Abstract
There are many studies suggesting an age-associated decline in the actin cytoskeleton, and this has been adopted as common knowledge in the field of aging biology. However, a direct identification of this phenomenon in aging multicellular organisms has not been performed. Here, we express LifeAct::mRuby in a tissue-specific manner to interrogate cytoskeletal organization as a function of age. We show for the first time in Caenorhabditis elegans that the organization and morphology of the actin cytoskeleton deteriorate at advanced age in the muscles, intestine, and hypodermis. Moreover, hsf-1 is essential for regulating cytoskeletal integrity during aging, so that knockdown of hsf-1 results in premature aging of actin and its overexpression protects actin cytoskeletal integrity in the muscles, the intestine, and the hypodermis. Finally, hsf-1 overexpression in neurons alone is sufficient to protect cytoskeletal integrity in nonneuronal cells.
INTRODUCTION
The cytoskeleton plays a fundamental role in cellular pathways, including chaperone function, trafficking, and autophagy (Berry et al., 1992; Young et al., 2003; Caviston and Holzbaur, 2006; Mooren et al., 2012; Higuchi et al., 2013). Loss of function of the actin cytoskeleton has been observed in clinical manifestations, including neurodegeneration and muscle myopathies (Acsadi et al., 1991; Alim et al., 2002, 2004). Recently, attention has been turned to the link between the actin cytoskeleton and aging. Cytoskeletal integrity has been shown to decline as a function of age, and preserving the cytoskeleton can have a beneficial impact on lifespan. Specifically, overexpression of the heat-shock transcription factor, HSF-1, can protect cytoskeletal integrity during stress and aging (Baird et al., 2014). However, a direct link of HSF-1 to preserving cytoskeletal integrity during aging is lacking. Rather, HSF-1 has been shown to transcriptionally up-regulate a subset of cytoskeletal components, and one of these targets, pat-10, has been shown to be responsible for HSF-1-mediated lifespan extension. Here, we show directly that overexpression or knockdown of HSF-1 can affect cytoskeletal organization and morphology during aging.
RESULTS AND DISCUSSION
The actin cytoskeleton shows a marked decline in structure and organization during aging in adult worms
Actin microfilaments are a primary cytoskeletal component that is important for cell viability and physiology. In addition to providing structural support, the actin cytoskeleton has long been understood to play important roles in intracellular organelle and cargo movement. Although several studies have shown that protection of the actin cytoskeleton can beneficially impact lifespan (Higuchi et al., 2013; Baird et al., 2014), a direct characterization of actin cytoskeletal organization and integrity as a function of age in multicellular organisms is lacking. This is likely due to the difficulty of visualizing the actin cytoskeleton in live organisms. Early studies revealed that actin-fusion proteins are functionally impaired, presumably due to the requirement for every surface of the actin monomer in protein–protein interactions to form filaments (Doyle and Botstein, 1996; Yamada et al., 2005). Here, we have adapted the use of LifeAct in Caenorhabditis elegans to visualize actin organization as a function of age in a tissue-specific manner. LifeAct is the first 17 amino acids of a yeast actin-binding protein, Abp140p, which binds to F-actin filaments and can be used to visualize the F-actin network in live cells by fusing it to a fluorescent protein (Riedl et al., 2008). We created stable transgenic lines expressing LifeAct::mRuby in various tissues.
