Abstract
Investigations into the development of new therapeutic agents for lung inflammatory disorders have led to the discovery of plant-based alternatives. The rhizomes of Anemarrhena asphodeloides have a long history of use against lung inflammatory disorders in traditional herbal medicine. However, the therapeutic potential of this plant material in animal models of lung inflammation has yet to be evaluated. In the present study, we prepared the alcoholic extract and derived the saponin-enriched fraction from the rhizomes of A. asphodeloides and isolated timosaponin A-III, a major constituent. Lung inflammation was induced by intranasal administration of lipopolysaccharide (LPS) to mice, representing an animal model of acute lung injury (ALI). The alcoholic extract (50–200 mg/kg) inhibited the development of ALI. Especially, the oral administration of the saponin-enriched fraction (10–50 mg/kg) potently inhibited the lung inflammatory index. It reduced the total number of inflammatory cells in the bronchoalveolar lavage fluid (BALF). Histological changes in alveolar wall thickness and the number of infiltrated cells of the lung tissue also indicated that the saponin-enriched fraction strongly inhibited lung inflammation. Most importantly, the oral administration of timosaponin A-III at 25–50 mg/kg significantly inhibited the inflammatory markers observed in LPS-induced ALI mice. All these findings, for the first time, provide evidence supporting the effectiveness of A. asphodeloides and its major constituent, timosaponin A-III, in alleviating lung inflammation.
Keywords: Anemarrhena asphodeloides, Timosaponin A-III, Lung inflammation, Cytokine
INTRODUCTION
Bronchitis and chronic obstructive pulmonary disease (COPD) are airway inflammatory diseases, which may be treated with several classes of antibiotics and anti-inflammatory agents. Clinically, drugs such as bronchodilators, antitussives, steroids and expectorants are used. In COPD, alveolar cell walls are destroyed mainly by long-term continual inflammation triggered, for example by smoking. The irreversible damage to alveolar sacs eventually diminishes the pulmonary gas exchange rate, which leads to frequent bouts of infection by pathogenic bacteria and viruses producing cough and sputum. These symptoms further exacerbate disease progression in COPD. The etiology of COPD is very complex (Al-Kassini and Alhamad, 2013). Continual inflammatory insults lead to the generation of proinflammatory cytokines/chemokines by lung epithelial cells, alveolar macrophages, and recruited neutrophils and macrophages. Nitric oxide (NO) and O2 radicals trigger oxidative stress. Matrix metalloproteinases such as elastase abrogate the elasticity and integrity of the alveolar sacs, resulting in dyspnea (Jeffery, 2001; Barnes, 2014). Despite the discovery of pathological factors, the key pathological mechanisms remain unknown, which explains the lack of effective treatment. Treatment with anti-TNF-α antibody and TNF receptor antagonists has not resulted in favorable outcomes in COPD (Dentener et al., 2008; Aaron et al., 2013). Phosphodiesterase 4 inhibitors showed only limited success (Reid and Pham, 2012). The need for new therapeutic agents in the field of COPD has prompted the investigation of several plant-based products worldwide for potential therapeutic application (Kim et al., 2017).
The rhizomes of Anemarrhena asphodeloides Bunge (Liliaceae) have been used in Chinese medicine to treat infectious, pyretic and inflammatory disorders (Kawasaki and Yamauchi, 1963; Park et al., 2003). Particularly, this plant material has a long history of therapeutic usage in lung inflammatory disorders (Bae, 2000). Many prescriptions containing the rhizomes of A. asphodeloides as a major component have been clinically used in traditional herbal medicine in treating bronchitis to reduce septum production and cough. Previously, the anti-allergic action of the rhizomes of A. asphodeloides was demonstrated in vitro and in vivo (Chai et al., 2013). The inhibitory action of the herbal mixture of A. asphodeloides and Coptis chin against the mouse colitis model was demonstrated (Jeong et al., 2014). Major constituents are terpenoid saponins. Many compounds were isolated from A. asphodeloides, and structurally identified including mangiferin, timosaponins and (−)-nya-sol (cis-hinokiresinol) (Lee et al., 1995; Kim et al., 2006; Bae et al., 2007). Mangiferin was found to inhibit the production of prostaglandin E2 and leukotriene B4 from J774 cells (Garrido et al., 2006) and this compound also showed anti-allergic effects including inhibition of IgE production, histamine release and lymphocyte proliferation (Rivera et al., 2006). In addition, timosaponin B-II was reported to inhibit lipopolysaccharide (LPS)-induced acute lung inflammation (Zhang et al., 2015). However, the therapeutic effects of the rhizomes derived from A. asphodeloides, the saponin-enriched fraction and its major saponin, timosaponin A-III, in animal models of lung inflammation have yet to be demonstrated. Thus, the present study was designed to investigate the therapeutic potential of these compounds against lung inflammation and to provide the scientific rationale for their use in herbal medicine.
