ABSTRACT
Introduction
Although topical antibiotics have been used as antimicrobial prophylaxis after ocular surgery, recent studies have determined that intracameral cefuroxime at the end of surgery significantly reduce the risk to suffer an infection and suggest that the use of topical antibiotics in the prophylaxis of infectious postoperative endophthalmitis (IPOE) is controversial. Moreover, there is no evidence to confirm the higher effectiveness of topical ciprofloxacin, considered the standard of care, or topical azithromycin in preventing IPOE of cataract surgeries.
Patients and methods
IPOE topical prophylaxis was performed with two different strategies: with azithromycin from January 1st, 2010 to December 31st, 2014 (group I) and with ciprofloxacin from January 1st, 2015 to January 31st, 2017 (group II). Patient characteristics and clinical signs and symptoms of IPOE from all consecutive cataract surgeries performed over a 7-year period were collected.
Results
A total of 15,146 cataract surgeries were conducted; 10,756 in group I and 4,390 in group II. Two cases of IPOE in each group were diagnosed, showing a 0.019% and 0.046% rate respectively, with no statistically significance. IPOE cases were related with aging, systemic and ocular comorbidities or with a complicated cataract surgery.
Conclusions
The benefit of the application of topical antibiotics after cataract surgery is questionable when intracameral cefuroxime prophylaxis is performed and no better effectiveness with ciprofloxacin or azithromycin was observed.
Key words: cataract surgery, infectious postoperative endophtalmitis, topical antibiotics
RESUMEN
Introducción
Aunque la aplicación de antibióticos tópicos se ha utilizado como profilaxis antibiótica tras cirugía ocular, estudios recientes han determinado que el uso de cefuroxima intracameral como profilaxis tras cirugía ocular ha reducido de forma significativa la aparición de infecciones y sugiere que el uso de antibióticos tópicos como profilaxis de endoftalmitis infecciosa post-operatoria (IPOE) es controvertido. Además, no existe evidencia que confirme la eficacia de ciprofloxacino tópico, considerado como la terapia estándar, o azitromicina tópica en la prevención de IPOE.
Pacientes y métodos
La profilaxis tópica para prevenir la IPOE, se realizó siguiendo dos estrategias: azitromicina tópica en el periodo entre 1 enero de 2010 al 31 de diciembre de 2014 (Grupo I) y ciprofloxacino en el periodo entre 1 de enero de 2015 al 31 de diciembre de 2017 (Grupo II). Se recogieron las características de los pacientes y los signos y síntomas clínicos de IPOE de las cirugías realizadas durante los 7 años.
Resultados
Se realizaron un total de 15.146 cataratas: 10.756 en el Grupo I y 4.390 en el Grupo II. Se diagnosticaron dos casos de IPOE en cada grupo, presentando una tasa de 0,019% y 0,046% respectivamente, sin diferencias estadísticamente significativas. Los casos de IPOE se relacionaron con edad avanzada, comorbilidades sistémicas u oculares o con cirugías complicadas.
Conclusiones
El beneficio de la aplicación de antibiótico tópico después de cirugía de cataratas es cuestionable siempre que se realice profilaxis con cefuroxima intracameral y no se encontró una mayor protección con ciprofloxacino o con azitromicina
Palabras clave: cirugía de cataratas, endoftalmitis infecciosa post-operatoria, antibióticos tópicos
INTRODUCTION
Cataract extraction with intraocular lens (IOL) implantation is the most commonly performed surgical procedure in the elderly population over the world, with 22 million of cataract surgeries per year [1]. Infectious postoperative endophthalmitis (IPOE) is the most dreaded complication of cataract surgery. It is also responsible for permanent and significant loss of vision, so huge efforts have to be done to avoid IPOE. Endophthalmitis is a serious intraocular inflammatory disorder resulting from infection of the vitreous cavity. Progressive vitritis is the hallmark of any form of endophthalmitis. Most present within 1-2 weeks, usually 3-5 days after the surgery. Initial symptoms are rapidly progressive, including pain, red eye, ocular discharge, and blurring. Most common signs include decreased visual acuity, lid swelling, conjunctival and corneal edema, anterior chamber cells + fibrin, hypopyon, vitreous inflammation, retinitis, and blunting of red reflex.
