Abstract
An efficient and practical method for the synthesis of indolyl-nitroalkane derivatives catalyzed by N-bromosuccinimide is described. The generality of this method was demonstrated by synthesizing an array of diverse 3-substituted indole derivatives by the reaction of different β-nitrostyrenes with various substituted indoles. Simple reaction conditions accompanied by good yields of indolyl-nitroalkanes are the merits of this methodology.
Keywords: indolyl-nitroalkane, N-bromosuccinimide, nitroalkenes, Friedel-Crafts alkylation
Introduction
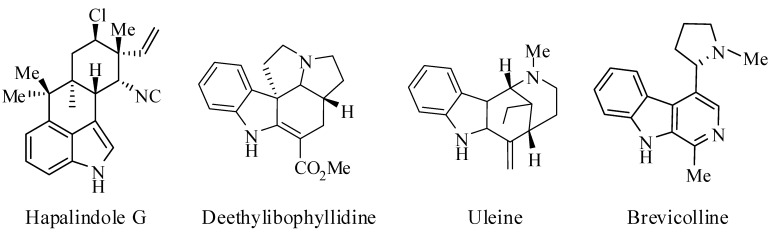
The indole nucleus is one of the most ubiquitous scaffolds found in natural products, pharmaceuticals, functional materials, and agrochemicals [1,2,3]. Several indole derivatives that occur in nature possess pharmacological activity. These include the hapalindole alkaloids, which exhibit significant antibacterial and antimycotic activity. Other indole alkaloids are uleine, aspidospermidine, the ibophyllidine alkaloids, brevicolline and numerous tryptamine derivatives which exhibit important biological activities (Figure 1) [4,5,6].
Figure 1.
Indole alkaloids.
Owing to the importance of the indole framework-containing compounds, the development of new strategies to synthesize indole derivatives remain a subject of interest in the present days. During the past several years, Michael addition was the most frequently employed tool for the synthesis of 3-substituted indole derivatives [7]. This is due to the fact that nitroalkenes are very good Michael acceptors and further the Michael adduct of the nitroalkenes are amenable to transformation into a wide range of different functionalized species. Similarly, Friedel-Crafts alkylation of indoles with nitroolefins in the presence of acidic catalysts is well documented in the literature [8,9,10,11,12,13,14,15,16,17,18,19,20,21,22]. Neverthless, most of the procedures reported suffer from several shortcomings such as the use of hazardous catalysts, tedious workup procedures and difficulties in product isolation. The most important drawback is the tendency of electron-rich heteroaromatic rings to undergo polymerization under acid catalyzed conditions. Hence the development of an effective method for the synthesis of 3-substituted indoles has still remained a problem far from resolution. In addition, recent progress in the stereoselective synthesis of these compounds was reported [23,24].
N-Bromosuccinimide (NBS), a mild source of bromine, is widely used in the presence of a catalytic amount of free-radical initiators for benzylic and allylic brominations with high regioselectivity [25]. In many instances, NBS have been used as an activator in stereoselective glycosidation [26], protection [27] and deprotection of ketals [28] or THP ethers [29] and in the synthesis of diindolylalkanes [30]. It is also widely employed as a mild oxidant [31] as well as for oxidative cyclizations [32,33]. Recently, we have reported synthesis of 1,5-benzodiazepine derivatives catalyzed by NBS under mild conditions [34]. In continuation of our research work on nitroolefins [35,36,37,38,39,40,41,42,43], we have developed a new route for the synthesis of 3-alkylated indoles via Friedel-Crafts alkylation of indole with β-nitrostyrene, catalyzed by N-bromosuccinimide.
Results and Discussion
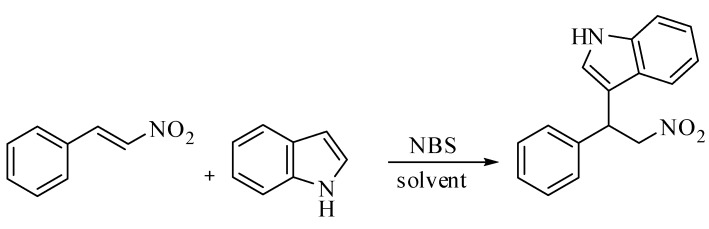
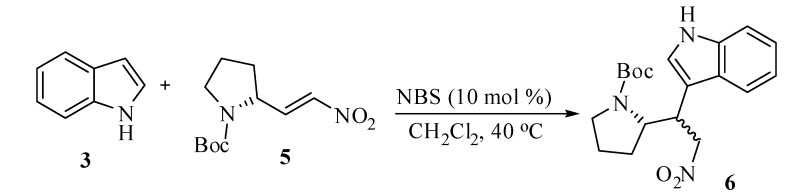
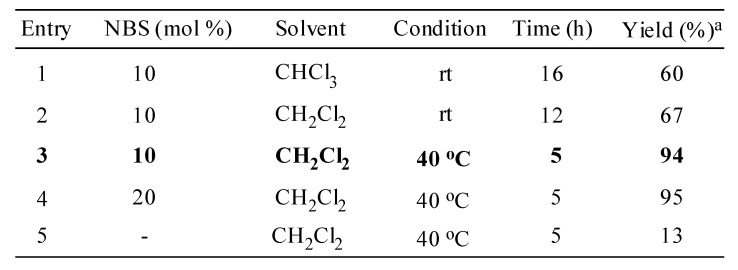
Initially, we examined the reaction between β-nitrostyrene and indole with 10 mol% of N-bromo-succinimide in dichloromethane at room temperature (Scheme 1). We were able to obtain only 67% of the corresponding alkylated adduct. Further, we examined the fate of the reaction at elevated temperatures. Conducting the reaction at 40 oC afforded the product in 94% yield, after 5 h (entry 3, Table 1). With 20 mol% of N-bromosuccinimide, the product yield remained the same, along with the same reaction time as when 10 mol% of NBS was utilized. Our optimization studies showed that 10 mol% of N-bromosuccinimide could be the best choice for this transformation, which is evident from Table 1.