MATERIALS AND METHODS
Chemicals
Dexamethasone, IL-1β and LPS (Escherichia coli 0127:B8) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Protein assay kit was purchased from Bio-Rad Lab. (Hercules, CA, USA). Antibodies against mitogen-activated protein kinases (MAPKs), signal transducer and activator of transcription 3 (STAT3), phospho-STAT3 (Tyr705), phospho-STAT3 (Ser727) and several transcription factors were purchased from Cell Signaling Technologies (Danvers, MA, USA). β-Actin antibody was purchased from Bethyl Laboratories, Inc (Montgomery, TX, USA). Lamin B1 antibody was purchased from Bioworld technology, Inc (Minneapolis, MN, USA).
Animals
Male ICR mice (male, 18–22 g, specific pathogen-free) were obtained from Orient Co (Seongnam, Korea). The animals were maintained in animal facility (Kangwon National University) at 20–22°C under 40–60% relative humidity and a 12 h/12 h (light/dark) cycle for at least 7 days prior to the experiment. The experimental design using the animals was approved by the local committee for animal experimentation, Kangwon National University (KW-160701-1). In addition, the ethical guideline described in the Korean Food and Drug Administration guide for the care and use of laboratory animals was followed throughout the experiments.
Preparation of 70% methanol extract and the saponin-enriched fraction of the rhizomes of A. asphodeloides
The dried rhizomes of A. asphodeloides were obtained from the local herbal market and were authenticated by one of the authors, Dr. Y.S. Kwon (Kangwon National University). The specimen was deposited at College of Pharmacy (Kangwon National University) under the name of KNU-123. The dried rhizomes (2 kg) were extracted in 70% methanol (10 L) at room temperature for 7 days. After filtration, the extract was dried under vacuo to yield 120.1 g of dried material (alcoholic extract). To obtain the saponin-enriched fraction, 70% methanol extract (100.0 g) was partitioned in water and n-butanol. And n-butanol fraction was obtained by evaporation to yield 21.8 g (n-butanol fraction, saponin-enriched fraction).
Isolation of timosaponin A-III
n-Butanol fraction (21.0 g) was chromatographed on silica gel with CHCl3-MeOH-H2O (52:28:8, lower layer) to yield 7 subfractions. The subfraction 5 was rechromatographed on silica gel with CHCl3-MeOH-H2O (7:3:1, lower layer) and the isolated compound was recrystallized with MeOH to obtain pure timosaponin A-III (608.8 mg). Its chemical structure was identified with the comparison of the previous report (Kawasaki and Yamauchi, 1963)
UPLC analysis of the 70% methanol extract and the saponin-enriched fraction
Timosaponin A-III was quantitatively analyzed by UPLC. Analysis was performed using an equipment ACQuity (Waters, Milford, MA, USA) with ELS detector. Aqilent C18 (4.6×150 mm, 5 μm) column was used as stationary phase. Acetonitrile-MeOH-H2O (55:35:10) was used as a mobile phase. Column temperature was 35°C and the flow rate was 0.5 ml/min.
