Abstract
Dementia, characterized by a progressive cognitive decline and a cumulative inability to behave independently, is highly associated with other diseases. Various cardiovascular disorders, such as coronary artery disease and atrial fibrillation, are well-known risk factors for dementia. Currently, increasing evidence suggests that sex factors may play an important role in the pathogenesis of diseases, including cardiovascular disease and dementia. Recent studies show that nearly two-thirds of patients diagnosed with Alzheimer’s disease are women; however, the incidence difference between men and women remains vague. Therefore, studies are needed to investigate sex-specific differences, which can help understand the pathophysiology of dementia and identify potential therapeutic targets for both sexes. In the present review, we summarize sex differences in the prevalence and incidence of dementia by subtypes. This review also describes sex differences in the risk factors of dementia and examines the impact of risk factors on the incidence of dementia in both sexes.
Keywords: Sex difference, Dementia incidence, Dementia prevalence, Cardiovascular risk factors
INTRODUCTION
Dementia is a chronic, progressive, and multifactorial neurodegenerative disorder characterized by cognitive decline. It has been a major public health problem, with 36 million people worldwide estimated to have dementia, and the global prevalence of dementia is expected to increase to more than 80 million by 2040 (Ferri et al., 2005). In addition to the substantial burden on patients and their families, dementia also affects the healthcare system worldwide. As a result, the high demand for medical care and treatment need for cumulative cognitive decline will have significant socioeconomic impacts.
Dementia has been demonstrated to be highly related to various risk factors, including age and many diseases. For example, various cardiovascular disorders (CVD), such as atrial fibrillation (AF) (Ott et al., 1997), heart failure (HF) (Qiu et al., 2006), and hypertension (HTN) (Kivipelto et al., 2001), are well–known risk factors for dementia; therefore, prevention of these diseases can help reduce the burden of dementia on people and the healthcare system (Wu et al., 2016). In fact, several cardiovascular (CV) drugs have been reported to reduce the risk of dementia (Kim et al., 2016; Mangmool et al., 2017; Xiao et al., 2017).
Increasing evidence indicates that sex factors can play an important role in the pathogenesis of diseases, including CVD and dementia. According to recent reports, almost two-thirds of patients with Alzheimer’s disease (AD), the most common type of dementia, are women (Hebert et al., 2013). However, the incidence studies suggest that sex differences in AD are still controversial. The Cache County Study in the United States (US) indicates a greater incidence of AD in men than in women before age 78, after which men have a lower incidence than women (Lethbridge et al., 2013). Notably, sex differences in the incidence of AD are not observed in most studies conducted in the US (Kukull et al., 2002). Therefore, it is critical to understand sex-related differences in the incidence of dementia, and the results would help to delineate the pathophysiology of dementia and suggest potential therapeutic strategies for men and women.
In this review, we discuss sex differences in the prevalence and/or incidence of different subtypes of dementia. Sex differences in various CVDs, well-known risk factors for dementia, are also summarized. In addition, we review the impact of risk factors on the incidence of dementia in men and women.
DEMENTIA IN MEN AND WOMEN
Incidence and prevalence are common terms used to describe disease epidemiology. In medicine, the incidence is generally the case for newly identified diseases and the frequency of disease is the actual number of cases alive. Thus, to explain the etiology of disease, incidence is more usually used than prevalence. The prevalence of AD has been shown to be significantly higher in women than in men (Mielke et al., 2014). The Italian Cohort study on dementia indicates that women account for more than 70% in a total of 213 consecutive dementia patients (Musicco, 2009). However, this clinical observation cannot be simply interpreted as a high dementia risk in women because the prevalence is determined by both the incidence of disease and the post-onset survival duration (Hebert et al., 2003). Despite the well-known great prevalence of AD in women, epidemiologic studies examining the incidence of AD suggest a different result of sex differences. Sex differences in the incidence of dementia seem to be complicated because many diseases can cause dementia (Musicco, 2009). Thus, although some subtypes of dementia may occur more frequently in women, the sex differences may not always apply to AD. In addition, the prevalence can increase as a function of disease duration. Indeed, AD is a long-lasting disease, and many patients die from other causes during illness. Because the average life expectancy is longer in women than in men, more women, especially at the older ages, survive with AD. Therefore, sex-specific risks of dementia should be considered from the studies that focus on incidence but not on prevalence.
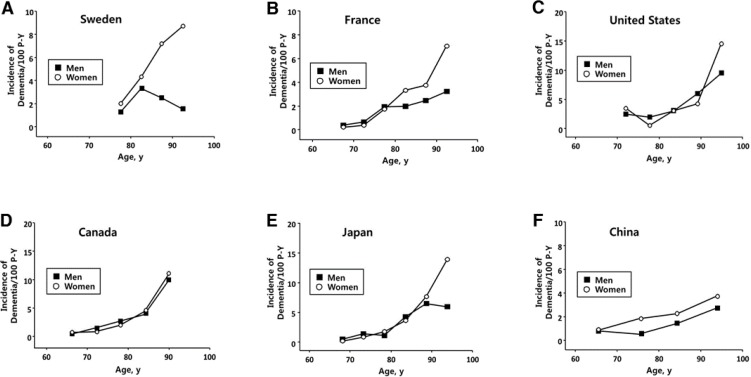
Many incidence studies carried out in Europe and the US are available in the scientific literature. Most of these studies have analyzed small populations, and the findings of sex differences show considerable variability. One large meta-analysis demonstrates that the risk of AD increases 1.6-fold in women (Gao et al., 1998), but the results have not been confirmed in recent US studies, which indicate the same incidence in both sexes. Fig. 1 shows the age- and sex-specific dementia incidence rates in studies in the US, Europe, and Asia. The incidence of dementia is higher in women especially aged more than 90 years in Europe and Japan. However, studies in the US and Canada show a little difference in the incidence between men and women. These results, although not well explained by methodological differences, suggest that some types of premorbid exposures, causally related to AD, and different patterns in various regions in the world may play different roles in both sexes.
Fig. 1.
Age- and sex-specific incidence/100 person-years of dementia in (A) Sweden (Fratiglioni et al., 1997), (B) France (Fratiglioni et al., 1997), (C) the United States (Katz et al., 2012), (D) Canada (The Canadian Study of Health and Aging Working Group, 2000), (E) Japan (Yamada et al., 2008), and (F) China (Chen et al., 2011).
Sex differences in prevalence also depend on dementia subtypes, such as AD, Lewy body dementia (LBD), vascular dementia (VD), and Parkinson’s dementia (PD). For example, women have a higher risk of developing AD, and men have a higher risk of developing VD (Podcasy and Epperson, 2016). Table 1 summarizes the sex differences in prevalence/incidence of different subtypes of dementia. AD is shown to be the most common form of dementia, accounting for up to 60–80% of dementia cases. Progression of AD may be more rapid among elderly women, but studies from the US and the United Kingdom (UK) demonstrate that women with AD have a longer lifespan (Kua et al., 2014). Women are frequently diagnosed earlier with AD than men, which can determine their postdiagnosis longevity. It is becoming increasingly important to consider sex together with other risk factors (such as apolipoprotein E genotype and depression) for dementia. The development of AD in women at a later age has been associated with a longer lifetime exposure to estrogens (Lin et al., 2011). However, women show a marked decrease in estradiol levels in the second half of menopause, while age-matched men retain either a lifelong gonadal steroid level or a relatively slower decline in testosterone synthesis (Mielke et al., 2014). VD is reported to account for 10–20% of dementia cases and results from hemorrhagic or ischemic insults in some regions of the brain, which is critical for cognitive functions (Gorelick et al., 2011). Studies conducted worldwide indicate that the prevalence of stroke, either ischemic or hemorrhagic stroke, is 44% higher in men than in women. However, other studies demonstrate that women have a greater risk for stroke possibly because of their longer life span and increased risk of thrombosis and stroke with AF (Cheng and Kong, 2016). LBD is clinically distinguished from Parkinson’s dementia because the onset of dementia precedes the onset of parkinsonism. The pathology of LBD is known as an abnormal deposition of α-synuclein, known as Lewy body. Autopsy analysis of patients who died with dementia reveals that Lewy bodies are shown almost three times more often in men than in women, regardless of age, smoking, or education (Nelson et al., 2010). PD is a movement disorder characterized by tremor at rest, rigidity, and difficulty with speech, and loss of midbrain dopaminergic neurons in the substantia is known to be the main cause of disease. The prevalence of PD is known to be between 0.3% and 3% of the population worldwide and is 2 times higher in men than in women at any given age (Elbaz et al., 2002). Among individuals who are treated in the early stages of PD, women are better in cognitive functioning than men (Augustine et al., 2015). Dementia from multiple causes (mixed dementia) is referred as cognitive impairment due to multiple central nervous system pathologies, which are most often a combination of AD pathology, β-amyloid deposits, and vascular damage, such as multiple microbleeds or infarcts (Jellinger, 2013). According to autopsy reports, vascular damage occurs in up to 28% of AD cases (Gearing et al., 1995). Creutzfeldt-Jakob disease (CJD) primarily occurs in individuals aged more than 60 years world-wide. Although little is known about the pathophysiology of the disease, researchers believe that the disease is caused by prions or misfolded proteins, which aggregate in the brain and lead to neuronal death and progressive dementia. The cases of CJD do not vary by sex, and no differences in the survival time after diagnosis are observed between men and women (Gubbels et al., 2012).
