Abstract
New 1,3,4-thiadiazole, 6, 7 and 1,2,4-triazole derivatives, 8, 9 containing a phenylalanine moiety have been synthesized by intramolecular cyclization of 1,4-disubstituted thiosemicarbazides, 4, 5, in acid and alkaline media, respectively; the thiosemicarbazides were obtained by reaction of hydrazide 3 with appropriate aromatic isothiocyanates. The toxicity of the synthesized compounds was evaluated and the anti-inflammatory study of the triazole compound 9 established an appreciable anti-inflammatory activity that is comparable with that of other nonsteroidal anti-inflammatory agents.
Keywords: phenylalanine; thiosemicarbazide; 1,3,4-thiadiazole; 1,2,4-triazole; anti-inflammatory activity
1. Introduction
The progress achieved in the synthesis of heterocyclic compounds with biological potential is due to improvement of the methodological study of tested substances too. It is known that many 1,3,4-thiadiazole and 1,2,4-triazole derivatives have biological activity, with their antibacterial [1,2,3], antimycobacterial [4,5], antimycotic [6], antifungal [7,8], antidepressive [9], and cardiotonic [10] action being notable. Recent research has also established for these heterocycles an analgesic [11] and anti-inflammatory [12,13] activity. Meanwhile, N-acylated amino acids are known for their hepatoprotective [14], antimicrobial [15,16] and antitumoral [17,18] action. Taking these data into account, in the present study, some new 1,3,4-thiadiazole and 1,2,4-triazole derivatives having a phenylalanine moiety have been synthesized and their structure confirmed by elemental and spectral (FT-IR, 1H-NMR, MS) analyses. The degree of toxicity of the compounds was established and the potential anti-inflammatory activity of the triazole compound 9 was also investigated.
2. Results and Discussion
2.1. Chemistry
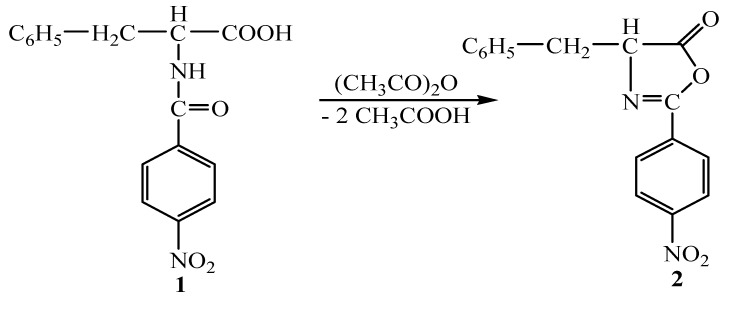
The synthesis of new 1,3,4-thiadiazole and 1,2,4-triazole compounds was performed in several steps. In the first step, 2-(p-nitrophenyl)-4-benzyl-Δ2-oxazolin-5-one (2) was obtained by heating N-(p-nitrobenzoyl)-L-phenylalanine (1) at 70-75°C for 60 minutes under the dehydrating action of excess acetic anhydride (1:10 molar rate) (Scheme 1).
Scheme 1.
Synthesis of oxazolone 2.
The structure of oxazolone 2 was established through by spectroscopic (IR, 1H-NMR, MS) as well as elemental analyses data. In the IR spectra the absorption bands characteristic for the >C=O lactonic group and >C=N bond were identified at 1,825 cm-1 and 1,620 cm-1, respectively, and in the 1H-NMR spectrum the proton signals due to the heterocyclic proton appeared at 4.85 ppm. In the MS spectra the base peak was observed at m/z = 297.09 and it was assigned as protonated compound having the monoisotopic mass 296.09. The [M+Na]+ species at m/z = 319.07 and Na adducts of two oxazolone molecules were also detected. The accurate mass measurements allowed the evaluation of the isotopic profile for the peaks situated at 297.09, 298.09 and 299.09.
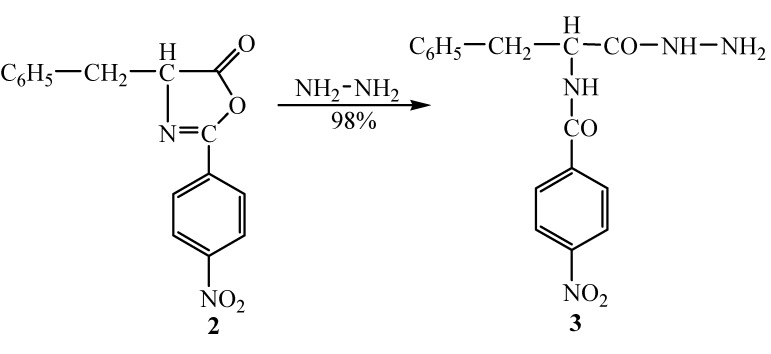
By reaction of Δ2-oxazolin-5-one 2 with hydrazine hydrate (98% solution) in anhydrous dioxane under reflux for 3 hours, N-(p-nitrobenzoyl)-D,L-phenylalanine hydrazide (3) was obtained (Scheme 2).
