Abstract
High-grade gliomas are especially difficult tumors to treat due to their invasive behavior. This has led to extensive research focusing on arresting glioma cell migration. Cell migration involves the sensing of a migratory cue, followed by polarization in the direction of the cue, and reorganization of the actin cytoskeleton to allow for a protrusive leading edge and a contractile trailing edge. Transmission of these forces to produce motility also requires adhesive interactions of the cell with the extracellular microenvironment. In glioma cells, transmembrane receptors such as CD44 and integrins bind the cell to the surrounding extracellular matrix that provides a substrate on which the cell can exert the requisite forces for cell motility. These various essential parts of the migratory machinery are potential targets to halt glioma cell invasion. In this review, we discuss the mechanisms of glioma cell migration and how they may be targeted in anti-invasion therapies.
INTRODUCTION
Major advances in the preceding decades have substantially improved the way cancer is treated. However, not every form of cancer has seen such profound benefits from the many advancements made. Successful treatment of high-grade gliomas (HGGs), which include glioblastomas (GBMs), remains particularly elusive. Current treatment typically involves surgical resection followed by tumor irradiation and chemotherapy. More than three decades of intensive research have produced only modest improvements in life expectancy (Sathornsumetee and Rich, 2006). Life expectancy remains dismal, with GBM harboring a median survival time of 15 months (Louis et al., 2016) and a 5-year survival of just 9.8% (Stupp et al., 2009). This poor prognosis is due in large part to the wide dissemination of tumor cells prior to diagnosis, which makes recurrence almost a certainty even after repeated resections (Barker et al., 1998). Conversely, less invasive gliomas typically result in long-term survival and are often curable (Hunter et al., 2003).
The resilience of GBM due to diffuse infiltration has increased investigation into mechanisms of motility and invasion, with the goal of arresting or slowing glioma spread. This therapeutic goal may allow for more successful localized treatment with reduced recurrence. Broadly, cell migration starts with an extrinsic migratory signal, including chemical (Carter, 1965), mechanical (Lo et al., 2000), electrical (Brown and Loew, 1994), and other cues. Cells then define a leading edge in the direction of the migratory cue and a trailing edge at the rear. After cell polarization, actin polymerization at the leading edge causes protrusion of the plasma membrane. At the surface of the cell, transmembrane receptors, such as integrins, allow the cells to transmit forces generated from the interactions of myosin and the cytoskeleton to the extracellular microenvironment. These processes are summarized in Figure 1. As such, valuable strategies for inhibiting glioma motility may include manipulation of migratory cues, cytoskeletal filaments and the myosin motors, transmembrane receptors, and the extracellular microenvironment. These can be grouped into extracellular and intracellular processes and factors, which will be reviewed in this article.
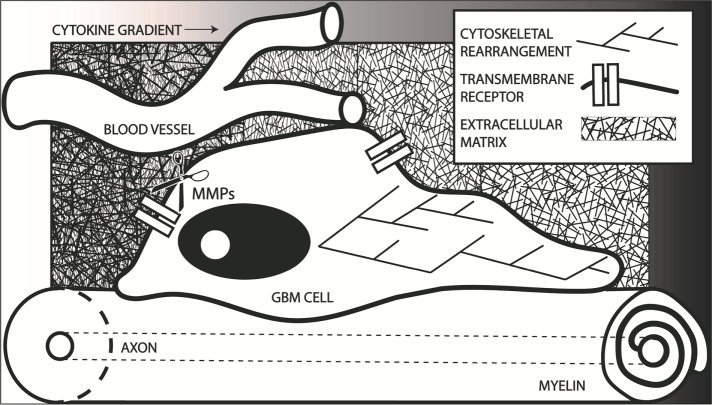
FIGURE 1:
Graphical summary of many of the factors involved in GBM invasion, presenting a GBM cell migrating away from the bulk tumor. GBM cells often migrate within perivascular spaces and along white matter tracts. Transmembrane receptors allow the cell to interact with both of these extracellular environments. The cytoskeleton rearranges to accommodate the directed migration down a migratory cue gradient, possibly including a chemokine gradient as depicted. Matrix metalloproteinases (MMPs) allow for remodeling of the extracellular matrix during migration. All of these factors present potential targets for halting GBM migration.
EXTRACELLULAR FACTORS
Migratory cues
Tumor cells have been shown to respond to a variety of migratory cues, including chemotactic, galvanotactic, and mechanical cues (Petrie et al., 2009). Glioma cells are no exception, and all of these cues are involved in glioma cell invasion. These migratory cues are being actively studied to increase the understanding of GBM migratory behavior and to find possible methods of intervention.
Chemotaxis is the process by which cells move in response to a chemical gradient, such as a cytokine, chemokine, or growth factor (Van Haastert and Devreotes, 2004). In cancer, these pathways can be hijacked to aid in dissemination of tumor cells (Condeelis et al., 2005). Glioma cells are more susceptible to chemotactic cues than the healthy brain tissue due to up-regulation of chemokine receptors and growth factor receptors. Epidermal growth factor (EGF) receptors (Ekstrand et al., 1991), platelet-derived growth factor (PDGF) receptors (Nazarenko et al., 2012), and fibroblast growth factor (FGF) receptor 2 (Brockman et al., 2003; The Cancer Genome Atlas Research Network, 2008) often have genomic amplification in GBMs, which, among other oncogenic functions, can all lead to chemotaxis in response to their ligands. A variety of drugs targeting these migratory signals are being evaluated in clinical trials (Roussos et al., 2011). The chemoattractant C-X-C ligand 12 (CXCL12, also known as stromal cell–derived factor 1, or SDF1) and its receptor C-X-C chemokine receptor 4 (CXCR4), which is required for brain development (Nagasawa et al., 1996), also contribute to malignancy in a wide range of cancer variants, including gliomas. CXCR4 is highly up-regulated in invasive glioma tumor cells compared with noninvasive tumor cells (Ehtesham et al., 2006). Treatment with a small molecular inhibitor of CXCR4 has been shown to suppress the growth of GBM tumors in vivo (Rubin et al., 2003). In a model in which applied fluid flow enhanced migratory behavior, CXCR4 appeared to modulate glioma migratory behavior independent of CXCL12 (Munson et al., 2013). This is especially significant, as convection enhanced delivery (described by Debinski and Tatter, 2009), an experimental therapy popular in clinical trials, flows drugs directly into the tumor mass, which could theoretically increase glioma invasion. CXCR4 is being targeted in an ongoing clinical trial.