Scheme 1.
Alkylation of indole with β-nitrostyrene.
Table 1.
Optimization studies of the alkylation reaction of indole and β-nitrostyrene.
|
a Yields were determined from 1H-NMR with toluene as internal standard.
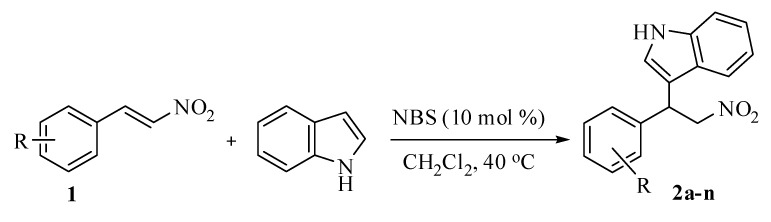
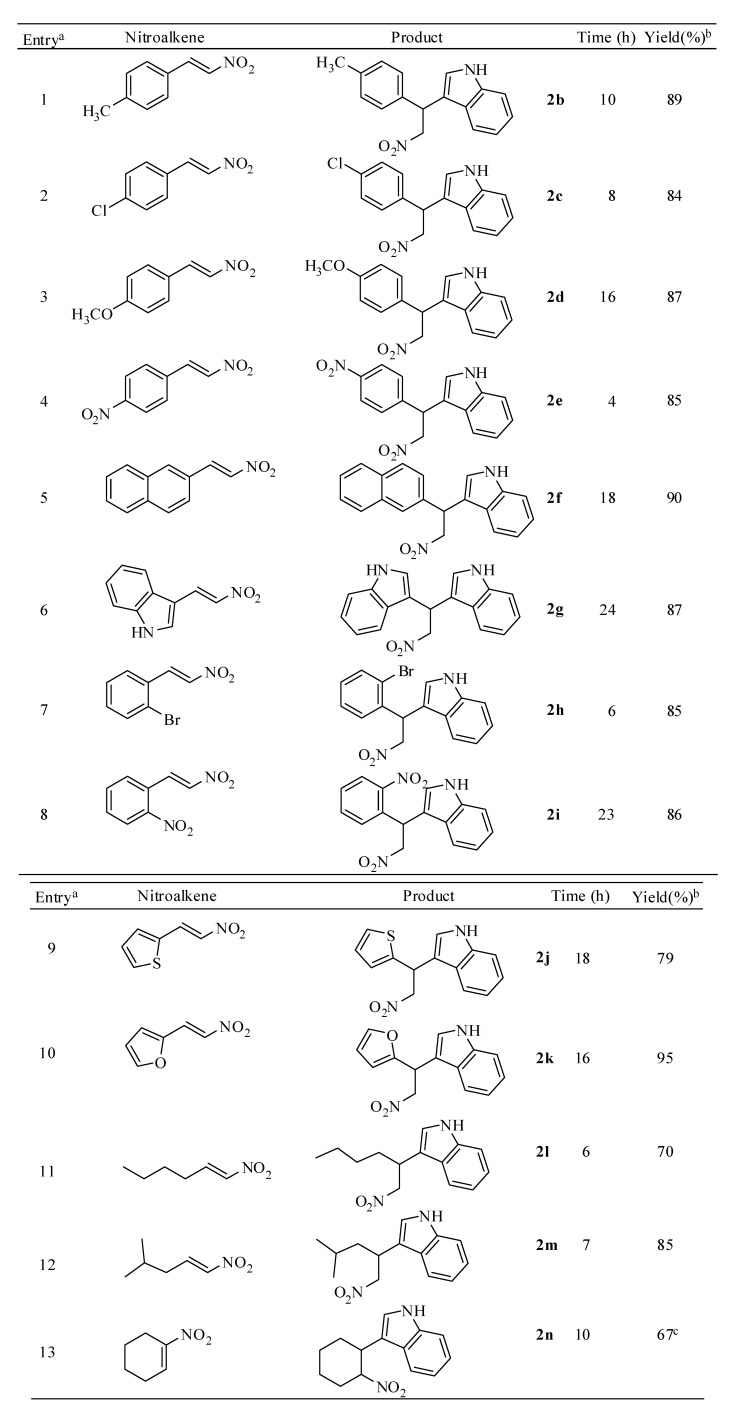
When 1 mmol of β-nitrostyrene 1 was reacted with 1.3 mmol of indole in the presence of 10 mole% of N-bromosuccinimide using dichloromethane as solvent at 40 °C, the reaction afforded exclusively the corresponding indolyl-nitroalkane in good to excellent yields. To further explore the scope and limitations of this methodology, we tested the alkylation reaction of indole with a wide array of structurally diverse nitroalkenes (Scheme 2). As seen from Table 2, the alkylation of indoles proceeds with various nitroolefins. The rate of the reaction is controlled by both electronic and steric factors of the substituent attached to the benzene ring of the β-nitrostyrene. For example, the nitroolefins containing electron-withdrawing groups (chloro and nitro, entries 2 and 4, Table 2) react well to afford the 3-alkylated indoles in excellent yields. However, nitroalkenes equipped with strong electron-donating groups such as methyl or methoxy (entries 1 and 3) required relatively longer reaction times. Moreover, sterically hindered substrates (entries 5-8) reacted smoothly under the present reaction conditions. Acid sensitive heterocyclic moieties substrates such as thienyl (entry 9) and furoyl (entry 10) and less reactive aliphatic nitroalkenes (entries 11 and 12) also reacted with equal ease to furnish the Friedel-Crafts products in high yields. 1-Nitrocyclohex-1-ene (entry 13), upon reaction with indole resulted in a mixture of two diastereomers in 7:3 ratio.
