Abstract
Representative benzimidazopyrimidinones were previously reported to be intercalating antitumor agents. In this work, we used 2-substituted 4,10-dihydrobenzo Dihydrobenzo[4,5]imidazo[1,2-a]pyriminin-4-ones for their diversification by regioselective alkylation. Under the conditions established, the alkylation gave 10-alkyl derivatives which permitted the parallel generation of a 500-member library of the title compounds.
Keywords: benzimidazole; Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one library; regioselective alkylation; anti-cancer agents
Introduction
The benzimidazole fragment is a frequent motif in recent publications devoted to drug design and molecular diversity oriented synthesis [1,2,3,4,5,6,7,8,9]. Fused polycyclic derivatives of benzimidazo[1,2-a] pyrimidines are often cited as anti-cancer and cytotoxic agents [10,11,12,13,14]. These molecules bind to DNA by stacking interactions of their π-electron rich planar fragments with the nucleic acid sandwiching in two consecutive base pairs of the double helix. This affects the DNA shape eventually prohibiting replication and causing the cell death [15,16].
Condensed benzimidazoles represent a suitable platform for the construction of such planar molecules applying the strategy of diversity oriented synthesis. We were interested in the generation of a library of diverse compounds based on the Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one core (Figure 1).
Figure 1.

Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one core.
We aimed to generate a 500-member library of Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-ones by introducing the initial diversity points in the positions C-2 and C-3 and then diversifying the obtained compounds by regioselective alkylation reactions using alkylating agents of different types in parallel format. In view of the scaffold structure (Figure 1) there are at least three products of monoalkylation that can be expected: the N1- and N10-substituted derivatives and the product of O-alkylation. Thus, uniform conditions for regioselective alkylation of Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-ones are required.
Results and Discussion
Several synthetic approaches to the Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one scaffold 1 have been found in the literature [17,18,19,20,21,22]. All of them utilize the reactivity of 2-aminobenzimidazole 2 with 1,3-dielectrophiles of several types. However, because of the commercial ability of different β-ketoesters 3, we have applied them [23] for the synthesis of starting compounds 1a–p. In contrast to the original protocol [23] where authors heated a neat mixture of 2-aminobenzimidazole 2 and some liquid β-ketoesters 3, we applied DMF as a solvent to make the procedure general for different starting materials including solid esters 3e–h (Table 1). Application of other solvents (EtOH, AcOH, dioxane) at reflux resulted in lower yields.
Table 1.
Synthesis of exampled starting Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-ones 1a–p.

| |||||
| Code | Structure | Yield,% | Code | Structure | Yield,% |
| 1a |  |
75 | 1i |  |
60 |
| 1b |  |
80 | 1j |  |
47 |
| 1c |  |
71 | 1k |  |
54 |
| 1d |  |
79 | 1l |  |
56 |
| 1e |  |
87 | 1m |  |
73 |
| 1f |  |
87 | 1n |  |
55 |
| 1g |  |
77 | 1o |  |
35 |
| 1h |  |
80 | 1p |  |
67 |
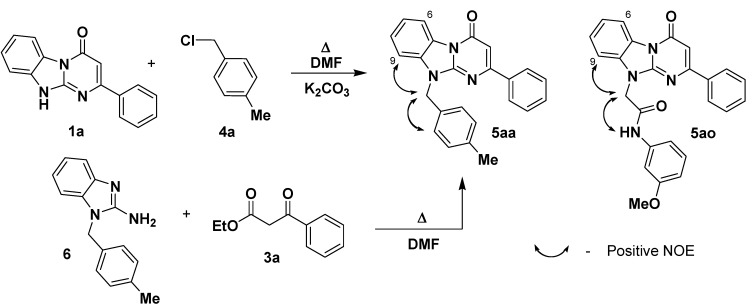
In our initial experiments on the alkylation we used the representative 1a in the reaction with 4-methylbenzyl chloride 4a under different reaction conditions. Strong alkali media (NaH or KOH in DMF, DMSO or dioxane) gave mixtures of alkylation products and therefore were not acceptable. Milder reaction conditions (NaHCO3 or K2CO3 in acetone, NEt3 in DMF) led to low yields or to products contaminated with the starting material 4a. However, application of K2CO3 (3 equiv) in DMF at 90 °C for 2h resulted in the formation of only one isomer 5aa with 81% yield following a simple aqueous workup (Scheme 1). Application of such conditions is very convenient for parallel synthesis because the reaction media does not reflux and the reaction can be carried out in a simple sealed vessel. These conditions were suitable for application of a representative of N-substituted 2-chloroacetamides 4o (Table 2). The product 5ao was obtained in the same manner with 77% yield (Scheme 1).