LPS-induced lung inflammation in mice
For measuring in vivo therapeutic effects, LPS-induced acute lung injury (ALI), a mouse model of airway inflammation, was used (Lim et al., 2013). The test compounds, including the reference drug, were dissolved in 0.3% carboxymethylcellulose (CMC) and were orally administered to mice at the indicated doses (n=10). The control and LPS treatment groups also received the same amount of CMC solution. For inducing bronchitis, LPS (E. coli 0127:B8, 2 mg/kg, PBS) was administered intranasally to mice (10 μl/mouse, 5 times) at 1 h after oral treatment with the test compounds according to the previously published procedures (Lim et al., 2013). At 16 h after LPS treatment, mice were sacrificed (n=7), and bronchoalveolar lavage fluid (BALF) was collected via intratracheal cannulation. In the BALF, the total cell number was counted with a haemocytometer, and the cells were differentially counted with fluorescence-activated cell sorter (FACS, BD Biosciences, San Jose, CA, USA). For FACS analysis, cells were stained with following antibodies; allophycocyanin (APC) conjugated anti-Ly-6C (BD Bioscience), allophycocyanin-Cy7 (APC-Cy7) conjugated anti-CD11c (BD Bioscience), fluorescein isothiocyanate (FITC) conjugated anti-CD11b (BD Bioscience), phyco-erythrin (PE) conjugated anti-F4/80 (eBioscience, San Diego, CA, USA), phycoerythrin–Cy7 (PE-Cy7) conjugated anti-Ly-6G (BD Bioscience). After incubation with antibody, cells were washed with 2% FBS RPMI media (Thermo Fisher Scientific, Waltham, MA, USA). Cell pellets were resuspended and analyzed. CD11b, Ly-6C and Ly-6G are essential markers of neutrophils. CD11c and F4/80 are markers of macrophages. For the histological analysis, the remaining mice (n=3) were sacrificed and lung tissues were excised. Histological examination was carried out using the conventional methods of H&E staining. ImageJ (National Institutes of Health, Bethesda, MD, USA) was used to measure the thickness of alveolar wall area.
Determination of proinflammatory cytokine concentrations in the BALF
LPS and timosaponin A-III were administered to mice as described above. Six hours later, mice were sacrificed (n=3) and BALF was obtained. From the BALF, the concentrations of IL-1β and IL-6 were determined with ELISA kit (BD Bioscience).
Action mechanism study of timosaponin A-III
For measuring the levels of MAPKs and STAT3 activation, timosaponin A-III was administered orally and, one hour later, LPS was intranasally administered in mice (n=3). After 2 h, mice were sacrificed and the lung tissues were excised. After separated the lobe of the lungs, about 20 mg of tissues in 400 μl of Pro-prep solution (iNtRON Biotechnology, Seongnam, Korea) containing 1 mM phenylmethanesulfonyl fluoride (PMSF), 1 mM sodium fluoride (NaF) and 1 mM sodium orthovanadate were homogenized, and centrifuged at 12,000 rpm for 10 min at 4°C. The supernatant was quantified and electrophoresis was performed in 10% sodium dodecyl sulfate polyacrylamide gels. On the other hand, for measuring the activation levels of transcription factors, about 50 mg of the remaining tissue were homogenized in 400 μl of buffer A (10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.9, 10 mM KCl, 0.1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM 1,4-dithiothreitol (DTT), 0.5 mM phenylmethyl-sulfonyl fluoride (PMSF), 1 mM sodium fluoride (NaF), 1 mM sodium orthovanadate) and added 25 μl of 10% NP-40, the solution was vortexed and centrifuged at 5,000 rpm for 2 min at 4°C. And the nuclear pellet was vigorously vortexed in buffer B (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, 1 mM sodium fluoride (NaF), 1 mM sodium orthovanadate) and centrifuged at 13,000 rpm for 10 min at 4°C. After the supernatant was quantified and electrophoresis was performed, transferred to polyvinylidene difluoride (PVDF) membranes. The transferred PVDF membranes were blocked with 5% skim milk for 1 h at room temperature and incubated with primary antibodies diluted 1:1000 in 5% skim milk overnight at 4°C. After membranes were incubated with secondary antibodies diluted 1:5000 in 5% skim milk for 1 h at room temperature. The bound antibodies were detected by enhanced chemiluminescence (West Femto Luminol/Enhancer solution, Thermo Scientific, Rockford, IL, USA).
Statistical analysis
Experimental values were represented as arithmetic mean ± SD. One way ANOVA followed by Dunnett’s test was used to determine the statistical significance.