Concentrations of autocrine chemotactic cues would decrease with distance from the bulk tumor, limiting its potential impact at the tumor edge. It has been suggested, however, that normal astrocytes can secrete a host of chemokines and other promigratory proteins that have been shown to enhance migration of glioblastoma stem-like cells (Rath et al., 2013), which could provide chemotactic cues to invasive cells distal to the tumor mass. Additional methods through which healthy brain tissue could aid in glioma invasion and survival have been reviewed elsewhere (Roos et al., 2017). GBM cell migration toward the vasculature has also been proposed to be induced by gradients of the chemotactic peptide bradykinin, which is released by the vascular endothelial cells in the brain (Montana and Sontheimer, 2011). Durotaxis, in which cells migrate down a substrate-rigidity gradient, has not been well characterized for glioma cells. The effects of other mechanical properties of the substrate, such as the arrangement of the physical features (nanotopography), have been well studied. Changes in topography can lead to changes in cytoskeletal organization, integrin expression, and cellular biophysical properties (Yim et al., 2010). Because of the importance of topography, it has been debated whether glioma cell migration paths are due to secreted biochemical cues or topography-related biomechanical cues. Both white matter tracts and the vasculature present, at least at the cellular scale, a linear pathway on which glioma cell migration can occur. Distant migration is less commonly found in the gray matter of the brain (Chicoine and Silbergeld, 1995), which is characteristically a random, nonlinear ECM of the same components as white matter (Bignami et al., 1992), so presenting a linear migratory pathway is thought to be able to induce the distant migration seen in the white matter. This theory was tested on electrospun poly(ε-caprolactone) (or PCL), nanofibers of linear or random organization. It was found that aligned fibers induced migration at over four times the velocity on randomly organized fibers (Johnson et al., 2009). Changing the mechanical properties of the nanofibers also induced large changes in migration speed (Sharma et al., 2013). Unexpectedly, at high cell densities on large width tracks velocity increases to the same level as seen on small linear tracks or in vivo. This calls into question whether mechanical confinement or the presence of linear tracks are the actual migratory cue (Monzo et al., 2016). Regardless, these linear tracks were harnessed as migratory cues to guide brain tumor cells into a cytotoxic hydrogel, significantly reducing tumor volume, displaying potential clinical value (Jain et al., 2014).
Electrical cues also play a role in directed migration of GBM cells. Direct-current electric fields (dcEFs) occur endogenously and regulate numerous biological processes such as wound healing and embryogenesis (Nuccitelli, 1988). One method by which dcEFs affect these processes is galvanotaxis, in which directed cell migration occurs in a physiologically comparable dcEF toward either the anode or cathode (Mycielska and Djamgoz, 2004). The mechanisms of galvanotaxis are still being explored, but it is postulated to depend on changes in intracellular Ca2+ concentrations and ion channel activities (Onuma and Hui, 1988). In the brain, an endogenous dcEF was found to guide migration of neuroblasts from the subventricular zone to the olfactory bulb in adult mice (Cao et al., 2013). Owing to the galvanotactic response of these and other brain cells, it was therefore hypothesized that galvanotaxis may play a role in GBM invasion (Huang et al., 2016). In two dimensions (2D), all GBM subtypes tested showed anode-directed migration while neural progenitor cells displayed cathodic directedness. Another group found, however, that GBM cells switch preferences in three dimensions (3D), migrating toward the cathode instead of the anode (Sato et al., 2009). A recently popularized clinical treatment method of alternating, intermediate frequency electric currents, called tumor treating fields, could possibly inhibit migration through galvanotactic mechanisms (Ahirwar et al., 2015).
Substrate
The brain parenchyma contains a complex network of vasculature that traverses both the white matter and gray matter. White matter is composed of myelinated neuronal axons. In contrast, gray matter contains mostly unmyelinated nerve cell bodies and associated dendritic processes. As mentioned in the preceding section, HGG cells have been shown to migrate distantly in the perivascular spaces or down the white matter tracts, two components of the histological secondary structures of Scherer that characterize glioma invasion patterns (Scherer, 1940). Histological images of glioma cells infiltrating perivascular and white matter microenvironments are seen in Figure 2. This demonstrates the morphological plasticity of GBM cells, allowing them to inhabit the diverse extracellular environments present in the brain. Oligodendrocytes contain membrane-bound inhibitors of cell attachment that prevent most cells from spreading or migrating along the white matter tracts (Caroni and Schwab, 1988). This may account for much of the static, nonmigratory composition of the adult brain (Schwab and Caroni, 1988). Glioblastoma cells, however, are able to attach to and migrate along these cell surfaces, a behavior that was inhibited by metalloprotease blockers (Paganetti et al., 1988). As for the extracellular matrix, the basement membrane of the vasculature usually consists of fibronectin, collagen, and laminin. The extravascular brain parenchyma lacks any significant quantities of these extracellular matrix proteins, but instead consists primarily of hyaluronic acid (HA, also referred to as hyaluronan; Rao, 2003; Bellail et al., 2004). The brain ECM and its effect on glioma invasion is further reviewed in Ferrer et al. (2018). The vastly different mechanisms required to traverse these different areas of the brain call into question whether glioma cells are intrinsically programmed to adapt to each environment or environmental selection leads to substrate-specific clonal evolution.
