Abstract
Epigenetic silencing is considered to be a major mechanism for loss of activity in tumor suppressors. Reversal of epigenetic silencing by using inhibitors of DNA methyltransferase (DNMT) or histone deacetylases (HDACs) such as 5-Aza-CdR and FK228 has shown to enhance cytotoxic activities of several anticancer agents. This study aims to assess the combinatorial effects of gene-silencing reversal agents (5-Aza-CdR and FK228) and oxaliplatin in gastric cancer cells, i.e., Epstein-Barr virus (EBV)-negative SNU-638 and EBV-positive SNU-719 cells. The doublet combinatorial treatment of 5-Aza-CdR and FK228 exhibited synergistic effects in both cell lines, and this was further corroborated by Zta expression induction in SNU-719 cells. Three drug combinations as 5-Aza-CdR/FK228 followed by oxaliplatin, however, resulted in antagonistic effects in both cell lines. Simultaneous treatment with FK228 and oxaliplatin induced synergistic and additive effects in SNU-638 and SNU-719 cells, respectively. Three drug combinations as 5-Aza-CdR prior to FK228/oxaliplatin, however, again resulted in antagonistic effects in both cell lines. This work demonstrated that efficacy of doublet synergistic combination using DNMT or HDACs inhibitors can be compromised by adding the third drug in pre- or post-treatment approach in gastric cancer cells. This implies that the development of clinical trial protocols for triplet combinations using gene-silencing reversal agents should be carefully evaluated in light of their potential antagonistic effects.
Keywords: Combinatorial index, EBV, Zta, Synergism, Antagonism
INTRODUCTION
Gastric cancer is one of the leading causes of cancer-related death worldwide, and Epstein-Barr virus (EBV)-associated gastric cancer comprises 1.3 to 20.1% of all cases of gastric cancer worldwide, making EBV one of the important causative agents for gastric cancer (Lee et al., 2009; Stanfield and Luftig, 2017). Combinatorial chemotherapy using cisplatin/5-fluorouracil (5-FU), docetaxel/cisplatin/5-FU (DCF), and epirubicin/cisplatin/5-FU (ECF) has been recommended as a standard treatment for gastric cancer (Morabito et al., 2009; Takayama et al., 2010). However, insufficient clinical efficacy of chemotherapy has been reported and attributed to drug resistance in gastric cancers (Shah and Ajani, 2010). EBV has also been known to induce chemoresistance in lymphoma and gastric cancer cells, as latent EBV gene products were related to impaired function of cell cycle check point and apoptosis (Leao et al., 2007; Banerjee et al., 2013; Kim et al., 2015; Yoon and Ko, 2017).
Oxaliplatin (EloxatinTM, LOHP) has been shown to possess potent cytotoxicity in vitro, and so is a promising candidate for treating advanced gastric cancer (Eriguchi et al., 2003; Chao et al., 2004; Bang et al., 2012). In fact, oxaliplatin showed a reduced toxicity and a decreased likelihood of developing resistance compared to another anticancer drug, cisplatin (Montagnani et al., 2011). Several previous studies have shown that combination treatment regimens that combine oxaliplatin with other anticancer agents can produce synergistic effects in treating human gastric cancer (Tanaka et al., 2005; Gu et al., 2006; Luo et al., 2010; Shi et al., 2013). New and effective treatment regimens for advanced gastric cancer, however, continue to lack in terms of treatment strategy, which is an important in improving treatment efficacy.
The loss of cellular tumor suppressing activity is now broadly accepted as an important step towards tumor initiation and growth. Furthermore, epigenetic silencing has also been shown to be a major mechanism causing the loss of tumor suppressor activity. Previous work has demonstrated the significance of altered gene expression by epigenetic silencing as many tumor suppressor genes are inactivated by hypermethylation and/or hypoacetylation in several cancers (Cameron et al., 1999; Fuks, 2005; Palii and Robertson, 2007). Epigenetic silencing is caused by integration of promoter hypermethylation with histone deacetylation, which is controlled by regulatory proteins such as DNA methyltransferase (DNMT), histone acetyltransferases (HATs), and histone deacetylases (HDACs) (Cameron et al., 1999; Baylin and Ohm, 2006; Jones and Baylin, 2007).
Several inhibitors, targeting DNMT and histone deacetylase, have been effectively utilized as anticancer drugs. For example, 5-aza-2′-deoxycytidine (5-Aza-CdR, DacogenTM, decitabine) is a DNMT inhibitor that successfully ameliorates cancer growth at a non-cytotoxic concentration in promyelocytic leukemia cells (Christman et al., 1983). Previous work has also shown that the cytotoxic activities of cisplatin and paclitaxel could be enhanced by co-treatment with 5-Aza-CdR, which acts to upregulate caspase-9 in lung cancer (Gomyo et al., 2004). It has also previously shown that 5-Aza-CdR induces apoptosis by both demethylating several apoptosis-related genes and through an unknown DNA methylation-independent mechanism, leading it to be approved by the FDA for myelodysplastic syndrome (MDS) treatment (Kuendgen and Lubbert, 2008; Li et al., 2014; Momparler et al., 2017). Other recent work has reported that low doses of DNMT inhibitors, such as Azacitidine and 5-Aza-CdR, cause antitumor effects for hematological neoplasms (Tsai et al., 2012). Among the class of histone deacetylase inhibitors (HDACIs), FK228 (Romidepsin, depsipeptide) has also previously been shown to possess potent anticancer activity by arresting the cell cycle as well as directly inducing cell death through various mechanisms (Mizutani et al., 2010; Ierano et al., 2013; Sun et al., 2017). Recently, combining bortezomib and 5-azacytidine provides a novel therapeutic method for treating EBV-associated gastric cancer (Fukayama, 2010).
This study aims to assess the combinatorial effects of gene silencing reversal agents, 5-Aza-CdR and FK228, and oxaliplatin in gastric cancer cells. We evaluated the combinatorial effect of these agents in an EBV-negative gastric cancer cell line (SNU-638) and an EBV-positive gastric cancer cell line (SNU-719). We combined these agents in doublet combination prior to or followed by the third agent. This approach was based on the recent studies in which doublet chemotherapy was suggested as a favored first-line therapy, and a sequential treatment (adding a third drug to a doublet chemotherapy) was recommended in terms of comparable overall survival and favorable toxicity profile in advanced gastric cancer patients (Bittoni et al., 2015; Laterza et al., 2017). We report varying degrees of synergistic or antagonistic combinational effects of 5-Aza-CdR, FK228, and oxaliplatin, which depends on the combinatorial regimen such as the dose and treatment schedule. These findings suggest that development of clinical trial protocols for triplet combinations of these three agents should be carefully designed and evaluated although combination of gene silencing reversal agents with oxaliplatin may offer therapeutic potential for treating gastric cancer.
