Abstract
One new alkaloid; (+)-N-(2-hydroxypropyl)lindcarpine (1), together with four known aporphine alkaloids, (+)-boldine (2) (+)-norboldine (3), (+)-lindcarpine (4) and (+)-methyllindcarpine (5) were isolated from the stem bark of Actinodaphne pruinosa Nees (Lauraceae). (+)-N-(2-Hydroxypropyl)lindcarpine (1) exhibited cytotoxic activity against P-388 murine leukemia cells with an IC50 value of 3.9 μg/mL. Structural elucidation of all the compounds were performed by spectral methods such as 1D- and 2D- NMR, IR, UV, and HRESIMS.
Keywords: Actinodaphne pruinosa, Lauraceae, aporphine alkaloid, cytotoxic
Introduction
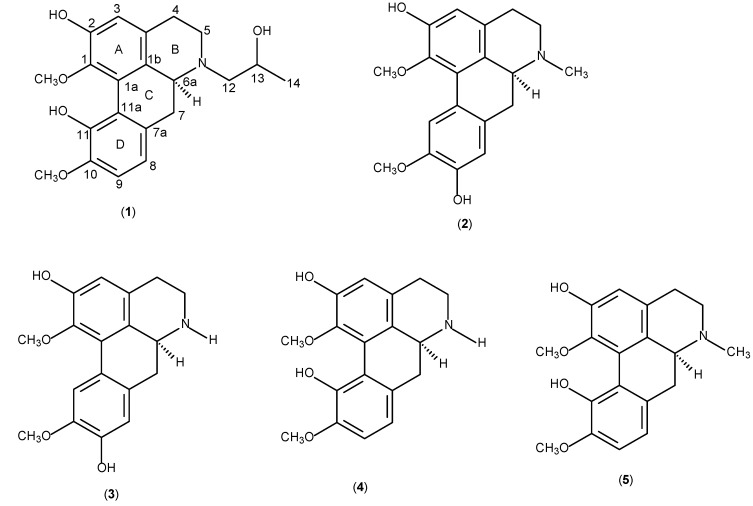
Actinodaphne pruinosa is a tree of moderate size (about 30-40 feet) found in Peninsular Malaysia and Jawa, Indonesia. Locally, Actinodapne is known as wuru (Indonesia) or medang kuning and medang kunyit (Malaysia) [1,2]. Actinodaphne plants of the family Lauraceae have been reported to produce isoquinoline alkaloids (aporphines, oxoaporphines) and lactones [3,4]. These alkaloids are of some pharmacological importance, as exemplified by liriodenine, an oxoaporphine, which was reported to have antitumor, antibacterial, and antifungal activities [5]. In addition, dicentrine, an aporphine, was known to have cytotoxic activity against P-388 murine cells [6]. In the present paper, the isolation and characterization of new aporphine; (+)-N-(2-hydroxypropyl)lindcarpine (1) is described. This alkaloid, together with four known alkaloids, (+)-boldine (2) [7], (+)-norboldine (3) [7], (+)-lindcarpine (4) [7,8] and (+)-methyllindcarpine (5) [8], were obtained from a CH2Cl2 extract of the stem bark of Actinodaphne pruinosa.
Results and Discussion
(+)-N-(2-Hydroxypropyl)lindcarpine (1; see Figure 1) exhibited a molecular formula of C21H26NO5 based on the HRESIMS spectrum (positive mode) which showed a pseudomolecular ion at m/z 372.1797 [M+H]+ (calcd. 372.1811, Δ-1.4 mmu). The IR spectrum revealed an absorption band at 3,180 cm-1 due to the OH stretching vibration. The overall physical properties and NMR spectral profile revealed its identity as a member of the aporphine group of isoquinoline, a characteristic and distinguishable chemical marker of Actinodaphne plants [3,4].
Figure 1.
Alkaloids 1- 5 isolated from Actinodaphne pruinosa.