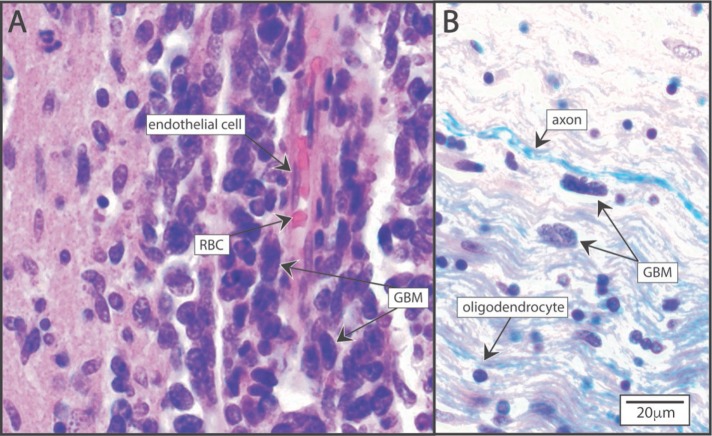
FIGURE 2:
Glioblastoma (GBM) migration patterns in brain parenchyma. (A) Perivascular migration. GBM cells adhere to and migrate along the canonical extracellular matrix proteins surrounding the vasculature. Hematoxylin and eosin (H&E) stained. (B) White matter infiltration of GBM cells showing elongated morphology between parallel bundles of axons in the white matter. Luxol fast blue myelin stain and H&E stain.
Owing to frequent perivascular migration, angiogenesis was previously thought to be elicited by GBM cells. Anti-angiogenic agents are being actively investigated as a possible treatment mechanism, and have been shown to provide clinical efficacy, at least transiently, in a multitude of cancer types. However, treatment of GBM cells with a popular antibody that functionally inhibits VEGFR2, bevacizumab, has been shown to lead to very short-term cessation of invasion followed by a long-term increase of invasive behavior (Páez-Ribes et al., 2009). Another group recapitulated these findings and showed that migration occurs on the preexisting brain microvessels, displacing the noncancerous cells in the perivascular space (Baker et al., 2014). This process was completely vascular endothelial growth factor (VEGF) independent.
To migrate efficiently through the dense extracellular space in the brain, GBM cells degrade and remodel the ECM. Matrix metalloproteinases (MMPs) are responsible for the degradation of the majority of ECM proteins, a process that has been previously reviewed in more detail (Birkedal-Hansen et al., 1993). GBM cells have been shown to overexpress MMPs 2 and 9 (Forsyth et al., 1999) compared with healthy brain tissue, and overexpression of MMPs has been linked to increased GBM invasion and poor prognosis (Rao, 2003). Additionally, inhibition of MMPs has led to reduced glioma invasion in vitro (Tonn and Goldbrunner, 2003). No clinical studies, however, have shown MMP inhibition to be effective in preventing glioma spread (Tonn et al., 1999). This could be due to GBM cells secreting other ECM proteins, such as tenascin-C, fibronectin, vitronectin, and collagen, while degrading the native ECM and causing increased glioma cell migration (Deryugina and Bourdon, 1996). Additionally, the clonal expansion of glioma cells in the cell culture environment may eliminate the adaptation to different environments found in primary tumor cells. GBM cells were also shown to respond to changes in their mechanical environment with differential ECM assembly and MMP or HA synthase expression (Wang et al., 2014). HA is also much more abundant in glioma ECM than in the normal brain (Delpech et al., 1993).
Even with degradation of the ECM, the brain parenchyma presents a severe mechanical challenge to migrating GBM cells. Cell processes are tightly packed in the brain parenchyma, with submicrometer pore sizes (Thorne and Nicholson, 2006). To migrate through these environments, GBM cells adopt a migratory behavior similar to neural progenitor cells in which they extend a long process that defines the migratory pathway, followed by the nucleus and cell body (Beadle et al., 2008). The nucleus of the cell deforms to allow migration through the pore. Movement of the nucleus through the pore, but not the leading process, was shown to require myosin II. In fact, they later showed that despite the abundance of promigratory factors involved in glioma migration, blocking myosin II effectively prevents migration of GBM in spatially constrained environments (Ivkovic et al., 2012).
Cell migration is sensitive to the mechanical properties of the substrate (Pelham et al., 1997), and the brain presents unique mechanical challenges to migrating GBM cells. The brain is one of the most compliant tissues in the body. The Young’s modulus ranges from several hundred pascals (Elkin et al., 2007) to several kilopascals (Hrapko et al., 2007), depending on factors such as testing conditions, anatomical origin of the sample, postmortem time, and donor characteristics (Hrapko et al., 2007). There are also regions with sharp variations in stiffness, presenting a severe mechanical challenge to migration (Franze, 2013). The basement membrane of the perivascular region has a much higher stiffness than the brain parenchyma (Candiello et al., 2007). GBM tumor cell lines have been shown to migrate rapidly on stiffer substrates, while failing to migrate effectively on compliant substrates with elastic moduli comparable to the brain parenchyma in 2D (Ulrich et al., 2009), and much lower migration velocities were seen in 3D (Ananthanarayanan et al., 2011). Another study, using a model of force transmission in cell migration, showed that the optimal stiffness for glioma cell migration was 100 kPa, 100-fold higher than that for optimal migration of healthy forebrain neurons and much higher than the brain parenchyma (Bangasser et al., 2017). The study showed that this increased response to stiff substrates was due to a much larger number of molecular motor clutches in glioma cells, and that softer substrates lead to a decrease in cell motility. These could explain the perivascular migratory behavior, but not how the cells can diffusely migrate through the much softer brain parenchyma. Engineered strategies for studying the effect of the microenvironment on glioblastoma have been extensively reviewed (Rape et al., 2014).