MATERIALS AND METHODS
Chemicals and reagents
5-Aza-2′-deoxycytidine (5-Aza-CdR, DacogenTM, decitabine) was purchased from Sigma-Aldrich (St. Louis, MO, USA). FK228 (Romidepsin, depsipeptide) and oxaliplatin (EloxatinTM, LOHP) was kindly provided by Sanofi-Aventis (Malvern, PA, USA). The tetrazolium compound (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) (MTS) was purchased from Promega (Madison, WI, USA). All cell culture reagents were purchased from Invitrogen (Carlsbad, CA, USA), while normal goat serum and the Zta antibody were purchased from Jackson ImmunoResearch Laboratory, Inc (West Grove, PA, USA) and Dako (Kyoto, Japan), respectively. Cy3-conjugated secondary antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). All other reagents including phenazine methosulfate (PMS) and trichostatin A (TSA) were purchased from Sigma-Aldrich unless otherwise noted.
Cell culture conditions
Both human gastric cancer cell lines, SNU-638 (the EBV-negative) and SNU-719 (the EBV-positive), were obtained from the Korea Cell Line Bank (Seoul, Korea). Cells were maintained in RPMI-1640 media supplemented with 10% FBS in a humidified 5% (v/v) CO2 atmosphere at 37°C.
Cytotoxicity measurements
Cell viability was assessed using the MTS assay according to the manufacturer’s instructions. Briefly, this involves growing cells exponentially before seeding in 96-well plates at either 500 cells/well (SNU-638) or 2000 cells/well (SNU-719). Cells were then incubated overnight and exposed to various concentrations and combinations of drugs for the time indicated. After drug exposure, 20 μL of MTS/PMS solution was added to each well. After an additional 2–3 h of incubation, we measured absorbance at 490 nm.
Immunofluorescence assay (IFA)
EBV lytic gene induction was assessed after single or combined drug exposure. TSA-treated cells were used as a reference for lytic induction. Briefly, SNU-719 cells were placed on eight-well microscope slides (4×105 cells/well) where they were treated with the drug/drug combination indicated. After 24 or 48 h, cells were harvested, fixed with 100% ice-cold methanol, and blocked with 20% normal goat serum for 20 min at room temperature. After incubating with Zta antibody (1:40) for 1 h at room temperature, cells were washed with PBS and then incubated with the Cy3-conjugated secondary antibody in the dark for 1 h at room temperature. The protein expression level of fluorescent-labeled Zta was determined using a confocal microscope (Bio-Rad MRC-1024, Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Data analysis
The IC50 was defined as the drug concentration at which cell viability is equal to 50% of the no drug control, and calculated using Eq. 1 and Eq. 2:
| (Eq. 1) |
| (Eq. 2) |
where D is the drug concentration, m is the Hill-type coefficient, R is the residual unaffected fraction (the resistance fraction), and Kd is the concentration of drug that produces a 50% of the drug’s maximum effect (Emax, 100-R) (Roell et al., 2017).
Drug interactions were characterized by using a combination index (CI) for various levels of cell death (Eq. 3). CI was calculated for an effect level of 50–80 %, i.e., CI50–CI80.
| (Eq. 3) |
where CIx is CI for a fixed effect level x identified for a combination of drug A and drug B. (DX)A and (DX)B are the concentrations of drug A or B necessary to produce effect x when applied in isolation. (D)A and (D)B are the concentrations of drug A or B required to produce effect x when applied in combination. α is 0 when A and B are mutually exclusive, and 1 when A and B are mutually non-exclusive (Chou and Talalay, 1984; Chung et al., 2009). CIx between 0.8 and 1.2 was defined as additive, ≤0.8 as synergistic, and ≥1.2 as antagonistic (Roell et al., 2017). The doublet or triplet combinations at fixed concentrations were analyzed by comparing experimental data to the reference additivity values calculated using Bliss independence model (Roell et al., 2017). The ratio of experimental survival rate to reference value between 0.8 and 1.2 was defined as additive, ≤0.8 as synergistic, and ≥1.2 as antagonistic.
RESULTS
Antiproliferative activity of 5-Aza-CdR, FK228, and oxaliplatin
We evaluated the antiproliferative activity of 5-Aza-CdR, FK228 and oxaliplatin treatment on both SNU-638 and SNU-719 gastric cell lines. We used model-fitting procedures to obtain the relevant pharmacodynamic parameters (Table 1). Cells were exposed to 5-Aza-CdR up to 25 μM, and high resistance fractions were shown ranging from 30.3% to 52.7% in both cell lines (Table 1). FK228 treatment produced strong antiproliferative effects with relatively low IC50 values in both cell lines (Table 1). It is noted, however, that EBV-positive SNU-719 cells were more resistant than SNU-638 cells as shown by significantly higher R values until 48 h exposure; R fraction was 55.3% and 25.5% in SNU-719 cells as compared to 31.7% and 4.11% in SNU-638 cells after 24 h and 48 h, respectively (p<0.01). Nonetheless, we found that the resistance was plummeted by prolonging the exposure time to 72 h (Table 1). Treatment with oxaliplatin also required long exposures to obtain significant antiproliferative activity. For example, IC50 values of oxaliplatin at 72 h exposure were lower by 10- and 300-folds compared to those of 48 h in SNU-638 and SNU-719 cells, respectively (Table 1). Despite the decreased IC50 at 72 h exposure, sustained resistance to oxaliplatin was observed in EBV-positive SNU-719 cells as shown by particularly high resistance fraction (36.6%) (Table 1).
Table 1.
