Abstract
Purpose of review
New insights into IgG4-related disease (IgG4-RD) have recently been obtained. A better understanding of the mechanisms underlying this disease is important for identification of therapeutic targets, which will lead to the development of specific strategies for treatment.
Recent findings
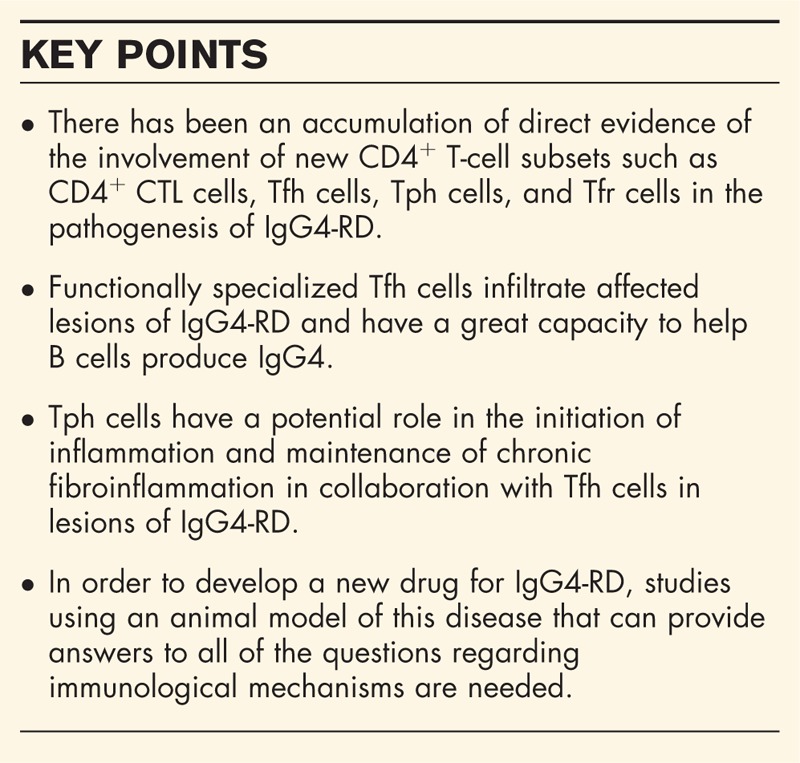
Infiltration of activated T follicular helper (Tfh) cells is observed in affected tissues of IgG4-RD. Such Tfh cells have a greater capacity than tonsillar Tfh cells to help B cells produce IgG4. Circulating PD-1hiCXCR5- peripheral T helper (Tph)-like cells are also increased in patients with IgG4-RD. Because Tph-like cells express high levels of chemokine receptors and granzyme A, they have the capacity to infiltrate affected tissues and exert a cytotoxic function. Tph-like cells can also produce CXCL13, and CXCR5+ Tfh cells and B cells are therefore preferentially recruited to form ectopic lymphoid structures in the sites. Tph cells may have a role to ignite inflammation and maintain persistent fibroinflammation in collaboration with Tfh cells in lesions of IgG4-RD.
Summary
Recent advances in understanding the pathogenesis of IgG4-RD are remarkable. In this review, we summarize and discuss the possible pathologic role of CD4+ T-cell subsets in IgG4-RD.
Keywords: CD4+ T-cell subsets, IgG4-related disease, Tfh cells, Tfr cells, Tph cells
INTRODUCTION
IgG4-related disease (IgG4-RD) is a chronic fibroinflammatory disease characterized by a significant elevation of serum IgG4 concentration and marked infiltration of IgG4-positive plasma cells in an affected organ or affected organs such as lacrimal glands, salivary glands, lymph nodes, pancreas, retroperitoneum, and lungs [1]. A dense lymphoplasmacytic infiltrate and the formation of ectopic lymphoid structures (ELSs) are characteristic histopathological findings in an IgG4-RD lesion [Figure 1]. These findings strongly suggest the preferential involvement of T and B lymphocytes in the development of this disease. Since the establishment of this disease entity, a number of studies have been performed for clarifying the immunological mechanisms of this disease. T cells and their subsets were focused on in the early period of IgG4-RD research. T helper 2 (Th2) cells [2–7] and regulatory T (Treg) cells [2,4,7–10] were considered as candidates of a main player in IgG4-RD. After such studies on CD4+ T cells, T follicular helper (Tfh) cells [11,12▪,13▪▪,14–16] and CD4+ cytotoxic T lymphocytes (CD4+ CTLs) [17▪▪,18▪] were suggested to play a cardinal role in the immunological settings of IgG4-RD. In addition to these CD4+ T cells, we have recently identified other new CD4+ T cell subsets including PD-1hiCXCR5- peripheral T helper (Tph)-like cells and T follicular regulatory (Tfr) cells in blood of patients with IgG4-RD and we found that they are significantly correlated with various clinical parameters [19▪▪] (F Ito, R Kamekura, unpublished data). Because of their function for secretion of chemokines such as CXCL13, which is a ligand of CXCR5, Tph cells have a potential role in the initiation of inflammation and subsequently lead to the characteristic immune responses in collaboration with CXCR5-expressing lymphocytes such as Tfh cells and B cells in lesions of IgG4-RD. There has been an accumulation of direct evidence regarding the involvement of CD4+ T-cell subsets in the immunological mechanisms of IgG4-RD [13▪▪,20]. In this review, we summarize the roles of CD4+ T-cell subsets and discuss the possible interplay between Tfh cells and Tph cells in the pathogenesis of IgG4-RD.