Povidone-iodine application of a 5% solution on the conjunctiva prior to surgery was found to reduce the rate of endophthalmitis by four fold [2].
The prospective randomized endophthalmitis prophylaxis trial of the European Society of Cataract and Refractive Surgeons (ESCRS) had reported a 5-fold reduction in endophthalmitis rates with direct intracameral injection of cefuroxime at the conclusion of cataract surgery (0.07% versus 0.34% without intracameral cefuroxime) [3,4]. Other studies in Europe have confirmed this reduction in the postoperative endophthalmitis rates with the systematic use of intracameral cefuroxime [5-7].
Topical antibiotics role in the prophylaxis of IPOE is controversial. Recent evidence suggests that topical antibiotics after intravitreal injection (IVI) may even be harmful by increasing antibiotic-resistant bacterial strains [8-12]. However, topical antibiotic long has been the standard of care after ocular surgery, and this practice is carried over to ocular injections after a preoperative topical instillation of povidone-iodine [2,13] and intracameral antibiotic injection at the end of cataract surgery [14,15].
It is not known if the use of different families of antibiotics at the end of cataract surgery could affect the incidence of IPOE. The use of ciprofloxacin eye drops as antibiotic prophylaxis has been the usual practice to prevent ocular infections. Also, it seems that fluoroquinolones like ciprofloxacin have good pharmacokinetics properties and its penetration to the aqueous humor after topical administration could allow to have enough antibiotic concentration to treat infections [16, 17]. Azithromycin is a broad spectrum antibiotic that covers most commonly found bacteria in the environment, and also could not increase antimicrobial resistance in conjunctive flora as much as ciprofloxacin. On the other hand, information about its penetration to the aqueous humor is lacking, but some studies suggest that macrolides like clarithromycin could achieve therapeutic levels after topical administration [18].
Even though the topical administration of antibiotics has been questioned, some developed countries still maintain this practice. The main purpose of this retrospective study was to assess the role of topical azithromycin or topical ciprofloxacin in addition to what is considered the standard of care in preventing IPOE, intracameral cefuroxime or vancomycin, in a long series of cataract surgeries.
PATIENTS AND METHODS
All surgeries were performed at the Hospital de la Esperanza (Barcelona, Catalonia, Spain). This institution belongs to the group Parc de Salut Mar, which is a 3rd level center and serves a population of approximately 330,000 inhabitants. This population has a mean age of 43 years and a life expectancy of 80 years for males and 85 for females.
All consecutive cataract surgeries performed between January 1, 2010 and January 31, 2017 were included. Topical antibiotic for IPOE prophylaxis was Azydrop (azithromycin dehydrate 15 mg/g. Thea lab. Clermont-Ferrand, France) in cases operated between January 1, 2010 and December 31, 2014 (group I). From January 1, 2015 to January 31, 2017 cataract surgeries were performed with Cetraflux eye drops (ciprofloxacin hydrochloride 3 mg/ml, Salvat Lab., Barcelona, Spain) as topical antibiotic IPOE prophylaxis (group II).
Institutional review board approval was obtained and the study adhered to the Declaration of Helsinki, and has followed the rules on confidentiality of data (Organic Law 15/1999 of 13 Of December of protection of data of personal character (LOPD)).
A database collection system for intravitreal antibiotic prepared in our hospital pharmacy was consulted. Database contains patient medical record number and date of IVI of antibiotic. Case-specific information was collected using the hospital electronic program for patient’s medical records. Endophthalmitis was diagnosed by the physician of record. Vitreous biopsy was performed immediately after diagnosis, and cultures were sent to the microbiology laboratory.