Scheme 2.
Reaction of indole with various nitroalkenes catalyzed by NBS.
Table 2.
Synthesis of indolyl-nitroalkane derivatives from various nitroalkenes.
|
a All reactions were performed by using 1 equiv. of nitroalkene and 1.3 equiv. of indole in the presence of 10 mol% of NBS; b Isolated yields; c A mixture of two diastereomers in 7:3 ratio.
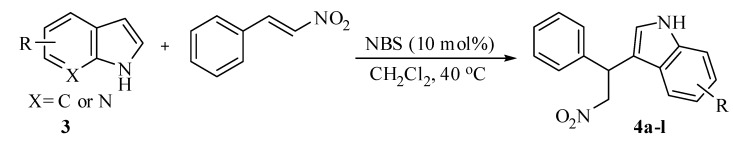
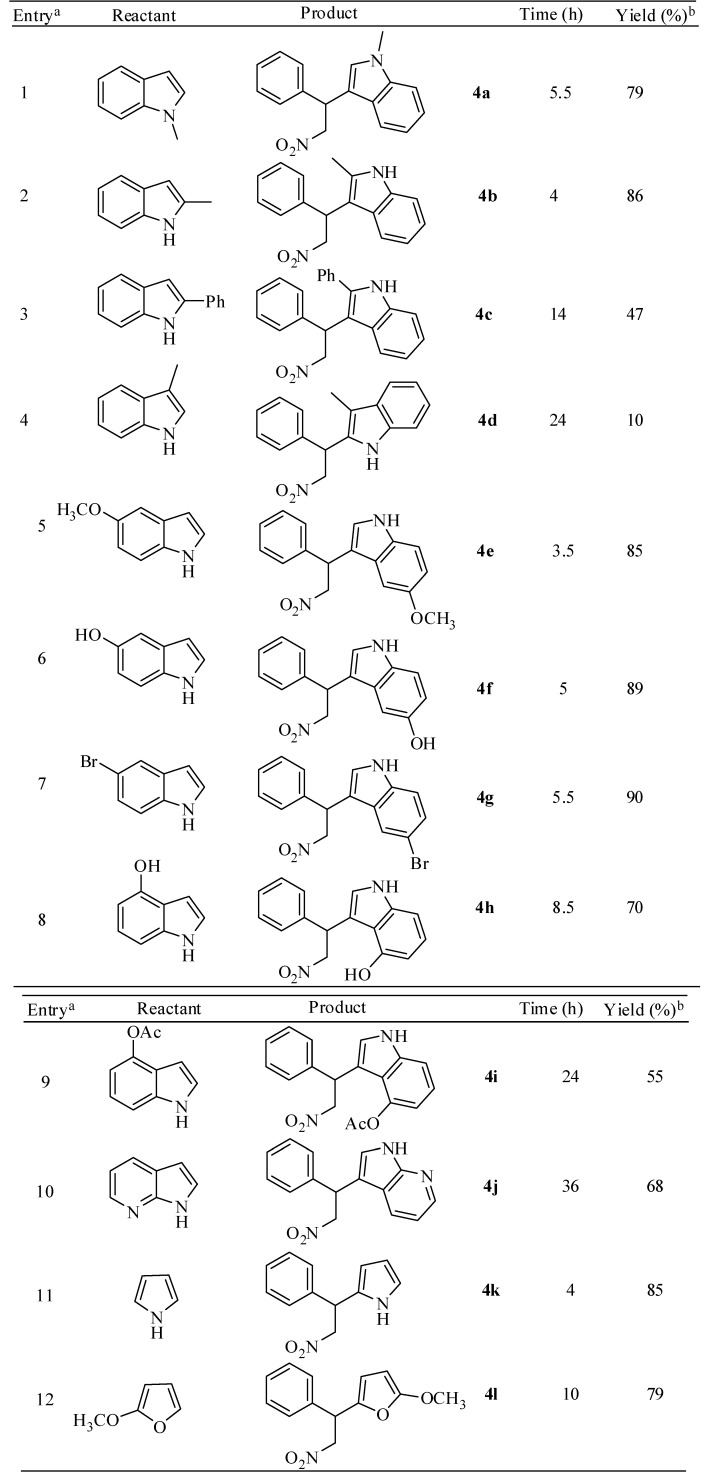
Furthermore, to study the effect of the substituents on the heterocyclic substrate, the reaction of β-nitrostyrene with various substituted indole and other heterocyclic derivatives was examined (Scheme 3). 2-Methylindole was reacted with β-nitrostyrene to furnish the product 4b in high yield. Similarly, 5-methoxyindole bearing an electron-donating group (entry 5, Table 3) was alkylated with β-nitrostyrene in a shorter time to give the desired product 4e in excellent yield. Moreover, increasing the size of the substituent on the indole nucleus, such as in the case of 2-phenylindole, gave only 47% yield. Generally, alkylation of hydroxyindoles with nitroalkenes often results in various side products, thereby decreasing the yields of alkylated product which may be due to the interaction of the hydroxyl group with the Lewis acid catalyst [44]. Indole containing free or protected hydroxyl group (entries 6, 8 and 9) reacted with the nitroolefin without any interference of the functional group. It is interesting to note that fused heteroarenes such as 7-azaindole (entry 10, Table 3) reacted with β-nitrostyrene to afford the corresponding adduct in moderate yield after prolonged reaction time. This method was found to be quite successful with the highly nucleophilic substrate pyrrole (entry 11) and the acid sensitive substrate 2-methoxyfuran (entry 12), producing the expected products in better yields compared to the earlier reported methods [45].