FIGURE 1.
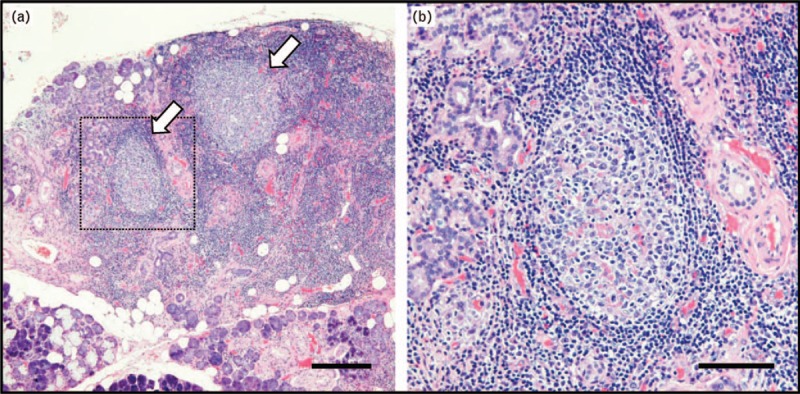
Histopathology of IgG4-related disease. (a) Formation of ectopic lymphoid structures (arrows) in a submandibular gland from a patient with IgG4-related dacryoadenitis and sialoadenitis. (b) High magnification image of the dotted square area in (a). A section of submandibular glands was stained with hematoxylin and eosin. Scale bars are 500 μm in (a) and 200 μm in (b).
Box 1.
no caption available
Th2 CELLS AND ALLERGIES
Patients with IgG4-RD often have allergic disorders such as bronchial asthma and allergic rhinitis [21]. Indeed, levels of Th2 cells and Th2 cytokines including interleukin (IL)-4, IL-5, and IL-10 are frequently increased in affected tissues or peripheral blood of patients with IgG4-RD [2–5,7]. Of note, Culver et al. reported associations of IgG4-RD with allergy, atopy, eosinophilia, increased serum levels of IgE, and IgE-positive mast cells in lymphoid and affected tissues. They concluded that levels of IgE could be used for diagnosis and predicting relapse [6]. Taken together, the results suggest that Th2 cells and IgE-mediated allergic response play a role in the pathogenesis of IgG4-RD.
However, several recent studies have shown controversial results. Mattoo et al. reported that circulating memory Th2 cells in IgG4-RD are detected in a limited population of subjects with atopy [22]. They also showed that CD4+GATA3+ Th2 cells were sparse in affected tissues of IgG4-related dacryoadenitis and sialoadenitis (IgG4-DS), which is an archetype of IgG4-RD postulated as Mikulicz disease [17▪▪,18▪]. In addition, the percentage of tissue CD4+GATA3+ Th2 cells in IgG4-RD does not seem to be correlated with clinical parameters such as serum IgG4 concentrations and the number of affected organs [17▪▪]. Our group has also provided evidence suggesting that clinical values indicating allergic status such as specific IgE against allergens are not important in the pathological mechanism of IgG4-DS (M. Yamamoto, R. Kamekura, unpublished data). Therefore, it is still not clear how classic Th2 cells and IgE-mediated allergy are involved in the pathogenesis of IgG4-RD.