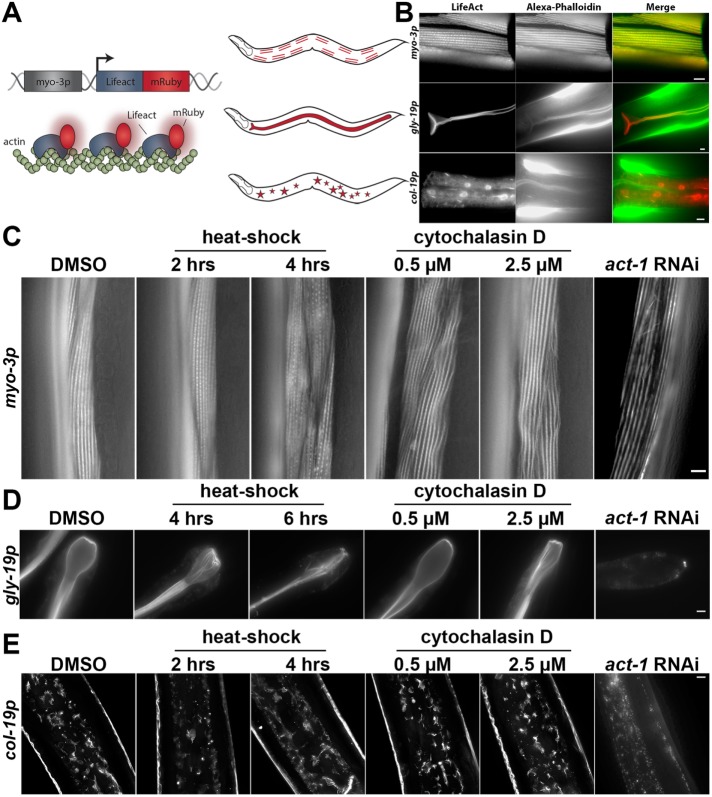
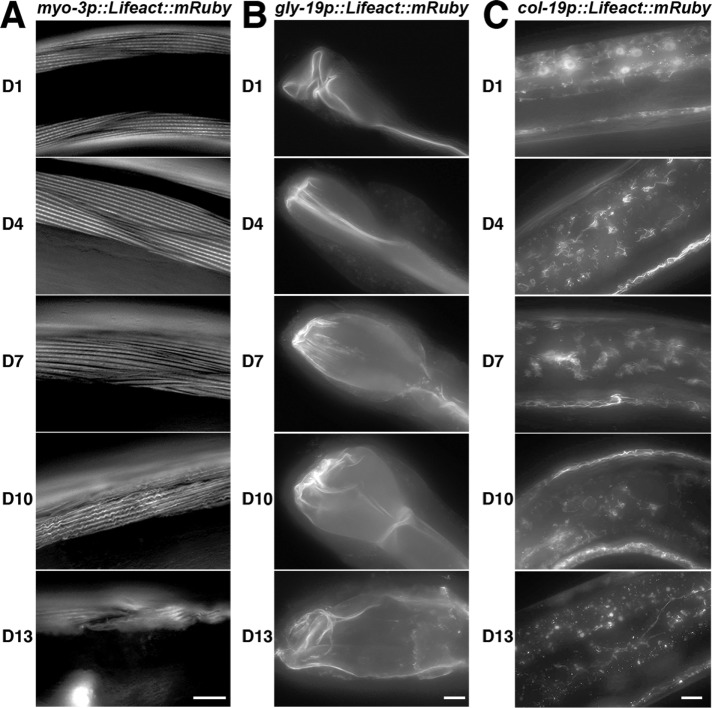
We used the mosSCI system to generate worms expressing a single copy of LifeAct::mRuby driven by tissue-specific promoters (Frøkjaer-Jensen et al., 2008). These transgenic worms exhibit robust staining of the actin in the muscles, intestine, and hypodermis (Figure 1, A and B). LifeAct::mRuby signal colocalizes with the traditional F-actin dye phalloidin, illustrating specific visualization of actin. This system overcomes phenotypic consequences of traditional visualization of actin in live cells, such as the toxic effects of phalloidin. Moreover, tissue-specific expression allows robust analysis of actin in specific populations of cells without bleed-through or interfering signal from neighboring cells with a stronger fluorescent signal. Destabilization of actin by knockdown of actin expression through RNA interference (RNAi), heat shock, or treatment with drugs that alter actin polymerization results in perturbations of LifeAct::mRuby structures, providing further evidence that LifeAct::mRuby labels bona fide actin structures in worms (Figure 1, C–E). The actin cytoskeleton in muscle cells can be visualized as linear striations of filamentous actin parallel to muscle fibers. These actin structures in the muscle show signs of disorganization beginning at day 7 of adulthood (D7), with progressive decline and decreased thickness through D10 and D13 (Figure 2A; Supplemental Figure S1A). Similarly, we find loss of cytoskeletal organization and structure within intestinal cells as early as D7, with significant aberrations in structural organization by D13 (Figure 2B). To our surprise, we have not been able to visualize significant cytoskeletal structures in the neurons or in the pharynx (unpublished data; strains are available and tested—see the Supplemental Resources and Methods).