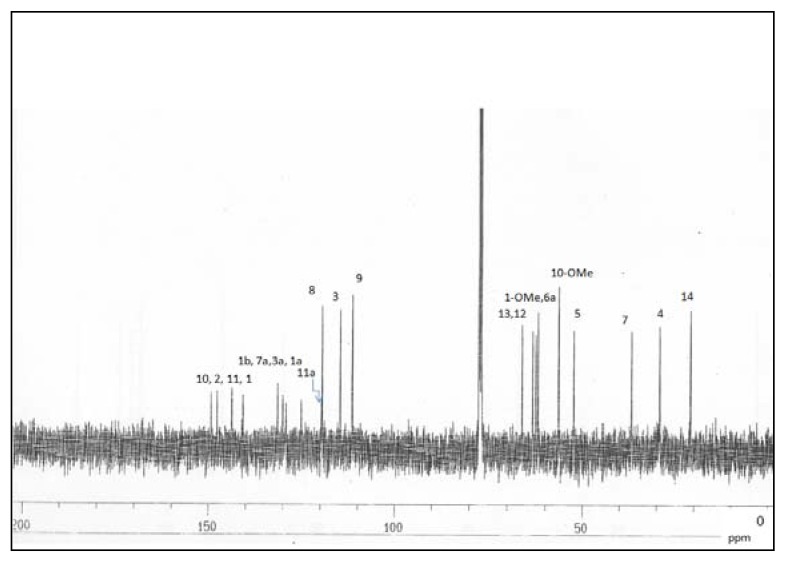
In the 1H-NMR spectrum (Table 1) the presence of a methyl group attached to -CH(OH)- signal at δ 1.22 (3H, d, J = 6.1 Hz); two methoxyl signals at δ 3.65 and δ 3.92; three aromatic protons at δ 6.79 (s, H-3), δ 6.84 (d, 8.0 Hz, H-8) and δ 6.86 (d, 8.0 Hz, H-9) were observed. The 13C-NMR (Table 1) and DEPT spectra, showed a total of 21 carbon signals; three methyls, four methylenes, one methine bearing hydroxyl group, four methines, and nine quaternary carbons in which four are aromatic oxygenated carbon signals.
Table 1.
1H-NMR (400 MHz) and 13C-NMR (100 MHz) spectral data of compound 1 in CDCl3 (δ in ppm, J in Hz).
| Position | δ 1H (Hz) | δ 13C | HMBC (2J, 3J) |
|---|---|---|---|
| 1 | 140.6 | ||
| 1a | 125.0 | ||
| 1b | 131.2 | ||
| 2 | 147.6 | ||
| 3 | 6.79 s | 114.0 | 1, 2, 3a, 4 |
| 3a | 129.2 | ||
| 4 | 2.67 m | 29.1 | |
| 2.71 m | 1b | ||
| 5 | 3.08 m | 52.3 | 12, 6a |
| 6a | 3.30 dd (13.1, 3.2) | 61.9 | 12 |
| 7 | 2.90 dd (13.1, 3.2) | 36.7 | 6a |
| 2.56 t (13.1) | |||
| 7a | 129.9 | ||
| 8 | 6.84 d (8.0) | 119.5 | 7, 11a, 10 |
| 9 | 6.86 d (8.0 | 111.4 | 7a, 11 |
| 10 | 149.2 | ||
| 11 | 143.2 | ||
| 11a | 119.6 | ||
| 12 | 2.80 m | 63.3 | 5, 6a |
| 2.38 dd (13.7, 9.0) |
5, 13 | ||
| 13 | 3.89 m | 66.1 | |
| 14 (Me) | 1.22 d (6.1) | 20.8 | 13 |
| 1-OMe | 3.65 s | 62.4 | 1 |
| 10-OMe | 3.92 s | 56.4 | 10 |
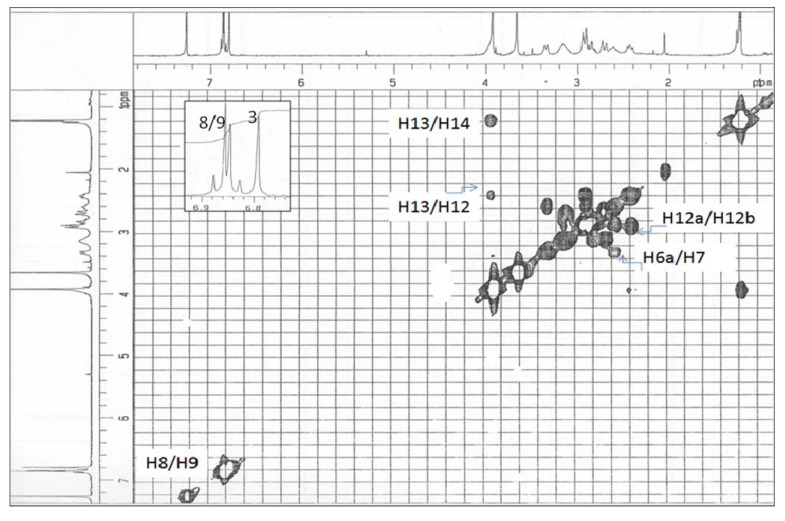
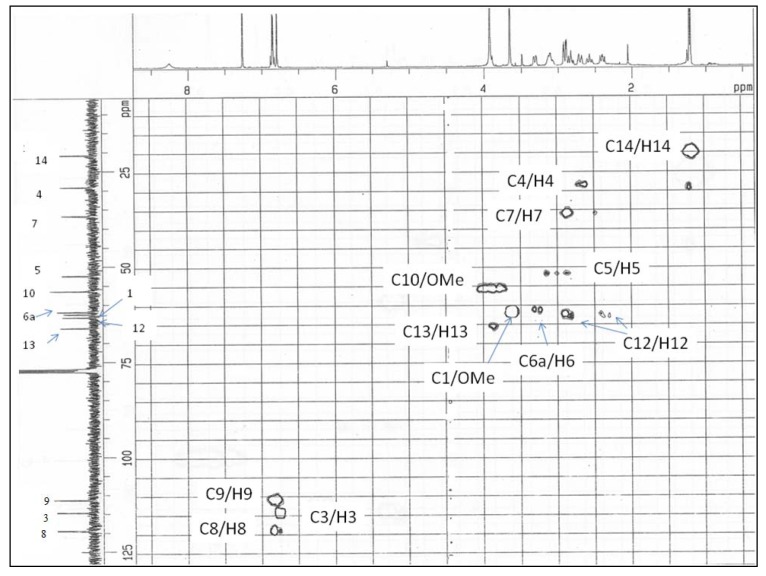