Treg CELLS
Histopathologically, infiltration of IgG4-positive plasma cells accompanied by storiform fibrosis is usually observed in affected tissues of IgG4-RD [1,23]. It is well known that IL-10 and TGF-β are key cytokines for IgG4 class-switching and fibrosis, respectively [24–26]. Therefore, regulatory T (Treg) cells have been focused on from the early period of IgG4-RD research as a pathognomonic source of IL-10 and TGF-β. Indeed, several studies have shown an increased number of Treg cells and increased expression level of their master regulator, Foxp3, in both affected sites and circulating leukocytes in patients with IgG4-RD [2,4,7–10]. We also found increased levels of Treg cells in blood and affected tissues of patients with IgG4-RD (F. Ito, R. Kamekura, unpublished data). Taken together, the results of these studies suggest that Treg cells are preferentially involved in IgG4 class-switching and fibrosis in lesions of IgG4-RD; however, no direct evidence regarding the function of Treg cells in IgG4-RD was shown in those reports. Further studies are probably required to clarify IgG4 class-switching and fibrosis caused by Treg cells in IgG4-RD.
CD4+ CYTOTOXIC T LYMPHOCYTES
CD4+ T cells with a cytotoxic function (named CD4+ CTLs) have been observed in various immunological conditions such as virus infection, autoimmune diseases, and cancer [27,28]. CD4+ CTLs are characterized by their unique function of secreting perforin, granzyme, and IFN-γ for killing target cells in an MHC class II-restricted fashion [27,28]. Recently, there has been an accumulation of experimental evidence suggesting the involvement of CD4+ CTLs in IgG4-RD. Mattoo et al. first reported the clonal expansion of CD4+ CTLs in inflamed tissue sites of IgG4-RD. These cells presented SLAMF7, granzyme A (GZMA), IL-1β, and TGF-β, suggesting their capacity related to tissue inflammation and fibrosis. Interestingly, clinical remission induced by rituximab-mediated B-cell depletion seems to be associated with a reduction in CD4+ CTLs in IgG4-RD [17▪▪]. In another report, the same group presented results showing an oligoclonal expansion of circulating plasmablasts (CD19+CD20-CD27+CD38+ cells) in patients with IgG4-RD [29]. These findings indicate that CD4+ CTLs collaborate with activated plasmablasts and play an important role in the pathogenesis of IgG4-RD. However, there has been no functional experiment on CD4+ CTLs in IgG4-RD because of the minor population in CD4+ T-cell subsets and the lack of specific surface markers for live cell sorting. Additional studies are required in the future to obtain direct evidence of cytotoxicity and fibrosis in affected tissues of IgG4-RD by these cells.
Tfh CELLS
As mentioned above, abundant infiltration of IgG4-positive plasma cells is usually observed in tissue lesions of IgG4-RD [1]. This suggests that dysregulation of the IgG4 class-switch underlies the pathogenesis of IgG4-RD. Tfh cells, which are postulated as a specialized class of effector helper CD4+ T cells, assist B cells to form germinal centers of lymphoid follicles, and Tfh cells thereby contribute to the class switch recombination of B cells and the selection of high-affinity B cells in germinal centers [30,31]. Importantly, Tfh cells have the capacity to secrete IL-4 and IL-10, which are key cytokines for IgG4 class-switching [24]. Tfh cells have been considered as a potential key player in the development of IgG4-RD.
It is well known that Tfh cells not only localize in lymphoid tissues but also exist in blood circulation and lesional sites of extra-lymphoid tissues [13▪▪,31]. Because of accessibility of blood samples, accumulating evidence has shown the role of circulating Tfh cells in IgG4-RD. Circulating Tfh cells comprise three subsets, Tfh1 cells, Tfh2 cells and Tfh17 cells, that can secrete restricted repertories of the cytokines IFN-γ, IL-4 and IL-17, respectively, as seen in conventional helper T-cell subsets such as Th1 cells, Th2 cells, and Th17 cells [32–34]. In IgG4-RD, increased circulating Tfh2 cells and activated Tfh2 cells with a high expression level of programmed cell death 1 (PD-1, i.e., PD-1hi Tfh2 cells) are able to help naïve B cells differentiate into plasmablasts and produce IgG4 [11,12▪,14]. Circulating activated Tfh1 cells were also shown to be increased in IgG4-RD and to be correlated with disease activity but not with serum IgG4 levels [12▪].