RESULTS
Effects of A. asphodeloides on LPS-induced lung inflammation in mice
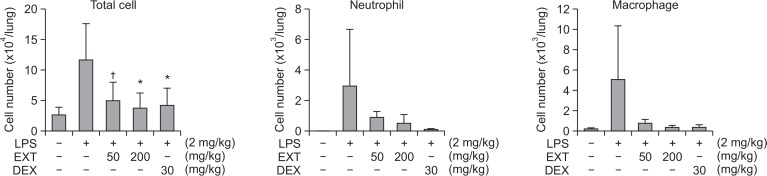
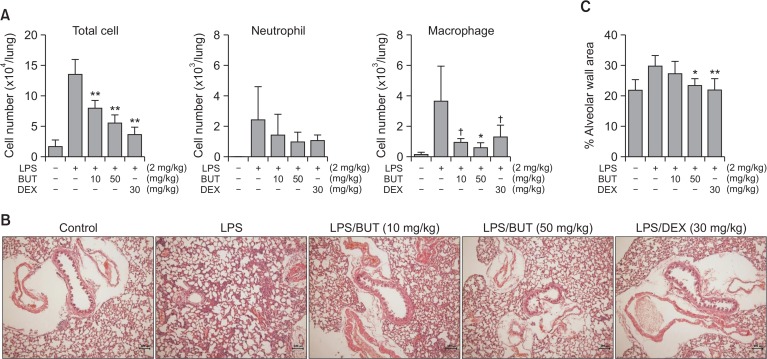
LPS treatment of mice induced lung inflammation. In the BALF, the total cell numbers increased approximately 4.5-fold. Oral administration of the alcoholic extract (50–200 mg/kg) in this animal model inhibited inflammatory response (Fig. 1). At 200 mg/kg, the alcoholic extract reduced the total number of cells in the BALF by 88.0%. To concentrate the active constituents, the saponin-enriched fraction was prepared by n-butanol fractionation. This fraction (10–50 mg/kg) showed much higher inhibitory action on lung inflammation than the alcoholic extract (Fig. 2). At 50 mg/kg, the saponin-enriched fraction inhibited cell recruitment in the BALF by 67.5%. The reduction of the inflammatory cell recruitment was also revealed by FACS analysis. Histological observations also showed that the fraction reduced inflammatory responses in the lung tissues. Dexamethasone (30 mg/kg) as a reference agent strongly inhibited these inflammatory parameters as expected.
Fig. 1.
Inhibition of LPS-induced ALI in mice by the alcoholic extract of A. asphodeloides. The extract was administered orally and, one hour later, LPS was intranasally administered. BALF was obtained 16 h later. From the BALF, total numbers of cells were counted and FACS was used to differentially count each cell type. EXT: alcoholic extract of Anemarrhena asphodeloides, DEX: dexamethasone, †p<0.1, *p<0.05, significantly different from the LPS-treated control group by ANOVA followed by Dunnett’s analysis (n=7).
Fig. 2.
Inhibition of LPS-induced ALI in mice by the n-butanol fraction of A. asphodeloides. The fraction was administered orally and, one hour later, LPS was intranasally administered. BALF was obtained 16 h later. (A) Effect on cell numbers, From the BALF, total numbers of cells were counted and FACS was used to differentially count each cell type. †p<0.1, *p<0.05, **p<0.01, significantly different from the LPS-treated control group by ANOVA followed by Dunnett’s analysis (n=7). (B) Histological observation, From the excised lungs, H&E staining gave histology. X100. Bar (100 μm). (C) Measurement of alveolar wall area, BUT: n-butanol fraction, DEX: dexamethasone.
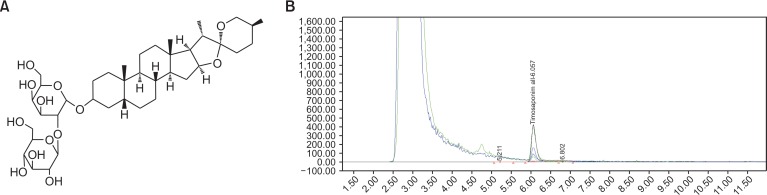
UPLC profile of A. asphodeloides and analysis of timosaponin A-III
Based on the above findings, timosaponin A-III (Fig. 3A) was successfully isolated as a major constituent in the saponin-enriched fraction. UPLC analysis was used to measure the concentration of timosaponin A-III. As shown in Fig. 3B, timosaponin A-III constituted 12.2 mg/g extract and 40.0 mg/g fraction of the 70% methanol extracts and n-butanol fraction, respectively.
Fig. 3.