Scheme 2.
Synthesis of hydrazide 3.
The structure of hydrazide 3 was established through by spectroscopic (IR, 1H-NMR) as well as elemental analyses data. The IR spectra showed two characteristic bands for NH groups at 2,857 cm-1 and 3,269 cm-1, respectively, and the absorption band for CO amide group appeared at 1,661 cm-1. In the 1H-NMR spectrum the NH groups appeared as two signals at 9.0 ppm and 10.40 ppm, while the proton signals of the NH2 group were identified at 5.37 ppm.
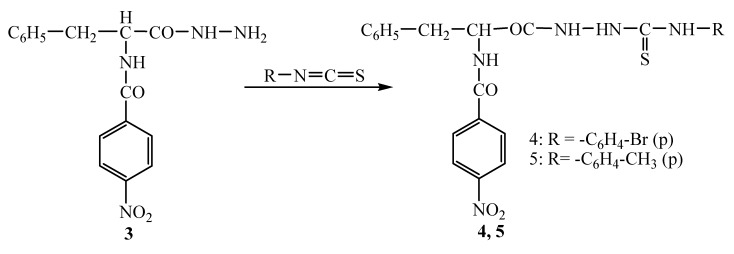
The new 1,4-disubstituted thiosemicarbazides 4, 5 were obtained through reaction of hydrazide 3 with p-bromophenyl- and p-tolylisothiocyanates in methanol media under reflux (Scheme 3).
Scheme 3.
Synthesis of thiosemicarbazides 4, 5.
The formation of thiosemicarbazides 4, 5 was indicated in the IR spectra by a shift in the CO amide group absorption of hydrazide 3 from 1,661 cm-1 to 1,654-1,683 cm-1 in the thiosemicarbazide derivatives and by the band of >C=S bond that appears at 1,234-1,299 cm-1. In the 1H-NMR spectra the protons linked to nitrogen appear at 9.00-10.48 ppm. In the MS spectra of thiosemicarbazide 4 the base peak was observed at m/z = 564.02 and it was assigned as its Na adduct (564 = 541 +23). Beside the Na adduct there were observed (M+H)+ and (M+K)+ adducts at m/z = 542 and 580, respectively. Two cluster molecules charged with Na were also observed at m/z = 1105 (541×2 + 23). For the MS spectra of thiosemicarbazide 5 the base peak was observed at m/z = 500.13 and it is associated with the presence of the Na adduct of this thiosemicarbazide (477+23). The peaks accompanying the base peak are (M+H)+ at m/z = 478 and (M+K)+ at 516. An additional peak at m/z = 977 representing the Na adduct of two thiosemicarbazide molecules (977=477×2+23) was also observed.
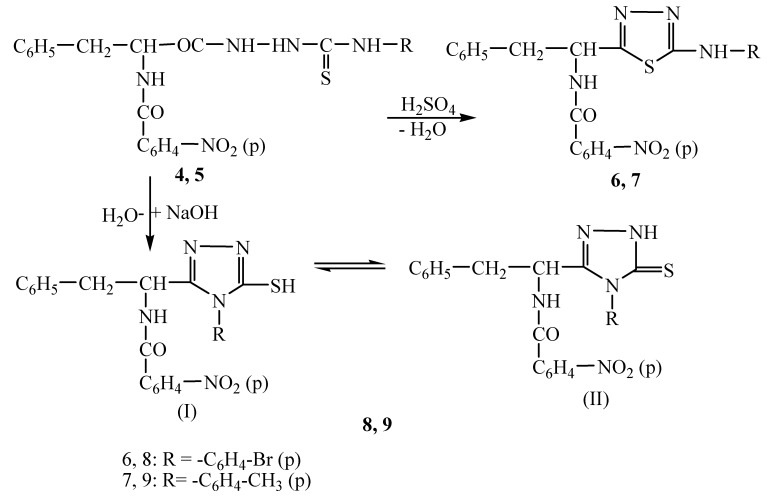
In the last step by intramolecular cyclization of thiosemicarbazides 4, 5 in acid and alkaline media, respectively, new 1,3,4-thiadiazole and 1,2,4-triazole compounds 6, 7 and 8, 9 were obtained (Scheme 4). The 1,2,4-triazole derivatives can exist in two tautomeric forms – thiole (I) and thione (II), the reaction conditions leading to the chemical stabilization of the tautomeric thione form [19,20].
Scheme 4.
Synthesis of 1,3,4-thiadiazole (6, 7) and 1,2,4-triazole (8, 9) compounds.