Scheme 1.
Model alkylation of the 2-phenyl-Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one 1a.
Table 2.
Selected examples 4a–u of alkylation agents used for the library 5 generation.
| Code | Structure | Code | Structure | Code | Structure |
|---|---|---|---|---|---|
| 4a |  |
4h |  |
4o |  |
| 4b |  |
4i |  |
4p |  |
| 4c |  |
4j |  |
4q |  |
| 4d |  |
4k |  |
4r |  |
| 4e |  |
4l |  |
4s |  |
| 4f |  |
4m |  |
4t |  |
| 4g |  |
4n |  |
4u |  |
The information on alkylation of the Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-ones 1 turned out to be absent in the literature, thus it was not possible to predict with certainty which isomer out of three mentioned above was formed. The NOE experiment, irradiation of the product 5aa with the resonance frequency of its CH2 protons at 5.58 ppm has demonstrated a close location of the methylene group and the upfield CH proton of the benzimidazole ring (doublet at 7.63 ppm) which agrees with either for O-alkylated or N10-alkylated product. To undoubtedly determine the structure of the product 5aa we have carried out a counter-synthesis (Scheme 1) of this compound starting from 2-amino-1-(4-methylbenzyl)benzimidazole 6 described previously [24]. The products obtained in these two alternative ways were identical. This means that the upfield doublet (at 7.63 ppm) of the benzimidazole ring belongs to the 9-C-H proton but not to the 6-C-H. Consequently, the latter gives its downfield doublet at 8.48 ppm. This alternative synthetic pathway leading to the compound 5aa gives lower yield (54% isolated yield). Also in this protocol the main diversity point (the alkylation agent) was introduced not at the last synthetic step, which was less convenient for the library generation. Alkylation of the starting derivative 1a using a representative of the N-substituted 2-chloroacetamides 4o led to one regioisomer 5ao. The discussed signal assignment for compound 5aa gives opportunity to determine structure of the product 5ao, the positive NOE between the protons of the CH2 group (5.29 ppm) and the upfield benzimidazole 9-C-H proton (doublet at 7.71 ppm) fully confirms the N10-alkylation.
The specified conditions allowed us to perform regioselective alkylation of the 2-(het)arylderivatives 1a–g with all the applied chemotypes of alkylation agents (representatives of benzyl clorides, 2-chloroacetic acid derivatives and representatives of alkylbromides or iodides, see Table 2 for selected alkylation agents 4a–u). However, a series of pilot experiments with the alkylation of 2-methyl-Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one 1i by representatives of the N-substituted 2-chloroacetamides 4l–u led to isolation of a mixture of two isomeric products. At the same time, the application of other alkylation agents was successful in this case. The same result was observed for the 2-tert-butyl derivative 1m. Also, in the case of the starting compounds 1o,p, we could not achieve the regioselectivity with alkylators of all chemotypes. Thus, we had to exclude the 2-alkyl derivatives 1i–n from the starting set used in the alkylation with the N-substituted 2-chloroacetamides 4l–u, and we did not use the derivatives 1o,p for the library generation.
The library was generated in parallel format (Scheme 2, Figure 2) and the structure and purity of every congener were checked by 1H-NMR. In about 85% cases the products were isolated with very good average yield (about 70%) and met the purity requirements (maximal total level of impurities less then 10% as determined by 1H-NMR). In those cases (about 10%) where the impurity level was higher than 10% the product purity was improved by heating in EtOH to remove soluble remains of the starting materials. In about 5% of cases the products did not satisfy the purity requirements either after the simple workup or after the additional purification.
Scheme 2.
Generation of a 500-member library of 10-alkyl-2-R1,3-R2-4,10-Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-ones 5.
Figure 2.
Selected members of the generated library 5 and their isolated yields.
As a result, the desired 500-member library contained different compound chemotypes whose distribution is illustrated in Figure 3. As one can see, the starting 2-(het)arylderivatives 1a–g contributed much more significantly to the total number of compounds because of their selectivity in the reaction with the diverse N-substituted 2-chloroacetamides 4l–u.
