Abstract
Background/Aims
Gastric varices (GVs) are a major cause of upper gastrointestinal bleeding in patients with liver cirrhosis. The current treatments of choice are balloon-occluded retrograde transvenous obliteration (BRTO) and the placement of a transjugular intrahepatic portosystemic shunt (TIPS). We aimed to compare the efficacy and outcomes of these two methods for the management of GV bleeding.
Methods
This retrospective study included consecutive patients who received BRTO (n=157) or TIPS (n=19) to control GV bleeding from January 2005 to December 2014 at a single tertiary hospital in Korea. The overall survival (OS), immediate bleeding control rate, rebleeding rate and complication rate were compared between patients in the BRTO and TIPS groups.
Results
Patients in the BRTO group showed higher immediate bleeding control rates (p=0.059, odds ratio [OR]=4.72) and lower cumulative rebleeding rates (log-rank p=0.060) than those in the TIPS group, although the difference failed to reach statistical significance. There were no significant differences in the rates of complications, including pleural effusion, aggravation of esophageal varices, portal hypertensive gastropathy, and portosystemic encephalopathy, although the rate of the progression of ascites was significantly higher in the BRTO group (p=0.02, OR=7.93). After adjusting for several confounding factors using a multivariate Cox analysis, the BRTO group had a significantly longer OS (adjusted hazard ratio [aHR]=0.44, p=0.01) and a longer rebleeding-free survival (aHR=0.34, p=0.001) than the TIPS group.
Conclusions
BRTO provides better bleeding control, rebleeding-free survival, and OS than TIPS for patients with GV bleeding.
Keywords: Balloon-occluded retrograde transvenous obliteration, Portasystemic shunt, transjugular intrahepatic, Variceal bleeding
INTRODUCTION
Gastric varices (GV) are one of the most common complications of liver cirrhosis, occurring in approximately 20% of the patients.1,2 GV bleeding is more severe (as reflected in transfusion requirement) than esophageal varices (EV) bleeding with higher mortality rates of up to 45% and 35% to 90% of patients were reported to rebleed after spontaneous hemostasis.3–5
Traditionally, it has been reported that endoscopic therapy such as endoscopic variceal ligation (EVL) or endoscopic variceal obturation (EVO) have important roles in treating GV bleeding.6 Those endoscopic approaches, however, is quite limited to apply for GV bleeding. Basically, EVL should be performed on small GV, because it is difficult to suction both mucosal and contralateral wall of the vessel into ligator due to its large diameter and thick vessel wall.7 In addition, EVL could be used to control GV bleeding only from GOV1, EV extending below gastric cardia along lesser curvature. EV extending into fundus along greater curvature (GOV2) and isolated GV located in fundus (IGV1), which commonly referred to as cardio-fundal varices, cannot be effectively treated by band ligation technically. Although GOV1 is most common type of GV (75% of GV), the risk of bleeding is relatively lower than other types of GV: IGV1 (78%)>GOV2 (55%)>GOV1 (10%).2,8 Those cardio-fundal varices which have higher risk of bleeding should be controlled by EVO instead. EVO has been proved fair safety and efficacy,9,10 however, the major adverse effect of EVO is systemic embolization including pulmonary embolism, stroke, and multi-organ infarction which may even cause death.11–13 Also premature extrusion of glue cast after EVO can lead to catastrophic re-bleeding. Meanwhile, lower rebleeding rates have been reported with transjugular intrahepatic portosystemic shunt (TIPS) and balloon-occluded retrograde transvenous obliteration (BRTO) compared with endoscopic therapy.14 Therefore, TIPS and BRTO are currently considered the treatments of choice for GV bleeding, especially for patients with massive, refractory, or recurrent bleeding.14–16 In clinics in the Western countries, TIPS is the first-line therapy for GV bleeding to decompress portal hypertension, whereas in Asia, especially South Korea and Japan, BRTO is the mainstay of hemostasis to treat GV.17,18
Intrahepatic shunts redirect portal blood flow to systemic circulation, thereby promptly relieving portal hypertension. For this reason, TIPS can minimize the associated complications, such as intractable ascites, portal hypertensive gastropathy (PHG), enteropathy, and various types of varices. However, TIPS may exacerbate portosystemic encephalopathy (PSE) as the portal venous blood bypasses the liver and does not undergo detoxification. Although this is avoided with BRTO,19 blockade of the gastrorenal shunt during this procedure increases the inflow of portal venous blood to the liver, which may aggravate portal hypertension.17 Thus, we conducted a study to compare these opposing approaches to GV bleeding management and determine their efficacy and associated complication rates.
MATERIALS AND METHODS
1. Study population
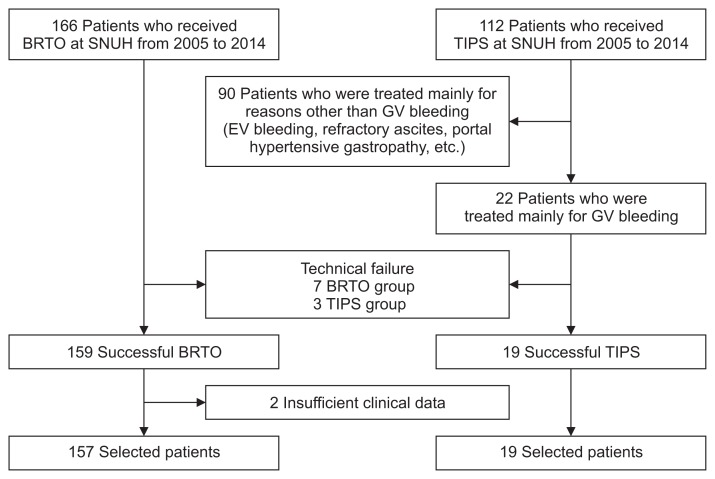
We retrospectively studied 278 consecutive patients who received either BRTO or TIPS for GV bleeding from January 2005 to December 2014 at a single tertiary hospital in Korea (Seoul National University Hospital). The GV bleeding are defined as the intersection of a symptom related to bleeding such as hematemesis, melena, or hematochezia (in case of massive bleeding), and identified stigma of recent bleeding in esophagogastroduodenoscopy following: (1) active spurting or oozing bleeding; (2) adherent clot, whit nipple sign or red nipple sign on GV; (3) no any other bleeding focus except large GV. Patients who were treated mainly for reasons other than GV bleeding, such as EV bleeding, refractory ascites, PHG, and so forth, were excluded. Further exclusion criteria were technical failure, insufficient clinical data, and death right after the procedure. Ultimately, 102 patients were excluded, leaving 176 patients for statistical analysis (Fig. 1).
