ABSTRACT
Introduction
Toxoplasma gondii is cause of a wide variety of infections in human. The aim of this study was; to evaluate the frequency of sero-positivity of the members in a family with a positive serologic person.
Methods
A case-control study with 170 enrolled units which was conducted in Iran in 2017. The units were settled into two group: I: the family members of seropositive individuals and II: the family members of seronegative individuals. The level of IgG and IgM anti-toxoplasma antibodies were evaluated by ELISA qualitative manner in both groups.
Results
Frequency of individuals with positive serology was 52.9% and 34.1% in group I and II, respectively (P value = 0.01).
Conclusion
Clusters of toxoplasma infection would be an event in the family members. Therefore evaluation of the family members (especially high risk persons) of a patient may be necessary.
Keywords: Toxopalsmosis, serology, family, community, transmission
RESUMEN
Introducción
Toxoplasma gondii es la causa de una gran variedad de infecciones en humanos. El objetivo de este estudio fue: evaluar la frecuencia de seropositividad de los miembros en una familia con una persona serológica positiva.
Material y métodos
Este estudio de casos y controles con 170 unidades inscritas fue realizado en Irán en 2017. Las unidades se dividieron en dos grupos: I: los miembros de la familia de individuos seropositivos y II: los miembros de la familia de los individuos seronegativos. El nivel de anticuerpos IgG e IgM anti-toxoplasma se evaluó por ELISA de manera cualitativa en ambos grupos.
Resultados
La frecuencia de individuos con serología positiva fue de 52,9% y 34,1% en el grupo I y II, respectivamente (p = 0,01).
Conclusión
Los grupos de infección por toxoplasma serían un evento en los miembros de la familia. Por lo tanto, la evaluación de los miembros de la familia (especialmente las personas de alto riesgo) de un paciente puede ser necesaria.
Palabras clave: Toxoplasmosis, serología, familia, comunidad, transmisión
INTRODUCTION
Toxoplasmosis is a public health problem all over the world. The cause of this disease is a parasite named Toxoplasma gondii [1].
This disease is one of the most common opportunistic infection in the world that is usually a self-limited disease in immune-competent hosts, but it may be a dangerous infection in immune-compromised individuals [2].
Toxoplasmosis in humans with intact immunity is asymptomatic in more than 80% of cases [3]; but in some other cases some symptoms such as cervical lymphadenopathy and fever may be seen. In other hands some patients may have some other nonspecific clinical signs and symptoms such as asthenia, myalgia which may persist for several weeks and mimicking infectious mononucleosis [3].
The parasite may be also a cause of visual impairment. In this kind of infection the parasite infect the retina and the choroid and may lead to retinal scarring [4, 5]. This form of infection may happen primarily or may be congenital. However, some authors believe that acquired infections are a more frequent cause of ocular toxoplasmosis than congenital infections [6].
In congenital toxoplasmosis a woman primarily get the infection during pregnancy and up to 20% of them developed trans-placental transmission to their baby [7]. It should recall that congenital infection is one of the sever form of infection in which the fetus may developed sever complications such as retinochoroiditis, brain calcifications, hydrocephalus, psychomotoric and neurologic disorders. In this setting fetal death is one of the outcomes [4].
In contrast to immune-competent patients, the infection in immune-compromised subjects is always life threatening. Therefore infection in HIV positive patients, transplant patients; specially heart and liver transplantation is very important and can be dangerous [8].
Nowadays we know three main pathway for transmission of this parasite to human: first; ingesting oocyte form through contaminated water and foods, second; eating an infected tissue and third; congenitally from the mother to her baby [9].
Transmission of this parasite to the human is primarily through ingestion its cyst form and / or its oocyst form. The cyst form of parasite may be present in previously infested and undercooked meat and its oocysts may be in contaminated soil, water, foods and vegetables [10].