Figure 3.
Distribution of different congener’s chemotypes in the generated 500-member library 5.
In summary, a 500-member library of 10-Alkyl-2-R1,3-R2-4,10-Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-ones was quickly generated under uniform conditions in liquid phase parallel format. Structural diversity of the library members was achieved by application of two rows of starting Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-ones containing different 2-(het)aryl (1a–h) or 2-alkyl (1i–n) substituents as well as three types of alkylation agents. The major part of congeners comprises conjugated planar molecules derived from 2-(het)arylDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-ones 1a–h that makes them potential anti-cancer DNA intercalators.
Experimental
General
The starting Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-ones 1a [25], 1e [26], 1i [25], 1o [27], 1p [27] have been previously described. The starting sets of ß-ketoesters and alkylation agents were supplied by Chemical Diversity Research Institute, Chimki, Moscow, Russia. All other reagents and solvents are commercially available and were used without additional purification. 1H, 13C and NOE NMR experiments were recorded at 200 MHz (50 MHz for 13C NMR) in DMSO-d6 solutions.
Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-ones (1a–p). An equimolar mixture of 2-amino-benzimidazole 2 and corresponding β-ketoester 3a–p (0.04 mol) in DMF (4 mL) was refluxed for 20–60 min. If a precipitate was formed or the mixture was solidified at heating, the mixture was kept at mild heating for another 5 min and then cooled down to the room temperature. In the cases when no precipitate was formed, the mixture was refluxed for 60 min and then cooled down to the room temperature. The solid reaction mixture was fluidized with EtOH, the precipitated was separated by filtration, washed with EtOH and dried on air. The products 1a-p obtained in this manner had adequate 1H NMR spectra and used for the next reaction step without additional purification.
2-(3-Chlorophenyl)dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (1b). 1H-NMR δ 6.70 (s, 1H), 7.31 (m, 1H), 7.41-7.62(m, 4H), 8.05(m, 1H), 8.14 (m, 1H), 8.44 (dd, J = 1.2 Hz, J = 7.9 Hz, 1H), 13.12 (br. s, 1H).
2-(3-Methoxyphenyl)dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (1c). 1H-NMR δ 3.83 (s, 3H), 6.64 (s, 1H), 7.06 (m, 1H), 7.20-7.57 (m, 4H), 7.57-7.83 (m, 2H), 8.46 (d, J = 7.9 Hz, 1H), 13.06 (br. s, 1H).
2-(4-Fluorophenyl)dihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (1d). 1H-NMR δ 6.61 (s, 1H), 7.22-7.42 (m, 3H), 7.47 (d, J = 4.0 Hz, 2H), 8.16 (m, 2H), 8.45 (dd, J = 0.7 Hz, J = 7.8 Hz, 1H), 13.10 (br. s, 1H).
2-(4-Metoxyphenyl)dihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (1e). 1H-NMR δ 3.82 (s, 3H), 6.50 (s, 1H), 7.05 (d, J = 8.7 Hz, 2H), 7.17-7.60 (m, 3H), 8.07 (d, J = 8.7 Hz, 2H), 8.44 (d, J = 7.9 Hz, 1H), 12.95 (br. s, 1H); 13C NMR δ 56.1, 96.6, 111.8, 114.8, 116.3, 122.4, 126.6, 126.8, 129.3, 130.2, 131.8, 150.3, 160.4, 161.1, 162.0.
2-(Pyridin-4-yl)dihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (1f). 1H-NMR δ 6.80 (s, 1H), 7.36 (m, 1H), 7.50 (d, J = 4.0 Hz, 2H), 8.04 (dd, J = 1.7 Hz, J = 5.2 Hz, 2H), 8.47 (d, J = 7.9 Hz, 1H), 8.71 (dd, J = 1.7 Hz, J = 5.2 Hz, 2H), 13.10 (br. s, 1H).
2-(Pyridin-3-yl)dihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (1g). 1H-NMR δ 6.73 (s, 1H), 7.33 (m, 1H), 7.42-7.60 (m, 3H), 8.33-8.56 (m, 2H), 8.66 (dd, J = 1.7 Hz, J = 4.7 Hz, 1H), 9.27 (dd, J = 0.8 Hz, J = 2.3 Hz, 1H).