Table 1.
Effects of sex on prevalence/incidence of dementia subtypes
| Subtypes of dementia | Sex differences in prevalence/incidence | References |
|---|---|---|
| Alzheimer disease (AD) |
|
Seshadri et al., 1997 Kua et al., 2014 |
| Vascular dementia (VD) |
|
Appelros et al., 2009 Pendlebury and Rothwell, 2009 |
| Lewy body dementia (LBD) |
|
Nelson et al., 2010 Savica et al., 2013 |
| Parkinson disease dementia (PD) |
|
Elbaz et al., 2002 Gillies et al., 2014 Augustine et al., 2015 |
| Due to multiple causes (mixed dementia) |
|
Martin-Laez et al., 2016 Siraj, 2011 |
| Creutzfeldt-Jakob disease (CJD) |
|
Gubbels et al., 2012 Skillback et al., 2014 |
Modified from Podcasy and Epperson (2016).
SEX DIFFERENCES IN CARDIOVASCULAR RISK FACTORS FOR DEMENTIA
Various CV disorders, such as coronary artery disease (CAD), AF, myocardial infarction (MI), HF, and HTN are known risk factors for dementia. Table 2 presents sex differences in the prevalence and incidence of these CV disorders. In addition, as shown in Table 3, the sex differences in the impact of CV disorders on the incidence of dementia are discussed here.
Table 2.
Sex differences in prevalence/incidence of cardiovascular diseases which are known as dementia risk factor
| CV Risk factors | Sex differences in prevalence/incidence/prognosis | References |
|---|---|---|
| Coronary artery disease (CAD) |
|
Bairey Merz et al., 2006 Shaw et al., 2006 Lerner and Kannel, 1986 Davis et al., 2015 |
| Atrial fibrillation (AF) |
|
Chugh et al., 2014 Heeringa et al., 2006 Svennberg et al., 2015 Piccini et al., 2012 |
| Myocardial infarction (MI) |
|
Albrektsen et al., 2016 de Torbal et al., 2006 Benjamin et al., 2018 Malacrida et al., 1998 |
| Heart failure (HF) |
|
Bleumink et al., 2004 Adams et al., 1996 Burns et al., 1997 |
| Hypertension (HTN) |
|
Roger et al., 2011 Ostchega et al., 2008 Lloyd-Jones et al., 2005 |
IR: incident rate, BP: blood pressure.
Table 3.
Sex differences in the impact of cardiovascular risk factors on dementia incidence
| Cardiovascular risk factors | Dementia risk | Note | References | |
|---|---|---|---|---|
|
| ||||
| Men | Women | |||
| Coronary artery disease (CAD) | + | + | Low cognitive scores in men and women (middle life) | Singh-Manoux et al., 2008 |
| Atrial Fibrillation (AF) | + | + | Increased incidence of dementia in men and women |
Chen et al., 2018 Miyasaka et al., 2007 |
| Myocardial Infarction (MI) | + | N.S. | Increased incidence of dementia in men but not in women with unrecognized MI | Ikram et al., 2008 |
| + | ++ | Greater dementia risk in women than in men | Aronson et al., 1990 | |
| + | + | Increased risk for vascular dementia but not for Alzheimer dementia in men and women | Sundboll et al., 2018 | |
| Heart failure (HF) | + | N.S. | Increased incidence of dementia in men but not in women | Noale et al., 2013 |
| + | + | Increased dementia risk in both men and women | Adelborg et al., 2017 | |
| Hypertension (HTN) | ||||
| All age | N.S. | + | Low cognitive scores in women but not in men | Arntzen et al., 2011 |
| + | + | Increased incidence of dementia in men and women |
Israeli-Korn et al., 2010 Kimm et al., 2011 |
|
| Mid-life | N.S. | + | Increased dementia risk in women but not in men |
Gilsanz et al., 2017 Joas et al., 2012 |
| Late- life | N.D. | N.S. | Risk for mild cognitive impairment or probable dementia is not significant in postmenopausal women | Johnson et al., 2008 |
+: increases dementia risk, ++: increases dementia risk to a great extent, N.D.: not mentioned, N.S.: not significant risk.
Coronary artery disease
CAD usually results from atherosclerosis and causes chest pain, shortness of breath during exercise, and heart attack. According to data from National Health and Nutrition Examination Survey (NHANES) 2011 to 2014 (National Center for Health statistics) in the US, the prevalence of CAD is higher in men than in women for all ages, 7.4% for men and 5.3% for women (Kivipelto et al., 2001). However, women with CAD show worse outcomes than men do when no adjustments are made for other characteristics and comorbidities. Women tend to present with CAD in life, and even in the young ages, they tend to have less evidence-based treatment for CAD than men do (Davis et al., 2015). CAD first presents approximately 10 years later in women than in men possibly because of the protective effect of estrogen. Upon reaching menopause, the incidence of CAD in women catches up with that in men (Yusuf et al., 2001).
The differences in CAD between women and men have been explained particularly by focusing on estrogen (Lawton, 2011). Estrogen is thought to be beneficial through vasodilation and the protective effects against atherosclerotic plaque, oxidative stress, and inflammation (Mendelsohn and Karas, 2005). Animal models have suggested that benefits conferred to females are due to estrogen (Patten et al., 2004). However, the cardiovascular advantages of exogenous estrogen have not been demonstrated clinically. Large randomized studies have reported that the estrogen treatment in postmenopausal women is not beneficial and even potentially harmful (Grady et al., 2002). Another reason for the greater incidence of CAD in men is the fact that men have an increased burden of atheroma than women. A study suggests that rupture of atheroma plaque in patients with sudden death is more often in men than in women (Patten et al., 2004). Men have more severe structural and functional abnormalities in the epicardial coronary arteries than women. In addition to physiological differences, other factors may be contributable to the clinical differences between women and men with CAD.
A strong association has been reported between CAD and dementia. The incidence of dementia is higher in those with prevalent CAD in the Cardiovascular Health Study cohort in the US (Newman et al., 2005), and several studies have confirmed that CAD is associated with cognitive impairment (Roberts et al., 2010) and hippocampal damage (Koschack and Irle, 2005). Recent studies have shown that CAD is observed more frequently in VD patients (Graban et al., 2009) and that the damaged region of the brain is strongly related to the atherosclerotic burden. Microvascular lesions in the brain are considered an important pathophysiological mechanism by which CAD acts as a risk factor for dementia (Rosano et al., 2005). Microvascular lesions in the brain disturb cerebral blood flow (CBF) regulation and perfusion, reduce cerebral circulation through blood-brain barrier disruption, and finally lead to brain tissue damage (Kovacic et al., 2011). In addition, several recent studies have found that the failure to clear excess Aβ (produced in cortical neurons) in the blood-brain barrier network contributes to cerebral hemorrhaging and AD pathology (Bell and Zlokovic, 2009). These aforementioned studies indicate a correlation between the CAD history and low cognitive scores and suggest that the longer the CAD period, the lower the cognitive scores. In men, those who had the first CAD event 10 years ago are more likely to have poor cognition. Therefore, future studies should be carried out to examine the correlation between the CAD history and cognition not only in men but also in postmenopausal women.
Atrial fibrillation
AF is the most common persistent cardiac arrhythmia and is recognized as one of the major public health problems (Naccarelli et al., 2009). Its prevalence is known to increase steadily with age, and the number of patients aged ≥65 is expected to reach 1.3 billion by 2040 worldwide (Dublin et al., 2011). The age-adjusted prevalence of AF is reported to be higher in men (10.3%) than in women (7.4%) in the US Medicare recipients (Piccini et al., 2012). Consistently, the incidence rates of AF are also shown to be higher in men than in women. Current evidence indicates that age and sex are the two most powerful predictors for the incidence of AF. In fact, the incidence of AF has been reported to be as high as 32.9 per 1,000 men and 30.4 per 1,000 women by age 85–89 years (Wilke et al., 2013). The lifetime risk of AF incidence has been slightly higher in men than in women (23.8% vs. 22.2% at 55 years of age in the Rotterdam study and 25.8% vs. 23.4% at 60 years of age in the Framingham study) (Lloyd-Jones et al., 2004). In addition, data from California administrative databases were analyzed for racial variation in the incidence of AF. After adjustment for AF risk factors, lower incidence rates have been found in blacks (Hazard Ratio (HR), 0.84; 95% Confidence Interval (CI), 0.82–0.85; p<0.001), Asians (HR, 0.78; 95% CI, 0.77–0.79; p<0.001), and Hispanics (HR, 0.78; 95% CI, 0.77–0.79; p<0.001) than in their white counterparts (Dewland et al., 2013). However, despite the high prevalence, incidence, and risk for AF in men, the count of women with AF is greater than that of men with AF due to the differences in life expectancy. In addition, the relative mortality rate of AF patients has been higher in women than in men. Although the findings in individual studies are still controversial, a meta-analysis study has demonstrated that the pooled ratio of relative risks for AF-associated all-cause mortality is 12% higher in women than in men (HR 1.12; 95% CI, 1.07–1.17) (Emdin et al., 2016).