One potential explanation is that ECM secretion by migratory cells can regulate the cell response to the mechanical environment by inserting elastic fibrils between cells and the fabricated substrate. Presenting an elastic fibril between the cell and the substrate can even mediate the cell-sensed stiffness of the substrate (Weinberg et al., 2017), but this has not been extensively explored in regard to gliomas. The ECM does stiffen in response to increasing pressure (Pogoda et al., 2015) resulting from tissue expansion during tumor growth and the resistance to this expansion by the skull (Ricard et al., 2003), and this in turn could also have profound effects on the stiffness sensed by the cells. Another explanation is that the established glioma cell lines do not accurately recapitulate the mechanosensitive behavior of primary tumor cells. In fact, of the three major subtypes of GBM, the majority of the commonly used cells lines are representative only of the mesenchymal subclass (Verhaak et al., 2010) and were used in the studies that showed an optimal substrate stiffness for migration to be higher than that of the brain parenchyma (Bangasser et al., 2017). Different primary glioma cells have diverse substrate migratory preferences, with some migrating dependently and others migrating independently of substrate stiffness (Grundy et al., 2016). It has been postulated that these differing mechanosensitive behaviors could be subclass specific; GBM cell lines that migrated independent of rigidity were of the proneural subclass, which may be consistent with the fact that neurons branch mostly in a rigidity-independent manner (Georges et al., 2006).
INTRACELLULAR FACTORS
Cytoskeleton
For cell migration to occur, the cytoskeletal structure must be conducive to migration. This is orchestrated by a combination of signaling pathways, which cause actin polymerization and myosin-based force that can be transmitted to the substrate. This process has been reviewed in more detail (Blanchoin et al., 2014), and how it pertains to tumor invasion has also been reviewed in other sources (Friedl and Wolf, 2003). As such, cytoskeletal proteins and involved signaling pathways may be attractive targets for inhibiting glioma cell motility.
Radial glia cells (Ge et al., 2006; Nguyen et al., 2006) and glioma cells (Manning et al., 2000; Slhia et al., 2005) both exhibit decreases in migration following increased Rho signaling. These developmentally normal cells also show increased migration along nanotopographic cues in the same manner as GBM cells, presenting evidence that glioma cells are migrating in a manner reminiscent of radial migration in corticogenesis, an idea that has been previously explored (Beadle et al., 2008). Radial glia cells in the developing brain present long, linear processes along which neural progenitor cells migrate to exit the stem cell niche and enter developmental areas of the brain. These similarities between radial glial and glioma migratory behavior are consistent with the notion that that cancer mirrors embryogenesis pathways (Kelleher et al., 2006) through the epigenetic reactivatioon of embryonically active genes (Ames et al., 2017; Zhou et al., 2018). Based on these similarities, it is possible that the down-regulation of RhoA that initiates cell migration in corticogenesis has similar mechanisms to the same phenomenon observed in glioma invasion. While RhoA down-regulation is required for migration in corticogenesis, RhoA is still required at low levels, implying a biphasic dependence on RhoA. Consistently, it was found that myosin II, which is activated downstream in the Rho-ROCK pathway, may biphasically affect migration in astrocytomas. Low concentrations of a myosin II inhibitor increased migration, whereas higher concentrations inhibited migration (Salhia et al., 2005). Another group found that glioma cells that can migrate on compliant substrates became sensitive to substrate stiffness when myosin-generated contractile force was increased through RhoA or ROCK activation (Wong et al., 2015), suggesting that the biphasic RhoA/myosin II dependence could underlie the different sensitivity of glioma subtypes to substrate stiffness that was discussed earlier. It was also found that mice with implanted tumors expressing constitutively active RhoA had a 30% increase in mean survival time, providing the basis of a possible clinical application (Wong et al., 2015).
The Arp2/3 complex is an actin nucleator involved in the cytoskeletal remodeling necessary for lamellipodia protrusion and is activated downstream from Rac. Formins, on the other hand, are involved in unbranched actin assembly, including stress fibers and filopodia. These different actin nucleators have been reviewed in more detail (Blanchoin et al., 2014). Not much evidence has been gathered to define the role of different actin nucleators in glioma invasion. The mechanical confinement theory for nanotopography-induced migration of glioblastoma cells was shown to be formin dependent, however, while Arp2/3 independent (Monzo et al., 2016). Another group showed that the Arp2/3 complex contributes significantly to glioma cell migration, although the drug used in this work only allowed observation for a short duration before cytotoxic effects (Liu et al., 2013). Finally, a recent paper showed that GBM invasion was hindered by a drug that disrupts actin polymerization in a method that was not reliant on Arp2/3, although formin reliance was not investigated (Hayashi et al., 2016).
Transmembrane receptors
Integrins mediate the adhesion to and migration along many common ECM proteins, such as collagens, fibronectin, and laminin (Barezyk et al., 2010), which are found in the perivascular region in the brain. In the brain parenchyma, CD44 is considered the transmembrane receptor held primarily responsible for cell adhesion to the HA-rich ECM (Merzak et al., 1994). Both integrins and CD44 have been extensively studied in cancer and play a significant role in the invasive behavior of malignant gliomas.
Integrin antagonists have been used to target HGGs and GBMs ever since cilengitide was first synthesized in the early 1990s as an RGD peptide-based competitive inhibitor of integrin binding to native RGD sequences in the ECM (Mas-Moruno et al., 2010). Cilengitide treatment alone did not result in any significant inhibition of tumor growth; however, combined treatment with cilengitide did enhance radiotherapy efficacy in vitro (Burke et al., 2002), showing its potential as a treatment component, if not a stand-alone treatment. Unfortunately, this potential did not translate to clinical testing, with no survival benefit being observed (Stupp et al., 2014). This has resulted in a string of new integrin inhibitors being studied, including several other RGD-based treatments (reviewed by Danhier et al., 2012) with promising in vitro results. Another recent integrin antagonist is GLPG0187, which was shown to cause detachment and cell death of mouse glioma tumor cells (Silginer et al., 2014). While clinical trial results for these new integrin antagonists have yet to be released, a possible explanation for cilengitide failure is that glioma cells are capable of migrating not just perivascularly, but also down the white matter tracts using CD44 as a transmembrane receptor for the HA-rich environment, which would not be inhibited by these integrin antagonists.