2-(Pyridin-2-yl)dihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (1h). 1H-NMR δ 7.03 (s, 1H), 7.35 (m, 1H), 7.41-7.63 (m, 3H), 7.98 (td, J = 0.8 Hz, J = 7.9 Hz, 1H), 8.32 (d, J = 7.9 Hz, 1H), 8.47 (d, J = 7.9 Hz, 1H), 8.70 (d, J = 0.5 Hz, 1H), 13.17 (br. s, 1H).
3-Benzyl-2-methyldihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (1j). 1H-NMR δ 2.30 (s, 3H), 3.88 (s, 2H), 7.03-7.35 (m, 6H), 7.35-7.60 (m, 2H) 8.39 (d, J = 7.8 Hz, 1H), 12.32 (br. s, 1H).
2-EthyldihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (1k). 1H-NMR δ 1.20 (t, J = 7.5 Hz, 3H), 2.56 (q,J = 7.5 Hz, 2H), 5.83 (s, 1H), 7.26 (td, J = 1.5 Hz, J = 7.9 Hz, 1H), 7.34-7.59 (m, 2H), 8.35 (d, 7.8 Hz, 1H), 12.66 (br. s, 1H).
2-PropyldihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (1l). 1H-NMR δ 0.90 (t, J = 7.2 Hz, 3H), 1.67 (hexet, J = 7.2 Hz, 2H), 2.42-2.55 (m, 2H), 5.82 (s, 1H), 7.25 (td, J = 1.5 Hz, J = 7.9 Hz, 1H), 7.33-7.60 (m, 2H), 8.35 (d, 7.9 Hz, 1H), 12.67 (br. s, 1H); 13C-NMR δ 14.0, 21.7, 38.1, 98.9, 113.8, 115.9, 122.0, 126.2, 127.5, 135.2, 149.4, 160.0, 164.1.
2-tert-ButyldihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (1m). 1H-NMR δ 1.27 (s, 9H), 5.95 (s, 1H), 7.27 (m, 1H), 7.33-7.53 (m, 2H), 8.37 (d, 7.9 Hz, 1H), 12.85 (br. s, 1H).
2-TrifluoromethtyldihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (1n). 1H-NMR δ 6.43 (s, 1H), 7.36 (m, 1H), 7.47-7.58 (m, 2H), 8.44 (dd, J = 0.8 Hz, J = 7.9 Hz, 1H).
Generation of 500-member Library 5 of 10-Alkyl-2-R1,3-R2-4,10-Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-ones
The equimolar mixture (0.6 mmol) of starting Dihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (1a–n...) and corresponding alkylation agent (4a–u…) was dissolved in DMF (2 mL) with mild heating. Then K2CO3 (1.8 mmol) was added and the reaction mixture was stirred at 90-100 °C for 2h. The mixture was cooled down and diluted with 50% aqueous EtOH (4 mL). The formed precipitate was removed by filtration, washed with H2O and 50% aqueous EtOH and dried on air at 90 °C. In those cases where 1H-NMR demonstrated more than 10 mol.% total impurity level, the product was additionally purified by heating in 1-2 mL of EtOH for 3-5 min, then cooling to rt, and isolation by filtration.
10-(4-Methylbenzyl)-2-phenyldihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (5aa). 1H-NMR δ 2.22 (s, 3H, Me), 5.58 (s, 2H, CH2), 6.72 (s, 1H, 3-CH), 7.13 (d, J = 8.1 Hz, 2H, Ar), 7.29-7.43 (m, 3H, Ar), 7.43-7.58 (m, 4H, Ar), 7.63 (d, J = 7.9 Hz, 1H, 9-CH), 8.07-8.33 (m, 2H, 7-CH and 8-CH), 8.48 (d, J = 7.9 Hz, 1H, 6-CH); 13C-NMR δ 21.2, 45.9, 98.4, 110.8, 116.4, 123.1, 125.9, 126.8, 127.8, 128.4, 129.3, 129.9, 131.0, 131.7, 133.5, 137.7, 137.9, 149.6, 160.4, 161.2.