The structure of the final compounds was confirmed by elemental and spectral (FT-IR, 1H-NMR and MS) analyses. In the IR spectra of the 1,3,4-thiadiazoles 6, 7 the >C=N group appears as an absorption band at 1,665-1,672 cm-1, and the C-S bond was identified at 640-652 cm-1. In the 1H-NMR spectra of 1,3,4-thiadiazoles 6, 7 the proton signal for the amine group appears as singlet at 6.00-6.05 ppm and that for the amide group appears at 9.08-9.10 ppm. The CH3 group is present as a singlet at 2.34 ppm. In the mass spectrum of thiadiazole 6 the base peak was observed at m/z = 524, corresponding to the [M-H]+ ions of the product. The base peak for thiadiazole 7 is situated at m/z = 460.13 corresponding to the [M-H]+ ions of the product.
In the IR spectra of 1,2,4-triazoles 8, 9 the >C=N group was identified at 1,566-1,595 cm-1 and the characteristic C=S function absorption band appeared at 1,300-1,320 cm-1 region. In the 1H-NMR spectra the proton signal for the NH from the triazole ring was observed as a singlet at 12.80 ppm. The mass spectra of triazole 8 showed the base peak at m/z = 524.03 corresponding to the [M-H]+ ions of the product with monoisotopic mass of 523.03 g/mol. The smaller peaks are assigned as Na and K adducts of the same molecule or species adducts formed by physical association of two molecules (e.g. 1069.01 = 2×523+23). The base peak observed in the case of triazole 9 is situated at m/z = 460.13 corresponding to the [M-H]+ ion of the product.
2.2. Biological Activities
The synthesized compounds were investigated for their toxicity (Table 1). It was ascertained that all tested compounds have low toxicity, the lowest toxic compounds being 1,3,4-thiadiazole 7 and 1,2,4-thiazole 9, both having a p-tolyl moiety in the structure.
Table 1.
DL50 values of the tested compounds.
| Comp. | DL50 (mg/kg body weight) | |||
|---|---|---|---|---|
| 24 hours | 48 hours | 7 days | Average | |
| 2 | 1,110 | 1,110 | 975 | 1,065 |
| 3 | 1,100 | 1,100 | 875 | 1,025 |
| 4 | 1,650 | 1,650 | 1,560 | 1,620 |
| 5 | 2,050 | 2,050 | 1,810 | 1,970 |
| 6 | 2,350 | 2,350 | 2,110 | 2,270 |
| 7 | 5,200 | 5,200 | 3,460 | 4,620 |
| 8 | 2,250 | 2,250 | 1,950 | 2,150 |
| 9 | 5,150 | 5,150 | 4,730 | 5,010 |
The potential anti-inflammatory activity of the 1,2,4-triazole (9) comparative with standard nonsteroidal anti-inflammatory drugs (indometacine, phenylbutazone, acetylsalicylic acid) was also investigated (Table 2).
Table 2.
The anti-inflammatory effects of tested compounds.
| Comp. | Dose (mg/kg body, p.o.) | Inflammatory oedema (mL/100 g body) | Inhibition (%) | |
|---|---|---|---|---|
| Control | Comp. | |||
| Indometacine | 5* | 0.280±0.110 | 0.111±0.008 | 60.30 |
| Phenylbutazone | 50* | 0.280±0.110 | 0.190±0.062 | 32.10 |
| Acetylsalicylic Acid | 150 * | 0.310±0.005 | 0.250±0.057 | 19.35 |
| 1,2,4-Triazole (9) | 80* | 0.280±0.110 | 0.186± 0.060 | 33.57 |
* = 1/10 DL50, **1/5DL50
The substances were administered in two concentrations, 1/10 of DL50 and 1/5 of DL50, respectively, relative to body weight of the animal tested. From the results presented in Table 2 it is observed that at small doses (1/10 DL50) only indometacine has an appreciable anti-inflammatory activity (60.30% inhibition of oedema) while at higher doses (1/5 DL50) all tested compounds reduced the nystatin-induced oedema (45.33-61.20% inhibition of oedema). The activity of the 1,2,4-triazole (9) is similar to the phenylbutazone’s activity (49.03% inhibition of oedema). This effect could be explained through both the lysosomal stabilization and the inhibition of prostaglandin biosynthesis [21,22,23].