Fig. 1.
Flowchart of patient population selection: 278 patients who received balloon-occluded retrograde transvenous obliteration (BRTO) or transjugular intrahepatic portosystemic shunt (TIPS) at Seoul National University Hospital (SNUH) from January 2005 to December 2014 were recruited. After excluding 102 patients based on the criteria above, 176 patients remained for statistical analysis.
EV, esophageal varices; GV, gastric varices.
2. BRTO procedure
The operator punctured right femoral vein under the ultrasonography and inserted sheath and Davis catheter. After selecting the left renal vein and inserting occlusion balloon catheter (Boston Scientific, Cork, Ireland, or Clinical Supply, Gifu, Japan), the operator performed angiography to check feeding veins, draining veins, and small variceal collaterals. These small vessels were embolized by microcoils or Gelform sponge particles to prevent leakage of the sclerosing agent. Then 5% of ethanolamine oleate (n=54; used until August 2009; Keuk Dong, Incheon, Korea) or 3% of sodium tetradecyl sulfate (n=103; used after September 2009; Tromboject, Omega Laboratories, Montreal, Canada) mixed with water-soluble contrast media (Pamiray; Dongkook, Jincheon, Korea) was injected to GV until it fully filled the gastric varix. The inflated balloon was maintained during overnight. In the early morning of the next day, the operator evaluated the presence of regurgitated fresh blood from the balloon catheter. If complete thrombosis was achieved without regurgitated blood, the balloon catheter was removed immediately in the angiography suite. If fresh blood was regurged from the balloon catheter, additional sclerosing agent was injected, and the balloon catheter was maintained for approximately 6 to 8 hours.
3. TIPS procedure
The operator punctured right internal jugular vein under the guidance of ultrasonography and inserted TIPS sheath and Davis catheter. After selecting right or middle hepatic vein, the operator checked right portal vein using CO2 portogram and then punctured right portal vein using the Colapinto needle (Cook Medical, Inc., Bloomington, IN, USA). Then preprocedural pressure gradient (mean±standard deviation [SD], 21.7±5.7) between inferior vena cava and portal vein was measured. Eight millimeter or 10 mm balloon delivered to a tract between RHV and RPV was used for dilation. The 10 mm covered (n=13; Taewoong, Seoul, Korea) or bare (n=6; Taewoong) Niti-S stent was placed in the dilated tract, followed by stent dilation if necessary. The postprocedural pressure gradient (mean±SD, 11.1±3.6) and the decrement in the pressure gradient (mean±SD, 10.6±3.7) was measured and the flow of blood in the shunt was checked using angiography.
4. Ethical consideration
The study was reviewed and approved by the Seoul National University Institutional Review Board (IRB number: H-1603-054-748). The study was conducted in accordance with the most recent ethical guidelines of the Declaration of Helsinki, modified during the 64th World Medical Association meeting in Brazil in 2013. The need for written informed consent was waived due to the retrospective nature of the study and because there were no study-specific interventions beyond routine clinical care. Patient records were anonymized and de-identified prior to analysis.
5. Study endpoints
The primary endpoint was the overall survival (OS) of the patients. The secondary endpoints were immediate bleeding control rate (bleeding controlled within one day after the procedure), cumulative rebleeding rate, and GV rebleeding-free survival. We also compared the rates of complications, including the aggravation of EV, PHG, pleural effusion, ascites, PSE, and the occurrence of spontaneous bacterial peritonitis (SBP) and hepatorenal syndrome (HRS). In addition, changes in liver function before and after the interventions were compared based on levels of albumin, total bilirubin, creatinine, prothrombin time/international normalized ratio, Child-Pugh scores and Model for End-Stage Liver Disease (MELD) scores.
Changes in GV after the procedure were evaluated by esophagogastroduodenoscopy at two points: before and within 3 months after the procedure. EV and PHG were evaluated by esophagogastroduodenoscopy after the intervention, and pleural effusion was evaluated by chest X-ray. Ascites was evaluated mainly by computed tomography at two points: before and within 1 month after the procedure, and PSE was evaluated from descriptive medical records. SBP and HRS were identified by laboratory findings.
6. Statistical analysis
The baseline characteristics and complication rates of the two groups were compared using two-sample t or Wilcoxon rank sum tests for continuous variables and chi-square or Fisher exact tests for categorical variables. Changes in liver function were assessed by paired t-tests. The OS and GV bleeding-free survival were estimated by the Kaplan-Meier method, and the cumulative probabilities of events were compared using a log-rank test. A Cox proportional hazards model was used to identify and adjust for independent risk factors for survival. Inverse probability weighting (IPW) based on propensity scores was conducted to balance the baseline characteristics of the two groups and Kaplan-Meier plots for survival analysis were fitted thereafter. Variables with a p-value of <0.05 in the univariate Cox regression analysis were used in the multivariate analysis. Differences with a p-value of <0.05 were considered statistically significant. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Baseline characteristics
Data from 176 patients (157 BRTO group and 19 TIPS group) who successfully underwent procedures were included in the study. The baseline characteristics of this population are shown in Table 1. Overall, there were no significant differences between the BRTO and TIPS groups except for patient age, the rate of concurrent hepatocellular carcinoma (HCC), presence of active bleeding and initial hemoglobin (Hb) level. Patients in the BRTO group were significantly older and had a significantly higher rate of concurrent HCC than those in the TIPS group. In addition, BRTO group showed lower rate of active bleeding and lower initial Hb level. The most common etiology of liver cirrhosis was hepatitis B virus-related cirrhosis followed by alcoholic cirrhosis. Baseline liver functions did not differ significantly between the two groups.