The chemical structure of timosaponin A-III and analysis of timosaponin A-III on UPLC. (A) Timosaponin A-III. (B) UPLC chromatogram. The conditions of analysis were described in the experimental section. The retention time for timosaponin A-III is 6.1 min.
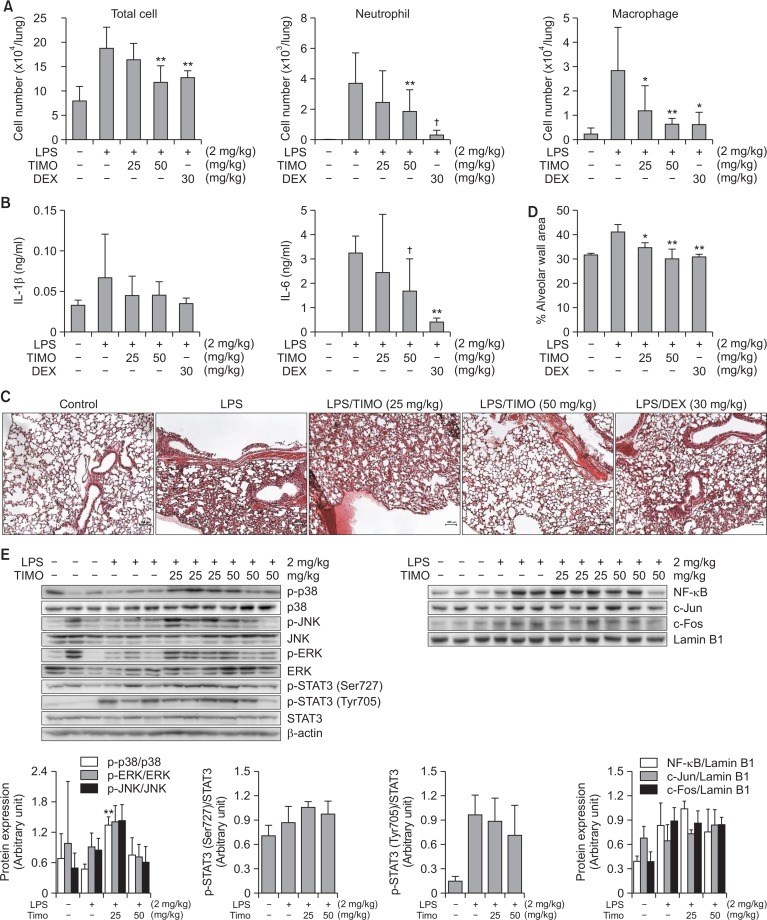
Effects of timosaponin A-III on LPS-induced lung inflammation in mice
Timosaponin A-III was orally administered to ALI mice. At a dose of 25–50 mg/kg, this compound significantly inhibited all the inflammatory markers investigated (Fig. 4). At 50 mg/kg, timosaponin A-III reduced the total number of cells recruited in BALF by 64.6%. The compound at a concentration of 50 mg/kg reduced inflammatory cell infiltration, neutrophil infiltration by 50.4% and macrophages by 84.6%. Timosaponin A-III also reduced proinflammatory cytokine production in the BALF, especially IL-6 production at 50 mg/kg. There was no significant reduction of TNF-α level by the treatment of timosaponin A-III (data not shown). Moreover, histological analysis of the lung tissues demonstrated substantial attenuation of inflammatory response: alveolar wall thickness and infiltration of inflammatory cells. When the action mechanism study was carried out using Western blotting analysis, timosaponin A-III did not affect the activation levels of MAPK including extracellular signal-regulated kinase (ERK), p38 MAPK and c-Jun N-terminal kinase (JNK), nuclear transcription factor-κB (NF-κB) and activator protein-1 (AP-1), which are important signaling molecules in this lung inflammatory animal model (Lv et al., 2015). Only STAT3 activation was slightly decreased (9.9% and 31.0% at 25 and 50 mg/kg, respectively), but not statistically significant (Fig. 4E). Thus, timosaponin A-III might inhibit lung inflammatory responses at least in part by interrupting STAT3 activation pathway. The more detailed action mechanism(s) need to be further investigated. All these findings clearly indicated the role of this major saponin as one of the active principles in A. asphodeloides.
Fig. 4.