In the study of CD44 in GBM invasion, a variety of models have been used, in addition to the widely used brain slice model. One group studied invasion in the astrocyte-rich brain stroma by culturing astrocytes to hyperconfluence before plating GBM cells (Gritsenko, et al., 2017). In this method, astrocytes develop a 3D scaffold that self-assembles an ECM that is presumably similar to that assembled in the brain. Other groups have modeled migration using HA-RGD hydrogels (Ananthanarayanan et al., 2011) and between an HA hydrogel and an ECM protein-coated surface (Rape and Kumar, 2014) as substrates. More recently, a method was developed that elegantly models multiple steps of invasion by embedding a cell reservoir in a 3D HA-RGD matrix featuring an open channel, representing bulk tumor, brain parenchyma, and the vasculature, respectively (Wolf et al., 2018). This model, though simple, was able to elicit the multiple-step invasive process described in vivo, with migration occurring through the matrix via long extended processes, followed by rapid migration along the vasculature.
Despite the multiple methods developed to study the importance of CD44 in GBM invasion, data has been reported on the importance of CD44 expression levels on glioma migration and its effects on patient survival, with different groups claiming it has positive, negative, or no impact on GBM invasion. These apparent contradictions were reconciled when a recent paper showed that survival, as well as glioma migration speed, depends biphasically on CD44 expression (Klank et al., 2017). As the authors of this study note, these findings correlate with previous work that showed that migration depends biphasically on cell-ECM adhesion strength (DiMilla et al., 1993). They also showed that CD44 expression varies for different glioma subtypes, with proneural subtypes expressing lower CD44 levels, intermediate expression found in proliferative subtypes, and highest expression in mesenchymal subtypes. Fastest migration occurs with intermediate expression levels. They also note that this complicates potential CD44 antagonist-based therapies, as decreasing CD44 levels or availability in the mesenchymal subtype (one of the most common glioblastoma subtypes [Phillips et al., 2006]) would lead to increased migratory speed and worsened patient prognosis. Clarifying the relative importance and different functions of CD44 and integrins in glioma cell migration could inform the development of glioma therapies that target these transmembrane receptors. Also, in addition to its role in adhesion, CD44 has been shown to promote the GBM stem-like phenotype and contribute to radiation resistance (Pietras et al., 2014). The role of CD44 in glioma invasion is reviewed in further detail by Mooney et al. (2016).
CONCLUSION
Although significant progress on blocking various mechanisms involved in cell migration has resulted in reduced glioma invasiveness in vitro, these findings have not translated to prolonged survival of HGG patients. This may be due to the fact that most studies in vitro explored only a single target, while many other mechanisms exist and are utilized in vivo. Approaches targeting combinations of varying migratory mechanisms, such as combining CD44 and integrin antagonists, have not been extensively explored to date and may produce a more effective treatment. Targeting multiple key players in migration concurrently, such as transmembrane receptors and cytoskeletal remodeling pathways, is another logical pathway to explore. Another reason for the lack of clinical success could be that diffuse migration of glioma cells commonly occurs before diagnosis, so that arresting migration would not lead to less invasion or increased success of localized treatments. Future work on early detection and targeting a full range of migratory mechanisms to halt the migration of heterogeneous glioma cells may lead to clinical success.
ACKNOWLEDGMENTS
We thank Hung-Ji Tsai for assisting in manuscript revisions.
Abbreviations used:
- dcEFs
direct-current electric fields
- ECM
extracellular matrix
- GBM
glioblastoma
- HA
hyaluronic acid
- HGGs
high-grade gliomas
- MMPs
matrix metalloproteinases.
Footnotes
REFERENCES
- Ahirwar DK, Nasser MW, Jones TH, Sequin EK, West JD, Henthorne TL, Javor J, Kaushik AM, Ganju RK, Subramaniam VV. (2015). Non-contact method for directing electrotaxis. Sci Rep , 11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames H, Halushka MK, Rodriquez FJ. (2017). miRNA regulation in gliomas: usual suspects in glial tumorigenesis and evolving clinical applications. J Neuropathol Exp Neurol , 246–254. [DOI] [PubMed] [Google Scholar]
- Ananthanarayanan B, Kim Y, Kumar S. (2011). Elucidating the mechanobiology of malignant brain tumors using a brain matrix-mimetic hyaluronic acid hydrogel platform. Biomaterials , 7913–7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker GJ, Yadav VN, Motsch S, Koschmann C, Calinescu AA, Mineharu Y, Camelo-Piragua SI, Orringer D, Bannykh S, Nichols WS, et al. (2014). Mechanisms of glioma formation: iterative perivascular glioma growth and invasion leads to tumor progression, VEGF-independent vascularization, and resistance to antiangiogenic therapy. Neoplasia : 543–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser BL, Shamsan GA, Chan CE, Opoku KN, Tüzel E, Schlichtmann BW, Kasim JA, Fuller BJ, McCullough BR, Rosenfeld SS, Odde DJ. (2017). Shifting the optimal stiffness for cell migration. Nat Commun , 15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barczyk M, Carracedo S, Gullberg D. (2010). Integrins. Cell Tissue Res , 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker FG, 2nd, SM Chang, Gutin PH, Malec MK, McDermott MW, Prados MD, Wilson CB. (1998). Survival and functional status after resection of recurrent glioblastoma multiforme. Neurosurgery , 709–720. [DOI] [PubMed] [Google Scholar]
- Beadle C, Assanah MC, Monzo P, Vallee R, Rosenfeld SS, Canoll P. (2008). The role of myosin II in glioma invasion of the brain. Mol Biol Cell , 3357–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. (2004). Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol , 1046–1069. [DOI] [PubMed] [Google Scholar]