Scheme 3.
Reaction of β-nitrostyrene with various indoles and other heterocyclic compounds catalyzed by NBS.
Table 3.
Synthesis of aryl-nitroalkane derivatives from various indoles and other heterocyclic derivatives.
|
a All reactions were carried out by using 1 equiv. of nitroalkene and 1.3 equiv. of indole in the presence of 10 mol% of NBS; b isolated yields.
Scheme 4.
Synthesis of the key precursor of (S)-brevicolline.
An interesting application of this methodology involves the synthesis of the compound 6, which is the key precursor for the synthesis of the natural product (S)-brevicolline. The nitroalkene 5 was prepared by the condensation of Boc-S-pyrrolidine-2-carboxyaldehyde with nitromethane according to the reported procedure [46]. Compound 6 was obtained as a mixture of two diastereomers in the ratio of 9:1 (HPLC) in 80% yield by the reaction of indole with nitroalkene 5 under the present reaction conditions in 16 h. The aforementioned example demonstrates the usefulness and versatility of this synthetic methodology.
Conclusion
In conclusion, we have developed a simple and efficient method for the synthesis of indolyl-nitroalkanes in good yields under catalytic conditions. This method is applicable to a wide range of nitroalkenes and various indoles. Low cost of the reagents and convenient isolation process of this reaction makes this method an attractive alternative to existing methods.
Experimental
General
All reagents and chemicals were purchased from Sigma-Aldrich Chemical Company, Acros Organics, Alfa Aesar or Merck and were used as received. Analytical thin layer chromatography was performed with E. Merck silica gel 60F glass plates and flash chromatography with E. Merck silica gel 60 (230-400 mesh). Melting points were determined with a microscope hot-stage apparatus and uncorrected. 1H- and 13C-NMR spectra were recorded on a Bruker Avance 400 instrument. Chloroform-d was used as the solvent and TMS (δ = 0.00 ppm) as an internal standard. Chemical shift values are reported in ppm relative to TMS in delta (δ) units. Chemical shifts are recorded in parts per million (ppm). For 1H-NMR spectra the residual solvent peak was used as an internal reference (CHCl3 7.26). For 13C-NMR spectra the central peak of the CDCl3 triplet was used as the internal reference (77.26). Multiplicities are recorded as s, singlet; d, doublet; t, triplet; q, quartet; dd, doublet of doublet; br broadened; m, multiplet. Coupling constants (J) are expressed in Hertz. MS and HRMS were measured on JEOL JMS-D300 and JEOL JMS-HX110 spectrometers, respectively.
General procedure for the synthesis of aryl-nitroalkanes
To a stirred solution of nitroalkene derivative (1 mmol) and indole (1.3 mmol) in CH2Cl2 (2 mL) was added NBS (0.1 mmol). The reaction mixture was then warmed to 40 °C with continuous stirring until the completion of the reaction as monitored by TLC. Upon completion, the reaction mixture was diluted with water and extracted with CH2Cl2 (3 x 15 mL), washed with brine, and then dried over MgSO4. The solvent was removed under reduced pressure and the crude residue was purified by flash chromatography on silica gel to afford pure product. The references of the known compounds are: 2a [14,15,17,19], 2b [36], 2c [15,22], 2d [13,15,17], 2e [36], 2f [36], 2g [22], 2j [15,22], 2k [15,36], cis-2n [14], trans-2n [14], 4a [15,17,19], 4b [15,17], 4c [12,36], 4d [15], 4e [14,17], 4f [14], 4g [17,19], 4k [15,35], 4l [45], 5 [46].
3-(1-(2-Bromophenyl)-2-nitroethyl)-1H-indole (2h): Colorless solid. mp 117-118 °C. 1H-NMR (400 MHz, CDCl3): δ 8.07 (brs, 1H), 7.61 (d, J = 7.8 Hz, 1H), 7.42 (d, J = 8.0 Hz, 1H), 7.33 (d, J = 8.2 Hz, 1H), 7.20-7.18 (m, 3H), 7.12-7.05 (m, 3H), 5.72 (t, J = 8.0 Hz, 2H), 4.99-4.90 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 138.3 (C), 136.7 (C), 133.7 (C), 129.4(CH), 129.3 (CH), 128.1 (CH), 126.4 (CH), 124.7 (CH), 123.0 (CH), 122.1 (C), 120.2 (CH), 119.2 (CH), 113.6 (C), 111.6 (CH), 78.0 (CH2), 40.8 (CH); MS (EI) m/z (relative intensity): 346 (14) [M+2]+, 344 (13) [M]+, 297 (28), 218 (100), 204 (27), 108 (20); HRMS-EI: m/z [M]+ calcd. for C16H13BrN2O2: 344.0160; found: 344.0155.