3. Experimental Section
3.1. Chemistry
3.1.1. General
All melting points were determined on a Melt-Temp R apparatus equipped with a digital thermometer and are uncorrected. The combustion analysis was performed on an Elemental Exeter Analytical CE 440 Apparatus. The IR spectra were measured as potassium bromide pellets on a Digilab Scimitar Series FT-IR Spectrophotometer; the wave numbers are given in cm-1. The 1H-NMR spectra were recorded in DMSO-d6 or CD3COCD3 solutions on Bruker ARX-300 spectrometer at ambient temperature. Chemical shifts were recorded as δ values in parts per millions (ppm) and were indirectly referenced to tetramethylsilane via residual solvent signal (2.49 for 1H). MS spectra were obtained using an instrument produced by Agilent Technologies, Wilmington, DE, USA. The instrument, Accurate Mass Q-TOF LC/MS 6520 was operated via the manufacture’s software, Mass Hunter. Samples were dissolved in acetonitrile/water mixture (95/5 v/v) to obtain a concentration of 10 μg/mL and 0.05 mL were directly injected into the electrospray source using the autosampler at a rate of 0.05 mL/min. The instrument was operated in High resolution mode with an acquisition rate of 4 GHz. The source voltage was set at 4,000 V, the spray gas flow at 5 L/min, heating gas temperature at 325 °C and the fragmentor potential at 215 V. All chemical reagents were obtained from the Aldrich Chemical Company.
3.1.2. Synthesis of 2-(p-nitrophenyl)-4-benzyl-Δ2-oxazolin-5-one (2)
N-(p-nitrobenzoyl)-L-phenylalanine (5.32 g, 0.018 mol) was dissolved in acetic anhydride (18 mL) and heated under reflux at 70-75ºC for 45-60 minutes. After cooling, the solution was added under stirring over a mixture of dried petroleum ether (55 mL) and dried ethyl ether (35 mL). The reaction mixture was stirred for 15 minutes, the ether layer was removed and the oily product obtained was washed many times with dried ethyl ether until a solid product was obtained that was filtered and dried for 8-10 hours under vacuum at 40-45 °C. Crystallization from anhydrous dioxane gave a pure yellow solid (383 mg, 72% yield), mp 120-123 °C; IR (cm-1) v: 1,825 (CO), 1,620 (C=N), 1,520 (asymmetric NO2 vibrations), 1,342 (symmetric NO2 vibrations), 786 (aromatic CH); 1H-NMR (CD3COCD3) δ: 3.15 (m, 1H, CH2), 3.25 (m, 1H, CH2), 4.85 (t, 1H, CH), 7.20 (m, 5H, Ph), 8.15 (d, 2H, C6H4), 8.35 (d, 2H, C6H4); MS (acetonitrile/water 95/5, v/v) m/z: 297.09 (base peak), 319.07 (14.5%), 615.11 (21%); Anal. calcd. for C16H12N2O4 (%): C, 64.86; H, 4.05; N, 9.45; found: C, 65.32; H, 4.26; N, 9.91.
3.1.3. N-(p-nitrobenzoyl)-D,L-phenylalanine hydrazide (3)
2-(p-Nitrophenyl)-4-benzyl-Δ2-oxazolin-5-one (2, 5.94 g, 0.02 mol) was dissolved in dioxane (50 mL) and hydrazine hydrate solution (98%, 0.5 mL, 0.02 mol) was added. The reaction mixture was heated at 65-70ºC for three hours, under reflux on a thermostated silicone oil bath. The solvent was removed by distillation under reduced pressure till 10-15 mL, and then water was added until a yellow semisolid product was separated. Crystallization from ethanol-water mixture gave a pure yellow solid (514 mg, 78.35% yield), mp 210-212 °C; IR (cm-1) v: 3,269, 2,857 (NH), 1,661 (CO), 1,526 (asymmetric NO2 vibrations), 1,346 (symmetric NO2 vibrations), 723, 868 (aromatic CH); 1H-NMR (DMSO-d6, 300 MHz) δ: 3.30 (m, 2H, CH2), 4.80 (m, 1H, CH), 5.37 (s, 2H, NH2), 7.40 (m, 5H, Ph), 8.05 (d, 2H, C6H4), 8.30 (d, 2H, C6H4), 9.0 (m, 1H, NH), 10.40 (s, 1H, NH); Anal. calcd. for C16H16N4O4 (%): C, 58.53; H, 4.87; N, 17.07; found: C, 58.75; H, 5.33; N, 17.46.
3.1.4. General procedure for synthesis of 1,4-disubstituted thiosemicarbazides 4, 5
N-(p-nitrobenzoyl)-D,L-phenylalanine hydrazide (3, 1.64 g, 0.005 mol) was dissolved in dried methanol (10 mL) and a solution of the corresponding isothiocyanate (0.005 mol) in dried methanol (10 mL) was added. The reaction mixture was heated under reflux at 70-80ºC for three hours. After cooling the solvent was evaporated under reduced pressure and the solid was dried under vacuum at room temperature. The rough product was purified by crystallization from ethanol.