FIGURE 1:
LifeAct is a robust and reliable reporter for tissue-specific visualization of actin in live animals. (A) Left: schematic for tissue-specific LifeAct expression in C. elegans. LifeAct is fused to the red fluorescent protein, mRuby, and expressed in worms using a tissue-specific promoter, such as myo-3p for muscle expression. Right: schematic of the location where LifeAct-mRuby is expressed and visualized in muscles, intestine, and hypodermis. (B) LifeAct-mRuby stained structures colocalize with Alexa Fluor 488 phalloidin staining. Transgenic worms expressing LifeAct-mRuby under tissue-specific promoters for muscles (myo-3p), intestine (gly-19p), and hypodermis (col-19p) were grown to day 1 on empty vector (EV) RNAi from hatch, fixed, and stained as described in the Supplemental Resources and Methods. All scale bars are 5 µm. (C–E) LifeAct-mRuby stained actin is susceptible to disruption by actin-destabilizing conditions, such as heat shock, cytochalasin D, and act-1 RNAi, in muscles (C), intestine (D), and hypodermis (E). Transgenic worms were grown to D1 for muscles and intestine and D4 for hypodermis on EV RNAi. Worms were heat-shocked at 34°C for 2–6 h on solid agar or treated with 0.5–2.5 µM cytochalasin D spinning in M9 solution for 4 h at 20°C. For actin RNAi, worms were grown from hatch until L4 on EV RNAi and then moved to act-1 RNAi for 24 h and imaged on D1. Images for act-1 RNAi were contrast-enhanced separately from other images for ease of visualization of phenotypes. Scale bars are 5 µm.
FIGURE 2:
The actin cytoskeleton shows loss of structure and integrity as a function of age. Cytoskeletal integrity was monitored in muscles (A), intestine (B), and hypodermis (C) of live C. elegans at various stages of adulthood (D1, D4, D7, D10, D13), using transgenic worms expressing LifeAct-mRuby under tissue-specific promoters as described in Figure 1. Worms were grown on EV RNAi from hatch and aged by three different methods: flotation, FUDR treatment, or hand picking, as described in the Supplemental Resources and Methods. All three methods show similar trends. Representative data from hand picking are shown. Scale bars are 5 µm.
A dramatically different phenomenon is apparent in the hypodermal actin during aging. The outer hypodermal layer under the cuticle of the worm has a large amount of actin throughout adulthood, but the inner hypodermal syncytium shows little to no cytoskeletal structure at D1. However, by D4, there are significant cytoskeletal structures in these cells that appear starlike (Figure 2C). These structures show loss of organization by D7 and complete resolution by D10. Using LAMPro technology, the fluorescent intensity of these starlike structures was quantified using positional information from a COPAS biosort (see Daniele et al., 2017, for detailed methods). Briefly, staged worms are run through a COPAS biosort to obtain fluorescent intensity along the anterior–posterior axis of the worm. This measurement is quantified and displayed as the median fluorescent intensity at multiple positions along the anterior–posterior axis, such that each line represents a specific segment of the worm. At D1, there is a high level of cytoplasmic LifeAct::mRuby signal. However, by D4, a majority of the fluorescent signal is represented by the starlike actin structures (see Figure 2C). In agreement with fluorescent microscopy, LAMPro quantification shows that the highest level of starlike actin structures exists in the hypodermis at D4, which show a rapid decline of these structures from D7 to D13, providing evidence that this is not just a feature of a small number of worms, but is representative across a large population (Supplemental Figure S1, B and C). Finally, RNAi knockdown of actin or actin-regulatory genes perturbs the formation of these structures (Supplemental Figure S2A). Moreover, treatment with the actin-destabilizing drugs, CK-666, cytochalasin D, latrunculin A, and SMIFH2, results in disruption of hypodermal actin structures (Supplemental Figure S2B). These data suggest that the starlike actin structures contain functional filamentous actin.