Inhibition of LPS-induced ALI in mice by timosaponin A-III. The compound was administered orally and, one hour later, LPS was intranasally administered. BALF was obtained 16 h later. (A) Effect on cell numbers, From the BALF, total numbers of cells were counted and FACS was used to differentially count each cell type (n=7). (B) Effect on cytokine production, For measuring cytokine concentrations in the lung tissue, mice were sacrificed at 6 h after LPS treatment as described in the experimental section. From the excised lungs, cytokine concentrations were measured using ELISA (n=3). (C) Histological observation, H&E staining of the lungs gave histology. X100. Bar (100 μm). (D) Measurement of alveolar wall area. For cellular mechanism study, timosaponin A-III was administered and one hour later, LPS was injected intranasally to mice (n=3). After two hours, mice were sacrificed and lung tissues were obtained. (E) Western blot, TIMO: timosaponin A-III, DEX: dexamethasone, †p<0.1, *p<0.05, **p<0.01, significantly different from the LPS-treated control group by ANOVA followed by Dunnett’s analysis.
DISCUSSION
The rhizomes of A. asphodeloides have been widely used in East Asia for lung diseases (Bae, 2000). This plant material is used in numerous prescriptions as a traditional remedy. However, the potential therapeutic properties have never been tested in animal model(s) of lung inflammation. Here, we have shown for the first time that the rhizomes of A. asphodeloides possess considerable inhibitory action against LPS-induced ALI in mice, following oral administration. Furthermore, the saponin-enriched fraction was associated with much higher activity, suggesting that saponins were the active ingredients. Indeed, the major saponin, timosaponin A-III exhibited strong inhibitory action in the same animal model, demonstrating the therapeutic potential of A. asphodeloides rhizomes and their saponins in lung inflammation. The inhibitory potency of timosaponin-AIII was slightly lower than that of dexamethasone used as positive reference. However, given that this compound is a natural product and dexamethasone has serious side-effects in long-term use, the therapeutic potential of timosaponin A-III is significant.
Timosaponin A-III was previously shown to inhibit colitis in a mouse model (Lim et al., 2015). The inhibition was mediated via inhibiting NF-κB and MAPK activation. Timosaponin A-III was also found to inhibit passive cutaneous anaphylaxis and pruritus (Lee et al., 2010). In addition, timosaponin A-III induced autophagy in HeLa cancer cells (Sy et al., 2008). All these previous findings indicate that this compound may regulate inflammatory and immune responses. The present investigation has shown that timosaponin A-III possesses strong inhibitory action against lung inflammation for the first time. This compound reduced proinflammatory cytokine production in the lung tissues. But, on the contrary to the previous finding (Lim et al., 2015), timosaponin A-III did not affect the activation of MAPK, NF-κB and AP-1 pathways. Instead, this compound slightly suppressed STAT3 activation, which is one of important signaling molecule in this animal model (Severgnini et al., 2004).
It is difficult to control COPD clinically. Currently, no therapeutic regimen is available to completely cure the disease, warranting the need for new agents. Due to the complex etiology of COPD and the role of numerous molecule(s) in COPD progression and exacerbation, it may not be appropriate to develop agent(s) targeting a single pathological pathway. In this respect, herbal drugs may be superior because of their complex chemical composition and multiple mechanisms of action. The inhibitory mechanisms of plant extracts in animal models of lung inflammation have been recently summarized (Kim et al., 2017). Several plant-derived drugs have been used to treat lung inflammatory disorders in Western remedies. For instance, Hedera helix (ivy leaf, Prospan®, Guo et al., 2006), Echinacea purpurea (Sharma et al., 2006; Agbabiaka et al., 2008) and Pelargonium sidoides (Umckamin syrup®, Matthys and Funk, 2008) represent major sources of ingredients in drugs prescribed for bronchitis treatment in Asian and European countries. In line with these previous findings, A. asphodeloides may be a potential candidate as a new plant-based therapeutic agent for the management of lung inflammatory diseases.
In conclusion, we demonstrated the inhibitory potential of A. asphodeloides rhizomes in lung inflammation, for the first time. We also showed the effect of its major saponin, timosaponin A-III in this study. Combined with previous finding of timosaponin B-II, these saponin derivatives may contribute to the pharmacological activity of A. asphodeloides. Timosaponin A-III represents a potential new therapeutic agent targeting lung inflammation.