The main reservoir of this parasite is cat. This domestic (and sometimes indoor) animal and other feline, are obligate hosts for T. gondii. The parasite reproduce sexually in the gastro- intestinal system of these animals and shed up to hundreds of millions of oocysts in their feces [9]. In environmental conditions the oocysts may undergo sporulation and contaminate the water and vegetables. Therefore by ingestion of these contaminated vegetables and water, infection will be happen in human and other warm blood animals [11]. In addition; in warm-blood livestock such as sheep, neat and pork, the parasite my developed to tissue cysts; and human by eating raw or undercooked infected meat may be infected by this parasite secondarily [12].
As said previously congenital transmission from mother to her baby is another route for transmission of the infection to the human [13].
Tachyzoite contaminated sperm and infected milk are other rare but potential routes of transmission [14]. There are also some reports of toxoplasma transmission following blood transfusion and organ transplantation [15].
There are some reports of common source outbreaks due to contaminated food and water in some communities [16]. Therefore it seems that incidence of T. gondii infection may be higher in some communities and even in some families because of the common source of infection in them.
Thus a clustering factor may play a role for higher incidence of infection in some communities and families.
Contopoulos et al. reported the higher incidence and prevalence of toxoplasmosis as a family clustering in fathers of congenitally infected children in North America; and he and his coworkers recommended that; it is better to investigate family clustering and community risk factors, when a recently infected person is identified in a family [17]. In addition; in a systematic evaluation; investigations in the United States have shown 18 household clusters in the families [18].
With due attention to the household clusters in anyplace in the world and the common source for infection in household members in a family; this question will be emerge that if a person in a family be seropositive for toxoplasma, how much is the chance for sero-positivity of another person in other household members in that family. Therefore we designed this study in Isfahan/Iran; to evaluate the frequency of seropositive persons which are household members of the index cases with toxoplasma sero-positivity.
MATERIAL AND METHODS
This is a case-control study in which 170 selected individuals enrolled in the study. This study is conducted in 2017 in Isfahan/Iran; by admission and support of Isfahan University of Medical Sciences (IUMS).
By referring to an authoritative laboratory in Isfahan/Iran; at first we found the name and addresses of all patients who had come to the laboratory for checking the toxoplasma serology (IgM and / or IgG) in the last 5 years (from January 2012 to January 2017). Then we categorized them into two groups on the results of their tests: positive (IgM and / or IgG) and negative (both IgM and IgG) groups. The patients with obscure test results had not enrolled in the study. After that we selected 85 patients by simple random sampling manner from each of the groups (total of 170 patients). After that we found the addresses and telephone numbers of them from the laboratory information file.
We telephoned to all the selected individuals in two groups and if they and their family consented to contribute in our study, that family would be selected for the final individual selection.
After this part, information about the household members of the selected family were collected and one of the family members selected by simple random manner as final selected individual. Then we invited the final selected individuals to the laboratory and after informed consent 5 ml blood was drown from each of them.
Some notes about the selection of the final selected individuals were as below:
We defined household members as; the individuals which live in the same location at least for one month and they consume from the same foods and water source in this time.
If a patient or his/her family didn’t consent to contribute in the study; one other patient would be selected
If a selected patient had not any other household member, he/she would be omitted from the study and another patient would be selected.
In the cases in which, the final selected individual didn’t agree for blood sampling and study; we picked up one another household member of the selected family or we should repeat selection once again from the first stage.
If selected individual has had an immunosuppressive disease or he/she was on the immunosuppressive drugs he/she would be omitted from the study and another one would be substituted.
After taking the blood from the selected individuals, serums were separated and stored in -20ºC at the laboratory within 1 hour after being obtained.
In this study for evaluating the IgG and IgM anti-toxoplasma quantitative method is used. In this study we used kit: “DIA.PRO diagnostic Bioprobes Srl. Italy”. This kit has sensitivity and specificity near 98% on the factory recommendations. At the time of lab test at first all the serum samples were exited from the freezing temperature and left at lab temperatures for 30 minutes. After that the test stages accomplished on kit guideline. At the end the results are read by Stat fax 2100 ELISA reader system. After collection the results of the serum samples; data were analyzed using Chi-square and independent t-tests by SPSS statistic program.