Antiproliferative activity of 5-Aza-CdR, FK228 and oxaliplatin in SNU-638 and SNU-719 human gastric cancer cells
| SNU-638 | 24 h | 48 h | 72 h | |
|---|---|---|---|---|
| 5-Aza-CdR | IC50 | 4.52 ± 0.27 | 1.01 ± 0.13 | 0.89 ± 0.21 |
| R | 36.1 ± 9.9 | 33.9 ± 4.2 | 30.3 ± 5.8 | |
| FK228 | IC50 | 16.4 ± 8.9 | 3.72 ± 0.03 | 2.68 ± 0.13 |
| R | 31.7 ± 1.9 | 4.11 ± 4.00 | 2.56 ± 2.24 | |
| Oxaliplatin | IC50 | 35.4 ± 7.3 | 6.63 ± 1.24 | 0.68 ± 0.15 |
| R | 39.6 ± 6.6 | 15.2 ± 18.2 | 13.0 ± 6.15 | |
| SNU-719 | 24 h | 48 h | 72 h | |
|---|---|---|---|---|
| 5-Aza-CdR | IC50 | ND | 7.72 ± 0.01 | 5.76 ± 3.44 |
| R | 52.7 ± 1.9 | 48.1 ± 3.1 | 47.8 ± 1.9 | |
| FK228 | IC50 | ND | 5.61 ± 0.08 | 3.06 ± 0.004 |
| R | 55.3 ± 4.2 | 25.5 ± 2.05 | 2.67 ± 2.03 | |
| Oxaliplatin | IC50 | ND | 328 ± 3.9 | 1.24 ± 0.07 |
| R | 72.7 ± 5.07 | 47.8 ± 2.0 | 36.6 ± 0.76 | |
Cell viability was determined using the MTS assay. IC50, the drug concentration that produces a 50% of the drug’s maximum effect following 48 or 72 h of continuous exposure; expressed in nM for FK228 and μM for 5-Aza-CdR and oxaliplatin. R, the residual unaffected fraction (resistance fraction); IC50 and R values were determined from dose-response curves analyzed using Eq. 1 and Eq. 2 (see Materials and Methods). Data are presented as the mean ± SD from at least three independent experiments. ND, not determined.
Combinatorial effects of 5-Aza-CdR and FK228
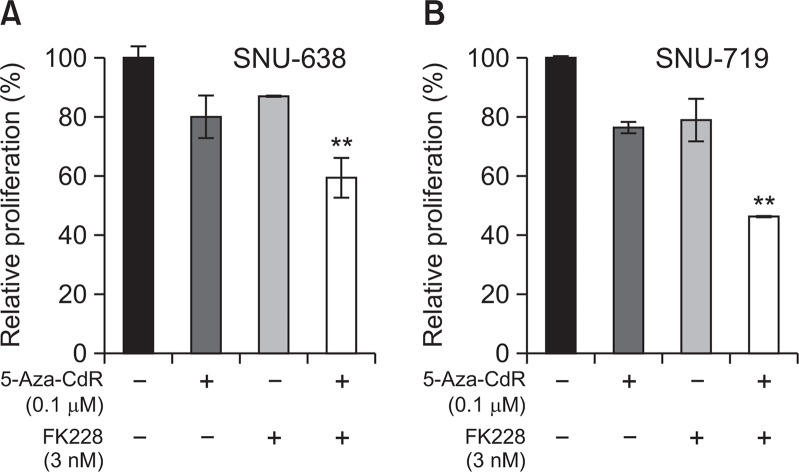
Doublet synergy of 5-Aza-CdR and FK228 was evaluated after 72 h post-exposure incubation following 24 h drug exposure (Fig. 1). Post-exposure period of 72 h was included in order to synchronize treatment schedule with the triplet combination where exposure to 5-Aza-CdR and FK228 doublet was followed by 72 h exposure to oxaliplatin. Simultaneous exposure to 0.1 μM of 5-Aza-CdR and 3 nM of FK228 produced additive to synergistic antiproliferative effects in both SNU-638 and SNU-719 cells (Fig. 1). Combination using a higher concentration of 5-Aza-CdR (0.25 μM) also showed synergistic effects in both cell lines (data not shown). However, the synergism was not obtained when 5-Aza-CdR was combined with FK228 at concentrations below 3 nM concentration (data not shown).
Fig. 1.
Effects of combined treatment with 5-Aza-CdR and FK228. SNU-638 (A) and SNU-719 cells (B) were treated with single agent of 5-Aza-CdR (0.1 μM) and FK228 (3 nM), or with doublet combination of 5-Aza-CdR/FK228 for 24 h. After removal of drug-containing media, cells were further incubated in drug-free media for an additional 72 h and were then subjected to MTS assay. Data are presented as the survival rate relative to the control, which was assigned a value of “100%”. Data are presented as the mean ± SD from three independent experiments. ** indicates synergistic effects.
Induction of Zta expression in EBV-positive gastric cancer cells
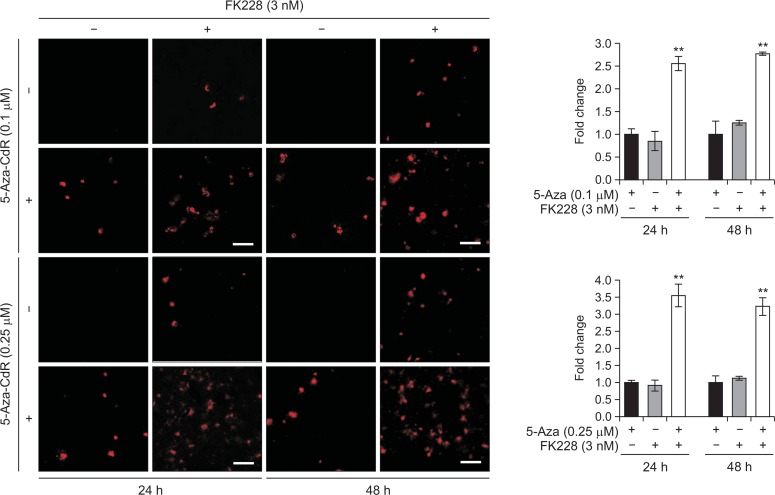
Previous study has reported that the expression of viral immediate-early antigens such as Zta (EB1, BZLF1, ZEBRA) can be inhibited by CpG methylation (Feng et al., 2002). Furthermore, it has been shown that the expression of these genes can induce the lytic reactivation and death of tumor cells (Cayrol and Flemington, 1996; Hui et al., 2012; Wildeman et al., 2012). As such, we next sought to investigate whether the antiproliferative effect of gene silencing reversal agents is associated with the induction of lytic EBV genes in EBV-positive cells (SNU-719 cells). We found that Zta protein expression was dramatically elevated up to 37.5% and 85.6% above the expected value for additive interaction between 5-Aza-CdR and FK228 for 24 h and 48 h, respectively (Fig. 2). Additionally, the combinatorial treatment of 5-Aza-CdR with another HDAC inhibitor, trichostatin A (TSA), showed similar synergistic effects in terms of Zta expression (data not shown). These data suggest that lytic reactivation may be one of underlying mechanisms for synergistic cytotoxicity induced by 5-Aza-CdR and FK228 in EBV-positive SNU-719 cells.