Recently, our group and others have demonstrated abundant infiltration of Tfh cells in affected submandibular glands of patients with IgG4-RD [13▪▪,14,15]. We further reported that lesional Tfh cells isolated from submandibular glands of patients with IgG4-DS showed high expression levels of B-cell lymphoma (Bcl) 6 and activation markers such as PD-1 and ICOS and had a greater capacity than tonsillar Tfh cells to help B cells produce IgG4 [13▪▪]. Moreover, Maehara et al.[16] recently reported that the expansion of IL-4+BAFF+ Tfh cells in lymphoid organs is linked to IgG4 class switching. Taken together, the results indicate that activated Tfh cells possessing unique functions abundantly infiltrate affected lesions of IgG4-RD and play an important role in the pathogenesis of IgG4-RD. Fundamental questions that remain to be answered are whether circulating Tfh cells and resident Tfh cells in affected tissues of patients with IgG4-RD have the same origin and, if so, how circulating Tfh cells migrate from or into the affected tissues. Further studies on Tfh cells in IgG4-RD are needed to answer these questions.
Tfr CELLS
Tfr cells have recently been characterized as a unique CD4+ T-cell subset that participates in the control of germinal center formation and class switch recombination of B cells in collaboration with Tfh cells [35–37]. Tfr cells express CXCR5, which is also shared by B cells and Tfh cells. Tfr cells are regulated by Bcl6, PD-1, and ICOS as well as forkhead box P3 (Foxp3), as observed in Treg cells [31,36]. To exert germinal center responses, Tfr cells produce IL-10 and TGF-β for the direct regulation of B cells and Tfh cells. Because most of the studies regarding Tfr cells in disease have mainly been performed in mouse models [38,39], functional roles of Tfr cells in human diseases are not fully understood. Recent studies have shown that Tfr cells proportionately and numerically proliferate during HIV infection and contribute to inefficient germinal center responses and then inhibit HIV clearance [40]. Other studies have demonstrated a decreased number of circulating Tfr cells and a significant correlation between the percentage of Tfr cells and clinical parameters in patients with systemic lupus erythematosus or multiple sclerosis [41]. In contrast, the pathological significance of Tfr cells in IgG4-RD has not been investigated. Therefore, we examined Tfr cells using clinical specimens to address the question of whether Tfr cells are associated with the pathogenesis of IgG4-RD. Our results showed that the number of Tfr cells was increased in blood and inflamed submandibular glands from patients with IgG4-DS (F. Ito, R. Kamekura, unpublished data). The percentage of Tfr cells was positively correlated with clinical parameters including serum level of IgG4 and number of involved organs in patients with IgG4-RD. Interestingly, the number of IL-10-producing circulating Tfr cells in patients with IgG4-RD was increased compared with that in healthy elderly patients, indicating the possible involvement of Tfr cells in IgG4-specific class-switch recombination in lesions of IgG4-RD. Collectively, these findings seem provide a novel insight into the role of Tfr cells in the disease pathogenesis.
Tph CELLS
A more recent study on rheumatoid arthritis (RA) has revealed an unidentified subset of CD4+ T cells named Tph cells (PD-1hiCXCR5-CD4+ cells) [42▪▪]. Tph cells present Tfh cell-like features to produce factors associated with B-cell help, including IL-21 and CXCL13 in the inflamed synovium of RA. Unlike Tfh cells, Tph cells in the synovium of RA do not express high levels of Bcl6 and instead show elevated levels of Blimp1, which opposes the actions of Bcl6 as a counter-regulator [43]. Tph cells also have a unique expression profile of chemokine receptors, such as CCR2, CCR5, and CX3CR1 (a fractalkine receptor), that ignite their migration to inflamed sites [42▪▪]. Thus, Tph cells show substantial differences from Tfh cells in their surface phenotypes, migratory capacity, and transcriptional regulation [42▪▪]. Recent studies have shown an increased percentage of Tph cells in blood from patients with primary Sjögren's syndrome [44]. In addition, Gu-Trantien et al.[45] reported that CD4+ T cells with a Tph cell-like phenotype were found in breast cancer tissues and that they have a possible regulatory function in immune responses against tumor cells. Based on these observations, we have first reported a possible pathological role of Tph cells in IgG4-RD [19▪▪]. Our results have shown that circulating PD-1+CXCR5− cells (including PD-1hiCXCR5− cells, thus collectively named Tph-like cells here) were significantly increased within CD4+ T cells in patients with IgG4-RD compared to those in healthy volunteers. We also found that their percentage was positively correlated with serum levels of IgG4 and soluble IL-2 receptor and with the number of involved organs in IgG4-RD patients. In addition, we found that such Tph-like cells frequently expressed GZMA, which is related to a cytotoxic property. Clinical remission achieved by treatment with glucocorticoids clearly led to a numerical reduction of Tph-like cells [19▪▪]. Taken together, our findings strongly suggest that circulating Tph-like cells play a pivotal role in the pathogenesis of IgG4-RD.