- Bignami A, Asher R, Perides G, Rahemtulla F. (1992). The extracellular matrix of cerebral gray matter: Golgi’s pericellular net and Nissl’s nervösen grau revisited. Int J Dev Neurosci , 291–299. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, Decarlo A, Engler JA. (1993). Matrix metalloproteinases: a review. Crit Oral Biol Med , 197–250. [DOI] [PubMed] [Google Scholar]
- Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. (2014). Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev , 235–263. [DOI] [PubMed] [Google Scholar]
- Brockmann MA, Ulbricht U, Gruner K, Fillbrandt R, Westphal M, Lamszus K. (2003). Glioblastoma and cerebral microvascular endothelial cell migration in response to tumor-associated growth factors. Neurosurgery , 1391–1399. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Loew LM. (1994). Electric field-directed fibroblast locomotion involves cell surface molecular reorganization and is calcium dependent. J Cell Biol , 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke PA, DeNardo SJ, Miers LA, Lamborn KR, Matzku S, DeNardo GL. (2002). Cilengitide targeting of αvβ3 integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res , 4263–4272. [PubMed] [Google Scholar]
- The Cancer Genome Atlas (TCGA) Research Network (2008). Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature , 1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candiello J, Balasubramani M, Schreiber EM, Cole GJ, Mayer U, Halfter W, Lin H. (2007). Biomechanical properties of native basement membranes. FEBS J , 2897–2908. [DOI] [PubMed] [Google Scholar]
- Cao L, Wei D, Reid B, Zhao S, Pu J, Pan T, Yamoah E, Zhao M. (2013). Endogenous electric currents might guide rostral migration of neuroblasts. EMBO Rep , 184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroni P, Schwab MA. (1988). Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J Cell Biol , 1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SB. (1965). Principles of cell motility: the direction of cell movement and cancer invasion. Nature , 1183–1187. [DOI] [PubMed] [Google Scholar]
- Chicoine MR, Silbergeld DL. (1995). Assessment of brain tumor cell motility in vivo and in vitro. J Neurosurg , 615–622. [DOI] [PubMed] [Google Scholar]
- Condeelis J, Singer RH, Segall JE. (2005). The great escape: when cancer cells hijack the genes for chemotaxis and motility. Annu Rev Cell Dev Biol , 695–718. [DOI] [PubMed] [Google Scholar]
- Danhier F, Le Breton A, Préat V. (2012). RGD-based strategies to target αvβ3 integrin in cancer therapy and diagnosis. Mol Pharm , 2961–2973. [DOI] [PubMed] [Google Scholar]
- Debinski W, Tatter SB. (2009). Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother , 1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpech B, Maingonnat C, Girard N, Chauzy C, Olivier A, Maunoury R, Olivier A, Tayot J, Creissard P. (1993). Hyaluronan and hyaluronectin in the extracellular matrix of human brain tumour stroma. Eur J Cancer , 1012–1017. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Bourdon MA. (1996). Tenascin mediates human glioma cell migration and modulates cell migration on fibronectin. J Cell Sci , 643–652. [DOI] [PubMed] [Google Scholar]
- DiMilla PA, Stone JA, Quinn JA, Albelda SM, Lauffenburger DA. (1993). Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol , 729–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehtesham M, Winston JA, Kabos P, Thompson RC. (2006). CXCR4 expression mediates glioma cell invasiveness. Oncogene , 2801–2806. [DOI] [PubMed] [Google Scholar]
- Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP. (1991). Genes for epidermal growth factor receptor, transforming growth factor α, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res , 2164–2172. [PubMed] [Google Scholar]
- Elkin BS, Azeloglu EU, Costa KD, 3rd Morrison B. (2007). Mechanical heterogeneity of the rat hippocampus measured by atomic force microscope indentation. J Neurotrauma , 812–822. [DOI] [PubMed] [Google Scholar]
- Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM, Sutherland G, Edwards DR. (1999). Gelatinase-A (MMP02), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer , 1828–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer VP, Moura Neto V, Mentlein R. (2018). Glioma infiltration and extracellular matrix: key players and modulators. Glia , 1542–1565. [DOI] [PubMed] [Google Scholar]
- Franze K. (2013). The mechanical control of nervous system development. Development , 3069–3077. [DOI] [PubMed] [Google Scholar]
- Friedl P, Wolf K. (2003). Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer , 362–374. [DOI] [PubMed] [Google Scholar]
- Ge X, He F, Kim KJ, Blanchi B, Coskun V, Nguyen L, Wu X, Zhao J, Heng JI, Martinowich K, et al (2006). Coupling of cell migration with neurogenesis by proneural bHLH factors. Proc Natl Acad Sci USA , 1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges PC, Miller WJ, Meaney DF, Sawyer ES, Janmey PA. (2006). Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures. Biophys J , 3012–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy TJ, De Leon E, Griffin KR, Stringer BW, Day BW, Fabry B, Cooper-White J, O’Neill GM. (2016). Differential response of patient-derived primary glioblastoma cells to environmental stiffness. Sci Rep , 23353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritsenko P, Leenders W, Friedl (2017). Recapitulating in vivo-like plasticity of glioma cell invasion along blood vessels and in astrocyte-rich stroma. Histochem Cell Biol , 395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Michiue H, Yamada H, Takata K, Nakayama H, Wei FY, Fujimura A, Tazawa H, Asai A, Ogo N, et al (2016). Fluvoxamine, an anti-depressant, inhibits human glioblastoma invasion by disrupting actin polymerization. Sci Rep , 23372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrapko M, van Dommelen JA, Peters GW, Wismans JS. (2007). The influence of test conditions on characterization of the mechanical properties of brain tissue. J Biomech Eng , 031003. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Hoffmann G, Wheeler B, Schiapparelli P, Quinones-Hinojosa A, Searson P. (2016). Cellular microenvironment modulates the galvanotaxis of brain tumor initiation cells. Sci Rep , 21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SB, Brat DJ, Olson JJ, Von Deimling A, Zhou W, Van Meir EG. (2003). Alterations in molecular pathways of diffusely infiltrating glial neoplasms: application to tumor classification and anti-tumor therapy. Int J Oncol , 857–869. [PubMed] [Google Scholar]