Fig. 2.
Zta induction after treatment with 5-Aza-CdR and/or FK228 in SNU-719 cells. Immunofluorescence was used to measure Zta. Representative fluorescence images are shown using anti-Zta primary antibody (1:40) after 24 h and 48 h of drug treatment. Bars, 100 μm. The data are expressed as relative fold change to control, 5-Aza-CdR single treatment. Data are presented as the mean ± SD from three independent experiments. ** indicates synergistic effects.
Effects of sequential treatment with 5-Aza-CdR/FK228 and oxaliplatin
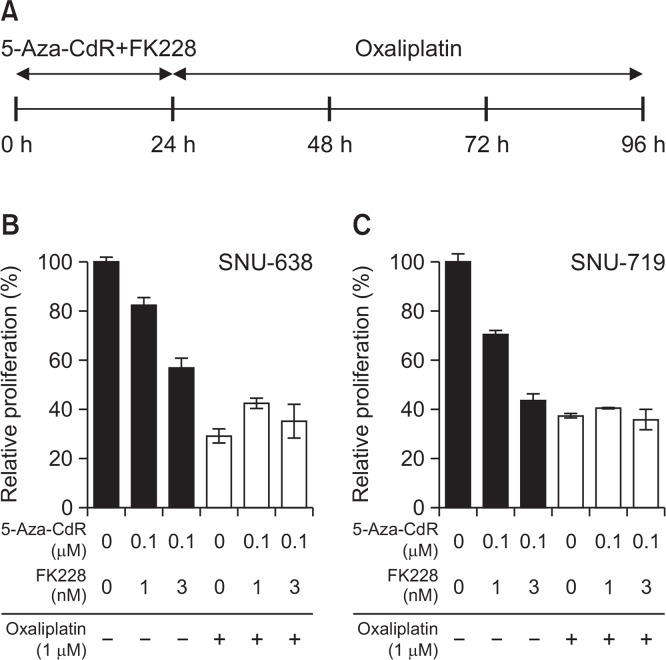
Having identified synergistic effects between 5-Aza-CdR and FK228, we next sought to investigate the combination effect when oxaliplatin was added in a sequential manner. Cells were exposed to a doublet pre-treatment using 0.1 μM of 5-Aza-CdR and 1 nM or 3 nM of FK228 for 24 h and then subsequently to 1 μM oxaliplatin for an additional 72 h (Fig. 3A). Survival rates were determined using the MTS assay at 96 h. Oxaliplatin (1 μM) alone resulted in about 29.1% survival in SNU-638 cells (Fig. 3B). When combined with pre-treatment of 5-Aza-CdR/FK228, severe antagonistic effects were induced as shown by 2 folds higher proliferation rates compared to the expected values at two different concentration levels of 5-Aza-CdR/FK228 in SNU-638 cells (Fig. 3B). Similar level of antagonism occurred in SNU-719 cells (Fig. 3C). Antagonistic effect was also observed when either using higher concentrations of oxaliplatin (5 μM and 25 μM) or by prolonging the pre-treatment doublet for 48 h followed by 48 h exposure to oxaliplatin in both cell lines (data not shown).
Fig. 3.
Pre-treatment with 5-Aza-CdR/FK228 antagonized the anti-proliferative effect of oxaliplatin. Cells were pre-treated with 5-Aza-CdR (0.1 μM)/FK228 (1 nM or 3 nM) for 24 h, washed and then exposed to 1 μM of oxaliplatin for subsequent 72 h (A). Relative rate of proliferation was determined in SNU-638 (B) and SNU-719 (C) cells using the MTS assay. Data are presented as the mean ± SD from three independent experiments. All triplet combinations showed antagonism in both cell lines.
Combinatorial effects of 5-Aza-CdR pre-treatment followed by simultaneous FK228/oxaliplatin treatment
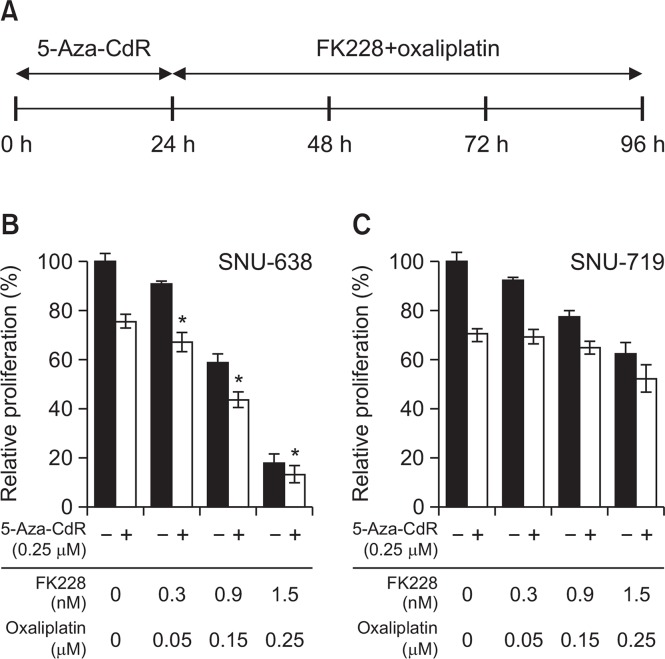
After unexpectedly identifying antagonistic interactions between gene silencing reversal agents (5-Aza-CdR/FK228) and oxaliplatin, we sought to modify the combination schedule. We first determined the combinatorial effect of FK228 and oxaliplatin given at the IC50 molar ratio (1:170 at 72 h in both cell lines). The combination indexes (CI) were between 0.6 and 0.73 in SNU-638 cells and close to 1 in SNU-719 cells, indicating synergistic and additive interaction, respectively (Fig. 4). To that end, we tested the combination effect of 0.25 μM of 5-Aza-CdR treatment for 24 h prior to the doublet combination of FK228 and oxaliplatin (Fig. 5A). Pre-treatment with 5-Aza-CdR exhibited additive effect in SNU-638 cells, whereas antagonistic interaction in SNU-719 cells at all concentration tested (Fig. 5B, 5C). Prolongation of pre-treatment duration up to 48 h showed similar results in both cell lines, suggesting that there would be little benefit to further pursuit these three-drug combinations in both cell lines (data not shown).
Fig. 4.