COLLABORATION OF Tph CELLS AND Tfh CELLS IN THE FORMATION OF ECTOPIC LYMPHOID STRUCTURES
In peripheral tissues of chronic inflammation such as IgG4-RD and RA, aggregations of T cells and B cells (so-called ectopic lymphoid structures, ELSs) frequently develop [46] [Figure 1]. In ELSs, T cell–B cell interactions result in uncontrolled somatic hypermutation, class switch recombination, and differentiation of plasma cells [46], the functional interplay of which accelerates the development of the disease. Our previous observations revealed abundant infiltration of PD-1hiICOShi Tfh cells in lesional ELSs of IgG4-DS [13▪▪], suggesting that activated Tfh cells interact and strongly induce B cells to produce IgG4 in ELSs of IgG4-RD. The appearance of a new player, Tph cells, in the research field of chronic inflammation, might lead to a deeper understanding of the immunological mechanisms of ELS formation in lesions of chronic inflammation including IgG4-RD. Based on results of previous studies and our recent findings regarding IgG4-RD, we presume the following relationship between Tph cells and Tfh cells in the pathogenesis of IgG4-RD. Owing to the high expression levels of chemokine receptors including CCR2, CCR5, and CX3CR1, Tph cells are preferentially prone to infiltration of inflamed tissues in patients with IgG4-RD. Production of CXCL13 by Tph cells may subsequently provide early stimuli for the recruitment of CXCR5+ immune cells including both Tfh cells and B cells. As a result, it is possible that Tfh cells and B cells accumulate to form ELSs in the lesions and eventually provide an immune microenvironment in which the production of IgG4 is further induced. Because fractalkine, which is a ligand of CX3CR1, is highly expressed by endothelial cells in submandibular glands of IgG4-DS (H. Yabe, R. Kamekura R, unpublished data), Tph cells might have a role in the initiation of inflammation and maintenance of chronic fibroinflammation in IgG4-RD, although influencing vascularities in the lesions [47]. Given that destructive inflammation is observed in IgG4-RD [Figure 1], our experimental evidence indicates that Tph-like (PD-1+CXCR5-CD4+) cells, which preferentially contain cytotoxic granules of GZMA, are responsible for such pathological changes in IgG4-RD [19▪▪]. In this study, we could not see abundant expression of IL21 mRNAs in Tph-like cells compared with those in Tfh cells from patients with IgG4-RD [19▪▪]. Taken together, the results suggest that Tph-like cells in IgG4-RD play a pathological role as CD4+ CTLs rather than as B cell helpers such as Tfh cells [Figure 2].
FIGURE 2.
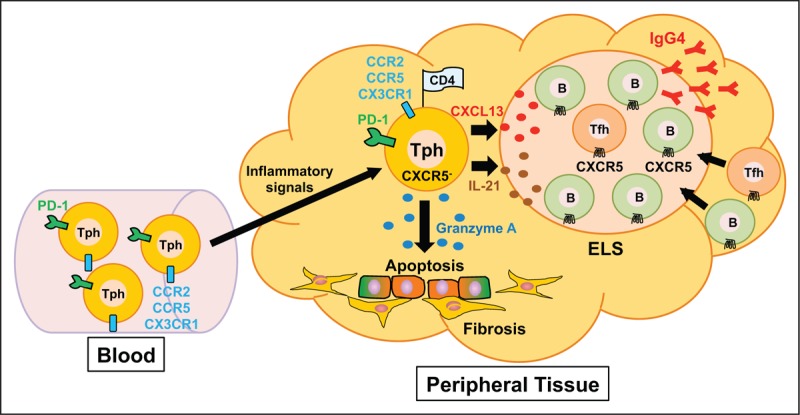
A model for the functional role of PD-1hiCXCR5- peripheral T helper (Tph) cells and T follicular helper (Tfh) cells in affected organs of IgG4-RD. Because Tph cells express high levels of chemokine receptors including CCR2, CCR5, and CX3CR1, Tph cells rapidly infiltrate inflamed tissues of IgG4-RD. Production of CXCL13 by Tph cells induces the recruitment of CXCR5+ Tfh cells and B cells. As a result, it is possible that the interaction of Tfh cells with B cells that subsequently accumulate and form ectopic lymphoid structures (ELSs) in the lesions provides an immune microenvironment in which production of IgG4 is induced. Tph cells also express a high level of cytotoxic granules such as granzyme A and exert cytotoxic activity in inflamed tissues.