During the study period all surgeons performed phacoemulsification using 2.75mm or 2.2 mm clear corneal approach and IOL implantation. From all cases, 88% were done under topical anesthesia and 12% with retrobulbar injection. Povidone–iodine solution 5% was started outside the operating theater and timed to be applied as 1 drop into the conjunctival sac and onto the cornea for a minimum of 3 minutes before surgery. Povidone-iodine 10% was used for surgical skin field antiseptic preparation. In patients with history of allergy to topical iodine the prophylactic antisepsis was done with chlorhexidine 0.05%. Eyelids and lashes were draped away from surgery field. Intracameral cefuroxime (1 mg/0.1 ml) or vancomycin (1 mg/0.1 ml) in case of β-lactam allergy were used in all procedures. Intracameral antibiotic solutions were compounded in our hospital pharmacy. Topical antibiotic eye drops were also prescribed, group one received azithromycin eye drops twice daily for three days, starting the day before surgery. Patients in group two received topical ciprofloxacin eye drops every 8 hours for one week, starting the same day of surgery. Patients received also steroid eye drops: dexamethasone 1mg/ml (Dexametasona, Alcon Cusí lab. Barcelona, Spain) every 2 h for the first 24h postoperative and then in a descending pattern (one drop every 6h first week, every 8h second week, every 12h the third and every 24h the fourth).
Postoperative endophthalmitis is an inflammatory condition of the eye, presumed to be due to an infectious process from bacteria, fungi or, on rare occasions, parasites that enter the eye during the perioperative period. If a patient presents with sudden decrease in visual acuity early after cataract surgery, often with pain and signs of diffuse intraocular inflammation (vitreous infiltration, hypopyon, red eye), infectious endophthalmitis should be suspected [19, 19b]
Surgeons did the follow-up of patients for at least 2 months. Scheduled visits were at first day postop, one week and 6-8 weeks.
Only 0.5% of patients were excluded for not attending any of the scheduled visits.
From the electronic medical record, data were collected regarding the comorbidity of the patient, such as immunodeficiency, diabetes or hypertension, the duration of the cataract surgery and the experience of the surgeon, since these factors could increase the risk of IPOE. Physician was defined as an “expert” surgeon when had more than 5 years of experience and more than 1000 cataract surgeries performed. If not, was considered a “novel” surgeon.
“Quantitative variables were described as median. Categorical variables were described as frequency (percentage). Possible associated factors to suffer IPOE were evaluated by the chi-squared test for categorical variables and Fisher’s exact test for continuous variables. Associations with a p-value <0.05 were considered statistically significant. Statistical analysis were done with the STATA 15.0 software (Stata Corp.; College Station, Texas, USA).”
RESULTS
Fifteen thousand one hundred and forty-six consecutive cataract surgeries were performed between January 1, 2010 and January 31, 2017. From January 1, 2010 to December 31, 2014, 10,756 cataract surgeries were performed with azithromycin eye drops for IPOE prophylaxis (group I). During this period, two cases of IPOE were diagnosed, what means a rate of 0.019%. One patient’s vitreous culture showed no growth but clinical presentation, patient signs and symptoms and loss of visual acuity (VA) were consistent for IPOE. Between January 1, 2015 and January 31, 2017, 4,390 cataract surgeries were performed with ciprofloxacin eye drops as topical antibiotic treatment for IPOE prophylaxis (group II). Two IPOE cases presented, that is a 0.046% rate. The differences in incidence of endophthalmitis were not statistically significant.
Overall, four IPOE cases were detected, what means an overall rate of 0.026%.
Three of our four IPOE cases were related with aging and systemic and ocular co-morbidities and were operated on, two by an expert cataract surgeon and the other one by a novel ophthalmologist. The fourth case did not have important comorbidities, but was a complicated cataract surgery done by a novel surgeon (table 1).
Table 1.
IPOE cases demographic and clinical data
| Topical antibiotic | Gender | Age | General background | Ophthalmic background | Surgery | IPOE | Vitreous Culture | Final VA |
|---|---|---|---|---|---|---|---|---|
| Azithromycin | Male | 85 | DMII- OAD Dyslipidaemia |
Pseudoexfoliation Glaucoma-prostaglandin treatment Myosis Pre-VA RE: 0.5 |
Expert surgeon IFIS 23 minutes surgery |
8 days’ post-surgery IV AB + Topical reinforced eye drops + posterior vitrectomy |
Coagulase negative Staphylococcus | 0.5 |
| Azithromycin | Male | 71 | Alcoholic liver disease, smoking, epilepsy, barbiturate intake | Mature cataract +++ Pre VA LE: 0.05 |
Novel surgeon 25 minutes surgery |
7 days’ post-surgery IV AB + Topical reinforced eye drops + posterior vitrectomy |
Negative | 0.6 |
| Ciprofloxacin | Female | 90 | Alzheimer Depression Arterial hypertension Obesity |
Pseudoexfoliation Phacodonesis Pre VA LE: CF |
Expert surgeon Stretching 28 minutes surgery |
14 days’ post-surgery IV AB + Topical reinforced eye drops + posterior vitrectomy |
Negative | HM |
| Ciprofloxacin | Male | 73 | Glaucoma Myopia Pre VA RE: 0.4 |
Novel surgeon Zonular dialysis Phenylephrine Iris hooks Anterior vitrectomy 31 minutes surgery |
2 days’ post-surgery IV AB + Topical reinforced eye drops + posterior vitrectomy |
Negative | 0.8 | |
IPOE: infectious postoperative endophthalmitis, DMII: Mellitus Diabetes type II, OAD: oral antidiabetics, Pre VA: previous Visual Acuity, RE: Right Eye, LE: Left Eye, IV AB: Intravitreal Antibiotics.