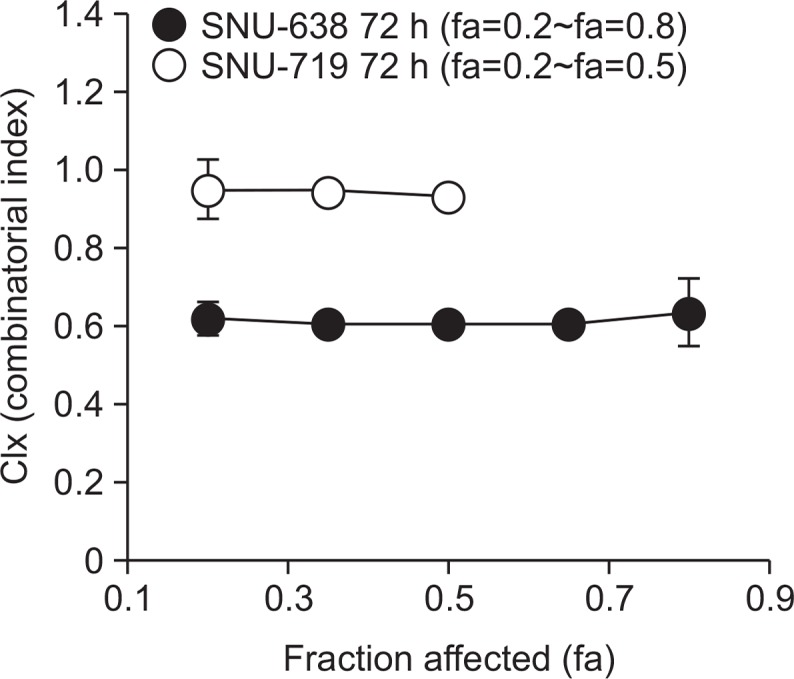
Combination indexes (CIx) versus affected fraction (fa) determined for combination of FK228 and oxaliplatin. Cells were treated with FK228/oxaliplatin for 72 h at fixed equitoxic ratios. CIx≤0.8, CIx≥1.2, and 0.8<CIx<1.2 indicate synergism, antagonism, and additivity, respectively (See methods for details).
Fig. 5.
Combination effects of 5-Aza-CdR pre-treatment combined with FK228/oxaliplatin treatment. Cells were treated with 0.25 μM of 5-Aza-CdR for 24 h, and then incubated for 72 h with FK228/oxaliplatin (A). SNU-638 (B) and SNU-719 (C) cells were exposed to drugs at the concentrations indicated. Relative rate of proliferation was determined using the MTS assay. Data are presented as the mean ± SD based on at least three replicate experiments. * indicates additive effects.
DISCUSSION
We investigated the effects of combined treatments involving 5-Aza-CdR, FK228, and oxaliplatin in both EBV-negative SNU-638 and EBV-positive SNU-719 cells. In drug combination studies, drugs are generally combined at the concentrations producing equal to or less than 50% of activity of each drug given alone, which may avoid potential toxic effects when translated into patient treatment. We used drug concentrations lower than IC50 of each drug, i.e., for doublet combination, 0.1–0.25 μM of 5-Aza-CdR was combined with 1–3 nM of FK228 compared to the IC50,72 h of 0.89–5.76 μM and 2.68–3.06 nM for 5-Aza-CdR and FK228, respectively (Table 1, Fig. 1, 2, 3). When FK228 was combined with oxaliplatin, even lower concentration as low as 0.3–1.5 nM of FK228 and 0.05–0.25 μM of oxaliplatin was used compared to the oxaliplatin IC50,72 h of 0.68–1.24 μM (Fig. 4, 5). In this work, we found that doublet combination of 5-Aza-CdR and FK228 exhibited synergistic effects in gastric cancer cell lines (Fig. 1). Combination of FK228 with other drugs at low concentration was reported to enhance the tumor response. Low concentration of 5-Aza-CdR is also known to sustain anti-tumor effects even after stopping a treatment due to the activation of immune response (Kanzaki et al., 2007; Wang et al., 2013).
Combinatorial treatment using 5-Aza-CdR/FK228, 5-Aza-CdR/cisplatin, and FK228/cisplatin exhibited synergistic effects when used to treat several different cancers (Karam et al., 2007; Shang et al., 2008; Wilson et al., 2012). The synergism between 5-Aza-CdR and FK228 has been measured in clear cell renal carcinoma cells and triple-negative breast cancer cells, for which induction of secreted frizzled-related protein 1 (sFRP1), a negative regulator of the Wnt signaling pathway, was detected (Cooper et al., 2012). On the other hand, combination therapy with gene silencing reversal agents was reported to cause antagonistic effects. For example, combined treatment with 5-Aza-CdR and hydroxy-carbamide has been shown to cause adverse effects on DNA methylation (Choi et al., 2007). Combined treatment of dimethoxycurcumin and 5-Aza-CdR exhibited antagonistic effects in primary leukemia cells (Hassan et al., 2016). Combination of FK228 with methotrexate and vincristine exhibited antagonistic effects when used on human leukemia and lymphoma cell lines (Kano et al., 2007). In our study, synergistic effects of 5-Aza-CdR and FK228 were shown and confirmed by Zta induction in SNU-719 cells (an EBV-positive cell line, Fig. 2). Zta expression in SNU-719 cells increased dramatically after combined treatment with 5-Aza-CdR and FK228 (Fig. 2). Since the activation of EBV lytic cycle is known to induce cell cycle arrest and apoptosis (Cayrol and Flemington, 1996; Hui et al., 2012; Wildeman et al., 2012), these findings suggest that combined treatment with 5-Aza-CdR and FK228 could provide a novel therapeutic strategy for treating EBV-positive gastric cancer. Additionally, the synergistic interaction of 5-Aza-CdR and FK228 in SNU-638 (EBV-negative cell line) can be due to the enhanced induction of sFRP1 (Cooper et al., 2012) and caspase-3 activation (Kalac et al., 2011).
In addition to 5-Aza-CdR/FK228 doublet combination, FK228 and oxaliplatin also demonstrated synergistic effects (Fig. 1, 4), and we next sought to assess the effects of triple combinations of 5-Aza-CdR, FK228 and oxaliplatin in gastric cancer cells. The triple combination of 5-Aza-CdR, FK228 and oxaliplatin not only fails to exhibit synergistic effects, but actually resulted in antagonistic effects; when 5-Aza-CdR/FK228 was given before oxaliplatin (Fig. 3) and when 5-Aza-CdR pre-treatment was combined with FK228 and oxaliplatin (Fig. 5). We speculated that the antagonistic effects measured for the triple combination treatment in our experiments may be a result of demethylating genes that are involved in tumor development (Ateeq et al., 2008), combined with the induction of P-glycoprotein by FK228 (Glaser, 2006; Ni et al., 2015). In addition, unexpected antagonism may be associated with cell cycle dependent interaction (Johnson et al., 1997; Sui et al., 2004; Xiong et al., 2007). Since 5-Aza-CdR and FK228 has been known to induce G0/G1 arrest in many types of cells (Lavelle et al., 2003; Son et al., 2010), 5-Aza-CdR/FK228 pre-treatment may interfere with antiproliferative effect of oxaliplatin, which is known to cause G2/M arrest (Xu et al., 2015).