Several questions remain to be answered to understand the mechanisms of the origin and differentiation of Tfh cell in lesions of IgG4-RD and the developmental relationship between Tfh cells and Tph cells.
CONCLUSION
Following the recent advances of our knowledge of human CD4+ T cells, IgG4-RD research has progressed remarkably. The appearance of Tph cells and Tfh cells as key players in the pathogenesis of IgG4-RD is one example of the progress in research. Accumulating evidence may lead to the development of specific therapeutic targets and a new strategy for IgG4-RD treatment. Treatment with glucocorticoids is still effective for IgG4-RD; however, it often causes side effects and relapse frequently occurs after tapering or discontinuing administration of glucocorticoids [48]. A new strategy needs to be developed to overcome the problem of relapse and refractory cases of IgG4-RD. Thus, it is important to consider experimental findings and knowledge regarding the pathogenesis of IgG4-RD from direct examination of inflamed tissues from patients with IgG4-RD. Because of the limited availability of clinical specimens, studies using lesional tissues and blood from patients with IgG4-RD are not always straightforward. Evidence obtained from animal models of this disease may also help to address the questions regarding immunological mechanisms.
Acknowledgements
The authors would like to thank Prof. Kenichi Takano (Department of Otolaryngology, Sapporo Medical University School of Medicine) and Dr. Tetsuo Himi (professor emeritus of Sapporo Medical University) for years of collaboration and advice. We also thank our colleagues for constructive discussion and technical support.
Financial support and sponsorship
This work was supported by grants-in-aid for scientific research from the Japanese Society for the Promotion of Science including #18K09323 (R.K.) and #18H02632 (S.I.) and from the Suhara Memorial Foundation (R.K.), the Takeda Science Foundation (R.K.) and the Bristol-Myers Squibb Foundation (S.I.).
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License 4.0 (CCBY-NC-ND), where it is permissible to download and share the work provided it is properly cited. The work cannot be changed in any way or used commercially without permission from the journal. http://creativecommons.org/licenses/by-nc-nd/4.0
REFERENCES
- 1.Stone JH, Zen Y, Deshpande V. IgG4-related disease. N Engl J Med 2012; 366:539–551. [DOI] [PubMed] [Google Scholar]
- 2.Zen Y, Fujii T, Harada K, et al. Th2 and regulatory immune reactions are increased in immunoglobin G4-related sclerosing pancreatitis and cholangitis. Hepatology 2007; 45:1538–1546. [DOI] [PubMed] [Google Scholar]
- 3.Kanari H, Kagami S, Kashiwakuma D, et al. Role of Th2 cells in IgG4-related lacrimal gland enlargement. Int Arch Allergy Immunol 2010; 152 suppl 1:47–53. [DOI] [PubMed] [Google Scholar]
- 4.Tanaka A, Moriyama M, Nakashima H, et al. Th2 and regulatory immune reactions contribute to IgG4 production and the initiation of Mikulicz disease. Arthritis Rheum 2012; 64:254–263. [DOI] [PubMed] [Google Scholar]
- 5.Muller T, Beutler C, Pico AH, et al. Increased T-helper 2 cytokines in bile from patients with IgG4-related cholangitis disrupt the tight junction-associated biliary epithelial cell barrier. Gastroenterology 2013; 144:1116–1128. [DOI] [PubMed] [Google Scholar]
- 6.Culver EL, Sadler R, Bateman AC, et al. Increases in IgE, eosinophils, and mast cells can be used in diagnosis and to predict relapse of IgG4-related disease. Clin Gastroenterol Hepatol 2017; 15:1444–1452.e1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heeringa JJ, Karim AF, van Laar JAM, et al. Expansion of blood IgG4+B, TH2, and regulatory T cells in patients with IgG4-related disease. J Allergy Clin Immunol 2018; 141:1831–1843.e1810. [DOI] [PubMed] [Google Scholar]
- 8.Kusuda T, Uchida K, Miyoshi H, et al. Involvement of inducible costimulator- and interleukin 10-positive regulatory T cells in the development of IgG4-related autoimmune pancreatitis. Pancreas 2011; 40:1120–1130. [DOI] [PubMed] [Google Scholar]
- 9.Kawamura E, Hisano S, Nakashima H, et al. Immunohistological analysis for immunological response and mechanism of interstitial fibrosis in IgG4-related kidney disease. Mod Rheumatol 2015; 25:571–578. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama M, Suzuki K, Kassai Y, et al. Resolution of elevated circulating regulatory T cells by corticosteroids in patients with IgG4-related dacryoadenitis and sialoadenitis. Int J Rheum Dis 2016; 19:430–432. [DOI] [PubMed] [Google Scholar]
- 11.Akiyama M, Suzuki K, Yamaoka K, et al. Number of circulating follicular helper 2 T cells correlates with IgG4 and interleukin-4 levels and plasmablast numbers in IgG4-related disease. Arthritis Rheumatol 2015; 67:2476–2481. [DOI] [PubMed] [Google Scholar]
- 12▪.Akiyama M, Yasuoka H, Yamaoka K, et al. Enhanced IgG4 production by follicular helper 2 T cells and the involvement of follicular helper 1 T cells in the pathogenesis of IgG4-related disease. Arthritis Res Ther 2016; 18:167. [DOI] [PMC free article] [PubMed] [Google Scholar]; This group reported that circulating type 2 Tfh cells induced the differentiation of naïve B cells into plasmablasts and enhanced the production of IgG4 in patients with active and untreated IgG4-related disease.