Next, we tested whether these structures are associated with endocytic vesicles. The actin cytoskeleton is a critical element for trafficking of endocytic vesicles, and endosomes found in Saccharomyces cerevisiae, called actin patches, have been shown to be decorated with a coat of F-actin, which is required for their trafficking along actin cables (Pruyne et al., 1998). Therefore, we tested whether the starlike actin cytoskeletal structures were endocytic vesicles coated with F-actin for trafficking. We treated worms with RNAi against genes essential for endocytosis (dyn-1, erp-1, sphk-1, unc-57) and found that knockdown of endocytic components perturbed the formation of these actin structures. This was not due to a developmental delay found in some endocytosis mutants, as these mutants also failed to form hypodermal actin structures through D7 (Supplemental Figure S2C). These data support the hypothesis that these starlike actin species found in hypodermal cells may be in contact with endocytic vesicles. Yet to be determined is why these cytoskeletal structures appear in hypodermal cells between D4 and D7. In S. cerevisiae, actin patches form in the bud and are believed to be essential for recycling membrane components (Pruyne et al., 1998). It is possible that these structures are endosomes that are critical for recycling components in these cells.
HSF-1 affects actin cytoskeletal integrity during aging
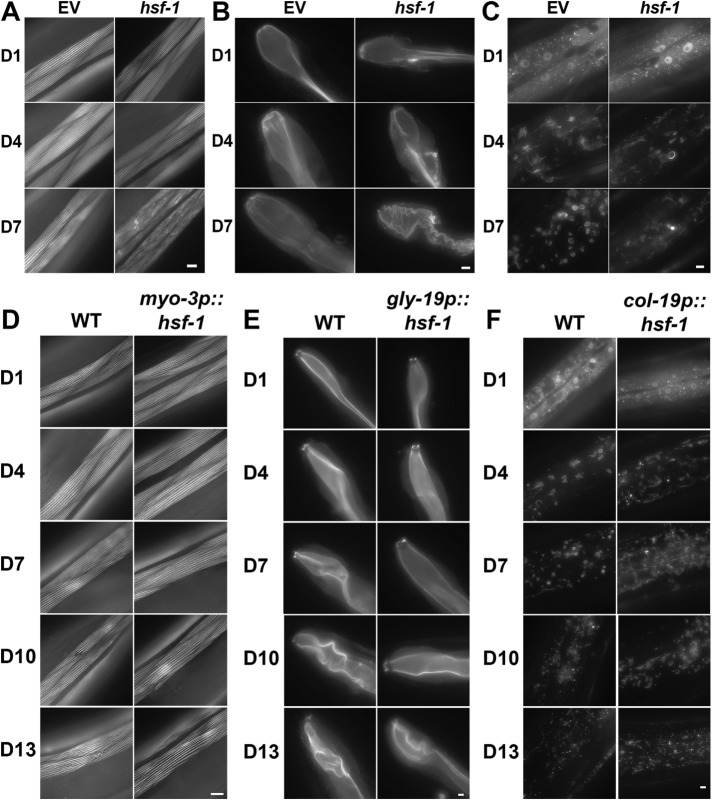
HSF-1 is a conserved master regulator for cytosolic heat shock response and its activation results in the induction of genes involved in protein refolding (e.g., chaperones) and protein degradation (e.g., proteasomes and autophagy), as reviewed in Labbadia and Morimoto (2015) and Higuchi-Sanabria et al. (2018). Moreover, HSF-1′s role in protein homeostasis is critical for lifespan regulation, and its down-regulation results in a decrease in lifespan (Garigan et al., 2002; Steinkraus et al., 2008), while its overexpression is sufficient to extend lifespan (Hsu et al., 2003; Morley and Morimoto, 2004). Beyond these canonical roles, additional studies have implicated HSF-1 in the maintenance of the cytoskeleton in C. elegans: HSF-1 promotes the induction of genes involved in cytoskeletal organization, including the troponin-like calcium-binding protein, PAT-10. Overexpression of hsf-1 or its downstream target, pat-10, is sufficient to promote thermotolerance and extend lifespan (Baird et al., 2014). However, a direct characterization of HSF-1′s role in cytoskeletal maintenance has yet to be performed. Here, we performed knockdown of hsf-1 in transgenic worms with tissue-specific LifeAct::mRuby expression to determine directly whether HSF-1 is essential for cytoskeletal maintenance in various tissues. We find that hsf-1 knockdown results in premature aging of the actin cytoskeleton in muscle cells. In D1 adults, hsf-1 knockdown worms have thinner actin cables, which show signs of disorganization as early as D4, with dramatic disruption by D7, compared with wild-type worms, which only begin to show subtle signs of disorganization at D7 (Figure 3A). Similarly, hsf-1 knockdown results in a disordered organization of actin in the intestine as early as D4, with complete loss of structural integrity by D7 (Figure 3B). Finally, hsf-1 knockdown results in loss of structural integrity of the starlike actin structures in hypodermal cells, with few or no visible cytoskeletal structures by D7 (Figure 3C). These data provide direct evidence that hsf-1 is essential for cytoskeletal maintenance during aging.