1-[N-(p-nitrobenzoyl)-D,L-phenylalanyl-4-(p-bromophenyl)-thiosemicarbazide (4): white solid, 184 mg (68.33% yield); mp 188-190°C; IR (cm-1) v: 3,541, 3,460, 3,169 (NH), 1,683 (CO), 1,573 (asymmetric NO2 vibrations), 1,379 (symmetric NO2 vibrations), 1,299 (C=S), 754, 796 (aromatic CH), 565 (C-Br); 1H-NMR (DMSO-d6, 300 MHz) δ: 3.04 (m, 1H, CH2), 3.10 (m, 1H, CH2), 4.70 (m, 1H, CH), 7.17-7.33 (m, 6H, Ar CH), 7.56 (m, 3H, Ar CH), 8.01 (d, 2H, Ar CH), 8.31 (m, 2H, Ar CH), 9.0 (d, 1H, NH), 9.91 (s, 1H, NH), 10.48 (s, 1H, NH); MS (acetonitrile/water 95/5, v/v) m/z: 564 (base peak) (541+23), 542 (541+1) (30%), 580 (541+39) (19%), 1105 (541×2+23) (4%); Anal. calcd. for C23H20N5O4BrS (%): C, 51.01; H, 3.69; N, 12.93; Br, 14.78; S, 5.91; found: C, 51.37; H, 3.88; N, 13.35; Br, 15.19; S, 6.27.
1-[N-(p-nitrobenzoyl)-D,L-phenylalanyl-4-(p-tolyl)-thiosemicarbazide (5): yellow solid, 186 mg (78.35% yield), mp 179-180°C; IR (cm-1) v: 3,321, 3,211 (NH), 1,654 (CO), 1558 (asymmetric NO2 vibrations), 1,357 (symmetric NO2 vibrations), 1,234 (C=S), 873 (aromatic CH); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.29 (s, 3H, CH3), 3.40 (m, 1H, CH2), 4.70 (m, 1H, CH), 7.14-7.50 (m, 8H, Ar CH), 7.95 (s, 1H, Ar CH), 8.01 (d, 2H, Ar CH), 8.30 (d, 2H, Ar CH), 9.19 (s, 2H, NH), 9.71 (s, 1H, NH), 10.44 (s, 1H, NH); MS (acetonitrile/water 95/5, v/v) m/z: 500.13 (base peak) (477+23), 478.15 (477+1) (39.5%), 516.11 (477+39) (13%), 977 (477×2+23) (30%). Anal. calcd. for C24H23N5O4S (%): C, 60.37; H, 4.82; N, 14.67; S, 6.70; found: C, 60.81; H, 5.01; N, 15.10; S, 7.19.
3.1.5. General procedure for synthesis of 1,3,4-thiadiazoles 6, 7
To the corresponding thiosemicarbazide 4, 5 (0.006 mol), concentrated H2SO4 (1 mL) was added under stirring. The reaction mixture was stirred at room temperature for one hour and then was added dropwise in cold water and stirred again till a solid product was obtained that was separated and dried under vacuum at 45-50ºC. The rough product was purified by crystallization from ethanol.
2-[1-(p-Nitrobenzoylamino)-2-phenyl]-ethyl-5-(p-bromophenylamino)-1,3,4-thiadiazole (6): light yellow solid, 223 mg (71.50% yield), mp 151-153°C; IR (cm-1) v: 3,263, 3,066 (NH), 1,672 (C=N), 1,620 (CO), 1,520 (asymmetric NO2 vibrations), 1,341 (symmetric NO2 vibrations), 812 (aromatic CH), 750 (C-Br), 640 (C-S); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.60 – 2.65 (m, 2 H, CH2), 4.76 (m, 1H, NH), 6.00 (m, 1H, CH), 6.56 (d, 2H, ArCH), 7.63 – 7.78 (m, 5H, ArCH), 8.30 (d, 2H, ArCH), 8.40 (d, 2H, ArCH), 8.80 (d, 2H, ArCH), 9.10 (s, 1H, -NH-CO-); MS (acetonitrile/water 95/5, v/v) m/z: 524 (base peak), 1047 (1.5%); Anal. calcd. for C23H18N5O3BrS (%): C, 52.67; H, 3.43; N, 13.35; Br, 15.26; S, 6.10; found: C, 53.02; H, 3.89; N, 13.84; Br, 15.67; S, 6.58.
2-[1-(p-Nitrobenzoylamino)-2-phenyl]-ethyl-5-(p-tolylamino)-1,3,4-thiadiazole (7): yellow solid, 195 mg (71.27% yield), mp 180-182°C; IR (cm-1) v: 3,259, 3,192 (NH), 1,665 (C=N), 1,620 (CO), 1,552 (asymmetric NO2 vibrations), 1,384 (symmetric NO2 vibrations), 815 (aromatic CH), 652 (C-S); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.34 (s, 3H, CH3), 2.60 – 2.63 (m, 2H, CH2), 4.76 (m, 1H, NH), 6.05 (m, 1H, CH), 6.58 (d, 2H, ArCH), 7.63-7.80 (m, 5H, ArCH), 8.33 (d, 2H, ArCH), 8.45 (d, 2H, ArCH), 8.75 (d, 2H, ArCH), 9.08 (s, 1H, -NH-CO-); MS (acetonitrile/water 95/5, v/v) m/z: 460.13 (base peak), 482.02 (1%), 919.14 (2%), 941.12 (1%); Anal. calcd. for C24H21N5O3S (%): C, 62.74; H, 4.57; N, 15.25; S, 6.97; found: C, 63.22; H, 4.91; N, 15.70; S, 7.47.