- Ivkovic S, Beadle C, Noticewala S, Massey SC, Swanson KR, Toro LN, Bresnick AR, Canoll P, Rosenfeld SS. (2012). Direct inhibition of myosin II effectively blocks glioma invasion in the presence of multiple motogens. Mol Biol Cell , 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Betancur M, Patel GD, Valmikinathan CM, Mukhatyar VJ, Vakharia A, Pai SB, Brahma B, MacDonald TJ, Bellamkonda RV. (2014). Guiding intracortical brain tumour cells to an extracortical cytotoxic hydrogel using aligned polymeric nanofibres. Nat Mater , 308–316. [DOI] [PubMed] [Google Scholar]
- Johnson J, Nowicki MO, Lee CH, Chiocca EA, Viapiano MS, Lawler SE, Lannutti JJ. (2009). Quantitative analysis of complex glioma cell migration on electrospun polycaprolactone using time-lapse microscopy. Tissue Eng Part C Methods , 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher FC, Fennelly D, Rafferty M. (2006). Common critical pathways in embryogenesis and cancer. Acta Oncol , 375–388. [DOI] [PubMed] [Google Scholar]
- Klank RL, Decker Grunck SA, Bangasser BL, Forster CL, Price MA, Odde TJ, SantaCruz KS, Rosenfeld SS, Canoll P, Turley EA, et al (2017). Biphasic dependence of glioma survival and cell migration on CD44 expression level. Cell Rep , 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Yang X, Chen C, Liu B, Ren B, Wang L, Zhao K, Yu S, Ming H. (2013). Expression of the Arp2/3 complex in human gliomas and its role in the migration and invasion of glioma cells. Oncol Rep , 2127–2136. [DOI] [PubMed] [Google Scholar]
- Lo C, Wang H, Dembo M, Wang Y. (2000). Cell movement is guided by the rigidity of the substrate. Biophys J , 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis DN, Perry A, Reifenberger G, Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. (2016). The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol , 803–820. [DOI] [PubMed] [Google Scholar]
- Manning TJ, Jr, Parker JC, Sontheimer H. (2000). Role of lysophosphatidic acid and rho in glioma cell motility. Cell Motil Cytoskeleton , 185–199. [DOI] [PubMed] [Google Scholar]
- Mas-Moruno C, Rechenmacher F, Kessler H. (2010). Cilengitide: the first anti-angiogenic small molecule drug candidate design, synthesis and clinical evaluation. Anticancer Agents Med Chem , 753–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzak A, Koocheckpour S, Pilkington GJ. (1994). CD44 mediates human glioma cell adhesion and invasion in vitro. Cancer Res , 3988–3992. [PubMed] [Google Scholar]
- Montana V, Sontheimer H. (2011). Bradykinin promotes the chemo-tactic invasion of primary brain tumors. J Neurosci , 4858–4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monzo P, Chong YK, Guetta-Terrier C, Krishnasamy A, Sathe SR, Yim EKF, Ng WH, Ang BT, Tang C, Ladoux B, et al (2016). Mechanical confinement triggers glioma liinear migration dependent on formin FHOD3. Mol Biol Cell , 1246–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney KL, Choy W, Sidhu S, Pelargos P, Bui TT, Voth B, Barnette N, Yang I. (2016). The role of CD44 in glioblastoma multiforme. J Clin Neurosci , 1–5. [DOI] [PubMed] [Google Scholar]
- Munson JM, Bellamkonda RV, Swartz MA. (2013). Interstitial flow in a 3D microenvironment increases glioma invasion by a CXCR4-dependent mechanism. Cancer Res , 1536–1546. [DOI] [PubMed] [Google Scholar]
- Mycielska ME, Djamgoz MB. (2004). Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J Cell Sci (Pt. 9), 1631–1639. [DOI] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. (1996). Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature , 635–638. [DOI] [PubMed] [Google Scholar]
- Nazarenko I, Hede SM, He X, Hedren A, Thompson J, Linstrom MS, Nister M. (2012). PDGF and PDGF receptors in glioma. Ups J Med Sci , 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Besson A, Heng JI, Schuurmans C, Teboul L, Parras C, Philpott A, Roberts JM, Guillemot F. (2006). p27kip1 independently promotes neuronal differentiation and migration in the cerebral cortex. Genes Dev , 1511–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccitelli R. (1988). Physiological electric fields can influence cell motility, growth and polarity. Adv Cell Biol , 213–233. [Google Scholar]
- Onuma EK, Hui SW. (1988). Electric field-directed cell shape changes, displacement, and cytoskeletal reorganization are calcium dependent. J Cell Biol , 2067–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Páez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. (2009). Cancer Cell , 220–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paganetti PA, Caroni P, Schwab ME. (1988). Glioblastoma infilitration into central nervous system tissue in vitro: Involvement of a metalloprotease. J Cell Biol , 2281–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham RJ, Jr, Wang Y. (1997). Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc Natl Acad Sci USA , 13661–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie RJ, Doyle AD, Yamada KM. (2009). Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol, , 538–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, et al (2006). Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progrssion, and resemble stages in neurogenesis. Cancer Cell , 157–173. [DOI] [PubMed] [Google Scholar]