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | BRTO group (n=157) | TIPS group (n=19) | p-value |
|---|---|---|---|
| Age, yr | 59.4±10.2 | 54.4±7.4 | 0.039 |
| Sex | 0.234 | ||
| Male | 122 (77.7) | 17 (89.5) | |
| Female | 35 (22.3) | 2 (10.5) | |
| Etiology of liver cirrhosis | 0.525 | ||
| HBV | 75 (49.3) | 12 (63.1) | |
| Alcoholic | 38 (25.0) | 4 (21.1) | |
| HCV | 24 (15.8) | 2 (10.5) | |
| Autoimmune | 12 (7.9) | 0 | |
| HBV and alcoholic | 2 (1.3) | 1 (5.3) | |
| HBV and HCV | 1 (0.7) | 0 | |
| Concurrent HCC | 0.014 | ||
| No | 77 (49.0) | 15 (78.9) | |
| Yes | 80 (51.0) | 4 (21.1) | |
| Liver transplantation | 0.964 | ||
| No | 141 (89.8) | 17 (89.5) | |
| Yes | 16 (10.2) | 2 (10.5) | |
| GV type | 0.884 | ||
| GOV 1 | 17 (10.8) | 1 (5.3) | |
| GOV 2 | 85 (54.1) | 10 (52.6) | |
| GOV 3 | 44 (28.0) | 5 (26.3) | |
| GOV 4 | 2 (1.3) | 0 | |
| Presence of active bleeding | 15 (9.6) | 8 (42.1) | 0.001 |
| Initial mean blood pressure, mm Hg | 83.2±15.9 | 79.8±17.1 | 0.385 |
| Initial hemoglobin, g/dL | 9.1±2.2 | 10.4±2.3 | 0.015 |
| Time to intervention, hr | 55.2±59.0 | 47.0±40.4 | 0.556 |
| Albumin, g/dL | 2.8±0.6 | 2.9±0.8 | 0.457 |
| Creatinine, mg/dL | 1.2±1.3 | 1.2±0.6 | 0.947 |
| Total bilirubin, mg/dL | 3.5±6.2 | 2.0±1.1 | 0.293 |
| Prothrombin time, INR | 1.5±0.5 | 1.6±0.6 | 0.391 |
| Ascites | 0.260 | ||
| None | 116 (73.9) | 17 (89.5) | |
| Mild to moderate | 27 (17.2) | 2 (10.5) | |
| Severe | 14 (8.9) | 0 | |
| Portosystemic encephalopathy | 0.236 | ||
| None | 136 (86.6) | 19 (100) | |
| Mild to moderate | 13 (8.3) | 0 | |
| Severe | 8 (5.1) | 0 | |
| Child-Pugh score | 8.0±2.0 | 7.3±2.0 | 0.160 |
| MELD score | 13.6±7.7 | 14.3±7.1 | 0.705 |
Data are presented as the mean±SD or number (%).
BRTO, balloon-occluded retrograde transvenous obliteration; TIPS, transjugular intrahepatic portosystemic shunt; HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; GV, gastric varices; INR, international normalized ratio; MELD, Model for End-Stage Liver Disease.
2. Overall survival and prognostic factors
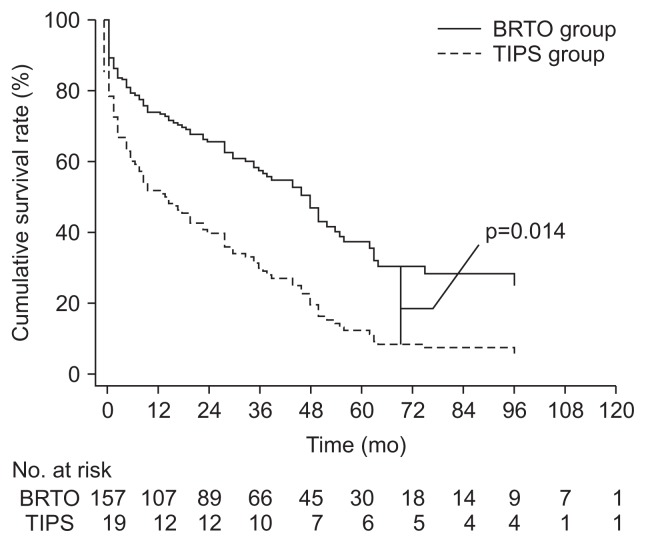
The 5-year survival rate was 38.5% in the BRTO group and 34.4% in the TIPS group. In the univariate analysis, there was no significant difference in OS between the two groups (hazard ratio [HR], 0.83; p=0.51). Some variables, such as sex (HR, 0.57; p=0.035), concurrent HCC (HR, 2.22; p<0.001), liver transplantation (HR, 0.25; p=0.006), albumin levels (HR, 0.50; p<0.001), total bilirubin levels (HR, 1.05; p<0.001), prothrombin time (HR, 1.92, p<0.001), ascites (HR, 1.72; p=0.030 for mild to moderate; HR, 2.03; p=0.038 for severe), presence of active bleeding, (HR, 1.80; p=0.02), and initial Hb (HR, 0.89; p=0.006) were associated with OS in the univariate analysis. In the multivariate analysis using the associated factors mentioned above, age and treatment method, the patients in the BRTO group had a significantly longer OS than those in the TIPS group (adjusted HR, 0.44; 95% confidence interval [CI], 0.23 to 0.84; p=0.01) (Table 2, Fig. 2). Young age, female sex, absence of concurrent HCC, post-liver transplantation status, high albumin levels, low bilirubin levels, and low prothrombin time were good prognostic factors for OS in GV bleeding patients in the multivariate analysis. After balancing age, gender, and baseline liver function by using IPW, patients in the BRTO group showed significantly longer OS than the TIPS group (HR, 1.80; 95% CI, 1.40 to 2.31; p<0.001).