The precise mechanisms that cause sex-related differences in AF are poorly explained, but several suggestions have been proposed. First, anthropomorphic differences (especially in lean body weight) between men and women cause a larger size and volume of left atrium in men than in women, and the changes could promote AF-maintenance, independently of the increase in AF incidence (Liu et al., 2004). Second, women with AF have a relatively greater burden of atrial fibrosis (Cochet et al., 2015). Third, variations in the X-linked genes may account for some sex differences in AF (Ravn et al., 2005). Further basic and clinical research are needed to clarify the pathophysiological aspect of sex differences in AF. In fact, the contribution of sex hormones has been examined in several studies. Testosterone deficiency has been shown to increase arrhythmogenicity possibly through increased calcium release from the sarcoplasmic reticulum, and this abnormality is reversed by testosterone therapy in an animal study using mice (Tsuneda et al., 2009). Clinically, it is observed that an increased risk of AF is associated with decreased testosterone levels in men (Magnani et al., 2014).
Recently, increasing evidence suggests that patients with AF have been associated with not only high rates of CAD and HF but also an increased risk of cognitive decline (Dublin et al., 2011). A meta-analysis study of eight studies including 77,668 patients has demonstrated that AF significantly increases the risk of dementia incidence by 42% (Santangeli et al., 2012). In addition, AF possibly causes modifiable ischemic stroke and leads to considerable physical and cognitive disability and dementia (Alonso and Arenas de Larriva, 2016). The relationship between AF and dementia in the absence of stroke is still uncertain (Kwok et al., 2011), but it is clear that AF can cause the onset of dementia (Barba et al., 2000; Rastas et al., 2007). Moreover, growing evidence supports AF as a risk factor for dementia without stroke (O’Connell et al., 1998). In fact, several studies have shown that AF is associated with brain abnormalities, more specifically, a small hippocampal volume and left ventricular hypertrophy (Wozakowska-Kaplon et al., 2009). Individuals suffering from AF also show poor learning, memory, attention, and performance (Knecht et al., 2008), as well as decreased scores on cognition tests (Schrader, 2004; Kermode-Scott, 2012).
Several mechanisms have been suggested to link AF and dementia. In AF patients, irregular rapid ventricular rates may cause a reduced cerebral perfusion because of low cardiac release (Petersen et al., 1989; Duron and Hanon, 2010). Another suggested mechanism is the increased risk of covert cerebral infarction and transient ischemic attack (Ezekowitz et al., 1995; Vermeer et al., 2003). AF can also result in a hypercoagulable state, which may give rise to subclinical cerebral embolism (Choudhury and Lip, 2003; Barber et al., 2004). Furthermore, cerebrovascular disease may precipitate neurodegenerative changes, manifesting as white matter hyperintensities on MRI (Kalaria, 2000). A recent study of sex differences in the relationship between AF and dementia suggests that age-adjusted rates of dementia are not different between men and women with AF (Miyasaka et al., 2007). Another sex-stratified analysis indicates that the risk of dementia associated with AF in women is comparable with that in men (p=0.96 for interaction by sex) (Chen et al., 2018). Therefore, further studies should focus on the sex differences in the relationship between AF and dementia.
Myocardial infarction
MI is defined as cardiomyocyte necrosis because of the sustained ischemia/hypoxia. It is frequently, but not always, an acute manifestation of atherosclerosis-related CAD (Mendis et al., 2011). Based on a UK national survey of self-reported MI, the prevalence of MI is approximately 4.1% in men and 1.7% in women in 2006, and the prevalence is age-dependent, extending from 1% in men aged <45 years to 17% in those aged ≥75 years. Similarly, the overall prevalence of MI is 3.0% in US adults aged ≥20 years according to data from NHANES 2011 to 2014. Men have a higher prevalence of MI than women in all age groups except those of 20 to 39 years (3.8% for men and 2.3% for women), and more women than men have MI in the highest age group because of women’s longevity (Benjamin et al., 2018). The sex difference in the risk of MI persists throughout life; the relative risk of MI diminishes with age, but the absolute sex differences increase (Albrektsen et al., 2016). The lower risk in women at premenopausal ages may be associated with a beneficial effect of female hormones on vascular function, lipid profile, or other cardioprotective molecules (Chou and Saw, 2014). Despite a positive role of female hormones on CV risk factors, a few previous studies have reported no or even an adverse effect of hormone treatment on the risk of MI (Coutinho, 2014). Actually, it is not easy to distinguish between effects of age and menopause (Gierach et al., 2006), but the age-incidence relationship with hormone-dependent diseases may be affected by hormone changes (Kalin and Zumoff, 1990). However, once a woman has MI, she loses the advantage and may become no better or worse than a man with MI at the same age (Rosengren et al., 2001). It is still controversial about the prognosis of women with MI. Several studies (Greenland et al., 1991; Maynard et al., 1992) indicate that women have a worse short-term prognosis than men; however, in general, the findings have been partly attributed to differences in age and prion diseases, mainly diabetes.
It is a growing interest to define the mechanism underlying the sex-related differences in MI-associated damage, but conflicting results have been found. Ischemic insults in the heart reduce blood flow and trigger apoptotic death, and loss of cardiac cells results in tissue damage. A primary clinical strategy is to restore blood flow, although the restored blood flow may further increase tissue damage because of myocardial ischemia/reperfusion (I/R) injury. An animal study has found that regional myocardial I/R in the heart produces a significantly larger size of infarct in male rats than in female rats (Le et al., 2014). Aggravated reperfusion injury correlates with increased apoptosis in the area at risk, and consistent with the smaller infarct size, significantly less apoptosis is observed in the female heart. In fact, testosterone has been shown to inversely affect myocardial remodeling after MI (Kanashiro-Takeuchi et al., 2009). Testosterone is known to activate nuclear factor-kB, which contributes to inflammatory mechanisms and the downregulation of fatty acid oxidation (Gardner et al., 2002).
Only a limited number of studies have examined the relationship between MI and dementia. Patients with MI have been shown to share a genetic background, including abnormal cholesterol metabolism and upregulated inflammation, with those with AD. Some studies have revealed a high risk of cognitive impairment after MI because of brain hypoperfusion (Zuccala et al., 2001), and a cross-sectional evaluation in the Rotterdam study reveals a positive relationship between MI and cognitive impairment and (Breteler et al., 1994). In addition, the Bronx Aging Study suggests that women with a history of MI have a 5-fold increase in the risk of dementia (Aronson et al., 1990). Another study indicates that the incidence of dementia is remarkably higher in women with than without a history of MI, but the results are not found in men (Aronson et al., 1990). The significant findings suggest that women with MI may be particularly susceptible for dementia (Aronson et al., 1990). However, the impact of MI on dementia remains controversial; for example, the Honolulu-Asia Aging Study has not demonstrated a link between later cognitive impairment and MI (Petrovitch et al., 1998). Therefore, further studies remain to improve understanding of the effect of MI on dementia.
Heart failure
HF is defined as “a complex clinical syndrome that can result from structural or functional heart injury that reduces the ability of the ventricle to pump blood” (Hunt et al., 2005). Because HF is a syndrome rather than a disease, the diagnosis can depend on a clinical test. The incidence of HF has been reported to be 14.4/1000 person-years (95% CI, 13.4–15.5) and is significantly higher in men (17.6/1000 man-years; 95% CI, 15.8–19.5) than in women (12.5/1000 woman-years; 95% CI, 11.3–13.8) (Bleumink et al., 2004). The incidence rate increases with age from 1.4/1000 person-years in those aged 55–59 years to 47.4/1000 person-years in those aged 90 years or older, and the age-associated increase is shown to be evident for both sexes. A Portugal study indicates that HF incidence per year is 1.3 cases per 1,000 people aged over 25 years, 8.8 cases per 1,000 people aged over 65 years, and 11.6 cases per 1,000 people aged over 85 years, and males have a 1.75-fold higher rate than females (Ceia et al., 2002). However, in the UK, the overall HF incidence rate per year is 4.4 cases per 1,000 people in men and 3.9 cases per 1,000 people in women and doubles every 5 years after the age of 55 years (Johansson et al., 2001).