2-(4-Oxo-2-phenylpyrimido[1,2-a]benzimidazol-10(4H)-yl)-N-(3-methoxyphenyl)acetamide (5ao).1H-NMR δ 3.68 (s, 3H, OMe), 5.29 (s, 2H, CH2), 6.65 (dd, J = 1.7 Hz, J = 7.9 Hz, 1H, Ar), 6.73 (s, 1H, 3-CH), 6.98-7.34 (m, 3H, Ar), 7.34-7.62 (m, 5H, Ar), 7.71 (d, J = 8.1 Hz, 1H, 9-CH), 8.00-8.27 (m, 2H, 7-CH and 8-CH), 8.51 (d, J = 8.1 Hz, 1H, 6-CH), 10.52 (s, 1H, NH).
2-[4-Oxo-2-(3-chlorophenyl)pyrimido[1,2-a]benzimidazol-10(4H)-yl]-N-(3-methylphenyl)acetamide
(5bl). 1H-NMR δ 2.25 (s, 3H), 5.29 (s, 2H), 6.76 (m, 1H), 6.89 (d, J = 7.3 Hz, 1H), 7.18 (t, J = 8.2 Hz, 1H), 7.27-7.64 (m, 6H), 7.70 (d, J = 7.9 Hz, 1H), 8.10 (d, J = 6.4 Hz, 1H), 8.20 (s, 1H), 8.53 (d, J = 7.9 Hz, 1H), 10.40 (br. s, 1H); 13C-NMR δ 21.6, 45.9, 99.1, 110.8, 116.2, 117.7, 121.0, 123.3, 125.1, 125.7, 126.3, 127.0, 127.5, 129.2, 130.7, 131.0, 131.1, 132.5, 134.4, 138.7, 139.0, 139.7, 149.6, 159.4, 160.2, 165.2.
10-(2-Chloro-6-fluorobenzyl)-2-(3-methoxyphenyl)dihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one
(5ch). 1H-NMR δ 3.84 (s, 3H), 5.74 (s, 2H), 6.71 (s, 1H), 7.04 (m, 1H), 7.19-7.78 (m, 9H), 8.50 (d, J = 7.9 Hz, 1H).
N-(Furan-2-ylmethyl)-2-[4-Oxo-2-(3-methoxyphenyl)pyrimido[1,2-a]benzimidazol-10(4H)-yl]-
acetamide (5ct). 1H-NMR δ 3.83 (s, 3H), 4.30 (d, J = 5.5 Hz, 1H), 5.11 (s, 2H), 6.23 (dd, J = 0.7 Hz, J = 3.3 Hz, 1H), 6.32 (dd, J = 1.8 Hz, J = 3.3 Hz, 1H), 6.75 (s, 1H), 7.07 (m, 1H), 7.31-7.47 (m, 2H), 7.47-7.59 (m, 2H), 7.59-7.79 (m, 3H), 8.49 (d, J = 8.1 Hz, 1H), 8.86 (t, J = 5.5 Hz, 1H).
N-(4-Carbomethoxyphenyl)-2-[4-oxo-2-(4-methoxyphenyl)pyrimido[1,2-a]benzimidazol-10(4H)-yl]-
acetamide (5er). 1H-NMR δ 3.78 (s, 6H), 5.31 (s, 2H), 6.63 (s, 1H), 6.98 (d, J = 8.9 Hz, 2H), 7.37 (t, J = 7.3 Hz, 1H), 7.51 (t, J = 7.3 Hz, 1H), 7.62-7.79 (m, 3H), 7.89 (d, J = 8.9 Hz, 2H), 8.09 (d, J = 8.9 Hz, 2H), 8.48 (d, J = 7.8 Hz, 1H), 10.70 (br. s, 1H); 13C-NMR δ 46.0, 52.4, 56.1, 97.2, 110.7, 114.8, 116.2, 119.8, 123.1, 125.4, 125.9, 126.7, 129.4, 129.8, 130.9, 132.5, 143.8, 149.6, 160.3, 160.9, 162.1, 166.0, 166.5.
N-(3-Methylphenyl)-2-[4-oxo-2-(pyridin-4-yl)pyrimido[1,2-a]benzimidazol-10(4H)-yl]-acetamide
(5fl). 1H-NMR δ 2.23 (s, 3H), 5.31 (s, 2H), 6.88 (d, J = 7.3 Hz, 1H), 6.93 (s, 1H), 7.18 (t, J = 7.8 Hz, 1H), 7.28-7.51 (m, 3H), 7.57 (t, J = 8.2 Hz, 1H), 7.73 (d, J = 8.2 Hz, 1H), 8.09 (d, J = 6.1 Hz, 2H), 8.52 (d, J = 7.8 Hz, 1H), 8.69 (d, J = 6.1 Hz, 2H), 10.49 (br. s, 1H).