Collectively, our findings suggest that the strategies for optimizing the dose and schedule are critical for developing effective drug combination therapies. Oxaliplatin can be used as part of combinatorial therapy when combined with gene silencing reversal agents; however, it is critical to carefully optimize the dose and time schedule. Although several recent studies have reported that triple combinations of anticancer drugs, including platin-based drugs, can be effective for treating several cancers, we found that the triple combination of 5-Aza-CdR, FK228 and oxaliplatin presented antagonistic interactions, which implies that the development of clinical trial protocols for triplet combinations using gene-silencing reversal agents should be carefully evaluated in light of their potential antagonistic effects.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea (NRF) funded by the Korean government (MEST) (No. 2016R1A2B2011832 and 2012R1A5A2047939) and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D-1A3B03035662).
Footnotes
CONFLICT OF INTEREST
The authors have no competing financial interests to declare.
REFERENCES
- Ateeq B, Unterberger A, Szyf M, Rabbani SA. Pharmacological inhibition of DNA methylation induces proinvasive and prometastatic genes in vitro and in vivo. Neoplasia. 2008;10:266–278. doi: 10.1593/neo.07947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee AS, Pal AD, Banerjee S. Epstein-Barr virus-encoded small non-coding RNAs induce cancer cell chemoresistance and migration. Virology. 2013;443:294–305. doi: 10.1016/j.virol.2013.05.020. [DOI] [PubMed] [Google Scholar]
- Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, Mok YJ, Kim YH, Ji J, Yeh TS, Button P, Sirzen F, Noh SH. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Ohm JE. Epigenetic gene silencing in cancer - a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- Bittoni A, Del Prete M, Scartozzi M, Pistelli M, Giampieri R, Faloppi L, Bianconi M, Cascinu S. Three drugs vs two drugs first-line chemotherapy regimen in advanced gastric cancer patients: a retrospective analysis. Springerplus. 2015;4:743. doi: 10.1186/s40064-015-1545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- Cayrol C, Flemington E. G0/G1 growth arrest mediated by a region encompassing the basic leucine zipper (bZIP) domain of the Epstein-Barr virus transactivator Zta. J Biol Chem. 1996;271:31799–31802. doi: 10.1074/jbc.271.50.31799. [DOI] [PubMed] [Google Scholar]
- Chao Y, Yeh KH, Chang CJ, Chen LT, Chao TY, Wu MF, Chang CS, Chang JY, Chung CY, Kao WY, Hsieh RK, Cheng AL. Phase II study of weekly oxaliplatin and 24-h infusion of high-dose 5-fluorouracil and folinic acid in the treatment of advanced gastric cancer. Br J Cancer. 2004;91:453–458. doi: 10.1038/sj.bjc.6601985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Byun HM, Kwan JM, Issa JP, Yang AS. Hydroxycarbamide in combination with azacitidine or decitabine is antagonistic on DNA methylation inhibition. Br J Haematol. 2007;138:616–623. doi: 10.1111/j.1365-2141.2007.06707.x. [DOI] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Christman JK, Mendelsohn N, Herzog D, Schneiderman N. Effect of 5-azacytidine on differentiation and DNA methylation in human promyelocytic leukemia cells (HL-60). Cancer Res. 1983;43:763–769. [PubMed] [Google Scholar]
- Chung WH, Sung BH, Kim SS, Rhim H, Kuh HJ. Synergistic interaction between tetra-arsenic oxide and paclitaxel in human cancer cells in vitro. Int J Oncol. 2009;34:1669–1679. [PubMed] [Google Scholar]
- Cooper SJ, von Roemeling CA, Kang KH, Marlow LA, Grebe SK, Menefee ME, Tun HW, Colon-Otero G, Perez EA, Copland JA. Reexpression of tumor suppressor, sFRP1, leads to antitumor synergy of combined HDAC and methyltransferase inhibitors in chemoresistant cancers. Mol Cancer Ther. 2012;11:2105–2115. doi: 10.1158/1535-7163.MCT-11-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriguchi M, Nonaka Y, Yanagie H, Yoshizaki I, Takeda Y, Sekiguchi M. A molecular biological study of anti-tumor mechanisms of an anti-cancer agent Oxaliplatin against established human gastric cancer cell lines. Biomed Pharmacother. 2003;57:412–415. doi: 10.1016/S0753-3322(03)00027-1. [DOI] [PubMed] [Google Scholar]
- Feng WH, Westphal E, Mauser A, Raab-Traub N, Gulley ML, Busson P, Kenney SC. Use of adenovirus vectors expressing Epstein-Barr virus (EBV) immediate-early protein BZLF1 or BRLF1 to treat EBV-positive tumors. J Virol. 2002;76:10951–10959. doi: 10.1128/JVI.76.21.10951-10959.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukayama M. Epstein-Barr virus and gastric carcinoma. Pathol Int. 2010;60:337–350. doi: 10.1111/j.1440-1827.2010.02533.x. [DOI] [PubMed] [Google Scholar]
- Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev. 2005;15:490–495. doi: 10.1016/j.gde.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Glaser KB. Defining the role of gene regulation in resistance to HDAC inhibitors--mechanisms beyond P-glycoprotein. Leuk Res. 2006;30:651–652. doi: 10.1016/j.leukres.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Gomyo Y, Sasaki J, Branch C, Roth JA, Mukhopadhyay T. 5-aza-2′-deoxycytidine upregulates caspase-9 expression cooperating with p53-induced apoptosis in human lung cancer cells. Oncogene. 2004;23:6779–6787. doi: 10.1038/sj.onc.1207381. [DOI] [PubMed] [Google Scholar]
- Gu J, Yamamoto H, Lu X, Ngan CY, Tsujino T, Konishi K, Takemasa I, Ikeda M, Nagata H, Hashimoto S, Matsuzaki T, Sekimoto M, Takagi A, Monden M. Low-dose oxaliplatin enhances the antitumor efficacy of paclitaxel in human gastric cancer cell lines. Digestion. 2006;74:19–27. doi: 10.1159/000095826. [DOI] [PubMed] [Google Scholar]