HF seems to have different characteristics in men and women. Some of the differences are attributed to age, ventricular function, and causes of HF. Women have a higher onset age of HF and have HF more often without left ventricular systolic dysfunction but less often due to ischemic heart disease than men. In addition to these effects, a pathophysiological specificity for sex is also observed. Left ventricular responses to pressure overload conditions, such as hypertension, can be modified by sex, and women are less likely to have ventricular dilatation (Mendes et al., 1997; Luchner et al., 2002). The relationships between hormones and the renin-angiotensin system are identified and may contribute to differences in remodeling, and the ventricular dilatation progresses less intensely in women (Fischer et al., 2002). Women have a great baseline activity of the vasodilator natriuretic peptide system, the fact that can contribute to the protective role. In contrast, women have increased the stiffness of vessel and ventricle even in the absence of heart disease (Redfield et al., 2005), and the combined ventricular–vascular stiffening may contribute to the increased prevalence of HF in women. Furthermore, women also tend to be more depressed than men do. Therefore, they have a lower threshold for perceiving and expressing their symptoms. In addition, social integration, another psychosocial factor, affects C-reactive protein concentration, a measure of inflammatory activation, in men but not in women (Ford et al., 2006). Thus, men tend to develop silent biological responses to such stimuli, and the responses may indirectly affect the cardiovascular risk. Therefore, clinical trials in women or at least with enough women are needed to guide management of HF.
The Heart-Mind Study suggests that participants with HF experience declines in cognitive function (Almeida et al., 2012). In addition, other studies demonstrate that HF is associated with both cognitive impairment and dementia (Trojano et al., 2003). A recent pilot case-control study indicates that HF patients have lower scores in cognitive functions, including visuospatial and executive function, visual memory, and verbal learning tasks (Beer et al., 2009). Investigation for pathophysiological mechanisms underlying the relationship between HF and dementia are still on going. Reduced CBF appears to worsen cognitive impairment related with HF, and those with heart transplantation have restored CBF and improved cognitive performance (Gruhn et al., 2001; Alves et al., 2005). In HF, low cardiac output combined with impaired cerebral autoregulatory systems may lead to reduced CBF and increased cognitive impairment and dementia (Jefferson et al., 2007, 2010). HF is also a risk factor for multiple cerebral emboli, which could cause cognitive impairment (Pullicino and Hart, 2001). HF is demonstrated to be a remarkable risk factor for dementia incidence in men (HR=2.58, 95% CI=1.11–5.99) but not in women (Noale et al., 2013). However, another study suggests that HF is a risk factor for dementia in both men and women, and slightly greater HR is found in men (HR=1.31; 95% CI, 1.26–1.36) than in women (HR=1.15; 95% CI, 1.11–1.18) (Adelborg et al., 2017). Although causes and outcomes of HF can differ between men and women (Taylor, 2015), the basis of the sex differences in the dementia risk is still unclear.
Hypertension
HTN is defined as a systolic blood pressure (BP) of 140 mmHg or a diastolic BP of 90 mmHg, and it has been reported that approximately 80 million US adults more than 20 years old have HTN (i.e., 32.6% prevalence) (Mozaffarian et al., 2016). HTN prevalence is shown to be the highest in non-Hispanic Blacks (33.5%), and it increases with age (65.4% among those aged ≥60 years) and tends to be higher in women than in men. The numbers of patients treated for HTN increase with time in both women and men and are more in women than in men. Although BP control has improved over time in women and men, fewer women than men have been able to control BP despite treatment, especially in the elderly. HTN is also more prevalent in women among the elderly (Roger et al., 2011), and about 80% of women aged over 70 years suffer from HTN (Roger et al., 2011). Women have a lower systolic blood pressure than men in early adulthood, but this trend is reversed after age 60 (Lloyd-Jones et al., 2005). Decreased survival in old hypertensive men can account for the higher prevalence of HTN in old women. Diastolic BP is slightly lower in women than in men, regardless of age. The prevalence of hypertension is significantly higher in men than in men (34% and 22%, respectively) (Ostchega et al., 2008). Previous NHANES data reveal a minimal difference in HTN incidence between women and men at all ages, but the incidence of HTN in black is almost twice that of white for almost all age- and sex-matched groups (Cornoni-Huntley et al., 1989). In the subgroup of adults under age 60, women (87%) are more likely to recognize and seek treatment for HTN than men (63%) (Ostchega et al., 2008). No differences in perception of HTN are found between hypertensive men (84%) and women (81%) over 60 years of age. Among HTN patients aged 18–59 years, men (47%) are less likely to be treated than women (74%), but no differences in treatment are observed between hypertensive men (78%) and women (75%) aged 60 years and older (Engberding and Wenger, 2012).
High BP is a well-known risk factor for cerebrovascular disorders, including cerebral infarct, stroke, and dementia (Vermeer et al., 2003; Celle et al., 2012). However, whether HTN is an independent risk factor for dementia is unknown. The association between HTN and the risk of dementia is prominent, and few studies have failed to show this relationship (Johnson et al., 2008). The high prevalence of HTN at the age of between 30 and 50 years indicates that understanding the relationship between HTN and dementia is important for the potential prevention of dementia. In fact, the Cache County study by Mielke et al. also indicates that systolic BP of 160 mmHg is related with greater rates of cognitive decline in the elderly than low systolic BP. Other studies have further shown that HTN is associated with not only VD but also AD (Staessen et al., 2007). In particular, elderly people with HTN rather than young people with HTN are shown to have an increased risk of VD (Yamada et al., 2003). The relationship between HTN and risk of AD has been also suggested by a recent study, which demonstrates a significant interaction between diastolic BP and plasma Aβ levels (Shah et al., 2012). In addition, mid-adulthood HTN is associated with increased dementia risk in women but not in men. It is predicted that women with onset HTN in the mid-adulthood have a 73% higher dementia risk than stable normotensive women (Gilsanz et al., 2017), but no evidence indicates that HTN increases dementia risk in men. In addition, it has been recently demonstrated that HTN is strongly associated with VD in both men and women similarly (Kimm et al., 2011). HTN has also been shown to be associated with AD, especially in men younger than 65 years. However, the association of HTN with AD is not observed in men or women over 65 years.
CONCLUSION
With aging of the population, the prevalence of dementia including AD is expected to reach an epidemic size worldwide. However, currently no cure is available for this devastating disease, and because currently approved medications are just symptomatic, they do not modify the underlying disease pathology. Although lots of preclinical studies and clinical trials for candidate medications to reduce amyloid and other targets for AD are ongoing, studies clarifying the factors related with the risk and progression of AD are needed to identify potential novel therapeutic targets. As described in this review, the prevalence/incidence of dementia and CV risk factors for dementia is quite different by sex. Because women live longer than men in most countries, the adverse impact of the risk factors may affect women in particular. In that context, drugs may have efficacy in only one sex or have different efficacy between men and women. Actually, both male and female patients were included in many studies, but most studies did not consider the issue of sex separately. Therefore, future clinical trials for new AD therapeutics should consider a deliberate stratification by sex, and an adequate sample size is needed to test the therapeutic efficacy in men and women separately. In addition, further studies are needed to understand sex-specific effects of the CV risk factors on dementia incidence and to examine the mechanisms underlying the sex differences. The resultant information may help to establish a new strategy for the development of individualized therapeutics and preventive medications for dementia.
Acknowledgments
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI18C0920); the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1A6A3A11935320 and 2018R1D1A1B07048729), Republic of Korea; and the Support Program for Women in Science, Engineering and Technology through the NRF funded by the Ministry of Science and ICT (No. 2016H1C3A1903202).