Ethyl N-(3-methylphenyl)-2-[4-oxo-2-(pyridin-3-yl)pyrimido[1,2-a]benzimidazol-10(4H)-yl]acetate
(5gk). 1H-NMR δ 1.21 (t, J = 7.0 Hz, 3H), 4.19 (q, 7.0 Hz, 2H), 5.36 (s, 2H), 6.87 (s, 1H), 7.32-7.66 (m, 3H), 7.74 (d, J = 7.9 Hz, 1H), 8.49 (d, J = 8.1 Hz, 2H), 8.67 (dt, J = 1.7 Hz, J = 4.7 Hz, 1H), 9.33 (m, 1H).
2-Methyl-10-(4-methylbenzyl)dihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (5ia). 1H-NMR δ 2.23 (s, 3H), 2.33 (s, 3H), 5.45 (s, 2H), 5.96 (s, 1H), 7.11 (d, J = 7.1 Hz, 2H), 7.19-7.66 (m, 5H), 8.45 (d, J = 7.9 Hz, 1H)
3-Benzyl-2-methyl-10-(3-trifluoromethylbenzyl)dihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one
(5jg). 1H-NMR δ 2.33 (s, 3H), 3.93 (s, 2H), 5.59 (s, 2H), 7.04-7.79 (m, 11H), 7.91 (s, 1H), 8.50 (d, J = 7.8 Hz, 1H).
10-(2-Fluorobenzyl)-2-ethyldihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (5kc). 1H NMR δ 1.19 (t, J = 7.5 Hz, 3H), 2.56 (q, J = 7.5 Hz, 2H), 5.55 (s, 2H), 5.97 (s, 1H), 7.11 (m, 1H), 7.19-7.41 (m, 4H), 7.41-7.65 (m, 2H), 8.45 (dq, J = 0.7 Hz, J = 7.9 Hz, 1H); 13C-NMR δ 13.0, 31.1, 100.3, 110.4, 116.0, 116.4, 123.0, 125.3, 125.4, 126.0, 126.7, 130.7, 130.8, 131.4, 149.4, 160.0, 163.4, 170.0.
2-tert-Butyl-10-(4-clorobenzyl)dihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (5me). 1H-NMR δ 1.27 (s, 9H), 5.48 (s, 2H), 6.03 (s, 1H), 7.13-7.59 (m, 6H), 7.66 (d, J = 8.1 Hz, 1H), 8.40 (d, J = 8.1 Hz, 1H).
10-Butyl-2-trifluoromethyldihydrobenzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidin-4-one (5nj). 1H-NMR δ 0.88 (t, J = 7.2 Hz, 3H), 1.31 (hexet, J = 7.5 Hz, 2H), 1.79 (pentet,J = 7.5 Hz, 2H), 4.32 (t, J = 7.2 Hz, 2H), 6.51 (s, 1H), 7.42 (t, J = 7.5 Hz, 1H), 7.60 (t, J = 7.6 Hz, 1H), 7.80 (d, J = 8.3 Hz, 1H), 8.50 (d, J = 8.0 Hz, 1H); 13C-NMR δ 13.9, 19.9, 30.2, 42.8, 100.1, 111.0, 116.6, 123.6, 124.6, 125.5, 127.6, 131.8, 149.6, 159.5, 160.2.
Acknowledgements
We thank ChemDiv, Inc. San Diego, CA, USA for supporting this work.
Footnotes
Samples Availability: Samples of all the compounds described are available from authors.