- 13▪▪.Kamekura R, Takano K, Yamamoto M, et al. Cutting edge: a critical role of lesional T follicular helper cells in the pathogenesis of IgG4-related disease. J Immunol 2017; 199:2624–2629. [DOI] [PubMed] [Google Scholar]; This is the first report showing that lesional Tfh cells isolated from submandibular glands of patients with IgG4-related disease had high expression levels of B-cell lymphoma 6 and activation markers such as PD-1 and ICOS and had a greater capacity than tonsillar Tfh cells to help B cells produce IgG4.
- 14.Chen Y, Lin W, Yang H, et al. Aberrant expansion and function of follicular helper T cell subsets in IgG4-related disease. Arthritis Rheumatol 2018; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kubo S, Nakayamada S, Zhao J, et al. Correlation of T follicular helper cells and plasmablasts with the development of organ involvement in patients with IgG4-related disease. Rheumatology (Oxford) 2018; 57:514–524. [DOI] [PubMed] [Google Scholar]
- 16.Maehara T, Mattoo H, Mahajan VS, et al. The expansion in lymphoid organs of IL-4+ BATF + T follicular helper cells is linked to IgG4 class switching in vivo. Life Sci Alliance 2018; 1: pii: e201800050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪▪.Mattoo H, Mahajan VS, Maehara T, et al. Clonal expansion of CD4+ cytotoxic T lymphocytes in patients with IgG4-related disease. J Allergy Clin Immunol 2016; 138:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first report showing clonal expansion of CD4+ CTLs in inflamed tissue sites of IgG4-related disease. The article highlights the pathological role of CD4+ CTLs that highly express granzyme A, IL-1β, and TGF-β and their capacity related to tissue inflammation and fibrosis.
- 18▪.Maehara T, Mattoo H, Ohta M, et al. Lesional CD4+ IFN-γ+ cytotoxic T lymphocytes in IgG4-related dacryoadenitis and sialoadenitis. Ann Rheum Dis 2017; 76:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that tissue infiltration by CD4+granzyme A+ cytotoxic T lymphocytes that secrete IFN-γ is associated with the pathogenesis of IgG4-related dacryoadenitis and sialoadenitis.
- 19▪▪.Kamekura R, Yamamoto M, Takano K, et al. Circulating PD-1+CXCR5-CD4+ T cells underlying the immunological mechanisms of IgG4-related disease. Rheumatol adv pract 2018; [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first report of a possible pathological role of Tph cells in IgG4-RD. This study showed increased circulating Tph-like (PD-1+CXCR5-CD4+) cells in patients with IgG4-RD and showed a cytotoxic function of Tph-like cells that preferentially contain cytotoxic granules of granzyme A.