- Hassan HE, Keita JA, Narayan L, Brady SM, Frederick R, Carlson S, Glass KC, Natesan S, Buttolph T, Fandy TE. The combination of dimethoxycurcumin with DNA methylation inhibitor enhances gene re-expression of promoter-methylated genes and antagonizes their cytotoxic effect. Epigenetics. 2016 doi: 10.1080/15592294.2016.1226452. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui KF, Ho DN, Tsang CM, Middeldorp JM, Tsao GS, Chiang AK. Activation of lytic cycle of Epstein-Barr virus by suberoylanilide hydroxamic acid leads to apoptosis and tumor growth suppression of nasopharyngeal carcinoma. Int J Cancer. 2012;131:1930–1940. doi: 10.1002/ijc.27439. [DOI] [PubMed] [Google Scholar]
- Ierano C, Chakraborty AR, Nicolae A, Bahr JC, Zhan Z, Pittaluga S, Bates SE, Robey RW. Loss of the proteins Bak and Bax prevents apoptosis mediated by histone deacetylase inhibitors. Cell Cycle. 2013;12:2829–2838. doi: 10.4161/cc.25914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Wang L, Miller MC, 3rd, Willingham MC, Fan W. 5-Fluorouracil interferes with paclitaxel cytotoxicity against human solid tumor cells. Clin Cancer Res. 1997;3:1739–1745. [PubMed] [Google Scholar]
- Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalac M, Scotto L, Marchi E, Amengual J, Seshan VE, Bhagat G, Ulahannan N, Leshchenko VV, Temkin AM, Parekh S, Tycko B, O’Connor OA. HDAC inhibitors and decitabine are highly synergistic and associated with unique gene-expression and epigenetic profiles in models of DLBCL. Blood. 2011;118:5506–5516. doi: 10.1182/blood-2011-02-336891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y, Akutsu M, Tsunoda S, Izumi T, Kobayashi H, Mano H, Furukawa Y. Cytotoxic effects of histone deacetylase inhibitor FK228 (depsipeptide, formally named FR901228) in combination with conventional anti-leukemia/lymphoma agents against human leukemia/lymphoma cell lines. Invest New Drugs. 2007;25:31–40. doi: 10.1007/s10637-006-9000-0. [DOI] [PubMed] [Google Scholar]
- Kanzaki M, Kakinuma H, Kumazawa T, Inoue T, Saito M, Narita S, Yuasa T, Tsuchiya N, Habuchi T. Low concentrations of the histone deacetylase inhibitor, depsipeptide, enhance the effects of gemcitabine and docetaxel in hormone refractory prostate cancer cells. Oncol Rep. 2007;17:761–767. [PubMed] [Google Scholar]
- Karam JA, Fan J, Stanfield J, Richer E, Benaim EA, Frenkel E, Antich P, Sagalowsky AI, Mason RP, Hsieh JT. The use of histone deacetylase inhibitor FK228 and DNA hypomethylation agent 5-azacytidine in human bladder cancer therapy. Int J Cancer. 2007;120:1795–1802. doi: 10.1002/ijc.22405. [DOI] [PubMed] [Google Scholar]
- Kim H, Choi H, Lee SK. Epstein-Barr virus miR-BART20-5p regulates cell proliferation and apoptosis by targeting BAD. Cancer Lett. 2015;356:733–742. doi: 10.1016/j.canlet.2014.10.023. [DOI] [PubMed] [Google Scholar]
- Kuendgen A, Lubbert M. Current status of epigenetic treatment in myelodysplastic syndromes. Ann Hematol. 2008;87:601–611. doi: 10.1007/s00277-008-0477-9. [DOI] [PubMed] [Google Scholar]
- Laterza MM, Pompella L, Petrillo A, Tirino G, Pappalardo A, Orditura M, Troiani T, Ciardiello F, Di Martino N, De Vita F. Efficacy of a triplet and doublet-based chemotherapy as first-line therapy in patients with HER2-negative metastatic gastric cancer: a retrospective analysis from the clinical practice. Med Oncol. 2017;34:186. doi: 10.1007/s12032-017-1046-7. [DOI] [PubMed] [Google Scholar]
- Lavelle D, DeSimone J, Hankewych M, Kousnetzova T, Chen YH. Decitabine induces cell cycle arrest at the G1 phase via p21(WAF1) and the G2/M phase via the p38 MAP kinase pathway. Leuk Res. 2003;27:999–1007. doi: 10.1016/S0145-2126(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Leao M, Anderton E, Wade M, Meekings K, Allday MJ. Epstein-barr virus-induced resistance to drugs that activate the mitotic spindle assembly checkpoint in Burkitt’s lymphoma cells. J Virol. 2007;81:248–260. doi: 10.1128/JVI.01096-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim SH, Han SH, An JS, Lee ES, Kim YS. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2009;24:354–365. doi: 10.1111/j.1440-1746.2009.05775.x. [DOI] [PubMed] [Google Scholar]
- Li Y, Geng P, Jiang W, Wang Y, Yao J, Lin X, Liu J, Huang L, Su B, Chen H. Enhancement of radiosensitivity by 5-Aza-CdR through activation of G2/M checkpoint response and apoptosis in osteosarcoma cells. Tumour Biol. 2014;35:4831–4839. doi: 10.1007/s13277-014-1634-5. [DOI] [PubMed] [Google Scholar]
- Luo HY, Wei W, Shi YX, Chen XQ, Li YH, Wang F, Qiu MZ, Li FH, Yan SL, Zeng MS, Huang P, Xu RH. Cetuximab enhances the effect of oxaliplatin on hypoxic gastric cancer cell lines. Oncol Rep. 2010;23:1735–1745. doi: 10.3892/or_00000819. [DOI] [PubMed] [Google Scholar]
- Mizutani H, Hiraku Y, Tada-Oikawa S, Murata M, Ikemura K, Iwamoto T, Kagawa Y, Okuda M, Kawanishi S. Romidepsin (FK228), a potent histone deacetylase inhibitor, induces apoptosis through the generation of hydrogen peroxide. Cancer Sci. 2010;101:2214–2219. doi: 10.1111/j.1349-7006.2010.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momparler RL, Cote S, Momparler LF, Idaghdour Y. Inhibition of DNA and histone methylation by 5-aza-2′-deoxycytidine (decitabine) and 3-Deazaneplanocin-A on antineoplastic action and gene expression in myeloid leukemic cells. Front Oncol. 2017;7:19. doi: 10.3389/fonc.2017.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnani F, Turrisi G, Marinozzi C, Aliberti C, Fiorentini G. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: a systematic review and meta-analysis. Gastric Cancer. 2011;14:50–55. doi: 10.1007/s10120-011-0007-7. [DOI] [PubMed] [Google Scholar]