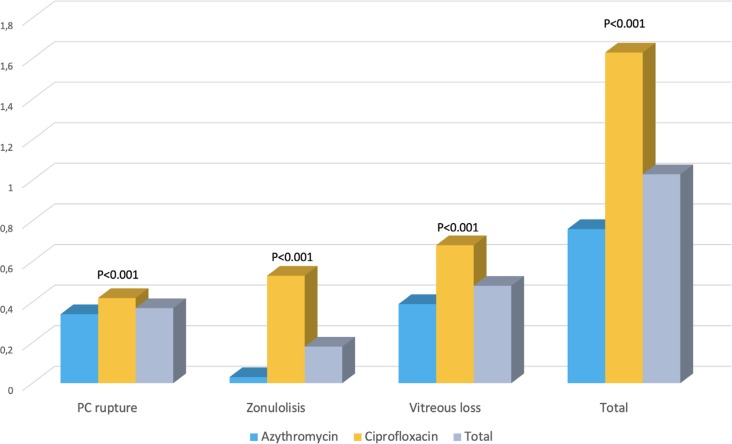
The clinical and demographic data of the patients are summarized in table 2. There were no differences between the groups in terms of age, laterality or incidence of arterial hypertension. There were a higher percentage of diabetics in group I (p <0.013). Perioperative complications and surgical time were also different, with a higher incidence of complications (p <0.001) and longer surgical time (p <0.0001) in group II (ciprofloxacin) (figure 1 and table 2).
Table 2.
Demographic and clinical data
| Group | Number |
Age (years) | Female Gender | RE | Diabetes | Arterial hypertension | Surgical complications |
Surgical time (minutes) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eyes | Patients | PC rupture | Zonulolysis | Vitreous loss | Total | |||||||
| Total | 15,146 | 12,847 | 74.9 | 59.2% | 49.85% | 25.96% | 61.4% | 0.37% | 0.18% | 0.48% | 1.03% | 18.36 |
| Azithromycin | 10,756 | 9,202 | 74.87 | 59.0% | 49.72% | 26.53% | 61.4% | 0.34% | 0.03% | 0.39% | 0.76% | 17.6 |
| Ciprofloxacin | 4,390 | 3,645 | 74.98 | 59.4% | 50.17% | 24.61% | 61.5% | 0.42% | 0.53% | 0.68% | 1.63% | 20.6 |
| P-value* | 0.5 | 0.6 | 0.013 | 0.9 | 0.001 | 0.001 | 0.001 | 0.001 | 0.0001 | |||
P-values refers to azithromycin versus ciprofloxacin comparison. RE: Right eye. PC rupture: Posterior capsule rupture.
Figure 1.
Surgical complications
PC rupture: Posterior capsule rupture.
In the azithromycin group 95% of patients received intracameral cefuroxime versus 96% in the ciprofloxacin group (no statistical differences). The rest of the patients in each group were treated with intracameral vancomycin.
DISCUSSION
The present study carried out on approximately 15,000 cataract procedures in two groups of antibiotics, azithromycin versus ciprofloxacin, were compared, did not show statistically significant differences in the appearance of IPOE.