FIGURE 3:
HSF-1 is required for maintenance of cytoskeletal integrity. Cytoskeletal integrity was monitored in muscles (A), intestine (B), and hypodermis (C) of transgenic worms expressing LifeAct::mRuby under tissue-specific promoters. Transgenic worms were grown on either EV or hsf-1 RNAi from hatch. Worms were aged by three different methods: flotation, FUDR treatment, or hand picking. Representative data from hand picking are shown. Imaging was stopped at D7 due to a large number of deaths in worms grown on hsf-1 RNAi beyond D10. (D) Cytoskeletal integrity was measured in muscles of wild-type and myo-3p::hsf-1 worms at D1, D4, D7, D10, and D13. (E) Cytoskeletal integrity was measured in the hypodermis of wild-type and col-19p::hsf-1 worms at D1, D4, D7, D10, and D13. (F) Cytoskeletal integrity was measured in the intestines of wild-type and gly-19p::hsf-1 worms at D1, D4, D7, D10, and D13. For A–C, worms were plated on EV RNAi from hatch and aged by flotation, growth on FUDR, or hand picking. Representative images from hand picking are shown. Scale bars are 5 µm.
To determine whether overexpression of hsf-1 was sufficient to protect cytoskeletal integrity during aging, we performed tissue-specific overexpression of hsf-1. Previous reports have shown that overexpression of hsf-1 in neurons was sufficient to promote beneficial effects on both thermotolerance and lifespan (Douglas et al., 2015). This is due to neurons signaling nonautonomously to activate transcription of HSF-1 targets in distal tissues. Because systemic overexpression of hsf-1 in all tissue would also include the effects of neuronal nonautonomous HSF-1 signaling, we created lines overexpressing hsf-1 in single tissues to determine the function of autonomous hsf-1 upon cytoskeletal regulation. hsf-1 was overexpressed in muscle, intestinal, and hypodermal cells using promoters identical to those used for tissue-specific LifeAct::mRuby expression. Overexpression of hsf-1 in muscles, intestine, and hypodermis was sufficient to protect cytoskeletal integrity autonomously in the same tissue (Figure 3, D–F). However, overexpression of hsf-1 in the intestine or hypodermis is not sufficient to protect cytoskeletal integrity in the muscles (Supplemental Figure S3A). Similarly, overexpression of hsf-1 in the muscles or hypodermis did not protect cytoskeletal integrity in intestinal cells (Supplemental Figure S3B), and overexpression of hsf-1 in the muscles or intestine did not protect cytoskeletal integrity in hypodermal cells (Supplemental Figure S3C). These data suggest that hsf-1 can function autonomously in the same tissue to protect cytoskeletal integrity in muscles, intestine, and hypodermis, but these cells do not communicate hsf-1 signaling to other tissues.