References and Notes
- 1.Charifson P.S., Grillot A.L., Grossman T.H., Parsons J.D., Badia M., Bellon S., Deininger D.D., Drumm J.E., Gross C.H., Letiran A., Liao Y., Mani N., Nicolau D.P., Perola E., Ronkin S., Shannon D., Swenson L.L., Tang Q., Tessier P.R., Tian S.K., Trudeau M., Wang T., Wei Yu., Zhang H., Stamos D. Novel dual-targeting benzimidazole urea inhibitors of DNA gyrase and topoisomerase IV possessing potent antibacterial activity: intelligent design and evolution through the judicious use of structure-guided design and stucture-activity relationships. J. Med. Chem. 2008;51:5243–5263. doi: 10.1021/jm800318d. [DOI] [PubMed] [Google Scholar]
- 2.Hasegawa M., Nishigaki N., Washio Y., Kano K., Harris P.A., Sato H., Mori I., West R.I., Shibahara M., Toyoda H., Wang L., Nolte R.T., Veal J.M., Cheung M. Discovery of novel benzimidazoles as potent inhibitors of TIE-2 and VEGFR-2 tyrosine kinase receptors. J. Med. Chem. 2007;50:4453–4470. doi: 10.1021/jm0611051. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A., Maurya R.A., Ahmad P. Diversity oriented synthesis of benzimidazole and benzoxa/(thia)zole libraries through polymer-supported hypervalent iodine reagent. J. Comb. Chem. 2009;11:198–201. doi: 10.1021/cc8001876. [DOI] [PubMed] [Google Scholar]
- 4.Podunavac-Kuzmanović S.O., Cvetković D.D., Barna D.J. QSAR Analysis of 2-amino or 2-methyl-1-substituted benzimidazoles against Pseudomonas aeruginosa. Int. J. Mol. Sci. 2009;10:1670–1682. doi: 10.3390/ijms10041670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Algul O., Kaessler A., Apcin Y., Yilmaz A., Jose J. Comparative studies on conventional and microwave synthesis of some benzimidazole, benzothiazole and indole derivatives and testing on inhibition of hyaluronidase. Molecules. 2008;13:736–748. doi: 10.3390/molecules13040736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y.S., Zeng C.C., Jiao Z.G., Hu L.M., Zhong R.G. Design, synthesis and anti-HIV integrase evaluation of 4-oxo-4H-quinolizine-3-carboxylic acid derivatives. Molecules. 2009;14:868–883. doi: 10.3390/molecules14020868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madhav J.V., Suresh B.K., Rajitha B. Dipyridine copper chloride as a mild and efficient catalyst for the solid state synthesis of 2-substituted benzimidazoles. ARKIVOC. 2008;xiii:145–150. [Google Scholar]
- 8.Vinodkumar R., Vaidya S. D., Kumar B.V., Bhise U.N., Bhirud S.B., Mashelkar U.C. Synthesis, anti-bacterial, anti-asthmatic and anti-diabetic activities of novel N-substituted 2-(4-styrylphenyl)-1H-benzimidazole and N- substituted-3[4-(1H-benzimidazole-2-yl)-phenyl]-acrylic acid tert-butyl ester. ARKIVOC. 2008;xiv:37–49. doi: 10.1016/j.ejmech.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Martínez-Palou R., Zepeda L.G, Höpfl H, Montoya1 A., Guzmán-Lucero D.J., Guzmán J. Parallel and automated library synthesis of 2-long alkyl chain benzoazoles and azole[4,5-b]pyridines under microwave irradiation. Mol. Divers. 2009;9:361–369. doi: 10.1007/s11030-005-6357-5. [DOI] [PubMed] [Google Scholar]
- 10.Abdel-hafez A.A.M. Benzimidazole condensed ring systems: new synthesis and antineoplastic activity of substituted 3,4-dihydro- and 1,2,3,4-tetrahydro-benzoDihydrobenzo[4,5]imidazo[1,2-a]pyrimidine derivatives. Arch. Pharm. Res. 2007;30:678–684. doi: 10.1007/BF02977627. [DOI] [PubMed] [Google Scholar]