3.1.6. General procedure for the synthesis of 1,2,4-triazoles 8, 9
To corresponding thiosemicarbazide 6, 7 (0.0014 mol), a solution of NaOH 2N (10 mL) was added. The reaction mixture was heated under reflux at 80-90 °C for four hours and then a solution of HCl 1N was added until it reached pH 4.5 when a solid product was formed. The rough product was separated and dried under vacuum at 55-60ºC and then it was recrystallized from ethanol.
4-(p-bromophenyl)-5-[1-(p-nitrobenzoylamino)-2-phenyl-ethyl]-3-thio-1,2,4-triazole (8): yellow-orange solid, 46 mg (64.45% yield), mp 147-149°C; IR (cm-1) v: 3,062 (NH), 1,664 (CO), 1,595 (C=N), 1,546 (asymmetric NO2 vibrations), 1,300 (C=S), 1,332 (symmetric NO2 vibrations), 925, 873 (aromatic CH), 752 (C-Br); 1H-NMR (DMSO-d6, 300 MHz) δ: 2.62 – 2.66 (m, 2H, CH2), 4.86 (m, 1H, CH), 7.25 – 7.30 (m, 5H, ArCH), 7.80 (d, 2H, ArCH), 8.39 ( d, 2H, ArCH), 8.50 (d, 2H, ArCH), 8.75 (d, 2H, ArCH), 9.05 (m, 1H, -NH-CO-), 12.80 (s, 1H, NH); MS (acetonitrile/water 95/5, v/v) m/z: 524.03 (base peak), 541.94 (33%), 563.92 (25%), 1069.01 (9%); Anal. calcd. for C23H18N5O3BrS (%): C, 52.67; H, 3.43; N, 13.35; Br, 15.26; S, 6.10; found: C, 53.08; H, 3.85; N, 13.85; Br, 15.26; S, 6.49.
5-[1-(p-nitrobenzoylamino)-2-phenyl-ethyl]-3-thio-4-(p-tolyl)-1,2,4-triazole (9): brown solid, 43 mg (69% yield), mp 172-174°C; IR (cm-1) v: 3,437 (NH), 1,670 (CO), 1,566 (C=N), 1,520 (asymmetric NO2 vibrations), 1,320 (C=S), 1,338 (symmetric NO2 vibrations), 962, 785 (aromatic CH); 1H-NMR (DMSO-d6, 300 MHz) δ: 2,32 (t, 3H, CH3), 2.57 – 3.35 (m, 2H, CH2), 7.14 – 7.19 (m, 4H, ArCH), 7.78 – 7.82 (m, 5 H, ArCH), 8.45 (d, 2H, ArCH), 8.75 (d, 2H, ArCH), 9.08 (s, 1H, -NH-CO-), 12.80 (s, 1H, NH); MS (acetonitrile/water 95/5, v/v) m/z: 460.13 (base peak), 482.04 (18%), 941.13 (11%). Anal. calcd. for C24H21N5O3S (%): C, 62.74; H, 4.57; N, 15.25; S, 6.97; found: C, 63.16; H, 5.06; N, 15.62; S, 7.41.
3.2. Toxicity study
The acute toxicity was estimated by intraperitoneal administration of the compounds 2-9 as a suspension in Tween 80 to groups of six male mice, each weighing 20-22 g, according to the classical laboratory methodology [24]. The animals were monitored and the death rate ascertained after 24 hours, 48 hours and 7 days. The DL50 was established using the Spearman-Karber method [25].
3.3. Anti-inflammatoty study
The nystatin-induced paw oedema in rats was realized according to the Niemegeers et al. method [24]. A group of six rats weighing 120-160 g housed at 21.5 °C with free-food and water for 24 hours was used. After the measurement of the initial volume of paw according to the Whitehouse method [19], the animals were injected in their left posterior paw with 0.1 mL nystatin suspension (3,500 U/mg) with concentration of 65 mg/mL in 0.9% NaCl solution. Two hours after injection, the triazole compound 9 and the standard anti-inflammatory drugs (indometacine, phenylbutazone and acetylsalicylic acid) were administered through tube-feeding, in 1/10 and 1/5 of DL50 dose as a suspension in CMC 0.5%, 20 ml/kg body. The control groups received only vehicle. The final volume of each paw was measured four hours later, and the difference between the initial and the final paw volume was established. This difference, expressed in mL/100 g body, represents the inflammatory oedema; the mean paw volume in the drug-treated and control groups respectively was also established. The percentage oedema was calculated, using student’s – t test for statistic interpretation.