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
REFERENCES
- Adams KF, Jr, Dunlap SH, Sueta CA, Clarke SW, Patterson JH, Blauwet MB, Jensen LR, Tomasko L, Koch G. Relation between gender, etiology and survival in patients with symptomatic heart failure. J. Am. Coll. Cardiol. 1996;28:1781–1788. doi: 10.1016/S0735-1097(96)00380-4. [DOI] [PubMed] [Google Scholar]
- Adelborg K, Horvath-Puho E, Ording A, Pedersen L, Toft Sorensen H, Henderson VW. Heart failure and risk of dementia: a Danish nationwide population-based cohort study. Eur. J. Heart Fail. 2017;19:253–260. doi: 10.1002/ejhf.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrektsen G, Heuch I, Lochen ML, Thelle DS, Wilsgaard T, Njolstad I, Bonaa KH. Lifelong Gender Gap in Risk of Incident Myocardial Infarction: The Tromso Study. JAMA Intern. Med. 2016;176:1673–1679. doi: 10.1001/jamainternmed.2016.5451. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Beer C, Lautenschlager NT, Arnolda L, Alfonso H, Flicker L. Two-year course of cognitive function and mood in adults with congestive heart failure and coronary artery disease: the Heart-Mind Study. Int. Psychogeriatr. 2012;24:38–47. doi: 10.1017/S1041610211001657. [DOI] [PubMed] [Google Scholar]
- Alonso A, Arenas de Larriva AP. Atrial fibrillation, cognitive decline and dementia. Eur. Cardiol. 2016;11:49–53. doi: 10.15420/ecr.2016:13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves TC, Rays J, Fraguas R, Jr, Wajngarten M, Meneghetti JC, Prando S, Busatto GF. Localized cerebral blood flow reductions in patients with heart failure: a study using 99mTc-HMPAO SPECT. J. Neuroimaging. 2005;15:150–156. doi: 10.1177/1051228404272880. [DOI] [PubMed] [Google Scholar]
- Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- Arntzen KA, Schirmer H, Wilsgaard T, Mathiesen EB. Impact of cardiovascular risk factors on cognitive function: the Tromso study. Eur. J. Neurol. 2011;18:737–743. doi: 10.1111/j.1468-1331.2010.03263.x. [DOI] [PubMed] [Google Scholar]
- Aronson MK, Ooi WL, Morgenstern H, Hafner A, Masur D, Crystal H, Frishman WH, Fisher D, Katzman R. Women, myocardial infarction, and dementia in the very old. Neurology. 1990;40:1102–1106. doi: 10.1212/WNL.40.7.1102. [DOI] [PubMed] [Google Scholar]
- Augustine EF, Perez A, Dhall R, Umeh CC, Videnovic A, Cambi F, Wills AM, Elm JJ, Zweig RM, Shulman LM, Nance MA, Bainbridge J, Suchowersky O. Sex differences in clinical features of early, treated Parkinson’s disease. PLoS ONE. 2015;10:e0133002. doi: 10.1371/journal.pone.0133002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study: part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J. Am. Coll. Cardiol. 2006;47:S21–S29. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]
- Barba R, Martinez-Espinosa S, Rodriguez-Garcia E, Pondal M, Vivancos J, Del Ser T. Poststroke dementia : clinical features and risk factors. Stroke. 2000;31:1494–1501. doi: 10.1161/01.STR.31.7.1494. [DOI] [PubMed] [Google Scholar]
- Barber M, Tait RC, Scott J, Rumley A, Lowe GD, Stott DJ. Dementia in subjects with atrial fibrillation: hemostatic function and the role of anticoagulation. J. Thromb. Haemost. 2004;2:1873–1878. doi: 10.1111/j.1538-7836.2004.00993.x. [DOI] [PubMed] [Google Scholar]
- Beer C, Ebenezer E, Fenner S, Lautenschlager NT, Arnolda L, Flicker L, Almeida OP. Contributors to cognitive impairment in congestive heart failure: a pilot case-control study. Intern. Med. J. 2009;39:600–605. doi: 10.1111/j.1445-5994.2008.01790.x. [DOI] [PubMed] [Google Scholar]
- Bell RD, Zlokovic BV. Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118:103–113. doi: 10.1007/s00401-009-0522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics-2018 update: a report from the american heart association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- Bleumink GS, Knetsch AM, Sturkenboom MC, Straus SM, Hofman A, Deckers JW, Witteman JC, Stricker BH. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure the rotterdam study. Eur. Heart J. 2004;25:1614–1619. doi: 10.1016/j.ehj.2004.06.038. [DOI] [PubMed] [Google Scholar]
- Breteler MM, Claus JJ, Grobbee DE, Hofman A. Cardiovascular disease and distribution of cognitive function in elderly people: the rotterdam study. BMJ. 1994;308:1604–1608. doi: 10.1136/bmj.308.6944.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns RB, McCarthy EP, Moskowitz MA, Ash A, Kane RL, Finch M. Outcomes for older men and women with congestive heart failure. J. Am. Geriatr. Soc. 1997;45:276–280. doi: 10.1111/j.1532-5415.1997.tb00940.x. [DOI] [PubMed] [Google Scholar]
- Ceia F, Fonseca C, Mota T, Morais H, Matias F, de Sousa A, Oliveira A. Prevalence of chronic heart failure in South-western Europe: the EPICA study. Eur. J. Heart Fail. 2002;4:531–539. doi: 10.1016/S1388-9842(02)00034-X. [DOI] [PubMed] [Google Scholar]
- Celle S, Annweiler C, Pichot V, Bartha R, Barthelemy JC, Roche F, Beauchet O. Association between ambulatory 24-hour blood pressure levels and brain volume reduction: a cross-sectional elderly population-based study. Hypertension. 2012;60:1324–1331. doi: 10.1161/HYPERTENSIONAHA.112.193409. [DOI] [PubMed] [Google Scholar]
- Chen LY, Norby FL, Gottesman RF, Mosley TH, Soliman EZ, Agarwal SK, Loehr LR, Folsom AR, Coresh J, Alonso A. Association of atrial fibrillation with cognitive decline and dementia over 20 Years: The ARIC-NCS (Atherosclerosis Risk in Communities Neurocognitive Study). J. Am. Heart Assoc. 2018;7:e007301. doi: 10.1161/JAHA.117.007301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Hu Z, Wei L, Ma Y, Liu Z, Copeland JR. Incident dementia in a defined older Chinese population. PLoS ONE. 2011;6:e24817. doi: 10.1371/journal.pone.0024817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EY, Kong MH. Gender differences of thrombo-embolic events in atrial fibrillation. Am. J. Cardiol. 2016;117:1021–1027. doi: 10.1016/j.amjcard.2015.12.040. [DOI] [PubMed] [Google Scholar]
- Chou AY, Saw J. Basis for sex-specific expression of Takotsubo cardiomyopathy, cardiac syndrome X, and spontaneous coronary artery dissection. Can. J. Cardiol. 2014;30:738–746. doi: 10.1016/j.cjca.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Choudhury A, Lip GY. Atrial fibrillation and the hypercoagulable state: from basic science to clinical practice. Pathophysiol. Haemost. Thromb. 2003;33:282–289. doi: 10.1159/000083815. [DOI] [PubMed] [Google Scholar]
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochet H, Mouries A, Nivet H, Sacher F, Derval N, Denis A, Merle M, Relan J, Hocini M, Haissaguerre M, Laurent F, Montaudon M, Jais P. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J. Cardiovasc. Electrophysiol. 2015;26:484–492. doi: 10.1111/jce.12651. [DOI] [PubMed] [Google Scholar]
- Cornoni-Huntley J, LaCroix AZ, Havlik RJ. Race and sex differentials in the impact of hypertension in the United States. The national health and nutrition examination survey i epidemiologic follow-up study. Arch. Intern. Med. 1989;149:780–788. doi: 10.1001/archinte.1989.00390040022005. [DOI] [PubMed] [Google Scholar]
- Coutinho T. Arterial stiffness and its clinical implications in women. Can. J. Cardiol. 2014;30:756–764. doi: 10.1016/j.cjca.2014.03.020. [DOI] [PubMed] [Google Scholar]
- Davis M, Diamond J, Montgomery D, Krishnan S, Eagle K, Jackson E. Acute coronary syndrome in young women under 55 years of age: clinical characteristics, treatment, and outcomes. Clin. Res. Cardiol. 2015;104:648–655. doi: 10.1007/s00392-015-0827-2. [DOI] [PubMed] [Google Scholar]
- de Torbal A, Boersma E, Kors JA, van Herpen G, Deckers JW, van der Kuip DA, Stricker BH, Hofman A, Witteman JC. Incidence of recognized and unrecognized myocardial infarction in men and women aged 55 and older: the Rotterdam Study. Eur. Heart J. 2006;27:729–736. doi: 10.1093/eurheartj/ehi707. [DOI] [PubMed] [Google Scholar]
- Dewland TA, Olgin JE, Vittinghoff E, Marcus GM. Incident atrial fibrillation among Asians, Hispanics, blacks, and whites. Circulation. 2013;128:2470–2477. doi: 10.1161/CIRCULATIONAHA.113.002449. [DOI] [PubMed] [Google Scholar]
- Dublin S, Anderson ML, Haneuse SJ, Heckbert SR, Crane PK, Breitner JC, McCormick W, Bowen JD, Teri L, McCurry SM, Larson EB. Atrial fibrillation and risk of dementia: a prospective cohort study. J. Am. Geriatr. Soc. 2011;59:1369–1375. doi: 10.1111/j.1532-5415.2011.03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron E, Hanon O. Atrial fibrillation and cognitive function. Psychol. Neuropsychiatr. Vieil. 2010;8:209–214. doi: 10.1684/pnv.2010.0222. [DOI] [PubMed] [Google Scholar]