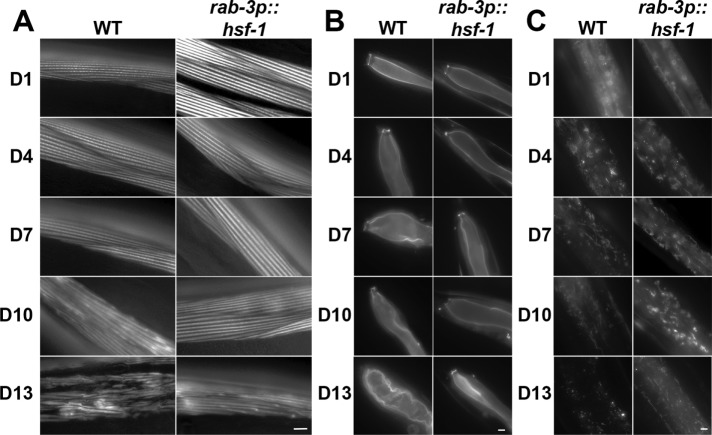
In contrast to other tissues, neuronal overexpression of hsf-1 has been shown to communicate HSF-1 signaling to distal tissue (Douglas et al., 2015; Tatum et al., 2015). Moreover, overexpression of hsf-1 in neurons alone is sufficient to promote thermotolerance and lifespan in C. elegans. Therefore, we tested whether neuronal overexpression of hsf-1 was also sufficient to protect cytoskeletal integrity in distal tissue. Indeed, neuronal overexpression of hsf-1 was sufficient to delay the age-associated decline of the actin cytoskeleton in distal tissue. Animals overexpressing hsf-1 in neurons only begin to show signs of cytoskeletal aging at D10, with clear disruption in the structure and organization at D13, in contrast to wild-type animals showing obvious defects at D10 (Figure 4A). Similarly, intestinal actin is preserved in animals overexpressing hsf-1 in neurons, with dysfunction starting at D7 and becoming readily apparent only after D10 (Figure 4B). Neuronal overexpression of hsf-1 does not delay the formation of the starlike actin structures in the hypodermal cells, which are readily apparent at D4, similarly to those in wild-type animals (Figure 4C). However, animals overexpressing hsf-1 in neurons results in a delay in the resolution of these structures, which begin at D10 and are still visible at D13, in contrast to wild-type animals at D7. This is different from overexpression of hsf-1 directly in the hypodermis (compare with Figure 3F). Animals with hypodermal hsf-1 overexpression still have starlike actin structures in the hypodermis at D4, but peak in the quantity of these structures at D7, whereas animals overexpressing hsf-1 in neurons do not increase the quantity of actin structures past D4. It is unclear what results in the subtle differences between autonomous and nonautonomous HSF-1 signaling in regulation of hypodermal actin structures, but it is clear that both result in preservation of these actin elements at a later age compared with that in wild-type animals.
FIGURE 4:
HSF-1 overexpression in neurons is sufficient to protect cytoskeletal integrity in nonneuronal tissue. Cytoskeletal integrity was monitored in muscles (A), intestine (B), and hypodermis (C) of transgenic worms expressing LifeAct::mRuby under tissue-specific promoters and overexpressing hsf-1 in neurons (rab-3p::hsf-1). Transgenic worms were grown on EV RNAi from hatch and were aged by three different methods: flotation, FUDR treatment, or hand picking. Representative data from hand picking are shown. Scale bars are 5 µm.
Considering that the nervous system is a major source of signals throughout an organism, it is not surprising that neurons can send stress signals to distal tissues. However, we were interested to find that a beneficial signal cannot be sent to distal tissue when hsf-1 is overexpressed in muscles, intestine, or hypodermis (Supplemental Figure S3, A–C). Taken together, these data suggest that hsf-1 is important in regulating cytoskeletal integrity, but can only act nonautonomously from neurons to distal tissue.