The complete 1H- and 13C-NMR (Figure 2) spectral assignment of 1 was accomplished by thorough analysis of DEPT, COSY (Figure 3), HMQC (Figure 4), and HMBC data. The 1H-1H COSY, combined with the HMQC spectrum revealed that 1 has the following partial structure: - CH2CH2 - (C4 and C5); -CHCH2- (C6a-C7); =CHCH= (C8-C9); -CH2CH- (C12-C13); and –CHCH3- (C13-C14). All of these segments were compatible for rings B, C, and D of a 1,2,9,10-tetrasubstituted aporphine type linked to –CH2CH(OH)CH3 unit. The HMBC spectrum of 1 provided conclusive evidence for the presence of the 2-hydroxypropyl chain unit. The HMBC spectrum showed cross peaks of H-3 with C1, C2, C3a and C4; H-12 with C5, C6a and C14; H-8 with C7, C11a and C-10; and H-9 with the C7a and C11.
Figure 2.
13C-NMR spectrum of alkaloid 1.
Figure 3.
COSY spectrum of alkaloid 1.
Figure 4.
HMQC spectrum of alkaloid 1.
The NOE differential measurements, showed enhancement of H-4 (δ 2.67) upon radiation of H-3 (δ 6.79). In addition the irradiation of H-9 showed enhancement of 10-OMe protons and H-8, suggesting that the methoxyl groups are placed at C-1 and C-10, respectively. The absolute configuration of the asymmetric carbon at C-13 was not determined due to the limited amount of compound available, so alkaloid 1 can be considered a racemic mixture.
To our knowledge, there has been no report on the phytochemical study and medicinal value of Actinodaphne pruinosa. This is the first report on the occurrence of N-(2-hydroxypropyl)aporphine type of alkaloid; (+)-N-(2-hydroxypropyl)lindcarpine (1) which exhibited significant cytotoxicity against P-388 murine leukemia cells.