3-(2-Nitro-1-(2-nitrophenyl)ethyl)-1H-indole (2i): Yellow solid. mp 117-119 °C. 1H-NMR (400 MHz, CDCl3): δ 8.20 (brs,1H), 7.89 (d, J = 7.8 Hz, 1H), 7.48-7.37 (m, 3H), 7.32 (dd, J = 12.7 and 8.4 Hz, 2H), 7.18 (t, J = 7.6 Hz, 1H), 7.13 (s, 1H), 7.03 (t, J = 7.3 Hz, 1H), 5.87 (t, J = 7.7 Hz, 1H), 5.09 (m, 2H). 13C-NMR (100 MHz, CDCl3): δ 149.8 (C), 136.7 (C), 133.9 (C), 133.4 (C), 130.1 (CH), 128.8 (CH), 126.1 (CH), 125.3 (CH), 123.2 (CH), 122.3 (CH), 120.4 (CH), 118.8 (CH), 113.0 (C), 111.7 (CH), 78.3 (CH2), 36.6 (CH); MS (EI) m/z (relative intensity): 311 (19) [M]+, 264 (29), 247 (33), 219 (100), 204 (39), 130 (57); HRMS-EI: m/z [M]+ calcd for C16H13N3O4: 311.0906; found: 311.0901.
3-(1-Nitromethylpentyl)-1H-indole (2l): 1H-NMR (400 MHz, CDCl3): δ 8.06 (brs, 1H), 7.61 (d, J = 7.8 Hz, 1H), 7.34 (d, J = 8.1 Hz, 1H), 7.23-7.18 (m, 1H), 7.15-7.11 (m, 1H), 6.98 (d, J = 2.0 Hz, 1H), 4.59 (m, 2H), 3.86 (m, 1H), 1.89 (m, 1H), 1.52 (m, 2H), 0.89 (d, J = 6.0 Hz, 3H), 0.87 (d, J = 6.0 Hz, 3H); 13C-NMR (100 MHz, CDCl3): δ 136.5 (C), 126.1 (C), 122.4 (CH), 122.1 (CH), 119.7 (CH), 118.7 (CH), 114.1 (C), 111.5 (CH), 81.0 (CH2), 41.4 (CH), 34.4 (CH2), 25.4 (CH2), 23.3 (CH2), 21.5 (CH3); MS (EI) m/z (relative intensity): 246 (19) [M]+, 178 (11), 157 (30), 144 (18), 143 (61), 130 (100), 115 (22), 77 (10); HRMS-EI: m/z [M]+ calcd for C14H18O2N2: 246.1368; found: 246.1370.
3-(3-Methyl-1-nitromethylbutyl)-1H-indole (2m): 1H-NMR (400 MHz, CDCl3): δ 8.07 (brs, 1H), 7.62 (d, J = 7.8 Hz, 1H), 7.33 (d, J = 8.1 Hz, 1H), 7.20 (m, 1H), 7.13 (m, 1H), 6.99 (d, J = 2.2 Hz, 1H), 4.59 (m, 2H), 3.87 (m, 1H), 1.89 (m, 1H), 1.51 (m, 2H), 0.88 (dd, J = 6.0 and 5.7 Hz, 6H); 13C-NMR (100 MHz, CDCl3): δ 136.5 (C), 126.1 (C), 122.3 (CH), 119.7 (CH), 118.7 (CH), 113.9 (C), 111.5 (CH), 80.9 (CH2), 41.4 (CH2), 34.4 (CH), 25.4 (CH), 23.3 (CH), 21.5 (CH3); MS (EI) m/z (relative intensity): 246 (24) [M]+, 157 (26), 144 (36), 143 (71), 130 (100), 115 (24), 84 (19), 57 (31), 55 (25); HRMS-EI: m/z [M]+ calcd for C14H18O2N2: 246.1368; found: 246.1365.
3-(2-Nitro-1-phenylethyl)-1H-indol-4-ol (4h): 1H-NMR (400 MHz, CDCl3): δ 7.94 (brs, 1H), 7.31-7.24 (m, 4H), 7.18 (d, J = 9.4 Hz, 1H), 6.93 (t, J = 7.8 Hz, 1H), 6.85 (d, J = 8.1 Hz, 1H), 6.64 (s, 1H), 6.32 (d, J = 7.5 Hz, 1H), 5.43 (t, J = 8.1 Hz, 1H), 5.23 (dd, J = 12.7 and 6.6 Hz, 1H), 5.11 (brs, 1H), 4.87 (t, J = 11.8 Hz, 1H); 13C-NMR (100 MHz, CDCl3): δ 149.7 (C), 139.7 (C), 138.9 (C), 128.8 (CH), 127.8 (CH), 127.4 (CH), 123.4 (CH), 121.8 (C), 115.5 (CH), 114.7 (C), 104.8 (CH), 104.5 (CH), 79.8 (CH2), 42.1 (CH); MS (EI) m/z (relative intensity): 282 (54) [M]+, 236 (47), 235 (100), 234 (32), 222 (70), 220 (45), 117 (14), 103 (17), 77 (12); HRMS-EI: m/z [M]+ calcd for C16H14O3N2: 282.1004; found: 282.1000.