- 11.Via L.D., Gia O., Magno S.M., Da Settimo A., Marini A.M., Primofiore G., Da Settimo F., Salerno S. Synthesis, in vitro antiproliferative activity and DNA-interaction of benzimidazoquinazoline derivatives as potential anti-tumor agents. Il. Farmaco. 2001;56:159–167. doi: 10.1016/s0014-827x(01)01079-5. [DOI] [PubMed] [Google Scholar]
- 12.Starčević K., Kralj M., Ester K., Karminski-Zamola G. Synthesis and cytostatic evaluation of pyridopyrimidobenzimidazole derivatives. Heterocycles. 2007;3:647–656. [Google Scholar]
- 13.Vaidyanathan V.G., Villalta P.W., Sturla S.J. Nucleobase-dependent reactivity of a quinone metabolite of pentachlorophenol. Chem. Res. Toxicol. 2007;20:913–919. doi: 10.1021/tx600359d. [DOI] [PubMed] [Google Scholar]
- 14.Chiba T., Shigeta S., Numazaki Y. Inhibitory effekt of piridobenzazoles on replication in Vitro. Biol. Pharm. Bull. 1995;18:1081–1083. doi: 10.1248/bpb.18.1081. [DOI] [PubMed] [Google Scholar]
- 15.Gupta S.P. Quantitative structure-activity relationship studies on anticancer drugs. Chem. Rev. 1994;94:1507–1551. [Google Scholar]
- 16.Gago F. Stacking Interactions and Intercalative DNA Binding. Methods. 1998;14:277–292. doi: 10.1006/meth.1998.0584. [DOI] [PubMed] [Google Scholar]
- 17.Papadopoulos K., Young W.D. Versatile synthesis of ibotenic acid analogues with potential for activity at glutamate receptors by use of a homochiral β-lactam template in our ‘ring switching’ strategy. Tetrahedron Lett. 2002;43:3951–3955. [Google Scholar]
- 18.Toche R.B., Ghotekar B.K., Muddassar A. Kazi M.A., Kendre D.B., Jachak M.N. Synthesis of fused pyrimidines from amines and cyclic β-formylesters. Tetrahedron. 2007;63:8157–8163. doi: 10.1016/j.tet.2007.05.123. [DOI] [Google Scholar]
- 19.Al-Awadi N.A., Abdelhamid I.A., Al-Etaibi A.M., Elnagdi M.H. Gas-phase pyrolysis in organic synthesis: rapid green synthesis of 4-quinolinones. Synlett. 2007;14:2205–2208. [Google Scholar]
- 20.Carpenter R.D., Lam K.S., Kurth M.J. Microwave-mediated heterocyclization to benzimidazo[2,1-b]quinazolin-12(5H)-ones. J. Org. Chem. 2007;72:284–287. doi: 10.1021/jo0618066. [DOI] [PubMed] [Google Scholar]
- 21.Metwally M.A., Desoky E.I., Fawzy R., Etman H.A. Ketene S,S-Acetals in the synthesis of some new fused pyrimidine derivatives. Chem. Heterocycl. Comp. 2007;43:382–386. [Google Scholar]
- 22.Wahe H., Asobo P.F., Cherkasov R.A., Fomum Z.T., Doepp D. Heterocycles of biological importance: Part 8. Formation of pyrimido[1,2-a]benzimidazoles and oxazolo[3,2-a]benzimidazoles by conjugate addition of 2-aminobenzimidazoles to 4-hydroxy-2-alkynenitriles. ARKIVOC. 2004;i:130–137. [Google Scholar]
- 23.Antaki H., Petrow V. New syntheses of heterocyclic compounds. Part XII. The condensation of ethyl β-aminocrotonate with some cyclic amidines. J. Chem. Soc. 1951:551–555. [Google Scholar]
- 24.Ramström H., Bourotte M., Philippe C., Schmitt M., Haiech J., Bourguignon J.-J. Heterocyclic bis-cations as starting hits for design of inhibitors of the bifunctional enzyme histidine-containing protein kinase/phosphatase from Bacillus subtilis. J. Med. Chem. 2004;47:2264–2275. doi: 10.1021/jm021043o. [DOI] [PubMed] [Google Scholar]
- 25.Ogura H., Kawano M., Itoh T. Studies on heterocyclic compounds. XIII. Reaction of 2-amino-benzazoles with acetylenic compounds. Chem. Pharm. Bull. 1973;21:2019–2025. [Google Scholar]
- 26.Niwa R., Katagiri N., Kato T. Studies on ketene and its derivatives. CXXII. Reaction of haloketenes with 1,3-diaza-1,3-diene compounds. Chem. Pharm. Bull. 1984;32:4149–4153. [Google Scholar]
- 27.De Cat A., van Dormael A. Heterocyclic derivatives of pyrimidobenzimidazoles. Bull.Soc. Chim. Belg. 1950;59:573–587. [Google Scholar]