Conclusions
In summary, we have observed that Actinodaphne pruinosa produces alkaloids closely related to those found in A. nitida, A. acutivena, A. abovata and A. sesquipedalis which were studied previously [4,9,10]. These species yielded noraporphine or N-methylaporphine types. The presence of N-(2-hydroxypropyl)aporphine type which significantly exhibited a potent cytotoxicity against P-388 [11] murine leukemia cells with IC50 3.9 μg/mL, suggesting its potential for further investigation as anti-cancer agent.
Experimental
General
The optical rotations were recorded on s Jasco (Japan) P1010 instrument equipped with a tungsten lamp. HRESIMS was obtained on a Thermo Finnigan Automass Multi. The ultraviolet spectra were obtained in MeOH on a Shimadzu UV-160A ultraviolet-visible spectrometer. The infrared spectra were recorded on a Perkin Elmer 1600 Double-Beam recording spectrometer, using chloroform as solvent. The 1H-NMR and 13C-NMR spectra were recorded in deuterated chloroform on a JEOL 400 MHz. Chemical shifts are reported in ppm on δ scale, and the coupling constants are given in Hz. Silica gel 60, 70-230 mesh ASTM (Merck 7734) and silica gel 60, 230-400 Mesh ASTM (Merck 9385) were used for column and flash chromatography, respectively. Mayer’s reagent was used for alkaloid screening.
Plant material
Stem bark of Actinodaphne pruinosa, collected at Bukit Bauk, Dungun, Terengganu, Malaysia, in May 2004 was identified by Mr. Teo Leong Eng. A voucher specimen (KL 5055) was deposited in the Herbarium of Department of Chemistry, University of Malaya, Malaysia and at the Herbarium of the Forest Research Institute, Kepong, Malaysia.
Extraction and isolation of the alkaloids
The dried stem bark of Actinodaphne pruinosa (2.0 kg) was ground and extracted exhaustively for 12 hours by Soxhlet extraction with hexane, followed by CH2Cl2. Extraction of alkaloids was carried out in the usual manner, which has been described in detail [12,13] and gave 43.0 g of crude alkaloid. The crude alkaloid was submitted to exhaustive column chromatography over silica gel using CH2Cl2 gradually enriched with methanol to yield 26 fractions. Fractions were combined on the basis of TLC behavior. Fractions 24-25 (3.0 g), afforded three alkaloids identified as (+)-N-(2-hydroxypropyl)-lindcarpine (1) (0.21%, PTLC; CH2Cl2-MeOH 98:2), (+)-lindcarpine (4) (1.51%, PTLC; CH2Cl2-MeOH 95:5), (+)-methyllindcarpine (5) (1.89%, PTLC; CH2Cl2-MeOH 97:3). Fraction 19-20 (1.3 g) produced alkaloid 2, identified as (+)-boldine (1.58%, PTLC; CH2Cl2-MeOH 95:5) [6]. (+)-Norboldine (3, 1.89%), was also separated by preparative TLC of fraction 26 (190 mg) over silica gel using CH2Cl2-MeOH; 95:5: saturated with NH4OH).
(+)-N-(2-Hydroxypropyl)lindcarpine (1, Figure 1): A colorless powder , [α]25D= + 120° (c = 0.02, MeOH), UV: λmethanol: 309 nm; IR νmax (KBr): 3,180, 3,014, 2,934, 2,360, 1,594 cm-1; HRESIMS (positive mode): m/z: 372.1797 [M+H]+ (Calcd. 372.1811, Δ-1.4 mmu for C21H26NO5); 1H-NMR (400 MHz, CDCl3) and 13C-NMR (100 MHz, CDCl3) see Table 1; Principle NOE’s (%) in CDCl3: OCH3-10 to H-9 (2.6%).
Cytotoxic assays
The cytotoxicity of (+)-N-(2-hydroxypropyl)lindcarpine (1) against P-388 murine leukemia cells was tested using MTT-microculture tetrazolim assay [11].
Acknowledgements
The authors thank the Malaysian Government through the vots: PPF/FP092/2005C, ScienceFund 12-02-03-2063 (UM) and the grant from Centre Nationale de la Recherche Scientifique (France).