The role of antibiotic prophylaxis of IPOE using different routes of administration is controversial. With the increasing adoption of topical anesthesia for cataract surgery, the use of sub-conjunctival antibiotic is bound to wane. Eight percent of surgeons in the 2014 ASCRS survey [19] reported use of irrigative antibiotic during cataract surgery although high-quality evidence either supporting or refuting this practice is lacking [14].
The efficacy of prophylactic intracameral cefuroxime in preventing endophthalmitis after cataract surgery was suggested by retrospective data from Sweden [20] and later by other studies carried out in Europe and the USA [15,20]. This finding was further substantiated by the ESCRS multicenter study of postoperative endophthalmitis that remains, to date, the only prospective, randomized, placebo-control trial of prophylactic antibiotic use in cataract surgery [3, 4]. Results showed that the use of intracameral cefuroxime reduces the risk of IPOE to approximately one-fifth the value observed without this prophylaxis [3, 4]. These results would reinforce the role of the intracameral administration of cefuroxime or vancomycin in cataract surgery and question the potential benefit of the subsequent instillation of eye drops with antibiotics. However, according to the 2014 ASCRS survey [19], perioperative topical antibiotic prophylaxis continues to be used by the overwhelming majority of surgeons in the USA. Actually, 97% of the surgeons uses a postoperative topical antibiotic and fourth generation quinolones (moxifloxacin and gatifloxacin) are the antibiotic most prescribed.
The most common strains from conjunctival isolates are found to be coagulase-negative Staphylococcus (CNS), Staphylococcus epidermidis and Staphylococcus aureus [12]. The Endophthalmitis Vitrectomy Study Group [18] revealed that 70% of infections were caused by CNS followed by 9.9% S. aureus. So organisms typically associated with conjunctival flora are those seen in IPOE after cataract surgery [21]. Moreover, some risk factors for endophthalmitis have been associated with more pathogenic conjunctival bacteria [22]. Age was associated with increased bacteria in general, S. aureus was associated with diabetes, lung disease, and renal and heart insufficiency, and Gram-negative rods with smoking habit [22]. In all cases, comorbidities considered as a risk factor to develop IPOE were observed.
Several studies have demonstrated that a large proportion of conjunctival bacteria, specifically pathogenic ones (CNS and S. aureus) are resistant to β-lactams, azithromycin or fluoroquinolones [7,23]. Moreover, there is evidence that the percentage of S. epidermidis isolated from the conjunctival surface significantly increases after repeated exposure to azithromycin or fluoroquinolones [9-11]. Furthermore, Miller et al [24] reported higher rates of fluoroquinolone resistance in CNS recovered from patients with endophthalmitis than would have been predicted based on surveillance studies.
Regional differences in conjunctival bacterial flora can not only determine different risk of incidence of endophthalmitis but worse outcome with poor final VA as suggested by Mesnard [25] that reports 8.5-fold reduction in postoperative endophthalmitis after introduce Aprokam for intracameral use, but finds more IPOE associated with Streptococcus sp. and gram-negative microorganisms which were found in series from USA or Europe.
We selected azithromycin as topical antibiotic prophylaxis in our patients because it is a broad spectrum antibiotic that covers most commonly found bacteria in the environment, and we thought fluoroquinolones must be reserved for ocular infections, in order to avoid increase antimicrobial resistance among conjunctival flora. Commercially available ophthalmic azithromycin is a preservative free formulation that allows for a short period treatment (twice daily only for 3 days). Residual azithromycin levels observed 7 days after the last topical administration were above the minimum inhibitory concentration of 0.5 μg/g in the conjunctiva and cornea. This permits topical administration twice daily for three days resulting in significant concentrations in the conjunctiva and cornea for at least 7 days after final administration [26]. These characteristics make azithromycin an AB with a very comfortable dosage for patients and no risk for allergies due to the preservatives. Due to regulations of the competent health authorities in our country, we had to replace azithromycin with ciprofloxacin in the prophylaxis of IPOE. Our results are according with other series using intracameral cefuroxime as unique antibiotic prophylaxis in cataract surgery [3-7, 27, 28]. To our knowledge, this is the first series using topical azithromycin plus intracameral cefuroxime for IPOE prophylaxis in cataract surgery.