4. Conclusions
New 1,3,4-thiadiazole (6-7) and 1,2,4-triazole (8-9) compounds having a D,L-phenylalanine moiety were synthesized by intramolecular cyclization of 1,4-disubstituted thiosemicarbazide in acid and alkaline medium, respectively. The corresponding thiosemicarbazides (4, 5) were obtained by addition of N-(p-nitrobenzoyl)-D,L-phenylalanine hydrazide to the aromatic isothiocyanates. The DL50 values for all synthesized compounds were established, all compounds having a low toxicity. The potential anti-inflammatory effects of the thiazole 9 by using the nystatin-induced paw oedema in rats was also studied. The triazole 9, at a dose of 160 mg/kg body (1/5 DL50), reduced the inflammatory oedema considerably, this action being comparable with that of the tested standard anti-inflammatory drugs.
Footnotes
Sample Availability: Samples of the compounds 2-9 are available from the authors.
References and Notes
- 1.Varvarason A., Tantili-Kakoulidou A., Siatra-Papastasikoudi T., Tiligada E. Synthesis and biological evaluation of indole containing derivatives of thiosemicarbazide and their cyclic 1,2,4-triazole and 1,3,4-thiadiazole analogs. Arzneim. Forsch. 2000;50:48–54. doi: 10.1055/s-0031-1300163. [DOI] [PubMed] [Google Scholar]
- 2.Gokce M., Cakir B., Earl K., Sahin M. Synthesis and antimicrobial activity of [(2-oxabenzothiazolin-3-yl)-methyl]-4-alkyl/aryl-1,2,4-triazoline-5-thiones. Arch. Pharm. 2001;334:279–283. doi: 10.1002/1521-4184(200109)334:8/9<279::AID-ARDP279>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 3.Pintilie O., Profire L., Sunel V., Popa M., Pui A. Synthesis and antimicrobial activity of some new 1,3,4-thiadiazole and 1,2,4-triazole compounds having a D,L-methionine moiety. Molecules. 2007;12:103–113. doi: 10.3390/12010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faroumadi A., Mirzaei M., Shafiee A. Synthesis and antituberculosis activity of 2-aryl-1,3,4-thiadiazole derivatives. Pharmazie. 2001;56:610–612. [PubMed] [Google Scholar]
- 5.Mamolo M.G., Falagiani V., Zanpier D., Vio L., Banfi F. Synthesis and antimycobacterial activity of [5-(pyridin-2-1,3,4-thiadiazol-2-yl-thio)]-acetic acid arylidene-hydrazide derivatives. Farmaco. 2001;56:587–592. doi: 10.1016/S0014-827X(01)01097-7. [DOI] [PubMed] [Google Scholar]
- 6.Zamani K., Faghifi K., Tefighi I., Sharlatzadeh R. Synthesis and potential antimycotic activity of 4-substituted 3-(thiophene-2-yl-methyl)-Δ2-1,2,4-triazoline-5-thiones. Turk. J. Chem. 2004;28:95–101. [Google Scholar]
- 7.Zan X.I., Lai L.H., Jin G.Y., Zhong Z.X. Synthesis, fungicide activity and 3D- QSAR of 1,3,4-oxadiazoles and 1,3,4-thiadiazoles. J. Agric. Food Chem. 2002;50:3757–3760. doi: 10.1021/jf0201677. [DOI] [PubMed] [Google Scholar]
- 8.Chem H., Li Z., Han Y. Synthesis and fungicidal activity against Rhizoctonia solani of 2-alkyl(alkylthio)-5-pyrazolyl-1,3,4-oxadiazoles (thiadiazoles) J. Agric. Food Chem. 2000;48:5312–5315. doi: 10.1021/jf991065s. [DOI] [PubMed] [Google Scholar]
- 9.Clerici F., Pocar D., Guido M., Loche A., Perlini V., Brufoni M. Synthesis of 2-amino-5-sulphonyl-1,3,4-thiadiazole derivatives and evaluation of their antidepressant and anxiolytic activity. J. Med. Chem. 2001;44:931–936. doi: 10.1021/jm001027w. [DOI] [PubMed] [Google Scholar]
- 10.Onkol T., Cakir B., Sahin M.F. Synthesis and antinociceptive activity of 2-[(2-oxabenzothiazolin-3-yl)-methyl]-5-aminoalkyl/aryl-1,3,4-thiadiazole. Turk. J. Chem. 2004;28:461–466. [Google Scholar]