Footnotes
Sample Availability: Samples of compounds 2-4 are available from the authors.
References
- 1.Burkill I.H. A Dictionary of the Economic Products of the Malay Peninsula. Ministry of Agriculture Malaysia; Kuala Lumpur, Malaysia: 1935. pp. 42–43. [Google Scholar]
- 2.Ridley H.N. The Flora of the Malay Peninsula. Reeve & Co. Ltd.; London, UK: 1924. pp. 107–112. [Google Scholar]
- 3.Kim M.R., Jung H.J., Min B.S., Oh S.R., Kim C.H., Ahn K.S., Kang W.S., Lee H.K. Constituents from the stems of Actinodaphne lancifolia. Phytochemistry. 2002;59:861–865. doi: 10.1016/S0031-9422(02)00018-3. [DOI] [PubMed] [Google Scholar]
- 4.Uprety H., Bhakuni D.S., Dhar M.M. Aporphine Alkaloids of Litsea Sebifera, L. Wightiana and Actinodaphne Obovata. Phytochemistry. 1972;11:3057–3059. doi: 10.1016/0031-9422(72)80105-5. [DOI] [Google Scholar]
- 5.Leboeuf M., Cavé A., Bhaumik P.K., Mukherjee B., Mukherjee R. The Phytochemistry of the Annonaceae. Phytochemistry. 1980;21:2783–2813. doi: 10.1016/0031-9422(80)85046-1. [DOI] [Google Scholar]
- 6.Kittisak L., Angerhofer C.K., Chai H., Pezzuto J.M., Cordell G.A., Ruangrungsi N. Cytotoxic and Antimalarial Alkaloids from the Tubers of Stephania pierrei. J. Nat. Prod. 1993;56:1468–1478. doi: 10.1021/np50099a005. [DOI] [PubMed] [Google Scholar]
- 7.Guinaudeau H., Leboeuf M., Cavé A. Aporphine alkaloids. Lloydia. 1975;38:275–288. [PubMed] [Google Scholar]
- 8.Shao W.S., Shoei S.L., Li Y.C., Chien K.C. Separation Of Aporphine Alkaloids By Micellar Electrokinetic Chromatography. J. Chromatogr. A. 1997;767:277–284. doi: 10.1016/S0021-9673(96)01093-X. [DOI] [Google Scholar]
- 9.Johns S.R., Lamberton J.A., Sioumis A.A. Alkaloids of Actinodaphne nitida (Lauraceae) Aust. J. Chem. 1969;22:2257–2257. doi: 10.1071/CH9692257. [DOI] [Google Scholar]
- 10.Lu S.T., Wang S.J., Lin F.S. Studies on the Alkaloids of Formosan Lauraceous Plants. XIV.: Alkaloids of Actinodaphne acutivena (Hayata) Nakai and Litsea hayatae. J. Pharm. Soc. Jap. 1969;89:1313–1317. doi: 10.1248/yakushi1947.89.9_1313. [DOI] [PubMed] [Google Scholar]
- 11.Alley M.C., Scudiero D.A., Monks A., Hursey M.L., Czerwinski M.J., Fine D.L., Abbott B. J., Mayo J.G., Shoemaker R.H., Boyd M.R. Feasibility of Drug Screening with Panels of Human Tumor Cell Lines Using a Microculture Tetrazolium Assay. Cancer Res. 1988;48:589–610. [PubMed] [Google Scholar]
- 12.Mukhtar M.R., Martin M.T., Domansky M., Pais M., Hadi A.H.A., Awang K. Phoebegrandines A and B, Proaporphine-Tryptamine Dimers, from Phoebe grandis. Phytochemistry. 1997;45:1543–1546. doi: 10.1016/S0031-9422(97)00189-1. [DOI] [Google Scholar]
- 13.Mukhtar M.R., Hadi A.H.A., Sevenet T., Martin MT., Awang K. Phoebegrandine C A Novel Proaporphine-Tryptamine Dimer from Phoebe grandis (Nees) Merr. Nat. Prod. Res. 2004;18:163–167. doi: 10.1080/14786410310001608082. [DOI] [PubMed] [Google Scholar]