Acknowledgments
The authors declare no conflict of interest. This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (NRF-2016R1A2B4007756), 2017 Research Grant from Kangwon National University (520170392) and BK21 PLUS program from the Ministry of Education, Republic of Korea.
Footnotes
CONFLICT OF INTEREST
All authors have no conflict of interest to declare.
REFERENCES
- Aaron SD, Vandemheen KL, Maltais F, Field SK, Sin DD, Bourbeau J, Marciniuk DD, FitzGerald JM, Nair P, Mallick R. TNFα antagonists for acute exacerbations of COPD: a randomized double-blind controlled trial. Thorax. 2013;68:142–148. doi: 10.1136/thoraxjnl-2012-202432. [DOI] [PubMed] [Google Scholar]
- Agbabiaka TB, Guo R, Ernst E. Pelargonium sidoides for acute bronchitis: a systematic review and meta-analysis. Phytomedicine. 2008;15:378–385. doi: 10.1016/j.phymed.2007.11.023. [DOI] [PubMed] [Google Scholar]
- Al-Kassini FA, Alhamad EH. A challenge to the seven widely believed concepts of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2013;8:21–30. doi: 10.2147/COPD.S38714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae G, Yu JR, Lee J, Chang J, Seo EK. Identification of the nyasol and structurally related compounds as the active principles from Anemarrhena asphodeloides against respiratory syncytial virus (RSV). Chem. Biodiver. 2007;4:2231–2235. doi: 10.1002/cbdv.200790181. [DOI] [PubMed] [Google Scholar]
- Bae K. The medicinal plants of Korea. Kyo-Hak Pub. Co.; Seoul, Korea: 2000. p. 546. [Google Scholar]
- Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin. Chest Med. 2014;35:71–86. doi: 10.1016/j.ccm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Chai OH, Shon DH, Han EH, Kim HT, Song CH. Effects of Anemarrhena asphodeloides on IgE-mediated passive cutaneous anaphylaxis, compound 48/80-induced systemic anaphylaxis and mast cell activation. Exp. Toxicol. Pathol. 2013;65:419–426. doi: 10.1016/j.etp.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Dentener MA, Creutzberg EC, Pennings HJ, Rijkers GT, Mercken E, Wouters EF. Effect of inflimab on local and systemic inflammation in chronic obstructive pulmonary disease: a pilot study. Respiration. 2008;76:275–282. doi: 10.1159/000117386. [DOI] [PubMed] [Google Scholar]
- Garrido G, Gonzalez D, Lemus Y, Delporte C, Delqado R. Protective effects of a standard extract of Mangifera indica L. (VIMANG) against mouse ear edema and its inhibition of eicosanoid production in J774 murine macrophages. Phytomedicine. 2006;13:412–418. doi: 10.1016/j.phymed.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Guo R, Pittier MH, Ernst E. Herbal medicines for the treatment of COPD: a systematic review. Eur. Respir. J. 2006;28:330–338. doi: 10.1183/09031936.06.00119905. [DOI] [PubMed] [Google Scholar]
- Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am. J. Respir. Crit. Care Med. 2001;164:S28–S38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- Jeong JJ, Jang SE, Hyam SR, Han MJ, Kim DH. The rhizome mixture of Anemarrhena asphodeloides and Coptidis chinensis ameliorates acute and chronic colitis in mice by inhibiting the binding of lipopolysaccharide to TLR4 and IRAK1 phosphorylation. Evid. Based Complement. Alternat. Med. 2014;2014:809083. doi: 10.1155/2014/809083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Yamauchi T. Saponins of timo (Anemarrhenae Rhizoma). II. Structure of timosaponin A-III. Chem. Pharm. Bull. 1963;11:1221–1224. doi: 10.1248/cpb.11.1221. [DOI] [PubMed] [Google Scholar]
- Kim CY, Ahn MJ, Kim J. Preparative isolation of mangiferin from Anemarrhena asphodeloides rhizomes by centrifugal partition chromatography. J. Liquid Chromat. Relat. Technol. 2006;29:869–875. doi: 10.1080/10826070500531391. [DOI] [Google Scholar]