HSF-1’s role in lifespan regulation is dependent on actin homeostasis
As a master regulator, hsf-1 functions in many different pathways beyond cytoskeletal maintenance, including immunity, autophagy, and the heat-shock response (Morley and Morimoto, 2004; Singh and Aballay, 2006; Steinkraus et al., 2008; Kumsta et al., 2017). Because hsf-1 is required for cytoskeletal maintenance, we tested whether perturbations of cytoskeletal integrity could abrogate the lifespan extension found in long-lived, neuronal hsf-1–overexpressing animals. We find that nonlethal actin knockdown results in a significant decline in lifespan, and that neuronal overexpression of hsf-1 has no effect on these animals (Supplemental Figure S4A). These data suggest that the beneficial effects of nonautonomous HSF-1 signaling from neurons to intestine are dependent on cytoskeletal maintenance.
Finally, we tested whether nonneuronal tissue-specific overexpression of hsf-1 was sufficient to extend lifespan. These animals can preserve cytoskeletal integrity autonomously in cells overexpressing hsf-1, but fail to signal nonautonomously to distal tissue. Intriguingly, worms overexpressing hsf-1 in muscles, intestine, or hypodermis have a mild extension in lifespan compared with those showing neuronal overexpression of hsf-1, suggesting that HSF-1 regulates lifespan by protecting actin cytoskeletal integrity in all tissues (Supplemental Figure S4B). This is consistent with previous work performing tissue-specific overexpression of hsf-1 using different promoters (Morley and Morimoto, 2004). Therefore, neuronal overexpression of hsf-1, which promotes cytoskeletal integrity in several distal tissues, has a more profound effect on lifespan than preservation of actin in a single tissue.
Concluding remarks
Here, we provide a platform for interrogating cytoskeletal integrity in multiple tissue types in C. elegans using LifeAct::mRuby. Using this system, we have characterized the decline in actin cytoskeletal structure and organization as a function of age in muscle, intestine, and hypodermal cells. Specifically, the muscles and intestine show signs of deterioration as early as D7, with significant loss of structural integrity by D10 and D13. However, hypodermal cells are unique and show formation of starlike actin structures at D4, which resemble endocytic vesicles decorated with actin. These structures begin to resolve by D7 and are completely absent after D10.
We also find that modulation of hsf-1 expression has a direct effect on cytoskeletal regulation during aging. Knockdown of hsf-1 results in premature aging of the actin cytoskeleton, while overexpression of hsf-1 can protect cytoskeletal integrity during aging. Moreover, overexpression of hsf-1 in neurons is sufficient to preserve cytoskeletal integrity in muscles, intestine, and the hypodermis during aging. However, overexpression of hsf-1 in nonneuronal cells can only protect cytoskeletal integrity autonomously in tissues harboring the overexpression, and is insufficient to protect cytoskeletal integrity in distal tissues. Still to be identified is how neuronal hsf-1 can function distally in protecting cytoskeletal integrity in peripheral tissue.
Supplementary Material
ACKNOWLEDGMENTS
We thank the Dillin lab for valuable assistance and discussion. Specifically, we thank Brant Webster for intellectual contributions to work involving endocytic vesicles. We also thank Larry Joe, Melissa Sanchez, and Anel Esquivel for significant administrative and technical assistance. Artwork was provided by Sarah Tronnes Scientific Illustration. This work was supported by Grant 5R01AG055891-02 from the National Institute of Aging (NIA) and the Howard Hughes Medical Institute to A.D., Grant 5F32AG053023-02 from the NIA to R.H.S., and a National Science Foundation Graduate Research Fellowship to J.W.P.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E18-06-0362) on August 22, 2018.