- Elbaz A, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Risk tables for parkinsonism and Parkinson’s disease. J. Clin. Epidemiol. 2002;55:25–31. doi: 10.1016/S0895-4356(01)00425-5. [DOI] [PubMed] [Google Scholar]
- Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, Odutayo AA. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ. 2016;532:h7013. doi: 10.1136/bmj.h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberding N, Wenger NK. Management of hypertension in women. Hypertens. Res. 2012;35:251–260. doi: 10.1038/hr.2011.210. [DOI] [PubMed] [Google Scholar]
- Ezekowitz MD, James KE, Nazarian SM, Davenport J, Broderick JP, Gupta SR, Thadani V, Meyer ML, Bridgers SL. Silent cerebral infarction in patients with nonrheumatic atrial fibrillation. The Veterans Affairs Stroke Prevention in Nonrheumatic Atrial Fibrillation Investigators. Circulation. 1995;92:2178–2182. doi: 10.1161/01.CIR.92.8.2178. [DOI] [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M, Baessler A, Schunkert H. Renin angiotensin system and gender differences in the cardiovascular system. Cardiovasc. Res. 2002;53:672–677. doi: 10.1016/S0008-6363(01)00479-5. [DOI] [PubMed] [Google Scholar]
- Ford ES, Loucks EB, Berkman LF. Social integration and concentrations of C-reactive protein among US adults. Ann. Epidemiol. 2006;16:78–84. doi: 10.1016/j.annepidem.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Viitanen M, von Strauss E, Tontodonati V, Herlitz A, Winblad B. Very old women at highest risk of dementia and Alzheimer’s disease: incidence data from the Kungsholmen Project, Stockholm. Neurology. 1997;48:132–138. doi: 10.1212/WNL.48.1.132. [DOI] [PubMed] [Google Scholar]
- Gao S, Hendrie HC, Hall KS, Hui S. The relationships between age, sex, and the incidence of dementia and Alzheimer disease: a meta-analysis. Arch. Gen Psychiatry. 1998;55:809–815. doi: 10.1001/archpsyc.55.9.809. [DOI] [PubMed] [Google Scholar]
- Gardner JD, Brower GL, Janicki JS. Gender differences in cardiac remodeling secondary to chronic volume overload. J. Card. Fail. 2002;8:101–107. doi: 10.1054/jcaf.2002.32195. [DOI] [PubMed] [Google Scholar]
- Gearing M, Mirra SS, Hedreen JC, Sumi SM, Hansen LA, Heyman A. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part X. Neuropathology confirmation of the clinical diagnosis of Alzheimer’s disease. Neurology. 1995;45:461–466. doi: 10.1212/WNL.45.3.461. [DOI] [PubMed] [Google Scholar]
- Gierach GL, Johnson BD, Bairey Merz CN, Kelsey SF, Bittner V, Olson MB, Shaw LJ, Mankad S, Pepine CJ, Reis SE, Rogers WJ, Sharaf BL, Sopko G. Hypertension, menopause, and coronary artery disease risk in the Women’s Ischemia Syndrome Evaluation (WISE) Study. J. Am. Coll. Cardiol. 2006;47:S50–S58. doi: 10.1016/j.jacc.2005.02.099. [DOI] [PubMed] [Google Scholar]
- Gillies GE, Pienaar IS, Vohra S, Qamhawi Z. Sex differences in Parkinson’s disease. Front. Neuroendocrinol. 2014;35:370–384. doi: 10.1016/j.yfrne.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilsanz P, Mayeda ER, Glymour MM, Quesenberry CP, Mungas DM, DeCarli C, Dean A, Whitmer RA. Female sex, early-onset hypertension, and risk of dementia. Neurology. 2017;89:1886–1893. doi: 10.1212/WNL.0000000000004602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graban A, Bednarska-Makaruk M, Bochynska A, Lipczynska-Lojkowska W, Ryglewicz D, Wehr H. Vascular and biochemical risk factors of vascular dementia after lacunar strokes (S-VaD) and after multiinfarcts in strategic areas (M-VaD). J. Neurol. Sci. 2009;283:116–118. doi: 10.1016/j.jns.2009.02.344. [DOI] [PubMed] [Google Scholar]
- Grady D, Herrington D, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N. Cardiovascular disease outcomes during 6.8 years of hormone therapy: Heart and Estrogen/progestin Replacement Study follow-up (HERS II) JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- Greenland P, Reicher-Reiss H, Goldbourt U, Behar S. In-hospital and 1-year mortality in 1,524 women after myocardial infarction. Comparison with 4,315 men. Circulation. 1991;83:484–491. doi: 10.1161/01.CIR.83.2.484. [DOI] [PubMed] [Google Scholar]
- Gruhn N, Larsen FS, Boesgaard S, Knudsen GM, Mortensen SA, Thomsen G, Aldershvile J. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–2533. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- Gubbels S, Bacci S, Laursen H, Hogenhaven H, Cowan S, Molbak K, Christiansen M. Description and analysis of 12 years of surveillance for Creutzfeldt-Jakob disease in Denmark, 1997 to 2008. Euro. Surveill. 2012;17:20142. [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch. Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeringa J, van der Kuip DA, Hofman A, Kors JA, van Herpen G, Stricker BH, Stijnen T, Lip GY, Witteman JC. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur. Heart J. 2006;27:949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American college of cardiology/American Heart association task force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure): developed in collaboration with the American college of chest physicians and the international society for heart and lung transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- Ikram MA, van Oijen M, de Jong FJ, Kors JA, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Unrecognized myocardial infarction in relation to risk of dementia and cerebral small vessel disease. Stroke. 2008;39:1421–1426. doi: 10.1161/STROKEAHA.107.501106. [DOI] [PubMed] [Google Scholar]
- Israeli-Korn SD, Masarwa M, Schechtman E, Abuful A, Strugatsky R, Avni S, Farrer LA, Friedland RP, Inzelberg R. Hypertension increases the probability of Alzheimer’s disease and of mild cognitive impairment in an Arab community in northern Israel. Neuroepidemiology. 2010;34:99–105. doi: 10.1159/000264828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, Gona P, Salton CJ, DeCarli C, O’Donnell CJ, Benjamin EJ, Wolf PA, Manning WJ. Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation. 2010;122:690–697. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson AL, Poppas A, Paul RH, Cohen RA. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiol Aging. 2007;28:477–483. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front. Aging Neurosci. 2013;5:17. doi: 10.3389/fnagi.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joas E, Backman K, Gustafson D, Ostling S, Waern M, Guo X, Skoog I. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. 2012;59:796–801. doi: 10.1161/HYPERTENSIONAHA.111.182204. [DOI] [PubMed] [Google Scholar]
- Johansson S, Wallander MA, Ruigomez A, Garcia Rodriguez LA. Incidence of newly diagnosed heart failure in UK general practice. Eur. J. Heart Fail. 2001;3:225–231. doi: 10.1016/S1388-9842(00)00131-8. [DOI] [PubMed] [Google Scholar]
- Johnson KC, Margolis KL, Espeland MA, Colenda CC, Fillit H, Manson JE, Masaki KH, Mouton CP, Prineas R, Robinson JG, Wassertheil-Smoller S. A prospective study of the effect of hypertension and baseline blood pressure on cognitive decline and dementia in postmenopausal women: the Women’s Health Initiative Memory Study. J. Am. Geriatr. Soc. 2008;56:1449–1458. doi: 10.1111/j.1532-5415.2008.01806.x. [DOI] [PubMed] [Google Scholar]
- Kalaria RN. The role of cerebral ischemia in Alzheimer’s disease. Neurobiol Aging. 2000;21:321–330. doi: 10.1016/S0197-4580(00)00125-1. [DOI] [PubMed] [Google Scholar]
- Kalin MF, Zumoff B. Sex hormones and coronary disease: a review of the clinical studies. Steroids. 1990;55:330–352. doi: 10.1016/0039-128X(90)90058-J. [DOI] [PubMed] [Google Scholar]
- Kanashiro-Takeuchi RM, Heidecker B, Lamirault G, Dharamsi JW, Hare JM. Sex-specific impact of aldosterone receptor antagonism on ventricular remodeling and gene expression after myocardial infarction. Clin. Transl. Sci. 2009;2:134–142. doi: 10.1111/j.1752-8062.2009.00094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz MJ, Lipton RB, Hall CB, Zimmerman ME, Sanders AE, Verghese J, Dickson DW, Derby CA. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer. Dis. Assoc. Disord. 2012;26:335–343. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermode-Scott B. Atrial fibrillation increases the risk of cognitive decline and need for long term care. BMJ. 2012;344:e1591. doi: 10.1136/bmj.e1591. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee J, Kang S, Moon H, Chung KH, Kim KR. Antiplatelet and antithrombotic effects of the extract of lindera obtusiloba leaves. Biomol. Ther (Seoul) 2016;24:659–664. doi: 10.4062/biomolther.2016.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimm H, Lee PH, Shin YJ, Park KS, Jo J, Lee Y, Kang HC, Jee SH. Mid-life and late-life vascular risk factors and dementia in Korean men and women. Arch. Gerontol. Geriatr. 2011;52:e117–122. doi: 10.1016/j.archger.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Soininen H, Tuomilehto J, Nissinen A. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Oelschlager C, Duning T, Lohmann H, Albers J, Stehling C, Heindel W, Breithardt G, Berger K, Ringelstein EB, Kirchhof P, Wersching H. Atrial fibrillation in stroke-free patients is associated with memory impairment and hippocampal atrophy. Eur. Heart J. 2008;29:2125–2132. doi: 10.1093/eurheartj/ehn341. [DOI] [PubMed] [Google Scholar]