- Pietras A, Katz AM, Ekström EJ, Wee B, Halliday JJ, Pitter KL, Werbeck JL, Amankulor NM, Huse JT, Holland EC. (2014). Osteopontin-CD44 signaling in the glioma perivascular niche enhances cancer stem cell phenotypes and promotes aggressive tumor growth. Cell Stem Cell , 257–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogoda K, Chin L, Georges PC, Byfield FJ, Bucki R, Kim R, Weaver M, Wells RG, Marcinkiewicz C, Janmey PA. (2015). Compression stiffening of brain and its effect on mechanosensing by glioma cells. Cancer Res , 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rape A, Kumar S. (2014). A composite hydrogel platform for the dissection of tumor cell migration at tissue interfaces. Biomaterials, , 8846–8853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rape A, Ananthanarayanan B, Kumar S. (2014). Engineering strategies to mimic the glioblastoma microenvironment. Adv Drug Deliv Rev , 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS. (2003). Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer , 489–501. [DOI] [PubMed] [Google Scholar]
- Rath BH, Fair JM, Jamal M, Camphausen K, Tofilon PJ. (2013). Astrocytes enhance the invasion potential of glioblastoma stem-like cells. PLoS One , e54752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY. (2003). Primary brain tumours in adults. Lancet , 323–331. [DOI] [PubMed] [Google Scholar]
- Roos A, Ding Z, Loftus JC, Tran NL. (2017). Molecular and microenvironmental determinants of glioma stem-like cell survival and invasion. Front Oncol , 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos ET, Condeelis JS, Patsialou A. (2011). Chemotaxis in cancer. Nat Rev Cancer , 573–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JB, Kung AL, Klein RS, Chan JA, Sun YP, Schmidt K, Kieran MW, Luster AD, Segal RA. (2003). A small-molecular antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci USA , 13513–13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhia B, Rutten F, Nakada M, Beaudry C, Berens M, Kwan A, Rutka JT. (2005). Inhibition of Rho-kinase affects astrocytoma morphology, motility, and invasion through activation of Rac1. Cancer Res , 8792–8800. [DOI] [PubMed] [Google Scholar]
- Sathornsumetee S, Rich JN. (2006). New treatment strategies for malignant gliomas. Expert Rev Anticancer Ther , 1087–1104. [DOI] [PubMed] [Google Scholar]
- Sato MJ, Kuwayama H, van Egmond WN, Takayama AL, Takagi H, van Haastert PJ, Yanagida T, Ueda M. (2009). Switching direction in electric-signal-induced cell migration by cyclic guanosine monophosphate and phosphatidylinositol signaling. Proc Natl Acad Sci USA , 6667–6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer HJ. (1940). The forms of growth in gliomas and their practical significance. Brain , 1–35. [Google Scholar]
- Schwab ME, Caroni P. (1988). Oligodendrocytes and CNS myelin are non-permissive substrate for neurite growth and fibroblasts spreading in vitro. J Neurosci , 2381–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Sheets K, Elankumaran S, Nain AS. (2013). The mechanistic influence of aligned nanofibers on cell shape, migration and blebbing dynamics of glioma cells. Integr Biol (Camb) , 1036–1044. [DOI] [PubMed] [Google Scholar]
- Silginer M, Weller M, Ziegler U, Roth P. (2014). Integrin inhibition promotes atypical anoikis in glioma cells. Cell Death Dis , e1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, Aldape KD, Lhermitte B, Pietsch T, Grujicic D, et al. (2014). Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol , 1100–1108. [DOI] [PubMed] [Google Scholar]
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al (2009). Effects of radiotherapy with concomitant and adjuvant temozolomide versus readiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol , 459–466. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Nicholson C. (2006). In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci USA , 5567–5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonn JC, Goldbrunner R. (2003). Mechanisms of glioma cell invasion. Acta Neurochir Suppl , 163–167. [DOI] [PubMed] [Google Scholar]
- Tonn JC, Kerkau S, Hanke A, Bouterfa H, Mueller JG, Wagner S, Vince GH, Roosen K. (1999). Effect of synthetic matrix- metalloproteinase inhibitors on invasive capacity and proliferation of human malignant gliomas in vitro. Int J Dev Neurosci , 764–772. [DOI] [PubMed] [Google Scholar]
- Ulrich TA, de Juan Pardo EM, Kumar S. (2009). The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res , 4167–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haastert PJ, Devreotes PN. (2004). Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol , 626–634. [DOI] [PubMed] [Google Scholar]
- Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al (2010). An integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell , 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Tong X, Yang F. (2014). Bioengineered 3D brain tumor model to elucidate the effects of matrix stiffness on glioblastoma cell behavior using PEG-based hydrogels. Mol Pharm , 2115–2125. [DOI] [PubMed] [Google Scholar]
- Weinberg SH, Mair DB, Lemmon CA. (2017). Mechanotransduction dynamics at the cell-matrix interface. Biophys J , 1862–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf KJ, Lee S, Kumar S. (2018). A 3D topographical model of parenchymal infiltration and perivascular invasion in glioblastoma. APL Bioeng , 031903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SY, Ulrich TA, Deleyrolle LP, MacKay JL, Lin JMG, Martuscello RT, Jundi MA, Reynolds BA, Kumar S. (2015). Constitutive activation of myosin-dependent contractility sensitizes glioma tumor-initiating cells to mechanical inputs and reduces tissue invasion. Cancer Res , 1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim EK, Darling EM, Kulangara K, Guilak F, Leong KW. (2010). Nanotopography-induced changes in focal adhesions, cytoskeletal organization, and mechanical properties of human mesenchymal stem cells. Biomaterials , 1299–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Alver BM, Li S, Hlady RA, Thompson JJ, Schroeder MA, Lee JH, Qiu J, Schwartz PH, Sarkaria JN, Robertson KD. (2018). Distinctive epigenomes characterize glioma stem cells and their response to differentiation cues. Genome Biol , 43. [DOI] [PMC free article] [PubMed] [Google Scholar]