Though this is a retrospective study, azithromycin did not cause any increase in IPOE cases, quite the opposite. Moreover, our hospital is a public educational institution and there are residents performing cataract surgery. The population we attend belongs to the most depressed area of the city, so that our patients have lower hygienic health level. And our IPOE rates are low indeed, so that is why we feel prophylaxis with azithromycin is equal in terms of preventing IPOE than that with ciprofloxacin.
As observed in our study, other studies have shown that experienced surgeons are more likely to be associated with IPOE cases because they use to be involved in more complicated cases [4]. Other authors found the more experienced surgeons associate a 5-fold difference in reducing the risk for IPOE [29]. The differences between both groups regarding perioperative complications and surgical time are related to the volume of surgeries performed by novel surgeons, which represents 15% in group I and near 40% in group II. Surgical time was somewhat longer in the ciprofloxacin group, but the difference (3 minutes), although statistically significant, would not be clinically relevant. In fact, although both intraoperative complications and surgical time are different in the two groups, the IPOE case was negligible, so we did not consider performing a statistical analysis more adjusted to the infection rate.
Fluoroquinolone-associated collagen toxicity has been described in previous studies with a long duration antimicrobial treatment (over 7 days) after oral or endovenous administration. [30, 31]. Nevertheless, since in our study ciprofloxacin application was topical this kind of toxicity could be discarded.
The role of topical antibiotic in the prophylaxis of IPOE is controversial. Herrinton [32] showed that risk for IPOE is double for patients that had no evidence of antibiotic prophylaxis, 42% reduction in risk associated with intracameral antibiotic administration and no differences in risk between intracameral agent alone and intracameral plus topical agent.
We found that the risk for IPOE was the same with perioperative topical ciprofloxacin than with topical azithromycin. Although conjunctival and lid margin isolates are not too sensitive to azithromycin [33], topical antibiotic are able to eradicate in vitro resistant strains in patients and provide the same cure rate for both susceptible and resistant strains [33] because of the high local concentrations that are reached and the local physicochemical conditions that may influence the overall activity of the agent at the site of application.
It has been proved for IVI that topical antibiotics drops not only do not reduce the risk for post injection endophthalmitis but it is increased [34,35]. It is remarkable that perioperative topical antibiotic prophylaxis continues to be used by the overwhelming majority of surgeons (97%) in the USA [21] and only 30% of US surgeons in the 2014 ASCRS survey were injecting intracameral antibiotic at the conclusion of surgery [36]. We suspect that cefuroxime labeled for intracameral use (Aprokam, Thea lab) approval will change this reality, because the published evidence for antibiotic prophylaxis is strongest for direct intracameral injection.
The main limitation of our study is its retrospective design and, despite being a one site large sample, the low incidence of IPOE makes it difficult to obtain definitive conclusions, but it seems that topical antibiotic prophylaxis with azithromycin is not better than the one with ciprofloxacin when associated with intracameral cefuroxime. Moreover, topical antibiotic administration is questionable and the low percentage of IPOE is probably attributable to the intracameral administration of cefuroxime or vancomycin.
In conclusion, as topical antibiotic administration is questionable for IPOE prophylaxis when intracameral administration of cefuroxime or vancomycin is performed, and azithromycin showed no benefit front ciprofloxacin, which has been the usual practice to prevent ocular infections, the application of topical antibiotic in addition to the intracameral antibiotic administration is a practice that should be avoided, since there is no benefit observed and could increase antibiotic-resistant bacterial strains.
FUNDING
None to declare.
CONFLICT OF INTEREST
The authors declare that have no conflict of interest.