- Koschack J, Irle E. Small hippocampal size in cognitively normal subjects with coronary artery disease. Neurobiol Aging. 2005;26:865–871. doi: 10.1016/j.neurobiolaging.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kovacic JC, Moreno P, Nabel EG, Hachinski V, Fuster V. Cellular senescence, vascular disease, and aging: part 2 of a 2-part review: clinical vascular disease in the elderly. Circulation. 2011;123:1900–1910. doi: 10.1161/CIRCULATIONAHA.110.009118. [DOI] [PubMed] [Google Scholar]
- Kua EH, Ho E, Tan HH, Tsoi C, Thng C, Mahendran R. The natural history of dementia. Psychogeriatrics. 2014;14:196–201. doi: 10.1111/psyg.12053. [DOI] [PubMed] [Google Scholar]
- Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L, Larson EB. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch. Neurol. 2002;59:1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]
- Kwok CS, Loke YK, Hale R, Potter JF, Myint PK. Atrial fibrillation and incidence of dementia: a systematic review and meta-analysis. Neurology. 2011;76:914–922. doi: 10.1212/WNL.0b013e31820f2e38. [DOI] [PubMed] [Google Scholar]
- Lawton JS. Sex and gender differences in coronary artery disease. Semin. Thorac. Cardiovasc. Surg. 2011;23:126–130. doi: 10.1053/j.semtcvs.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Le TY, Ashton AW, Mardini M, Stanton PG, Funder JW, Handelsman DJ, Mihailidou AS. Role of androgens in sex differences in cardiac damage during myocardial infarction. Endocrinology. 2014;155:568–575. doi: 10.1210/en.2013-1755. [DOI] [PubMed] [Google Scholar]
- Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am. Heart J. 1986;111:383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- Lethbridge L, Johnston GM, Turnbull G. Co-morbidities of persons dying of Parkinson’s disease. Prog. Palliat Care. 2013;21:140–145. doi: 10.1179/1743291X12Y.0000000037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Kroenke CH, Epel E, Kenna HA, Wolkowitz OM, Blackburn E, Rasgon NL. Greater endogenous estrogen exposure is associated with longer telomeres in postmenopausal women at risk for cognitive decline. Brain Res. 2011;1379:224–231. doi: 10.1016/j.brainres.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XK, Jahangir A, Terzic A, Gersh BJ, Hammill SC, Shen WK. Age- and sex-related atrial electrophysiologic and structural changes. Am. J. Cardiol. 2004;94:373–375. doi: 10.1016/j.amjcard.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, D’Agostino RB, Massaro JM, Beiser A, Wolf PA, Benjamin EJ. Lifetime risk for development of atrial fibrillation: the framingham heart study. Circulation. 2004;110:1042–1046. doi: 10.1161/01.CIR.0000140263.20897.42. [DOI] [PubMed] [Google Scholar]
- Luchner A, Bröckel U, Muscholl M, Hense H-W, Döring A, Riegger GAJ, Schunkert H. Gender-specific differences of cardiac remodeling in subjects with left ventricular dysfunction: a population-based study. Cardiovasc. Res. 2002;53:720–727. doi: 10.1016/S0008-6363(01)00510-7. [DOI] [PubMed] [Google Scholar]
- Magnani JW, Moser CB, Murabito JM, Sullivan LM, Wang N, Ellinor PT, Vasan RS, Benjamin EJ, Coviello AD. Association of sex hormones, aging, and atrial fibrillation in men: the Framingham Heart Study. Circ. Arrhythm. Electrophysiol. 2014;7:307–312. doi: 10.1161/CIRCEP.113.001322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malacrida R, Genoni M, Maggioni AP, Spataro V, Parish S, Palmer A, Collins R, Moccetti T. A comparison of the early outcome of acute myocardial infarction in women and men. The Third international study of infarct survival collaborative group. N. Engl. J. Med. 1998;338:8–14. doi: 10.1056/NEJM199801013380102. [DOI] [PubMed] [Google Scholar]
- Mangmool S, Denkaew T, Parichatikanond W, Kurose H. Beta-adrenergic receptor and insulin resistance in the heart. Biomol. Ther (Seoul) 2017;25:44–56. doi: 10.4062/biomolther.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Laez R, Caballero-Arzapalo H, Valle-San Roman N, Lopez-Menendez LA, Arango-Lasprilla JC, Vazquez-Barquero A. Incidence of Idiopathic Normal-Pressure Hydrocephalus in Northern Spain. World Neurosurg. 2016;87:298–310. doi: 10.1016/j.wneu.2015.10.069. [DOI] [PubMed] [Google Scholar]
- Maynard C, Litwin PE, Martin JS, Weaver WD. Gender differences in the treatment and outcome of acute myocardial infarction. Results from the Myocardial Infarction Triage and Intervention Registry. Arch. Intern. Med. 1992;152:972–976. doi: 10.1001/archinte.1992.00400170062012. [DOI] [PubMed] [Google Scholar]
- Mendelsohn ME, Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- Mendes LA, Davidoff R, Cupples LA, Ryan TJ, Jacobs AK. Congestive heart failure in patients with coronary artery disease: the gender paradox. Am. Heart J. 1997;134:207–212. doi: 10.1016/S0002-8703(97)70126-1. [DOI] [PubMed] [Google Scholar]
- Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mahonen M, Ngu Blackett K, Lisheng L. World Health Organization definition of myocardial infarction: 2008–09 revision. Int. J. Epidemiol. 2011;40:139–146. doi: 10.1093/ije/dyq165. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: assessing sex and gender differences. Clin. Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka Y, Barnes ME, Petersen RC, Cha SS, Bailey KR, Gersh BJ, Casaclang-Verzosa G, Abhayaratna WP, Seward JB, Iwasaka T, Tsang TS. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a community-based cohort. Eur. Heart J. 2007;28:1962–1967. doi: 10.1093/eurheartj/ehm012. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics-2016 update: a report from the American heart association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- Musicco M. Gender differences in the occurrence of Alzheimer’s disease. Funct. Neurol. 2009;24:89–92. [PubMed] [Google Scholar]
- Naccarelli GV, Varker H, Lin J, Schulman KL. Increasing prevalence of atrial fibrillation and flutter in the United States. Am. J. Cardiol. 2009;104:1534–1539. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Nelson PT, Schmitt FA, Jicha GA, Kryscio RJ, Abner EL, Smith CD, Van Eldik LJ, Markesbery WR. Association between male gender and cortical Lewy body pathology in large autopsy series. J. Neurol. 2010;257:1875–1881. doi: 10.1007/s00415-010-5630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Fitzpatrick AL, Lopez O, Jackson S, Lyketsos C, Jagust W, Ives D, Dekosky ST, Kuller LH. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J. Am. Geriatr. Soc. 2005;53:1101–1107. doi: 10.1111/j.1532-5415.2005.53360.x. [DOI] [PubMed] [Google Scholar]
- Noale M, Limongi F, Zambon S, Crepaldi G, Maggi S. Incidence of dementia: evidence for an effect modification by gender. The ILSA study. Int. Psychogeriatr. 2013;25:1867–1876. doi: 10.1017/S1041610213001300. [DOI] [PubMed] [Google Scholar]
- O’Connell JE, Gray CS, French JM, Robertson IH. Atrial fibrillation and cognitive function: case-control study. J. Neurol. Neurosurg Psychiatry. 1998;65:386–389. doi: 10.1136/jnnp.65.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostchega Y, Yoon SS, Hughes J, Louis T. Hypertension awareness, treatment, and control--continued disparities in adults: United States, 2005–2006. NCHS Data Brief. 2008;(3):1–8. [PubMed] [Google Scholar]
- Ott A, Breteler MM, de Bruyne MC, van Harskamp F, Grobbee DE, Hofman A. Atrial fibrillation and dementia in a population-based study. The Rotterdam study. Stroke. 1997;28:316–321. doi: 10.1161/01.STR.28.2.316. [DOI] [PubMed] [Google Scholar]
- Patten RD, Pourati I, Aronovitz MJ, Baur J, Celestin F, Chen X, Michael A, Haq S, Nuedling S, Grohe C, Force T, Mendelsohn ME, Karas RH. 17beta-estradiol reduces cardiomyocyte apoptosis in vivo and in vitro via activation of phospho-inositide-3 kinase/Akt signaling. Circ. Res. 2004;95:692–699. doi: 10.1161/01.RES.0000144126.57786.89. [DOI] [PubMed] [Google Scholar]
- Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- Petersen P, Kastrup J, Videbaek R, Boysen G. Cerebral blood flow before and after cardioversion of atrial fibrillation. J. Cereb. Blood Flow Metab. 1989;9:422–425. doi: 10.1038/jcbfm.1989.62. [DOI] [PubMed] [Google Scholar]