RESULTS
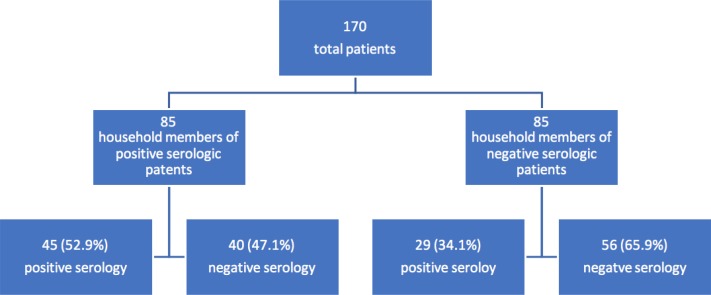
In this study 170 patients were enrolled. They settled into two groups: first the patients which were household member of seropositive patients (85 units) (group I), and second the patients which were household member of seronegative patients (85 units) (group II). Mean age of the patients were 32.04±7.4 years. Mean age in men were 36.6±8 and in women were 29.8±6.
In group I; 29 out of 85 (34%) were male and 56 out of them (66%) were female. In group II; 25 out of 85 (29.5%) were male and 60 out of them (70.5%) were female.
There was not significant statistical difference in ages and sexes between group I and group II (P = 0.098)
In our study 74 out of 170 patients (43.5%) were positive and 96 ones (56.5%) were negative for toxoplasma serology. The frequency of serology results was different between two groups (P= 0.01) (figure 1).
Figure 1.
The frequency of patients in every groups (I and II)
As it showed in table 1, 45 units out of 74 (60.8%) patients with positive serology are from group I and 29 units (39.2%) are from group II.
Table 1.
Frequency of toxoplasma sero-positivity in household members of studied patients
| Group I Seropositive patients (n=85) | Group II Seronegative patients (n=85) | OR (CI95%) | P- value | |
|---|---|---|---|---|
| Age, media (± SD | 30.8 ± 6.6 | 32.9 ± 7.8 | 0.93 | 0.07 |
| Sex, male (n, %) | 29 (34%) | 25 (29.5%) | 1.2 | 0.09 |
| Sero-positivity (n, %) | 45 (53.9%) | 29 (34.1%) | 2.17 | 0.01 |
SD: standard deviation; CI: Confidence interval 95%; OR : Odds ratio
On the other hand 52.9% of patients in group I are seropositive but 34.1% of patients in group II are seropositive (P = 0.01) (table 1).
DISCUSSION
As we know; transmission of toxoplasma does not occur directly between the family members which are living in the same home. But because of the common source of water and food, infection may happen in them at the same time. Therefore infection in one of the family members may be a key to think the hazard of infection in any other family members which is living with the index case.
Balasundaram et al. reported a big outbreak of toxoplasmosis as the largest acquired ocular toxoplasmosis in south India in which the suspected source of infection has been the common municipal drinking water [19]. They believe that large numbers of people of any age are at risk during an outbreak in these situations. They emphasized that when a case of toxoplasmosis is appeared in a community, some other cases would be present in the same community at the same time [19].
In another study in turkey which is conducted by Doganci et al a large outbreak of toxoplasmosis is reported in 171 students from a boarding school in turkey. They suggest that this outbreak possibly happened following exposure to cat litter [20].
By attention to the pathways of toxoplasma transmission, infection may happen in the human everywhere in which a group of them living together in one place and consume from the same water and food sources.
Therefore this event also may happen in a smaller community such as a family; and the family members which consume from the same source of food and water.
In addition it should emphasize that the life and health style in a community such as a family, is usually without changes. Therefore infection is not necessarily occur at the same time; and the members of a family may infected at different times. Thus all identified family/household members can be categorized as acutely infected (before last 6 months), recently infected (between before 6-12 months) and chronically infected (more than before 12 months) [18].