REFERENCES
- Acsadi G, Dickson G, Love DR, Jani A, Walsh FS, Gurusinghe A, Wolff JA, Davies KE. (1991). Human dystrophin expression in mdx mice after intramuscular injection of DNA constructs. Nature , 815–818. [DOI] [PubMed] [Google Scholar]
- Alim MA, Hossain MS, Arima K, Takeda K, Izumiyama Y, Nakamura M, Kaji H, Shinoda T, Hisanaga S, Ueda K. (2002). Tubulin seeds alpha-synuclein fibril formation. J Biol Chem , 2112–2117. [DOI] [PubMed] [Google Scholar]
- Alim MA, Ma Q-L, Takeda K, Aizawa T, Matsubara M, Nakamura M, Asada A, Saito T, Kaji H, Yoshii M, et al. (2004). Demonstration of a role for alpha-synuclein as a functional microtubule-associated protein. J Alzheimers Dis , 435–442; discussion 443–449. [DOI] [PubMed] [Google Scholar]
- Baird NA, Douglas PM, Simic MS, Grant AR, Moresco JJ, Wolff SC, Yates JR, Manning G, Dillin A. (2014). HSF-1-mediated cytoskeletal integrity determines thermotolerance and life span. Science , 360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AJ, Rinaldi MG, Graybill JR. (1992). Use of high-dose fluconazole as salvage therapy for cryptococcal meningitis in patients with AIDS. Antimicrob Agents Chemother , 690–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviston JP, Holzbaur ELF. (2006). Microtubule motors at the intersection of trafficking and transport. Trends Cell Biol , 530–537. [DOI] [PubMed] [Google Scholar]
- Daniele JR, Esping DJ, Garcia G, Parsons LS, Arriaga EA, Dillin A. (2017). High-throughput characterization of region-specific mitochondrial function and morphology. Sci Rep , 6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas PM, Baird NA, Simic MS, Uhlein S, McCormick MA, Wolff SC, Kennedy BK, Dillin A. (2015). Heterotypic signals from neural HSF-1 separate thermotolerance from longevity. Cell Rep , 1196–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle T, Botstein D. (1996). Movement of yeast cortical actin cytoskeleton visualized in vivo. Proc Natl Acad Sci USA , 3886–3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frøkjaer-Jensen C, Davis MW, Hopkins CE, Newman BJ, Thummel JM, Olesen S-P, Grunnet M, Jorgensen EM. (2008). Single-copy insertion of transgenes in Caenorhabditis elegans. Nat Genet , 1375–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garigan D, Hsu A-L, Fraser AG, Kamath RS, Ahringer J, Kenyon C. (2002). Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics , 1101–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R, Vevea JD, Swayne TC, Chojnowski R, Hill V, Boldogh IR, Pon LA. (2013). Actin dynamics affect mitochondrial quality control and aging in budding yeast. Curr Biol , 2417–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi-Sanabria R, Frankino PA, Paul JW, Tronnes SU, Dillin A. (2018). A futile battle? protein quality control and the stress of aging. Dev Cell , 139–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu A-L, Murphy CT, Kenyon C. (2003). Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science , 1142–1145. [DOI] [PubMed] [Google Scholar]
- Kumsta C, Chang JT, Schmalz J, Hansen M. (2017). Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat Commun , 14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbadia J, Morimoto RI. (2015). The biology of proteostasis in aging and disease. Annu Rev Biochem , 435–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooren OL, Galletta BJ, Cooper JA. (2012). Roles for actin assembly in endocytosis. Annu Rev Biochem , 661–686. [DOI] [PubMed] [Google Scholar]
- Morley JF, Morimoto RI. (2004). Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell , 657–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne DW, Schott DH, Bretscher A. (1998). Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J Cell Biol , 1931–1945. [DOI] [PubMed] [Google Scholar]
- Riedl J, Crevenna AH, Kessenbrock K, Yu JH, Neukirchen D, Bista M, Bradke F, Jenne D, Holak TA, Werb Z, et al. (2008). Lifeact: a versatile marker to visualize F-actin. Nat Methods , 605–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Aballay A. (2006). Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proc Natl Acad Sci USA , 13092–13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinkraus KA, Smith ED, Davis C, Carr D, Pendergrass WR, Sutphin GL, Kennedy BK, Kaeberlein M. (2008). Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell , 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatum MC, Ooi FK, Chikka MR, Chauve L, Martinez-Velazquez LA, Steinbusch HWM, Morimoto RI, Prahlad V. (2015). Neuronal serotonin release triggers the heat shock response in C. elegans in the absence of temperature increase. Curr Biol , 163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. (2005). Deconstructing the cadherin-catenin-actin complex. Cell , 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Barral JM, Ulrich Hartl F. (2003). More than folding: localized functions of cytosolic chaperones. Trends Biochem Sci , 541–547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.