REFERENCES
- 1.Market scope , 2013. Comprehensive Report of the Global Cataract Surgical Equipment Market. [Google Scholar]
- 2.Speaker MG, Menikoff JA, Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology 1991; 98: 1769-1775. PMID [DOI] [PubMed] [Google Scholar]
- 3.Barry P, Seal DV, Gettinby G et al. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery. Preliminary report of principal results from a European multicenter study. J cataract Refract Surg 2006; 32:407-410. PMID [DOI] [PubMed] [Google Scholar]
- 4.ESCRS Endophthalmitis Study Group Prophylaxis of postoperative endophthalmitis following cataract surgery: Results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg 2007;33: 978-988. PMID [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez Caravaca G, García-Sáenz MC, Villar-del-Campo MC et al. Incidence of endophthalmitis and impact of prophylaxis with cefuroxime on cataract surgery. J Catarac Refract Surg 2013; 39: 1399-1403. PMID [DOI] [PubMed] [Google Scholar]
- 6.Behnding A, Cochener B, Güell JL, et al. Endophthalmitis porphylaxis in cataract surgery: Oveview of current practice patterns in 9 European countries. J CataractRefract Surg 2013; 39: 1421-1431. PMID [DOI] [PubMed] [Google Scholar]
- 7.Friling E, Lundström M, Stenevi U, Montan P. Six-year incidence of endophthalmitis after cataract surgery: Swedish national study. J Cataract Ref Surg 2013; 39: 15-21. PMID [DOI] [PubMed] [Google Scholar]
- 8.Haas W, Pillar Ch M, Torres M, et al. Monitoring antibiotic resistance in ocular microorganisms: Results from the antibiotic resistance monitoring in ocular microorganisms (ARMOR) 2009 surveillance study. Am J Ophthalmol 2011; 152: 567-574. PMID [DOI] [PubMed] [Google Scholar]
- 9.Kim SJ, Toma HS. Ophthalmic antibiotics and antimicrobial resistance. A randomized, controlled study of patients undergoing intravitreal injections. Ophthalmology 2011; 118: 1358-1363. PMID [DOI] [PubMed] [Google Scholar]
- 10.Dave SB, Toma HS, Kim SJ. Ophthalmic antibiotic use and multidrug-resistant staphylococcus epidermidis. Ophthalmology 2011; 118: 2035-2040. PMID [DOI] [PubMed] [Google Scholar]
- 11.Dave SB, Toma HS, Kim SJ. Changes in ocular flora in eyes exposed to ophthalmic antibiotics. Opthalmology 2013; 120: 937-941. PMID [DOI] [PubMed] [Google Scholar]
- 12.Hsu HY, Lind JT, Tseng L and Miller D. Ocular flora and their antibiotic resistance patterns in the Midwest: A prospective study of patients undergoing cataract surgery. Am J Ophthalmol 2013; 155: 36-44. PMID [DOI] [PubMed] [Google Scholar]
- 13.Carrim ZI, Mackie G, Gallacher G and Wykes W. The efficacy of 5% povidone-iodine for 3 minutes prior to cataract surgery. Eur J Ophthalmol 2009; 19: 560-564. PMID [DOI] [PubMed] [Google Scholar]
- 14.Vazirani J, Basu S. Role of topical, subconjunctival, intracameral and irrigative antibiotics in cataract surgery. Curr Opin Ophthalmol 2013; 24: 60-65. PMID [DOI] [PubMed] [Google Scholar]
- 15.Yu Ch Q, Ta Ch N. Prevention of postcataract endophthalmitis: evidence-based medicine. Curr Opin Ophthalmol 2012; 23: 19-25. PMID [DOI] [PubMed] [Google Scholar]
- 16.Clode Alison B, Davis Jennifer L, Salmon Jacklyn, et al. Aqueous humor and plasma concentrations of ciprofloxacin and moxifloxacin following topical ocular administration in ophthalmologically normal horses. Am J Vet Res. 2010. May;71(5):564-9. PMID [DOI] [PubMed] [Google Scholar]
- 17.Cantor L, WuDunn D, Yung C, et al. Ocular penetration of levofloxacin, ofloxacin and ciprofloxacin in eyes with functioning filtering blebs: investigator masked, randomised clinical trial. Br J Ophthalmol. 2008. March;92(3):345-7. PMID [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Wang L, Zhou J, et al. Ocular penetration and pharmacokinetics of topical clarithromycin eye drops to rabbits. J Ocul Pharmacol Ther. 2014. February;30(1):42-8. PMID [DOI] [PubMed] [Google Scholar]
- 19.Chang DF, Braga-Mele R, Henderson BA et al. Antibiotic prophylaxis of postoperative endophthalmitis after cataract surgery: Results of the 2014 ASCRS member survey. J Cataract Ref Surg 2015; 41: 1300-1305. PMID [DOI] [PubMed] [Google Scholar]