- Petrovitch H, White L, Masaki KH, Ross GW, Abbott RD, Rodriguez BL, Lu G, Burchfiel CM, Blanchette PL, Curb JD. Influence of myocardial infarction, coronary artery by-pass surgery, and stroke on cognitive impairment in late life. Am. J. Cardiol. 1998;81:1017–1021. doi: 10.1016/S0002-9149(98)00082-4. [DOI] [PubMed] [Google Scholar]
- Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993–2007. Circ. Cardiovasc. Qual Outcomes. 2012;5:85–93. doi: 10.1161/CIRCOUTCOMES.111.962688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podcasy JL, Epperson CN. Considering sex and gender in Alzheimer disease and other dementias. Dialogues. Clin. Neurosci. 2016;18:437–446. doi: 10.31887/DCNS.2016.18.4/cepperson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullicino PM, Hart J. Cognitive impairment in congestive heart failure?: Embolism vs hypoperfusion. Neurology. 2001;57:1945–1946. doi: 10.1212/WNL.57.11.1945. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch. Intern. Med. 2006;166:1003–1008. doi: 10.1001/archinte.166.9.1003. [DOI] [PubMed] [Google Scholar]
- Rastas S, Verkkoniemi A, Polvikoski T, Juva K, Niinisto L, Mattila K, Lansimies E, Pirttila T, Sulkava R. Atrial fibrillation, stroke, and cognition: a longitudinal population-based study of people aged 85 and older. Stroke. 2007;38:1454–1460. doi: 10.1161/STROKEAHA.106.477299. [DOI] [PubMed] [Google Scholar]
- Ravn LS, Hofman-Bang J, Dixen U, Larsen SO, Jensen G, Haunso S, Svendsen JH, Christiansen M. Relation of 97T polymorphism in KCNE5 to risk of atrial fibrillation. Am. J. Cardiol. 2005;96:405–407. doi: 10.1016/j.amjcard.2005.03.086. [DOI] [PubMed] [Google Scholar]
- Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age- and gender-related ventricular-vascular stiffening: a community-based study. Circulation. 2005;112:2254–2262. doi: 10.1161/CIRCULATIONAHA.105.541078. [DOI] [PubMed] [Google Scholar]
- Roberts RO, Knopman DS, Geda YE, Cha RH, Roger VL, Petersen RC. Coronary heart disease is associated with non-amnestic mild cognitive impairment. Neurobiol Aging. 2010;31:1894–1902. doi: 10.1016/j.neurobiolaging.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American heart association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosano C, Naydeck B, Kuller LH, Longstreth WT, Jr, Newman AB. Coronary artery calcium: associations with brain magnetic resonance imaging abnormalities and cognitive status. J. Am. Geriatr. Soc. 2005;53:609–615. doi: 10.1111/j.1532-5415.2005.53208.x. [DOI] [PubMed] [Google Scholar]
- Rosengren A, Spetz CL, Koster M, Hammar N, Alfredsson L, Rosen M. Sex differences in survival after myocardial infarction in Sweden; data from the Swedish national acute myocardial infarction register. Eur. Heart J. 2001;22:314–322. doi: 10.1053/euhj.2000.2368. [DOI] [PubMed] [Google Scholar]
- Santangeli P, Di Biase L, Bai R, Mohanty S, Pump A, Cereceda Brantes M, Horton R, Burkhardt JD, Lakkireddy D, Reddy YM, Casella M, Dello Russo A, Tondo C, Natale A. Atrial fibrillation and the risk of incident dementia: a meta-analysis. Heart Rhythm. 2012;9:1761–1768. doi: 10.1016/j.hrthm.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol. 2013;70:1396–1402. doi: 10.1001/jamaneurol.2013.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. The significance of arterial hypertension for the development of dementia. MMW Fortschr. Med. 2004;146:40–42. [PubMed] [Google Scholar]
- Seshadri S, Wolf PA, Beiser A, Au R, McNulty K, White R, D’Agostino RB. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the framingham study. Neurology. 1997;49:1498–1504. doi: 10.1212/WNL.49.6.1498. [DOI] [PubMed] [Google Scholar]
- Shah NS, Vidal JS, Masaki K, Petrovitch H, Ross GW, Tilley C, DeMattos RB, Tracy RP, White LR, Launer LJ. Midlife blood pressure, plasma beta-amyloid, and the risk for Alzheimer disease: the honolulu asia aging study. Hypertension. 2012;59:780–786. doi: 10.1161/HYPERTENSIONAHA.111.178962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G. Insights from the NHLBI-sponsored women’s ischemia syndrome evaluation (WISE) study: part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J. Am. Coll. Cardiol. 2006;47:S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Sabia S, Lajnef M, Ferrie JE, Nabi H, Britton AR, Marmot MG, Shipley MJ. History of coronary heart disease and cognitive performance in midlife: the Whitehall II study. Eur. Heart J. 2008;29:2100–2107. doi: 10.1093/eurheartj/ehn298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siraj S. An overview of normal pressure hydrocephalus and its importance: how much do we really know? J. Am. Med. Dir. Assoc. 2011;12:19–21. doi: 10.1016/j.jamda.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Skillback T, Rosen C, Asztely F, Mattsson N, Blennow K, Zetterberg H. Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: results from the Swedish Mortality Registry. JAMA Neurol. 2014;71:476–483. doi: 10.1001/jamaneurol.2013.6455. [DOI] [PubMed] [Google Scholar]
- Staessen JA, Richart T, Birkenhager WH. Less atherosclerosis and lower blood pressure for a meaningful life perspective with more brain. Hypertension. 2007;49:389–400. doi: 10.1161/01.HYP.0000258151.00728.d8. [DOI] [PubMed] [Google Scholar]
- Sundboll J, Horvath-Puho E, Adelborg K, Schmidt M, Pedersen L, Botker HE, Henderson VW, Toft Sorensen H. Higher risk of vascular dementia in myocardial infarction survivors. Circulation. 2018;137:567–577. doi: 10.1161/CIRCULATIONAHA.117.029127. [DOI] [PubMed] [Google Scholar]
- Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation. 2015;131:2176–2184. doi: 10.1161/CIRCULATIONAHA.114.014343. [DOI] [PubMed] [Google Scholar]
- Taylor AL. Heart failure in women. Curr. Heart Fail. Rep. 2015;12:187–195. doi: 10.1007/s11897-015-0252-x. [DOI] [PubMed] [Google Scholar]
- The Canadian Study of health and Aging Working Group The incidence of dementia in Canade. Neurology. 2000;55:66–71. doi: 10.1212/WNL.55.1.66. [DOI] [PubMed] [Google Scholar]
- Trojano L, Antonelli Incalzi R, Acanfora D, Picone C, Mecocci P, Rengo F. Cognitive impairment: a key feature of congestive heart failure in the elderly. J. Neurol. 2003;250:1456–1463. doi: 10.1007/s00415-003-0249-3. [DOI] [PubMed] [Google Scholar]
- Tsuneda T, Yamashita T, Kato T, Sekiguchi A, Sagara K, Sawada H, Aizawa T, Fu LT, Fujiki A, Inoue H. Deficiency of testosterone associates with the substrate of atrial fibrillation in the rat model. J. Cardiovasc. Electrophysiol. 2009;20:1055–1060. doi: 10.1111/j.1540-8167.2009.01474.x. [DOI] [PubMed] [Google Scholar]
- Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N. Engl. J. Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- Wilke T, Groth A, Mueller S, Pfannkuche M, Verheyen F, Linder R, Maywald U, Bauersachs R, Breithardt G. Incidence and prevalence of atrial fibrillation: an analysis based on 8.3 million patients. Europace. 2013;15:486–493. doi: 10.1093/europace/eus333. [DOI] [PubMed] [Google Scholar]
- Wozakowska-Kaplon B, Opolski G, Kosior D, Jaskulska-Niedziela E, Maroszynska-Dmoch E, Wlosowicz M. Cognitive disorders in elderly patients with permanent atrial fibrillation. Kardiol. Pol. 2009;67:487–493. [PubMed] [Google Scholar]
- Wu YT, Fratiglioni L, Matthews FE, Lobo A, Breteler MM, Skoog I, Brayne C. Dementia in western Europe: epidemiological evidence and implications for policy making. Lancet Neurol. 2016;15:116–124. doi: 10.1016/S1474-4422(15)00092-7. [DOI] [PubMed] [Google Scholar]
- Xiao X, Liu HX, Shen K, Cao W, Li XQ. Canonical transient receptor potential channels and their link with cardio/cerebro-vascular diseases. Biomol. Ther (Seoul) 2017;25:471–481. doi: 10.4062/biomolther.2016.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Kasagi F, Sasaki H, Masunari N, Mimori Y, Suzuki G. Association between dementia and midlife risk factors: the radiation effects research foundation adult health study. J. Am. Geriatr. Soc. 2003;51:410–414. doi: 10.1046/j.1532-5415.2003.51117.x. [DOI] [PubMed] [Google Scholar]
- Yamada M, Mimori Y, Kasagi F, Miyachi T, Ohshita T, Sudoh S, Ikeda J, Matsui K, Nakamura S, Matsumoto M, Fujiwara S, Sasaki H. Incidence of dementia, Alzheimer disease, and vascular dementia in a Japanese population: radiation effects research foundation adult health study. Neuroepidemiology. 2008;30:152–160. doi: 10.1159/000122332. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- Zuccala G, Onder G, Pedone C, Carosella L, Pahor M, Bernabei R, Cocchi A. Hypotension and cognitive impairment: selective association in patients with heart failure. Neurology. 2001;57:1986–1992. doi: 10.1212/WNL.57.11.1986. [DOI] [PubMed] [Google Scholar]