On the other hands sometimes the evidence of infection in family members may be only a positive toxoplasma serology.
By attention to the above facts, the existence of clusters of patient in a community (and family) will be debatable. Of course in these communities (and families) except for situations of sharing common food, other risk factors are not different; therefore only on epidemiologic and clinical information; clusters of cases within a family can be predictable [17].
Contopoulos et al. in their study evaluate clustering of toxoplasma infections within families of congenitally infected children and they conclude that in these cases; many fathers and mothers became infected at the same time.
All of these studies and our study emphasized that in a community or family the risk for toxoplasmosis is nearly equivalent for all the members.
In this study we identified that the frequency of seropositive toxoplasma persons is higher in family members of a seropositive individual than the family members of a seronegative one.
In this study there were some limitations. In this research the family groups of the patients who has been attended to the laboratory were evaluated. If the patient selection were from the community, it would be better, but the budget of the study was limited and we had to select the patients from the laboratory. In addition, because of limited budget we had to select one patient from every selected family, it would be better to evaluate all the members of each selected family. It should be say that satisfying the persons “who had not any complaint” for blood test was very troublesome and we had to refer many times to the selected persons for satisfying them for blood test.
The conclusion of our data is that the chance of the members of a family which are living in a same place, is nearly the same for toxoplasma infection. This fact show the potential of T. gondii to cause clusters of infections within families.
This is because of this fact that family members frequently share common exposures to water and food or other environmental sources which may be contaminated with T. gondii.
Therefore it is better to screen the family members of persons with toxoplasmosis especially when a pregnant woman and /or an immune-compromised person is living in the same family.
On the other hand identification of a person with toxoplasma infection in a family could lead us to earlier implementation of appropriate preventive strategies and /or therapeutic interventions to prevent transmission and/or to treat the infection in other members of family especially high-risk persons, such as pregnant women, HIV positive patients and other immune-compromised persons.
ACKNOWLEDGEMENT
This paper is the outcome of thesis number 396757 which was supported by Isfahan University of medical sciences. We thank our colleagues from infectious diseases and tropical medicine research center in Isafahan University of medical sciences, who provided insight and expertise that greatly assisted the study.
FUNDING
This work was supported by Isfahan University of Medical Sciences. This paper is the outcome of thesis number 396757.
CONFLICT OF INTEREST
All authors declare that they have no conflict of interest.
REFERENCES
- 1.Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. Am J Epidemiol 2001; 154 (4):357-65. 10.1093/aje/154.4.357 [DOI] [PubMed] [Google Scholar]
- 2.Matsuura J, Fujii A, Mizuta I, Norose K, Mizuno T. Cerebral Toxoplasmosis Diagnosed by Nested-Polymerase Chain Reaction in a Patient with Rheumatoid Arthritis. Intern Med J 2018:0139-17. 10.2169/internalmedicine.0139-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert-Gangneux F, Darde M-L. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 2018; 25(2):264-96. doi: 10.1128/CMR.05013-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilking H, Thamm M, Stark K, Aebischer T, Seeber F. Prevalence, incidence estimations, and risk factors of Toxoplasma gondii infection in Germany: a representative, cross-sectional, serological study. Sci Rep 2016; 6:22551. doi; 10.1038/srep22551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maenz M, Schluter D, Liesenfeld O, Schares G, Gross U, Pleyer U. Ocular toxoplasmosis past, present and new aspects of an old disease. Prog Retin Eye Res 2014; 39 : 77 –106. 