- Kim HP, Lim H, Kwon YS. Therapeutic potential of medicinal plants and their constituents on lung inflammatory disorders. Biomol. Ther (Seoul) 2017;25:91–104. doi: 10.4062/biomolther.2016.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Trinh HT, Jung K, Han SJ, Kim DH. Inhibitory effects of steroidal timosaponins isolated from the rhizomes of Anemarrhena asphodeloides against passive cutaneouos anaphylaxis reaction and pruritus. Immunopharmacol. Immunotoxicol. 2010;32:357–363. doi: 10.3109/08923970903383889. [DOI] [PubMed] [Google Scholar]
- Lee SH, Ryu SY, Choi SU, No Z, Kim SK, Lee CO, Ahn JW. Antitumor agent from the rhizome of Anemarrhena asphodeloides. Saengyak Hakhoechi. 1995;26:47–50. [Google Scholar]
- Lim HJ, Jin H-K, Woo E-R, Lee SK, Kim HP. The root barks of Morus alba and the flavonoid constituents inhibit airway inflammation. J. Ethnopharmacol. 2013;149:169–175. doi: 10.1016/j.jep.2013.06.017. [DOI] [PubMed] [Google Scholar]
- Lim SM, Jeong JJ, Kang GD, Kim KA, Choi HS, Kim DH. Timosaponin AIII and its metabolite sarsasapogenin ameliorate colitis in mice by inhibiting NF-κB and MAPK activation and restoring Th17/Treg cell balance. Int. Immunopharmacol. 2015;25:493–503. doi: 10.1016/j.intimp.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Lv H, Zhu C, Liao Y, Gao Y, Lu G, Zhong W, Zheng Y, Chen W, Ci X. Tenuigenin ameliorates acute lung injury by inhibiting NF-κB and MAPK signalling pathways. Respir. Physiol. Neurobiol. 2015;216:43–51. doi: 10.1016/j.resp.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Matthys H, Funk P. EPs 7630 improves acute bronchitic symptoms and shortens time to remission. Results of a randomized, double-blind, placebo-controlled, multicenter trial. Planta Med. 2008;74:686–692. doi: 10.1055/s-2008-1074519. [DOI] [PubMed] [Google Scholar]
- Park HJ, Lee JY, Moon SS, Hwang BK. Isolation and anti-oomycete activity of nyasol from Anemarrhena asphodeloides rhizomes. Phytochem. 2003;64:997–1001. doi: 10.1016/S0031-9422(03)00462-X. [DOI] [PubMed] [Google Scholar]
- Reid DJ, Pham NT. Rofumilast: a novel treatment for chronic pulmonary disease. Ann. Pharmacother. 2012;46:521–529. doi: 10.1345/aph.1Q646. [DOI] [PubMed] [Google Scholar]
- Rivera DG, Balmaseda IH, Leon AA, Hernandez BC, Montiel LM, Garrido GG, Cuzzocrea S, Hernandez RD. Anti-allergic properties of Mangifera indica L. extract (VIMANG) and contribution of its glucosylxanthone mangiferin. J. Pharm. Pharmacol. 2006;58:385–395. doi: 10.1211/jpp.58.3.0014. [DOI] [PubMed] [Google Scholar]
- Severgnini M, Takahashi S, Rozo LM, Homer RJ, Kuhn C, Jhung JW, Perides G, Steer M, Hassoun PM, Fanburg BL, Cochran BH, Simon AR. Activation of the STAT pathway in acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;286:L1282–L1292. doi: 10.1152/ajplung.00349.2003. [DOI] [PubMed] [Google Scholar]
- Sharma M, Arnason JT, Burt A, Hudson JB. Echinacea extracts modulate the pattern of chemokine and cytokine secretion in rhinovirus-infected and uninfected epithelial cells. Phytother. Res. 2006;20:147–152. doi: 10.1002/ptr.1824. [DOI] [PubMed] [Google Scholar]
- Sy LK, Yan SC, Lok CN, Man RY, Che CM. Timosaponin A-III induces autophagy preceding mitochondria-mediated apoptosis in HeLa cancer cells. Cancer Res. 2008;68:10229–10237. doi: 10.1158/0008-5472.CAN-08-1983. [DOI] [PubMed] [Google Scholar]
- Zhang T, Wang J, Wang S, Ma C. Timosaponin B-II inhibits lipopolysaccharide-induced acute lung toxicity via TLR/NF-κB pathway. Toxicol. Mech Methods. 2015;25:665–671. doi: 10.3109/15376516.2015.1045652. [DOI] [PubMed] [Google Scholar]