3-(2-Nitro-1-phenylethyl)-1H-indol-4-yl acetate (4i): 1H-NMR (400 MHz, CDCl3): δ 8.16 (brs, 1H), 7.27-7.06 (m, 7H), 6.79 (d, J = 6.8 Hz, 1H), 6.65 (s, 1H), 5.19 (m, 1H), 5.01 (dd, J = 12.8 and 6.5 Hz, 1H), 4.85 (dd, J = 11.8 and 9.9 Hz, 1H), 2.21 (s, 3H); 13C-NMR (100 MHz, CDCl3): δ 170.3 (C), 143.8 (C), 139.0 (C), 138.9 (C), 129.0 (CH), 127.8 (CH), 127.7 (CH), 124.0 (CH), 122.8 (CH), 118.5 (CH), 113.2 (C), 112.6 (CH), 109.7 (CH), 79.9 (CH2), 42.0 (CH), 21.3 (CH3); MS (EI) m/z (relative intensity): 324 (16) [M]+ , 282 (68), 236 (55), 235 (100), 222 (49); HRMS-EI: m/z [M]+ calcd for C18H16N2O4: 324.1110; found: 324.1111.
3-(2-Nitro-1-phenylethyl)-1H-pyrrolo[2,3-b]pyridine (4j): 1H-NMR (400 MHz, CDCl3): δ 8.31 (d, J = 3.5 Hz, 1H), 7.87 (dd, J = 7.7 and 1.1 Hz, 1H), 7.33-7.30 (m, 5H), 7.20 (d, J = 3.5 Hz, 1H), 7.07 (dd, J = 7.7 and 4.7 Hz, 1H), 6.71 (dd, J = 9.2 and 6.1 Hz, 1H), 6.47 (d, J = 3.6 Hz, 1H), 5.37 (dd, J = 13.4 and 9.2 Hz, 1H), 5.16 (dd, J = 13.4 and 6.1 Hz, 1H). 13C-NMR (100 MHz, CDCl3): δ 147.6 (C), 143.2 (CH), 135.9 (C), 129.3 (CH), 129.1 (CH), 129.0 (CH), 128.8 (CH), 127.0 (CH), 126.0 (CH), 121.0 (C), 116.6 (CH), 101.4 (C), 56.7 (CH2); MS (EI) m/z (relative intensity): 267 (5) [M]+, 221 (21), 149 (11), 118 (100), 102 (18), 91 (70), 84 (25), 77 (45), 63 (21), 51 (28); HRMS-EI: m/z [M]+ calcd for C15H13O2N3: 267.1008; found: 267.1005.
Acknowledgements
Financial support of this work by the National Science Council of the Republic of China is gratefully acknowledged.
Footnotes
Samples Availability: Samples of the compounds are available from the authors.
References
- 1.Lim K.H., Hiraku O., Komiyama K., Koyano T., Hayashi M., Kam T.S. Biologically active indole alkaloids from Kopsia arborea. J. Nat. Prod. 2007;70:1302–1307. doi: 10.1021/np0702234. [DOI] [PubMed] [Google Scholar]
- 2.Sundberg R.J. Indoles. Academic Press; London, UK: 1996. [Google Scholar]
- 3.Faulkner D.J. Marine natural products. Nat. Prod. Rep. 2001;18:1–49. doi: 10.1039/b006897g. [DOI] [PubMed] [Google Scholar]
- 4.Fukuyama T., Chen X. Stereocontrolled synthesis of (-)-Hapalindole G. J. Am. Chem. Soc. 1994;116:3125–3126. doi: 10.1021/ja00086a053. [DOI] [Google Scholar]
- 5.Moore R.E., Cheuk C., Yang X.Q.G., Patterson G.M.L., Bonjouklian R., Smitka T.A., Mynderse J.S., Foster R.S., Jones N.D. Hapalindoles, antibacterial and antimycotic alkaloids from the cyanophyte Hapalosiphon fontinalis. J. Org. Chem. 1987;52:1036–1043. [Google Scholar]
- 6.Moore R.E., Cheuk C., Patterson G.M.L. Hapalindoles: New alkaloids from the blue-green alga Hapalosiphon fontinalis. J. Am. Chem. Soc. 1984;106:6456–6457. [Google Scholar]
- 7.Wang S.Y., Ji S.J., Loh T.P. The Michael addition of indole to α,β-unsaturated ketones catalyzed by iodine at room temperature. Synlett. 2003:2377–2379. [Google Scholar]
- 8.Olah G.A., Krishnamurthy R., Prakash G.K.S. In: Comprehensive Organic Synthesis, 1st. Trost B.M., Fleming I., editors. Vol. 3. Pergamon; Oxford, UK: 1991. pp. 293–339. [Google Scholar]
- 9.Harrington P.E., Kerr M.A. Reaction of indoles with electron deficient olefins catalyzed by Yb(OTf)3·3H2O. Synlett. 1996:1047–1048. [Google Scholar]
- 10.Kumar V.P., Sridhar R., Srinivas B., Narender M., Rao K.R. Friedel-Crafts alkylation of indoles with nitroolefins in the presence of beta-cyclodextrin in water under neutral conditions. Can. J. Chem. 2008;86:907–911. doi: 10.1139/v08-118. [DOI] [Google Scholar]
- 11.Yadav J.S., Abraham S., Reddy B.V.S., Sabitha G. InCl3-catalysed conjugate addition of indoles with electron-deficient olefins. Synthesis. 2001:2165–2169. [Google Scholar]
- 12.Bandini M., Melchiorre P., Melloni A., Umani-Ronchi A. A practical indium tribromide catalysed addition of indoles to nitroalkenes in aqueous media. Synthesis. 2002:1110–1114. [Google Scholar]