- 11.Shenone S., Bruno O., Ranise A., Bondavalli W., Falcone G., Giordano L., Vitelli M. 3-Arylsulphonyl-5-arylamino-1,3,4-thiadiazol-2(3H)ones as anti-inflammatory and analgesic agents. Bioorg. Med. Chem. 2001;9:2149–2153. doi: 10.1016/S0968-0896(01)00121-3. [DOI] [PubMed] [Google Scholar]
- 12.Labanauskas L., Kalcas V., Uderenaite E., Gaidelis P., Brukstus A., Dauksas V. Synthesis of 3-(3,4-dimethoxyphenyl)-1H-1,2,4-triazole-5-thiol and 2-amino-5-(3,4-dimethoxyphenyl)-1,3,4-thiadiazole derivatives exhibiting anti-inflammatory activity. Pharmazie. 2001;56:617–619. [PubMed] [Google Scholar]
- 13.Palaska E., Sahin G., Kelincen P., Durlu N.T., Altionax G. Synthesis and anti-inflammatory activity of 1-acyl thiosemicarbazides, 1,3,4-oxadiazoles, 1,3,4-thiadiazoles and 1,2,4-triazole-3-thiones. Farmaco. 2002;57:101–107. doi: 10.1016/S0014-827X(01)01176-4. [DOI] [PubMed] [Google Scholar]
- 14.Sunel V., Lionte C., Popa M., Pintilie O., Mungiu P., Teleman S. Synthesis of new methionine derivatives for the treatment of paracetamol-inducet hepatic injury. Eur. Chem. Tech. J. 2002;4:201–205. [Google Scholar]
- 15.Pintilie O., Sunel V., Profire L., Pui A. Synthesis and antimicrobial activity of some new (sulfonamidophenyl)-amides of N-(metanitrobenzoyl)-D,L-methionine. Farmacia. 2007;55:345–251. [Google Scholar]
- 16.Moise M., Sunel V., Profire L., Popa M., Lionte C. Synthesis and antimicrobial activity of some new (sulfon-amidophenyl)-amide derivatives of N-(4-nitrobenzoyl)-phenylglycine and N-(4-nitrobenzoyl)-phenylalanine. Farmacia. 2008;56:283–289. [Google Scholar]
- 17.Sunel V., Lionte C., Basu C., Cheptea C. New antitumour alkylating compounds with N-[m-(arylthiocarbamoyl)-aminobenzoyl]-asparagic acids support. Chem. Indian J. 2005;2:1–6. [Google Scholar]
- 18.Sunel V., Popa M., Desbrieres J., Profire L., Pintilie O., Lionte C. New di-(β-chloroethyl)-α-amides on N-(m-acylaminobenzoyl)-D, L-aminoacid supports with antitumoral activity. Molecules. 2008;13:177–189. doi: 10.3390/molecules13010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaker R.M. The chemistry of mercapto- and thione- substituted 1,2,4-triazole and their utility in heterocyclic synthesis. ARKIVOC. 2006 (ix):59–112. [Google Scholar]
- 20.Serwar M., Akhtar T., Hameed S, Khan K.M. Synthesis, urease inhibition and antimicrobial activities of some chiral 5-aryl-4-(1-phenylpropyl)-2H-1,2,4-triazole-3(H)-thiones. ARKIVOC. 2009 (vii):59–112. [Google Scholar]
- 21.Whitehouse W. Anti-inflammatory agents. Volume 2. Academic Press; San Diego, CA, USA: 1974. pp. 210–221. [Google Scholar]
- 22.Seymar P.A., Larson D., Browne P. The effect of piroxicam and nystatin on locomotor activity in rats with adjuvants arthritis. Drug Dev. Res. 1986;7:165–172. doi: 10.1002/ddr.430070207. [DOI] [Google Scholar]
- 23.Selph L., Boncek V., Soroko E., Harris T., Cochran R. The pharmacologic evaluation of locomotor activity versus inflammatory parameters in rat adjuvant arthritis. Agents Actions. 1993;39:201–203. doi: 10.1007/BF01972766. [DOI] [PubMed] [Google Scholar]
- 24.Czajkowska T., Graczyk J., Krysiak B., Stetkiewicz J. Acute toxic effects of trimethyl and triethyl phosphates. J. Med. Pharm. 1978;29:393–398. [PubMed] [Google Scholar]
- 25.Hamilton M.A., Russo R.C., Thurston R.V. Trimmed Sperman Käber method for estimating median lethal concentrations in toxicological bioassays. Envir. Sci. Technol. 1978;12:417–422. [Google Scholar]
- 26.Niemegeers E., Awouters F., Lenacrts F.M., Janseen P.A. The activity of suprofen on nystatin-induced paw oedema in rats. Arzneim. Forsch. 1975;25:1516–1519. [PubMed] [Google Scholar]