Table 2.
The Results of a Cox Proportional Hazards Models for the Overall Survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age, yr | 1.018 (0.999–1.038) | 0.069 | 1.027 (1.003–1.050) | 0.024 |
| Female sex | 0.571 (0.339–0.963) | 0.035 | 0.523 (0.298–0.918) | 0.024 |
| Etiology of liver cirrhosis | - | 0.085 | - | - |
| Concurrent HCC | 2.217 (1.488–3.301) | <0.001 | 2.053 (1.290–3.267) | 0.002 |
| Liver transplantation | 0.246 (0.090–0.670) | 0.006 | 0.249 (0.085–0.733) | 0.012 |
| GV type | 0.856 | - | - | |
| GOV 2 | 0.891 (0.478–1.663) | 0.718 | ||
| IGV 1 | 0.771 (0.392–1.518) | 0.453 | ||
| IGV 2 | 1.200 (0.156–9.255) | 0.861 | ||
| Presence of active bleeding | 1.799 (1.079–2.998) | 0.024 | 1.524 (0.858–2.705) | 0.150 |
| Initial mean blood pressure | 1.002 (0.989–1.014) | 0.811 | - | - |
| Initial hemoglobin | 0.885 (0.811–0.965) | 0.006 | 0.948 (0.855–1.051) | 0.313 |
| Time to intervention | 0.999 (0.995–1.003) | 0.674 | - | - |
| Albumin, g/dL | 0.502 (0.363–0.694) | <0.001 | 0.463 (0.304–0.707) | <0.001 |
| Creatinine, mg/dL | 1.063 (0.952–1.187) | 0.277 | - | - |
| Total bilirubin, mg/dL | 1.053 (1.026–1.082) | <0.001 | 1.056 (1.065–1.026) | 0.001 |
| Prothrombin time, INR | 1.923 (1.401–2.641) | <0.001 | 1.410 (0.862–2.306) | 0.171 |
| Ascites | 0.021 | 0.141 | ||
| Mild to moderate | 1.718 (1.055–2.798) | 0.030 | 1.470 (0.870–2.482) | 0.150 |
| Severe | 2.025 (1.038–3.951) | 0.038 | 1.782 (0.862–3.685) | 0.119 |
| Portosystemic encephalopathy | 0.815 | - | - | |
| Mild to moderate | 1.257 (0.609–2.594) | 0.536 | ||
| Severe | 1.097 (0.444–2.712) | 0.841 | ||
| BRTO group | 0.825 (0.468–1.454) | 0.505 | 0.435 (0.225–0.844) | 0.014 |
HR, hazard ratio; CI, confidential interval; HCC, hepatocellular carcinoma; GV, gastric varices; INR, international normalized ratio; BRTO, balloon-occluded retrograde transvenous obliteration.
Fig. 2.
Plots of the cumulative overall survival in the balloon-occluded retrograde transvenous obliteration (BRTO) group and transjugular intrahepatic portosystemic shunt (TIPS) group from the Cox regression model. Note a significantly higher rate after BRTO compared with TIPS (p=0.014).
3. Immediate bleeding control rate, rebleeding rate, and GV rebleeding-free survival
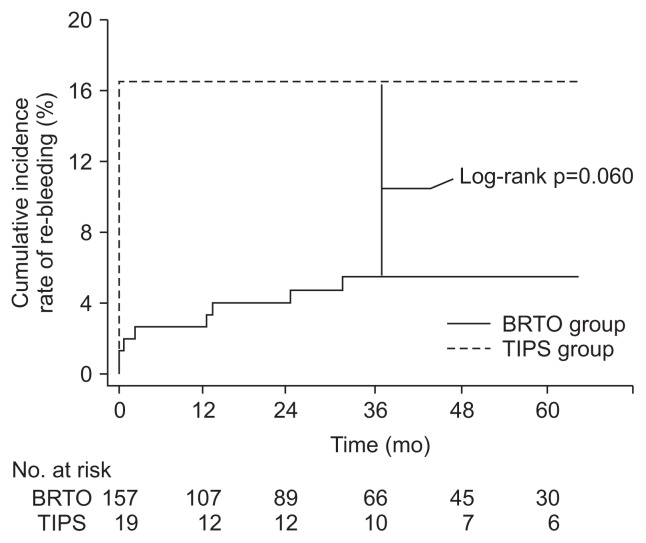
Patients in the BRTO group had somewhat higher immediate bleeding control rates (96.2% vs 84.2%; odds ratio [OR], 4.72; 95% CI, 1.08 to 20.70; p=0.06) and lower trend of rebleeding rates (HR, 0.60; log-rank p=0.06) (Fig. 3) than those in the TIPS group without statistical significance.
Fig. 3.
Kaplan-Meier plots for the cumulative incidence rate of re-bleeding in the balloon-occluded retrograde transvenous obliteration (BRTO) group and transjugular intrahepatic portosystemic shunt (TIPS) group. Note the higher rate after TIPS compared with BRTO with marginal significance (p=0.060 by log-rank test).
The 5-year GV rebleeding-free survival rate was 38.6% in the BRTO group and 23.4% in the TIPS group. In the univariate analysis, there was no significant difference in GV rebleeding-free survival (HR, 0.60; 95% CI, 0.35 to 1.03; p=0.061) (Table 3) between the BRTO group and the TIPS group. In the multivariate analysis, however, GV rebleeding-free survival was significantly longer in the BRTO group (adjusted HR, 0.34; 95% CI, 0.18 to 0.63; p=0.001) (Table 3).