- 13.Zhan Z.P., Yanga R.F., Langa K. Samarium triiodide-catalyzed conjugate addition of indoles with electron-deficient olefins. Tetrahedron Lett. 2005;46:3859–3862. [Google Scholar]
- 14.Bartoli G., Bosco M., Giuli S., Giuliani A., Lucarelli L., Marcantoni E., Sambri L., Torregiani E. Efficient preparation of 2-indolyl-1-nitroalkane derivatives employing nitroalkenes as versatile Michael acceptors: New practical linear approach to alkyl 9H-β-carboline-4- carboxylate. J. Org. Chem. 2005;70:1941–1944. doi: 10.1021/jo048776w. [DOI] [PubMed] [Google Scholar]
- 15.Lin C., Hsu J., Sastry M.N.V., Fang H., Tu Z., Liu J.T., Yao C.F. I2-catalyzed Michael addition of indole and pyrrole to nitroolefins. Tetrahedron. 2005;61:11751–11757. doi: 10.1016/j.tet.2005.09.038. [DOI] [Google Scholar]
- 16.Firouzabadi H., Iranpoor N., Nowrouzi F. The facile and efficient Michael addition of indoles and pyrrole to α,β-unsaturated electron-deficient compounds catalyzed by aluminium dodecyl sulfate trihydrate [Al(DS)3]·3H2O in water. Chem. Commun. 2005:789–791. doi: 10.1039/b412653j. [DOI] [PubMed] [Google Scholar]
- 17.Azizi N., Arynasab F., Saidi M.R. Efficient Friedel-Crafts alkylation of indoles and pyrrole with enones and nitroalkene in water. Org. Biomol. Chem. 2006:4275–4277. doi: 10.1039/b610263h. [DOI] [PubMed] [Google Scholar]
- 18.Ballini R., Clemente R.R., Palmieri A., Petrini M. Conjugate addition of indoles to nitroalkenes promoted by basic alumina in solventless conditions. Adv. Synth. Catal. 2006;348:191–196. [Google Scholar]
- 19.An L.T., Zou J.P., Zhang L.L., Zhang Y. Sulfamic acid-catalyzed Michael addition of indoles and pyrrole to electron-deficient nitroolefins under solvent-free condition. Tetrahedron Lett. 2007;48:4297–4300. doi: 10.1016/j.tetlet.2007.04.011. [DOI] [Google Scholar]
- 20.Wu P., Wan Y., Cai J. Carbohydrate-Based Tolylsulfonyl Hydrazines: Effective catalysts for Michael addition of indoles to electron-deficient olefins in water. Synlett. 2008:1193–1198. [Google Scholar]
- 21.Chakrabarty M., Basak R., Ghosh N., Harigaya Y. Michael reaction of indoles with 3-(2’- nitrovinyl)indole under solvent-free conditions and in solution. An efficient synthesis of 2,2- bis(indolyl)nitroethanes and studies on their reduction. Tetrahedron. 2004;60:1941–1949. [Google Scholar]
- 22.Kusurkar R.S., Alkobati N.A.H., Gokule A.S., Chaudhari P.M., Waghchaure P.B. Thermal and microwave-assisted conjugate additions of indole on electron-deficient nitro-olefins. Synth. Commun. 2006;36:1075–1081. doi: 10.1080/00397910500498788. [DOI] [Google Scholar]
- 23.Itoh J., Fuchibe K., Akiyama T. Chiral phosphoric acid catalyzed enantioselective Friedel-Crafts alkylation of indoles with nitroalkenes: Cooperative effect of 3 Å molecular sieves. Angew. Chem. Int. Ed. 2008;47:4016–4018. doi: 10.1002/anie.200800770. [DOI] [PubMed] [Google Scholar]
- 24.Ganesh M., Seidel D. Catalytic enantioselective additions of indoles to nitroalkenes. J. Am. Chem. Soc. 2008;130:16464–16465. doi: 10.1021/ja8063292. [DOI] [PubMed] [Google Scholar]
- 25.Zhang P., Liu R., Cook J.M. Regiospecific bromination of 3-methylindoles with N- bromosuccinimide. Tetrahedron Lett. 1995;36:3103–3106. doi: 10.1016/0040-4039(95)00478-U. [DOI] [Google Scholar]
- 26.Fukase K., Hasuoka A., Kinoshita I., Aoki Y., Kasumoto S. Regiospecific bromination of 3- methylindoles with N-bromosuccinimide. Tetrahedron. 1995;51:4923–4932. doi: 10.1016/0040-4020(95)98690-J. [DOI] [Google Scholar]
- 27.Karimi B., Hazarkhani H., Maleki J. N-Bromosuccinimide (NBS) catalyzed highly chemoselective acetalization of carbonyl compounds using silylated diols and pentaerythritol under neutral aprotic conditions. Synthesis. 2005:279–285. [Google Scholar]
- 28.Iranpoor N., Firouzabadi H., Shaterian H.R. Catalytic and chemoselective deprotection of S,S- and S,O-acetals and ketals in the presence of their O,O-analogs with electrophilic halogens under neutral conditions. Tetrahedron Lett. 2003;44:4769–4773. [Google Scholar]
