang Y.L., Jia S.Y., Li Z.R., Pei S.J., Qiu M.H., Michael L.G., Samuel X.Q. Cimicifoetisides A and B, two cytotoxic cycloartane triterpenoid glycosides from the rhizomes of Cimicifuga foetida, inhibit proliferation of cancer cells. Beilstein J. Org. Chem. 2007;3(No.3) doi: 10.1186/1860-5397-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun L.R., Yan J., Nian Y., Zhou L., Zhang H.J., Qiu M.H. New triterpene diglycosides from the rhizome of Cimicifuga foetida. Molecules. 2008;13:1712–1721. doi: 10.3390/molecules13081712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haralampidis K, Trojanowska M, Osbourn A.E. Biosynthesis of Triterpenoid Saponins in Plant. In: Scheper T., editor. Advances in Biochemical Engineering/Biotechnology. volume 75. Springer-Verlag; Berlin, Heidelberg, New York: 2002. pp. 31–44. [DOI] [PubMed] [Google Scholar]