Table 3.
The Results of a Cox Proportional Hazards Models for Gastric Variceal Rebleeding-Free Survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age, yr | 1.015 (0.996–1.034) | 0.126 | 1.022 (0.999–1.045) | 0.061 |
| Female sex | 0.535 (0.318–0.901) | 0.019 | 0.537 (0.308–0.936) | 0.028 |
| Etiology of liver cirrhosis | 0.092 | - | - | |
| Concurrent HCC | 2.252 (1.519–3.338) | <0.001 | 2.102 (1.331–3.318) | 0.001 |
| Liver transplantation | 0.234 (0.086–0.636) | 0.004 | 0.234 (0.080–0.684) | 0.008 |
| GV type | 0.933 | - | - | |
| GOV 2 | 0.953 (0.512–1.775) | 0.880 | ||
| IGV 1 | 0.843 (0.430–1.652) | 0.618 | ||
| IGV 2 | 1.117 (0.153–9.077) | 0.876 | ||
| Presence of active bleeding | 1.669 (1.003–2.777) | 0.049 | 1.383 (0.781–2.446) | 0.266 |
| Initial mean blood pressure | 0.999 (0.986–1.011) | 0.829 | ||
| Initial hemoglobin | 0.895 (0.822–0.974) | 0.010 | ||
| Time to intervention | 1.000 (0.996–1.003) | 0.790 | ||
| Albumin, g/dL | 0.567 (0.410–0.783) | 0.001 | 0.570 (0.375–0.866) | 0.009 |
| Creatinine, mg/dL | 1.058 (0.947–1.182) | 0.319 | - | - |
| Total bilirubin, mg/dL | 1.049 (1.022–1.078) | <0.001 | 1.059 (1.021–1.099) | 0.002 |
| Prothrombin time, INR | 1.812 (1.320–2.488) | <0.001 | 1.440 (0.893–2.322) | 0.135 |
| Ascites | 0.042 | 0.203 | ||
| Mild to moderate | 1.651 (1.016–2.683) | 0.043 | 1.408 (0.835–2.375) | 0.199 |
| Severe | 1.850 (0.951–3.601) | 0.070 | 1.694 (0.817–3.510) | 0.156 |
| Portosystemic encephalopathy | 0.933 | - | - | |
| Mild to moderate | 1.147 (0.557–2.364) | 0.710 | ||
| Severe | 1.021 (0.413–2.523) | 0.963 | ||
| BRTO group | 0.601 (0.352–1.025) | 0.061 | 0.336 (0.178–0.633) | 0.001 |
HR, hazard ratio; CI, confidential interval; HCC, hepatocellular carcinoma; GV, gastric varices; INR, international normalized ratio; BRTO, balloon-occluded retrograde transvenous obliteration.
4. Reduction in the GV after the procedure
After the procedure, GV was reduced in the BRTO group with proportion of 24.8% and in the TIPS group with proportion of 9.1%. However, there was no significant difference between the two groups (p=0.24).
5. Complications after procedures
There were no significant differences between the two groups in terms of aggravation of EV, PHG, and pleural effusion after treatment (p=0.48, p=0.61, and p=0.07, respectively) (Table 4). The two groups showed similar rates for the post-procedural development of SBP and HRS (log-rank p=0.43 and p=0.21, respectively). The grade of ascites was significantly aggravated one month after BRTO (p<0.001) with proportion of 30.6% but not after TIPS (p=0.32) with proportion of 5.3%. With regard to PSE, the most concerning complication from TIPS, there was no significant change after the procedure in the BRTO group (p=0.10) and the TIPS group (p=1.00); 2.5% of the BRTO group patients and none of the TIPS group patients developed or got aggravated PSE.
Table 4.
Complications after Procedure, Compared by Chi-Square or Fisher Exact Test
| BRTO group (n=157) | TIPS group (n=19) | p-value | |
|---|---|---|---|
| Aggravation of EV | 14.0 | 5.3 | 0.48 |
| Aggravation of PHG | 7.0 | 0 | 0.61 |
| Aggravation of pleural effusion | 31.2 | 10.5 | 0.07 |
| Development of SBP | 3.2 | 0 | 0.43 |
| Development of HRS | 7.6 | 15.8 | 0.21 |
Data are presented as percentage.
BRTO, balloon-occluded retrograde transvenous obliteration; TIPS, transjugular intrahepatic portosystemic shunt; EV, esophageal varices; PHG, portal hypertensive gastropathy; SBP, spontaneous bacterial peritonitis; HRS, hepatorenal syndrome.