- 29.Narender M., Reddy M.S., Rao K.R. A mild and efficient oxidative deprotection of THP ethers with NBS in the presence of β-cyclodextrin in water. Synthesis. 2004:1741–1743. [Google Scholar]
- 30.Koshima H., Matsusaka W. N-Bromosuccinimide catalyzed condensations of indoles with carbonyl compounds under solvent-free conditions. J. Heterocycl. Chem. 2002;39:1089–1092. doi: 10.1002/jhet.5570390539. [DOI] [Google Scholar]
- 31.Surendra K., Krishnaveni N.S., Kumar V.P., Sridhar R., Rao K.R. Selective and efficient oxidation of sulfides to sulfoxides with N-bromosuccinimide in the presence of β-cyclodextrin in water. Tetrahedron Lett. 2005;46:4581–4583. [Google Scholar]
- 32.Ohno M., Spande T.F., Witkop B. Cyclization of tryptophan and tryptamine derivatives to pyrrolo[2,3-b]indoles. J. Am. Chem. Soc. 1968;90:6521–6522. doi: 10.1021/ja01025a055. [DOI] [PubMed] [Google Scholar]
- 33.Sakurai O., Takashahi M., Ogiku T., Hayashi M., Horikawa H., Iwasaki T. A new synthesis of 1β-methylcarbapenems using NBS-promoted cyclization as a key step. Tetrahedron Lett. 1994;35:6317–6320. doi: 10.1016/S0040-4039(00)73421-6. [DOI] [Google Scholar]
- 34.Kuo C.W., More S.V., Yao C.F. NBS as an efficient catalyst for the synthesis of 1,5- benzodiazepine derivatives under mild conditions. Tetrahedron Lett. 2006;47:8523–8528. [Google Scholar]
- 35.Tu Z., Raju B.R., Liou T.R., Kavala V., Kuo C.W., Jang Y., Shih Y.H., Wang C.C., Yao C.F. An efficient method for the synthesis of α-arylated nitroalkanes and α-arylated hydroximoyl chlorides mediated by AlCl3. Tetrahedron. 2009;65:2436–2442. doi: 10.1016/j.tet.2009.01.075. [DOI] [Google Scholar]
- 36.Habib P.M., Kavala V., Kuo C.W., Yao C.F. Catalyst-free aqueous-mediated conjugative addition of indoles to β-nitrostyrenes. Tetrahedron Lett. 2008;49:7005–7007. doi: 10.1016/j.tetlet.2008.09.109. [DOI] [Google Scholar]
- 37.Tu Z., Jang Y., Lin C., Liu J.T., Hsu J., Sastry M.N.V., Yao C.F. The study of reaction mechanism for the transformation of nitronate into nitrile by phosphorus trichloride. Tetrahedron. 2005;61:10541–10551. [Google Scholar]
- 38.Yan M.C., Jang Y.J., Wu J., Lin Y.F., Yao C.F. Radical reactions in esters with alkoxy functionality chemistry an unusual alcohol moiety hydrogen abstraction. Tetrahedron Lett. 2004;45:3685–3688. [Google Scholar]
- 39.Yao C.F., Jang Y.J., Yan M.C. An easy and efficient synthesis of 3-nitrochromans. Tetrahedron Lett. 2003;44:3813–3816. [Google Scholar]
- 40.Yan M.C., Tu Z., Lin C., Yao C.F. An easy and efficient method for the synthesis of hydroximoyl chloride from nitro olefin and silyl enol ether. Tetrahedron Lett. 2002;43:7991–7994. [Google Scholar]
- 41.Liu J.T., Yao C.F. One-pot synthesis of trans-β-alkylstyrenes. Tetrahedron Lett. 2001;42:6147–6150. [Google Scholar]
- 42.Yan M.C., Jang Y.J., Yao C.F. An easy and efficient method for the synthesis of 2,2-dialkyl-3- nitrochromene. Tetrahedron Lett. 2001;42:2717–2721. [Google Scholar]
- 43.Liu J.Y., Liu J.T., Yao C.F. Novel synthesis of alkenes via triethylaluminum-induced free radical reactions of alkyl iodides and β-nitrostyrenes. Tetrahedron Lett. 2001;42:3613–3615. doi: 10.1016/S0040-4039(01)00519-6. [DOI] [Google Scholar]
- 44.Yamamoto H. Lewis Acid Reagents. Oxford University Press; New York, NY, USA: 1999. [Google Scholar]
- 45.Itoh K., Kishimoto S. The reaction of β-nitrostyrenes with 2-methoxyfuran: a novel formation of isoxazoline N-oxide together with Michael adducts. New J. Chem. 2000;24:347–349. [Google Scholar]
- 46.Mahboobi S., Popp A., Burgemeister T., Schollmeyer D. Diastereoselective synthesis of (−)-1- methyl-(3S,4R)-3,4-bis((2S)-N-(tert-butyloxycarbonyl)pyrrolidin-2-yl)-2-pyrrolidinone by an asymmetric Michael reaction. TetrahedronAsymmetry. 1998;9:2369–2376. [Google Scholar]