- Morabito A, Carillio G, Longo R. Systemic treatment of gastric cancer. Crit Rev Oncol Hematol. 2009;70:216–234. doi: 10.1016/j.critrevonc.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Ni X, Li L, Pan G. HDAC inhibitor-induced drug resistance involving ATP-binding cassette transporters (review). Oncol Lett. 2015;9:515–521. doi: 10.3892/ol.2014.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palii SS, Robertson KD. Epigenetic control of tumor suppression. Crit Rev Eukaryot Gene Expr. 2007;17:295–316. doi: 10.1615/CritRevEukarGeneExpr.v17.i4.40. [DOI] [PubMed] [Google Scholar]
- Roell KR, Reif DM, Motsinger-Reif AA. An introduction to terminology and methodology of chemical synergy-perspectives from across disciplines. Front Pharmacol. 2017;8:158. doi: 10.3389/fphar.2017.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MA, Ajani JA. Gastric cancer--an enigmatic and heterogeneous disease. JAMA. 2010;303:1753–1754. doi: 10.1001/jama.2010.553. [DOI] [PubMed] [Google Scholar]
- Shang D, Liu Y, Matsui Y, Ito N, Nishiyama H, Kamoto T, Ogawa O. Demethylating agent 5-aza-2′-deoxycytidine enhances susceptibility of bladder transitional cell carcinoma to Cisplatin. Urology. 2008;71:1220–1225. doi: 10.1016/j.urology.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Shi M, Lou B, Ji J, Shi H, Zhou C, Yu Y, Liu B, Zhu Z, Zhang J. Synergistic antitumor effects of dasatinib and oxaliplatin in gastric cancer cells. Cancer Chemother Pharmacol. 2013;72:35–44. doi: 10.1007/s00280-013-2166-1. [DOI] [PubMed] [Google Scholar]
- Son DS, Wilson AJ, Parl AK, Khabele D. The effects of the histone deacetylase inhibitor romidepsin (FK228) are enhanced by aspirin (ASA) in COX-1 positive ovarian cancer cells through augmentation of p21. Cancer Biol Ther. 2010;9:928–935. doi: 10.4161/cbt.9.11.11873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield BA, Luftig MA. Recent advances in understanding Epstein-Barr virus. F1000Res. 2017;6:386. doi: 10.12688/f1000research.10591.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui M, Dziadyk JM, Zhu X, Fan W. Cell cycle-dependent antagonistic interactions between paclitaxel and gamma-radiation in combination therapy. Clin Cancer Res. 2004;10:4848–4857. doi: 10.1158/1078-0432.CCR-03-0707. [DOI] [PubMed] [Google Scholar]
- Sun WJ, Huang H, He B, Hu DH, Li PH, Yu YJ, Zhou XH, Lv Z, Zhou L, Hu TY, Yao ZC, Lu MD, Shen X, Zheng ZQ. Romidepsin induces G2/M phase arrest via Erk/cdc25C/cdc2/cyclinB pathway and apoptosis induction through JNK/c-Jun/caspase3 pathway in hepatocellular carcinoma cells. Biochem Pharmacol. 2017;127:90–100. doi: 10.1016/j.bcp.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Takayama S, Wakasugi T, Funahashi H, Takeyama H. Strategies for gastric cancer in the modern era. World J Gastrointest Oncol. 2010;2:335–341. doi: 10.4251/wjgo.v2.i9.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka R, Ariyama H, Qin B, Shibata Y, Takii Y, Kusaba H, Baba E, Mitsugi K, Harada M, Nakano S. Synergistic interaction between oxaliplatin and SN-38 in human gastric cancer cell lines in vitro. Oncol Rep. 2005;14:683–688. [PubMed] [Google Scholar]
- Tsai HC, Li H, Van Neste L, Cai Y, Robert C, Rassool FV, Shin JJ, Harbom KM, Beaty R, Pappou E, Harris J, Yen RW, Ahuja N, Brock MV, Stearns V, Feller-Kopman D, Yarmus LB, Lin YC, Welm AL, Issa JP, Minn I, Matsui W, Jang YY, Sharkis SJ, Baylin SB, Zahnow CA. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell. 2012;21:430–446. doi: 10.1016/j.ccr.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LX, Mei ZY, Zhou JH, Yao YS, Li YH, Xu YH, Li JX, Gao XN, Zhou MH, Jiang MM, Gao L, Ding Y, Lu XC, Shi JL, Luo XF, Wang J, Wang LL, Qu C, Bai XF, Yu L. Low dose decitabine treatment induces CD80 expression in cancer cells and stimulates tumor specific cytotoxic T lymphocyte responses. PLoS ONE. 2013;8:e62924. doi: 10.1371/journal.pone.0062924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildeman MA, Novalic Z, Verkuijlen SA, Juwana H, Huitema AD, Tan IB, Middeldorp JM, de Boer JP, Greijer AE. Cytolytic virus activation therapy for Epstein-Barr virus-driven tumors. Clin Cancer Res. 2012;18:5061–5070. doi: 10.1158/1078-0432.CCR-12-0574. [DOI] [PubMed] [Google Scholar]
- Wilson AJ, Lalani AS, Wass E, Saskowski J, Khabele D. Romidepsin (FK228) combined with cisplatin stimulates DNA damage-induced cell death in ovarian cancer. Gynecol Oncol. 2012;127:579–586. doi: 10.1016/j.ygyno.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Sui M, Fan W, Kraft AS. Cell cycle dependent antagonistic interactions between paclitaxel and carboplatin in combination therapy. Cancer Biol Ther. 2007;6:1067–1073. doi: 10.4161/cbt.6.7.4323. [DOI] [PubMed] [Google Scholar]
- Xu K, Chen Z, Cui Y, Qin C, He Y, Song X. Combined olaparib and oxaliplatin inhibits tumor proliferation and induces G2/M arrest and gamma-H2AX foci formation in colorectal cancer. Onco Targets Ther. 2015;8:3047–3054. doi: 10.2147/OTT.S89154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon H, Ko YH. LMP1+SLAMF1high cells are associated with drug resistance in Epstein-Barr virus-positive Farage cells. Oncotarget. 2017;8:24621–24634. doi: 10.18632/oncotarget.15600. [DOI] [PMC free article] [PubMed] [Google Scholar]