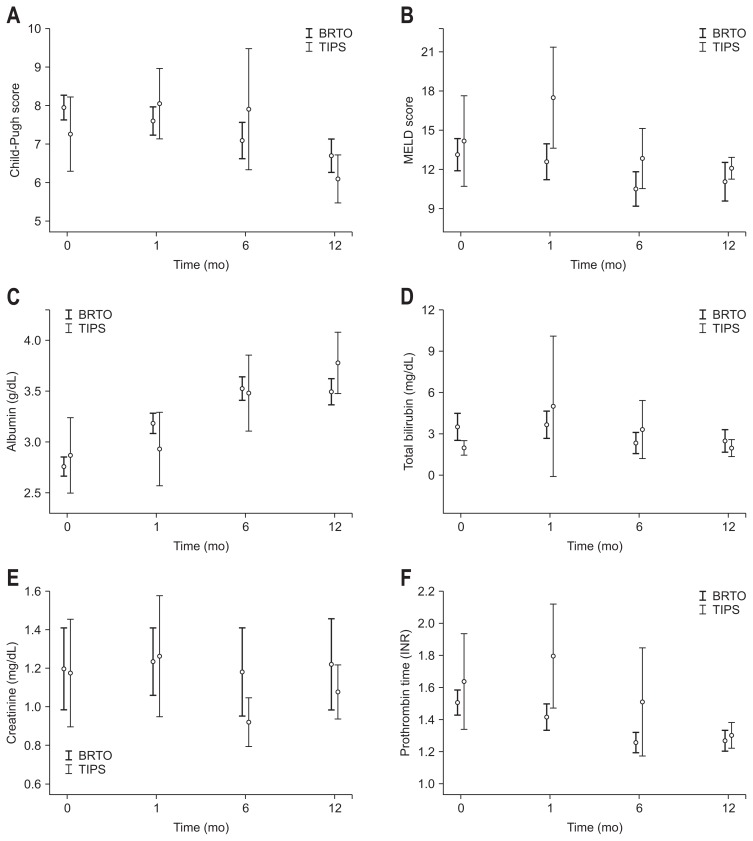
6. Changes in liver function
Changes in liver function after each procedure are shown in Table 5 and Fig. 4. As shown in Fig. 4, Child-Pugh and MELD scores gradually decreased in patients who received BRTO, indicating an improvement in liver function. In contrast, these scores increased in patients who received TIPS for one month immediately after the procedure, and then decreased thereafter. The results of the paired t-tests comparing variables measured before and after each procedure are shown in Table 5. In the BRTO group, the levels of albumin (p<0.001 at 1 month, 6 months, and 12 months) and prothrombin times (p=0.024 at 1 month and p<0.001 at 6 months and 12 months) improved significantly after the procedure, which made Child-Pugh and MELD scores better. In the TIPS group, only the albumin levels at 12 months improved significantly (p=0.009).
Table 5.
Change in Liver Function (Paired t-Test)
| Pair | Time, mo | BRTO | TIPS | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Mean±SD | p-value | Mean±SD | p-value | |||
| Child-Pugh score | 1 | 0 | 7.9±2.0 | 0.004 | 7.3±2.0 | 0.024 |
| 1 | 7.6±2.3 | (n=156) | 8.1±1.9 | (n=19) | ||
| 2 | 0 | 7.4±1.8 | 0.132 | 6.1±1.1 | 0.064 | |
| 6 | 7.1±2.3 | (n=97) | 7.9±2.3 | (n=11) | ||
| 3 | 0 | 7.4±1.8 | 0.001 | 6.2±1.1 | 0.847 | |
| 12 | 6.7±2.1 | (n=91) | 6.1±0.9 | (n=10) | ||
| MELD score | 1 | 0 | 13.1±7.8 | 0.273 | 14.2±7.1 | 0.038 |
| 1 | 12.6±8.8 | (n=156) | 17.5±8.1 | (n=19) | ||
| 2 | 0 | 11.3±5.5 | 0.169 | 10.7±3.2 | 0.209 | |
| 6 | 10.5±6.6 | (n=100) | 12.8±3.6 | (n=12) | ||
| 3 | 0 | 11.1±5.5 | 0.998 | 11.4±2.3 | 0.351 | |
| 12 | 11.1±7.1 | (n=91) | 12.1±1.2 | (n=10) | ||
| Albumin, g/dL | 1 | 0 | 2.8±0.6 | <0.001 | 2.9±0.8 | 0.530 |
| 1 | 3.1±0.6 | (n=156) | 2.9±0.8 | (n=19) | ||
| 2 | 0 | 2.9±0.6 | <0.001 | 3.3±0.5 | 0.220 | |
| 6 | 3.5±0.6 | (n=105) | 3.5±0.6 | (n=12) | ||
| 3 | 0 | 2.9±0.6 | <0.001 | 3.3±0.5 | 0.009 | |
| 12 | 3.5±0.6 | (n=97) | 3.8±0.4 | (n=10) | ||
| Total bilirubin, mg/dL | 1 | 0 | 3.5±6.2 | 0.672 | 2.0±1.1 | 0.245 |
| 1 | 3.7±6.2 | (n=156) | 5.0±10.6 | (n=19) | ||
| 2 | 0 | 2.1±3.4 | 0.636 | 1.6±0.7 | 0.140 | |
| 6 | 2.3±3.9 | (n=105) | 3.3±3.3 | (n=12) | ||
| 3 | 0 | 2.1±3.5 | 0.460 | 1.7±0.8 | 0.523 | |
| 12 | 2.5±4.0 | (n=97) | 2.0±0.9 | (n=10) | ||
| Creatinine, mg/dL | 1 | 0 | 1.2±1.3 | 0.464 | 1.2±0.6 | 0.512 |
| 1 | 1.2±1.1 | (n=156) | 1.3±0.7 | (n=19) | ||
| 2 | 0 | 1.2±1.4 | 0.813 | 1.0±0.2 | 0.353 | |
| 6 | 1.2±1.2 | (n=105) | 1.0±0.2 | (n=12) | ||
| 3 | 0 | 1.1±1.3 | 0.080 | 1.0±0.2 | 0.311 | |
| 12 | 1.2±1.2 | (n=97) | 1.1±0.2 | (n=10) | ||
| Prothrombin time, INR | 1 | 0 | 1.5±0.5 | 0.024 | 1.6±0.6 | 0.067 |
| 1 | 1.4±0.5 | (n=156) | 1.8±0.7 | (n=19) | ||
| 2 | 0 | 1.4±0.3 | <0.001 | 1.4±0.1 | 0.374 | |
| 6 | 1.3±0.3 | (n=100) | 1.5±0.5 | (n=12) | ||
| 3 | 0 | 1.4±0.3 | <0.001 | 1.3±0.2 | 0.196 | |
| 12 | 1.3±0.3 | (n=91) | 1.3±0.1 | (n=10) | ||
BRTO, balloon-occluded retrograde transvenous obliteration; TIPS, transjugular intrahepatic portosystemic shunt; MELD, Model for End-Stage Liver Disease; INR, international normalized ratio.
Fig. 4.