10.1016/j.preteyeres.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 6.Delair E, Monnet D, Grabar S, Dupouy-Camet J, Yera H, Brezin AP. Respective roles of acquired and congenital infections in presumed ocular toxoplasmosis. Am J ophthalmol 2008;146(6):851-5. 10.1016/j.ajo.2008.06.027 [DOI] [PubMed] [Google Scholar]
- 7.Moncada PA, Montoya JG. Toxoplasmosis in the fetus and newborn: an update on prevalence, diagnosis and treatment. Expert Rev Anti Infec ther 2012; 10 (7):815-28. https://doi/abs/10.1586/eri.12.58 [DOI] [PubMed] [Google Scholar]
- 8.Botterel F, Ichai P, Feray C, Bouree P, Saliba F, Raspa RT, et al. . Disseminated toxoplasmosis, resulting from infection of allograft, after orthotopic liver transplantation: usefulness of quantitative PCR. J Clin Microbiol 2002; 40(5):1648-50. 10.1128/JCM.40.5.1648-1650.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.VanWormer E, Fritz H, Shapiro K, Mazet JA, Conrad PA. Molecules to modeling: Toxoplasma gondii oocysts at the human-animal-environment interface. Comp Immunol Microbiol Infect dis 2013; 36(3):217-31. 10.1016/j.cimid.2012.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubey J, Hill D, Jones J, Hightower A, Kirkland E, Roberts J, et al. . Prevalence of viable Toxoplasma gondii in beef, chicken, and pork from retail meat stores in the United States: risk assessment to consumers. J Parasitol 2005; 91(5):1082-93. 10.1645/GE-683.1 [DOI] [PubMed] [Google Scholar]
- 11.Jones JL, Dubey J. Foodborne toxoplasmosis. Clin Infec Dis 2012; 55(6):845-51. 10.1093/cid/cis508 [DOI] [PubMed] [Google Scholar]
- 12.Schluter D, Daubener W, Schares G, Grob U, Pleyer U, Luder C. Animals are key to human toxoplasmosis. Int J Med Microbiol 2014; 304(7):917-29. 10.1016/j.ijmm.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 13.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J parasitol 2000; 30(12-13):1217-58. 10.1016/S0020-7519(00)00124-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arantes TP, Lopes WDZ, Ferreira RM, Pieroni JSP, Pinto VM, Sakamoto CA, et al. . Toxoplasma gondii: Evidence for the transmission by semen in dogs. Exp parasitol 2009; 123(2):190-4. 10.1016/j.exppara.2009.07.003 [DOI] [PubMed] [Google Scholar]
- 15.Derouin F, Pelloux H. Prevention of toxoplasmosis in transplant patients. Clin Microbiol Infect 2008; 14(12):1089-101. 10.1111/j.1469-0691.2008.02091.x [DOI] [PubMed] [Google Scholar]
- 16.Boyer K, Hill D, Mui E, Wroblewski K, Karrison T, Dubey J, et al. . Unrecognized ingestion of Toxoplasma gondii oocysts leads to congenital toxoplasmosis and causes epidemics in North America. Clin Infect Dis 2011; 53(11):1081-9. 10.1093/cid/cir667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Contopoulos-Ioannidis D, Wheeler KM, Ramirez R, Press C, Mui E, Zhou Y, et al. . Clustering of Toxoplasma gondii infections within families of congenitally infected infants. Clin Infect Dis 2018; 61(12):1815-24. 10.1093/cid/civ721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Contopoulos-Ioannidis DG, Maldonado Y, Montoya JG. Acute Toxoplasma gondii infection among family members in the United States. Emerg Infect Dis 2013; 19(12):1981. doi: 10.3201/eid1912.121892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balasundaram MB, Andavar R, Palaniswamy M, Venkatapathy N. Outbreak of acquired ocular toxoplasmosis involving 248 patients. Arch Ophthalmol 2010; 128(1):28-32. doi: 10.1001/archophthalmol.2009.354 [DOI] [PubMed] [Google Scholar]
- 20.Doganci L, Tanyuksel M, Araz E, Besirbellioglu B, Erdem U, Ozoguz C, et al. . A probable outbreak of toxoplasmosis among boarding school students in Turkey. Clin Microbiol Infect 2006; 12(7):672-4. 10.1111/j.1469-0691.2006.01449.x [DOI] [PubMed] [Google Scholar]