- 19b.Barry P, Cordovés L, Gardner S. ESCRS Guidelines for Prevention and Treatment of Endophthalmitis Following Cataract surgery. Available at: http://www.escrs.org/downloads/Endophthalmitis-Guidelines.pdf
- 20.Montan PG, Wejde G, Koranyi G and Rylander M. Prophylactic intracameral cefuroxime. Efficacy in preventing endophthalmitis after cataract surgery. J Cataract Refract Surg 2002; 28: 977-981. PMID [DOI] [PubMed] [Google Scholar]
- 21.Endophthalmitis Vitrectomy Study Group Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Arch Ophthalmol 1995; 113: 1479-1496. PMID [PubMed] [Google Scholar]
- 22.Fernández-Rubio ME, Cuesta-Rodriguez T, Urcelay-Segura JL and Cortés-Valdés C. Pathogenic conjunctival bacteria associated with systemic co-morbidities of patients undergoing cataract surgery. Eye 2013; 27: 915-923. PMID [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanco AR, Roccaro AS, Spoto CG and Papa V. Susceptibility of methicillin-resistant Staphylococci clinical isolates to Netilmicin and other antibiotics commonly used in ophthalmic therapy. Current Eye Research 2013; 38: 811-816. PMID [DOI] [PubMed] [Google Scholar]
- 24.Miller D, Flynn PM, Scott IV et al. In vitro fluoroquinolone resistance in staphylococcal endophthalmitis isolates. Arch Opthalmol 2006; 124: 479-483. PMID [DOI] [PubMed] [Google Scholar]
- 25.Mesnard Ch, Beral L, Merle H et al. Endophthalmitis after cataract surgery despite intracameral antibiotic prophylaxis with licensed cefuroxime. J Cataract Refract Surg 2016; 42: 1318-1323. PMID [DOI] [PubMed] [Google Scholar]
- 26.Javitt JC. Intracameral antibiotics reduce the risk of endofthalmitis after cataract surgery: Does the preponderance of the evidence mandate a global change in practice?. Editorial. Ophthalmology 2016; 123: 226-230. PMID [DOI] [PubMed] [Google Scholar]
- 27.Lundström M, Wejde G, Stenevi U et al. Endophthalmitis after cataract surgery. A nationwide prospective study evaluating incidence in relation to incision type and location. Ophthalmology 2007; 114: 866-870. PMID [DOI] [PubMed] [Google Scholar]
- 28.Shorstein NH, Kevin L, Winthrop L and Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a northern California eye department. J Cataract Refract Surg 2013; 39: 8-14. PMID [DOI] [PubMed] [Google Scholar]
- 29.Raen M, Sandvik GF, Droslum L. Endophthalmitis following cataracta surgery: the role of prophylactic postoperative chloramphenicol eye drops. Acta Ophthalmol 2013; 91: 118-122. PMID [DOI] [PubMed] [Google Scholar]
- 30.Björn P, Malin I and Henrik S. Fluoroquinolone use and risk of aortic aneurysm and dissection: nationwide cohort study. BMJ 2018; 360:k678. PMID [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Daneman N, Lu H, Redelmeier DA. Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study. BMJ 2015;5:e010077. PMID [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrinton LJ, Shorstein NH, Paschal JF et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology 2016; 123: 287-294. PMID [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papa V, Blanco AR, Santocono M. Ocular flora and their antibiotic susceptibility in patients having cataract surgery in Italy. J Cataract Refract Surg 2016; 42: 1312-1317. PMID [DOI] [PubMed] [Google Scholar]
- 34.Cheung CSY, Wong AWT, Lui A et al. Incidence of endophthalmitis and use of antibiotic prophylaxis after intravitreal injections. Ophthalmology 2012; 119: 1609-1614. PMID: [DOI] [PubMed] [Google Scholar]
- 35.Storey P, Dollin M, Pitcher J et al. The role of topical antibiotic prophylaxis to prevent endopthalmitis after intravitreal injection. Ophthalmology 2014; 121: 283-289. [DOI] [PubMed] [Google Scholar]
- 36.Hsu J, Gerstenblith AT, Garg SJ and Vander JF. Conjunctival flora antibiotic resistance patterns after serial intravitreal injections without postinjection topical antibiotics. Am J Ophthalmol 2014; 157: 514-518. PMID [DOI] [PubMed] [Google Scholar]