- 20.Akiyama M, Suzuki K, Yasuoka H, et al. Follicular helper T cells in the pathogenesis of IgG4-related disease. Rheumatology (Oxford) 2018; 57:236–245. [DOI] [PubMed] [Google Scholar]
- 21.Masaki Y, Dong L, Kurose N, et al. Proposal for a new clinical entity, IgG4-positive multiorgan lymphoproliferative syndrome: analysis of 64 cases of IgG4-related disorders. Ann Rheum Dis 2009; 68:1310–1315. [DOI] [PubMed] [Google Scholar]
- 22.Mattoo H, Della-Torre E, Mahajan VS, et al. Circulating Th2 memory cells in IgG4-related disease are restricted to a defined subset of subjects with atopy. Allergy 2014; 69:399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umehara H, Okazaki K, Masaki Y, et al. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol 2012; 22:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeannin P, Lecoanet S, Delneste Y, et al. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol 1998; 160:3555–3561. [PubMed] [Google Scholar]
- 25.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-β signaling in fibrosis. Growth Factors 2011; 29:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsuboi H, Matsuo N, Iizuka M, et al. Analysis of IgG4 class switch-related molecules in IgG4-related disease. Arthritis Res Ther 2012; 14:R171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheroutre H, Husain MM. CD4 CTL: living up to the challenge. Semin Immunol 2013; 25:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeuchi A, Saito T, CD4 CTL. A cytotoxic subset of CD4+ T cells, their differentiation and function. Front Immunol 2017; 8:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mattoo H, Mahajan VS, Della-Torre E, et al. De novo oligoclonal expansions of circulating plasmablasts in active and relapsing IgG4-related disease. J Allergy Clin Immunol 2014; 134:679–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King C, Tangye SG, Mackay CR. T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol 2008; 26:741–766. [DOI] [PubMed] [Google Scholar]
- 31.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014; 41:529–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morita R, Schmitt N, Bentebibel SE, et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011; 34:108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ueno H, Banchereau J, Vinuesa CG. Pathophysiology of T follicular helper cells in humans and mice. Nat Immunol 2015; 16:142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamekura R, Shigehara K, Miyajima S, et al. Alteration of circulating type 2 follicular helper T cells and regulatory B cells underlies the comorbid association of allergic rhinitis with bronchial asthma. Clin Immunol 2015; 158:204–211. [DOI] [PubMed] [Google Scholar]
- 35.Wollenberg I, Agua-Doce A, Hernandez A, et al. Regulation of the germinal center reaction by Foxp3+ follicular regulatory T cells. J Immunol 2011; 187:4553–4560. [DOI] [PubMed] [Google Scholar]
- 36.Sage PT, Sharpe AH. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol 2015; 36:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laidlaw BJ, Lu Y, Amezquita RA, et al. Interleukin-10 from CD4+ follicular regulatory T cells promotes the germinal center response. Sci Immunol 2017; 2: pii: eaan4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding Y, Li J, Yang P, et al. Interleukin-21 promotes germinal center reaction by skewing the follicular regulatory T cell to follicular helper T cell balance in autoimmune BXD2 mice. Arthritis Rheumatol 2014; 66:2601–2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SY, Jung YO, Ryu JG, et al. Intravenous immunoglobulin attenuates experimental autoimmune arthritis by inducing reciprocal regulation of Th17 and Treg cells in an interleukin-10-dependent manner. Arthritis Rheumatol 2014; 66:1768–1778. [DOI] [PubMed] [Google Scholar]
- 40.Miles B, Miller SM, Folkvord JM, et al. Follicular regulatory T cells impair follicular T helper cells in HIV and SIV infection. Nat Commun 2015; 6:8608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhaeze T, Peelen E, Hombrouck A, et al. Circulating follicular regulatory T cells are defective in multiple sclerosis. J Immunol 2015; 195:832–840. [DOI] [PubMed] [Google Scholar]
- 42▪▪.Rao DA, Gurish MF, Marshall JL, et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017; 542:110–114. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first report showing the role of peripheral T helper cells in rheumatoid arthritis. The article highlights the role of these cells as a B-cell helper in extranodal sites of rheumatoid joints.
- 43.Johnston RJ, Poholek AC, DiToro D, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009; 325:1006–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verstappen GM, Meiners PM, Corneth OBJ, et al. Attenuation of follicular helper T cell-dependent B cell hyperactivity by abatacept treatment in primary Sjogren's syndrome. Arthritis Rheumatol 2017; 69:1850–1861. [DOI] [PubMed] [Google Scholar]
- 45.Gu-Trantien C, Migliori E, Buisseret L, et al. CXCL13-producing TFH cells link immune suppression and adaptive memory in human breast cancer. JCI Insight 2017; 2: [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pitzalis C, Jones GW, Bombardieri M, et al. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol 2014; 14:447–462. [DOI] [PubMed] [Google Scholar]
- 47.Manes TD, Pober JS. Identification of endothelial cell junctional proteins and lymphocyte receptors involved in transendothelial migration of human effector memory CD4+ T cells. J Immunol 2011; 186:1763–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto M, Yajima H, Takahashi H, et al. Everyday clinical practice in IgG4-related dacryoadenitis and/or sialadenitis: results from the SMART database. Mod Rheumatol 2015; 25:199–204. [DOI] [PubMed] [Google Scholar]