Dynamics of liver function after procedures: changes in (A) Child-Pugh score, (B) MELD score, (C) albumin, (D) total bilirubin, (E) creatinine and (F) prothrombin time differed by procedure over time. Variables expressed as the mean±95% confidence interval. BRTO, balloon-occluded retrograde transvenous obliteration; TIPS, transjugular intrahepatic portosystemic shunt; MELD, Model for End-Stage Liver Disease; INR, international normalized ratio.
DISCUSSION
In this study, we found that BRTO for GV bleeding significantly prolonged overall and GV rebleeding-free survival compared with TIPS. In addition, BRTO showed considerable trend of better immediate bleeding control and a lower chance of rebleeding although it failed to reach the statistical significance. Complication rates were similar between both procedures, though BRTO tended to aggravate ascites more than TIPS. The liver function in patients who underwent BRTO gradually improved after the procedure.
Recently, several studies have suggested that BRTO is a better treatment option for patients with GV.19–21 Although Choi et al.22 also concluded that BRTO is a good alternative to the placement of TIPS due to immediate hemostasis, absence of rebleeding, and improved liver function, their sample sizes were too small to establish statistical significance. Ninoi et al.23 reported that patients who received BRTO had lower rebleeding rates and higher 5-year survival than those who received TIPS. However, the OS of patients with poor liver function (Child-Pugh class B or C) did not differ between the treatments. A meta-analysis by Wang et al.19 revealed that BRTO had more advantages than TIPS, including lower rates for rebleeding and PSE. Nevertheless, the authors recommended BRTO only as an alternative method rather than a treatment of choice for GV because of the quality of the original studies. Most recently, Lee et al.24 showed better OS in patients who underwent BRTO, but this did not remain significant in the multivariate analysis, as the baseline liver function was better in the BRTO group than in the TIPS group.
Our findings provide evidence worthy to be considered evidence that BRTO is indeed superior to TIPS placement for treating GV bleeding especially in patients with PSE or decreased liver function. Most importantly, our study included a larger population of patients than previous studies and compared groups with similar baseline liver functions. In addition, our data were obtained from a single center, and thus the quality control for the procedures was similar. Another advantage of our study is that we also assessed bleeding control rate and various complications. For example, we identified that BRTO and TIPS groups had similar risks for EV, PHG, and pleural effusion after each procedure. The increase in portal hypertension with BRTO does not contraindicate its use. However, BRTO did increase the development of ascites and should thus be applied carefully in patients who have refractory ascites. The blood flow to the portal vein increases after BRTO because the procedure obliterates the gastrorenal shunt which can be a sort of bypass route of the portal vein. Therefore, portal hypertension could be aggravated, resulting in development of PHG (occurs in 5% to 13%), ascites (0% to 44%), and hydrothorax/pleural effusion (0% to 8%).25 Nevertheless, hepatic function in patients who underwent BRTO gradually improved over 12 months. It is well known that patients with major shunts such as GV have decreased portal blood flow, resulting in gradually decreased hepatic functional reservoir. Conversely, by obliterating these shunts, the decreased portal blood flow can be recovered, which leads to liver function improvement.22,26,27 This fact was demonstrated in our study, and other studies have shown similar evidence for sustained improvement of hepatic function after BRTO.24,27
In summary, it is essential to individualize the treatment plans for patients with GV. BRTO would be a treatment of choice particularly for patients with hepatic encephalopathy or decreased liver function. On the other hand, TIPS would be better than BRTO for patients with refractory ascites or without gastrorenal shunts.
There are some limitations of our study that should be noted. First, this was a retrospective study of procedures performed in Korea, resulting in a larger number of patients who underwent BRTO and fewer who received TIPS. Since the treatment modality should be selected in each patient individually considering the particular medical condition of the patient, it is practically impossible to design a prospective study such as a randomized controlled study. Second, the development of PSE, a well-established major complication of TIPS, could not be definitively verified. This was due to the small sample size of the TIPS group as well as a selection bias resulting from the tendency of physicians to avoid placing TIPS in patients with a high risk of PSE. Third, there would be some problems due to the long study period. There had been some changes in materials of procedures during the study period. The obliterating material used in BRTO was changed from 5% of ethanolamine oleate to 3% of sodium tetradecyl sulfate from September 2009. The type of stent used in TIPS was also changed from the covered type to the bare type from May 2010. Besides, the number of patients of the BRTO group tended to increase as time goes on while that of the TIPS group decreased. Those changes during the study period inevitably could have affected the outcomes. As a result, a further well-designed prospective study is needed to confirm our findings.
In conclusion, we found that BRTO is superior to TIPS with regard to OS and liver function improvement in patients with GV bleeding. For this reason, BRTO can be considered preferentially for GV bleeding if feasible, especially in patients with hepatic encephalopathy or decreased liver function.
Footnotes
See editorial on page 611.
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Nakano R, Iwao T, Oho K, Toyonaga A, Tanikawa K. Splanchnic hemodynamic pattern and liver function in patients with cirrhosis and esophageal or gastric varices. Am J Gastroenterol. 1997;92:2085–2089. [PubMed] [Google Scholar]
- 2.Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343–1349. doi: 10.1002/hep.1840160607. [DOI] [PubMed] [Google Scholar]
- 3.de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. 2001;5:645–663. doi: 10.1016/S1089-3261(05)70186-0. [DOI] [PubMed] [Google Scholar]
- 4.Triantafyllou M, Stanley AJ. Update on gastric varices. World J Gastrointest Endosc. 2014;6:168–175. doi: 10.4253/wjge.v6.i5.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wani ZA, Bhat RA, Bhadoria AS, Maiwall R, Choudhury A. Gastric varices: classification, endoscopic and ultrasonographic management. J Res Med Sci. 2015;20:1200–1207. doi: 10.4103/1735-1995.172990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girotra M, Raghavapuram S, Abraham RR, Pahwa M, Pahwa AR, Rego RF. Management of gastric variceal bleeding: role of endoscopy and endoscopic ultrasound. World J Hepatol. 2014;6:130–136. doi: 10.4254/wjh.v6.i3.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 8.Kim T, Shijo H, Kokawa H, et al. Risk factors for hemorrhage from gastric fundal varices. Hepatology. 1997;25:307–312. doi: 10.1002/hep.510250209. [DOI] [PubMed] [Google Scholar]
- 9.Kahloon A, Chalasani N, DeWitt J, et al. Endoscopic therapy with 2-octyl-cyanoacrylate for the treatment of gastric varices. Dig Dis Sci. 2014;59:2178–2183. doi: 10.1007/s10620-014-3148-9. [DOI] [PubMed] [Google Scholar]
- 10.ASGE Technology Committee. Bhat YM, Banerjee S, et al. Tissue adhesives: cyanoacrylate glue and fibrin sealant. Gastrointest Endosc. 2013;78:209–215. doi: 10.1016/j.gie.2013.04.166. [DOI] [PubMed] [Google Scholar]
- 11.Berry PA, Cross TJ, Orr DW. Clinical challenges and images in GI: pulmonary embolization of histoacryl “glue” causing hypoxia and cardiovascular instability. Gastroenterology. 2007;133:1413–1748. doi: 10.1053/j.gastro.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 12.Saracco G, Giordanino C, Roberto N, et al. Fatal multiple systemic embolisms after injection of cyanoacrylate in bleeding gastric varices of a patient who was noncirrhotic but with idiopathic portal hypertension. Gastrointest Endosc. 2007;65:345–347. doi: 10.1016/j.gie.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 13.Türler A, Wolff M, Dorlars D, Hirner A. Embolic and septic complications after sclerotherapy of fundic varices with cyanoacrylate. Gastrointest Endosc. 2001;53:228–230. doi: 10.1067/mge.2001.111561. [DOI] [PubMed] [Google Scholar]
- 14.Chang CJ, Hou MC, Liao WC, et al. Management of acute gastric varices bleeding. J Chin Med Assoc. 2013;76:539–546. doi: 10.1016/j.jcma.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Olson JC, Saeian K. Gastrointestinal issues in liver disease. Crit Care Clin. 2016;32:371–384. doi: 10.1016/j.ccc.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Fukui H, Saito H, Ueno Y, et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J Gastroenterol. 2016;51:629–650. doi: 10.1007/s00535-016-1216-y. [DOI] [PubMed] [Google Scholar]
- 17.Saad WE, Darcy MD. Transjugular intrahepatic portosystemic shunt (TIPS) versus balloon-occluded retrograde transvenous obliteration (BRTO) for the management of gastric varices. Semin Intervent Radiol. 2011;28:339–349. doi: 10.1055/s-0031-1284461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim MY, Um SH, Baik SK, et al. Clinical features and outcomes of gastric variceal bleeding: retrospective Korean multicenter data. Clin Mol Hepatol. 2013;19:36–44. doi: 10.3350/cmh.2013.19.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YB, Zhang JY, Gong JP, Zhang F, Zhao Y. Balloon-occluded retrograde transvenous obliteration versus transjugular intra-hepatic portosystemic shunt for treatment of gastric varices due to portal hypertension: a meta-analysis. J Gastroenterol Hepatol. 2016;31:727–733. doi: 10.1111/jgh.13248. [DOI] [PubMed] [Google Scholar]
- 20.Park JK, Saab S, Kee ST, et al. Balloon-occluded retrograde transvenous obliteration (BRTO) for treatment of gastric varices: review and meta-analysis. Dig Dis Sci. 2015;60:1543–1553. doi: 10.1007/s10620-014-3485-8. [DOI] [PubMed] [Google Scholar]
- 21.Jang SY, Kim GH, Park SY, et al. Clinical outcomes of balloon-occluded retrograde transvenous obliteration for the treatment of gastric variceal hemorrhage in Korean patients with liver cirrhosis: a retrospective multicenter study. Clin Mol Hepatol. 2012;18:368–374. doi: 10.3350/cmh.2012.18.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi YH, Yoon CJ, Park JH, Chung JW, Kwon JW, Choi GM. Balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding: its feasibility compared with transjugular intra-hepatic portosystemic shunt. Korean J Radiol. 2003;4:109–116. doi: 10.3348/kjr.2003.4.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ninoi T, Nakamura K, Kaminou T, et al. TIPS versus transcatheter sclerotherapy for gastric varices. AJR Am J Roentgenol. 2004;183:369–376. doi: 10.2214/ajr.183.2.1830369. [DOI] [PubMed] [Google Scholar]
- 24.Lee SJ, Kim SU, Kim MD, et al. Comparison of treatment outcomes between balloon-occluded retrograde transvenous obliteration and transjugular intrahepatic portosystemic shunt for gastric variceal bleeding hemostasis. J Gastroenterol Hepatol. 2017;32:1487–1494. doi: 10.1111/jgh.13729. [DOI] [PubMed] [Google Scholar]
- 25.Saad WE. Balloon-occluded retrograde transvenous obliteration of gastric varices: concept, basic techniques, and outcomes. Semin Intervent Radiol. 2012;29:118–128. doi: 10.1055/s-0032-1312573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato T, Uematsu T, Nishigaki Y, Sugihara J, Tomita E, Moriwaki H. Therapeutic effect of balloon-occluded retrograde transvenous obliteration on portal-systemic encephalopathy in patients with liver cirrhosis. Intern Med. 2001;40:688–691. doi: 10.2169/internalmedicine.40.688. [DOI] [PubMed] [Google Scholar]
- 27.Kumamoto M, Toyonaga A, Inoue H, et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric fundal varices: hepatic deterioration links to portosystemic shunt syndrome. J Gastroenterol Hepatol. 2010;25:1129–1135. doi: 10.1111/j.1440-1746.2010.06262.x. [DOI] [PubMed] [Google Scholar]